In vivo analysis of Yorkie phosphorylation sites (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 30.
Published in final edited form as: Oncogene. 2009 Mar 30;28(17):1916–1927. doi: 10.1038/onc.2009.43
Abstract
The co-activator Yorkie mediates transcriptional regulation effected by the Drosophila Fat-Warts-Hippo pathways. Yorkie is inhibited by Warts-mediated phosphorylation, and a Warts phosphorylation site at Ser168 has been identified. Here we identify two additional Warts phosphorylation sites on Yorkie, and examine the respective contribution of all three sites to Yorkie nuclear localization and activity. Our results show that while Ser168 is the most critical site, all three phosphorylation sites influence Yorkie localization and activity in vivo, and can be sites of regulation by Warts. Thus investigations of the role of Yorkie and its mammalian homologue YAP in development and oncogenesis should include evaluations of additional sites. The WW domains of Yorkie are not required for its phosphorylation, but instead are positively required for its activity. We also identify two potential sites of phosphorylation by an unknown kinase, which could influence phosphorylation of Ser168 by Warts, suggesting that there are additional mechanisms for regulating Yorkie/YAP activity.
INTRODUCTION
Investigations of how growth is controlled during development in model systems have resulted in the identification of genes that can act as tumor suppressors or oncogenes in humans (reviewed in Vidal and Cagan, 2006). Fat-Hippo-Warts signaling comprises a set of genes that function within the interconnected Fat-Warts and Hippo-Warts pathways to regulate growth (reviewed in Reddy and Irvine, 2008). Mutation or dysregulation of these genes can result in tumor formation, both in Drosophila and in mammals (reviewed in Reddy and Irvine, 2008; Zeng and Hong, 2008). A transcriptional co-activator protein, called Yorkie (Yki) in Drosophila and Yes-Associated Protein (YAP) in mammals, acts as the key effector of Fat-Hippo-Warts signaling. YAP has been implicated as an oncogene in liver, mammary, and prostate tumors, and upstream regulators of YAP have been implicated as tumor suppressors in soft tissue sarcomas, ovarian tumors, neurofibromas, melanomas, and mammary carcinomas. Understanding the mechanisms that regulate Yki/YAP activity is thus important to a wide range of cancers.
Upstream components of Fat-Hippo-Warts signaling converge on a kinase, known as Warts in Drosophila and Lats1 and Lats2 in mammals (reviewed in Reddy and Irvine, 2008). Warts is made in an inactive form, and is activated by phosphorylation and association with a cofactor, Mob as tumor suppressor (Mats). Activation of Warts is promoted by another kinase, Hippo (Hpo), which phosphorylates Warts, Mats, and a scaffolding protein called Salvador (Sav). The activity of Hpo is promoted by two membrane-associated FERM domain proteins, Merlin and Expanded (Ex).
Yki/YAP is a non-DNA binding transcriptional co-activator, but interacts with the Sd protein in Drosophila, and homologues of Sd in mammals, the TEF/TEAD proteins (Goulev et al., 2008; Wu et al., 2008; Zhang et al., 2008; Zhao et al., 2008). Sd/TEAD provides DNA binding activity, and also promotes nuclear localization of unphosphorylated Yki/YAP. Investigations of the regulation of Yki and YAP by Warts/Lats identified a critical Ser residue, Ser168 of Yki (Ser127 of YAP) (Dong et al., 2007; Hao et al., 2008; Oh and Irvine, 2008; Zhang et al., 2008; Zhao et al., 2007). Phosphorylation of this Ser creates a binding site for 14-3-3 proteins (Basu et al., 2003; Dong et al., 2007; Oh and Irvine, 2008; Zhao et al., 2007), a class of proteins that anchor phosphoproteins in the cytoplasm (Mackintosh, 2004). Thus, Wts inhibits Yki activity at least in part by excluding it from the nucleus. Although Ser168 is a critical Wts phosphorylation site, several observations indicate that additional sites contribute to Yki regulation. Direct examination of Yki phosphorylation in vivo revealed that there are multiple Wts sites (Oh and Irvine, 2008). Similarly, studies of YAP phosphorylation in cultured cells identified multiple Lats sites (Hao et al., 2008; Zhao et al., 2007). Moreover, mutation of Yki Ser168 or YAP Ser127 reduced, but did not eliminate, their sensitivity to Wts/Lats (Hao et al., 2008; Oh and Irvine, 2008; Zhao et al., 2007). Studies in mammalian cells identified a consensus phosphorylation sequence, HX(R/H/K)XX(S/T), and mutation of all five consensus sites further reduced the sensitivity of YAP to Lats in cultured cell assays (Hao et al., 2008; Zhao et al., 2007). However, the respective contributions and mechanism of action of the additional sites have not been examined in detail. Here, we use cell culture and in vivo assays in Drosophila to characterize the contributions of all of the Wts phosphorylation sites to Yki activity, to evaluate the roles of the WW domains, and to identify a potential novel mechanisms of Yki regulation.
RESULTS
Ser111 and Ser250 of Yki are phosphorylated by Wts in S2 cells
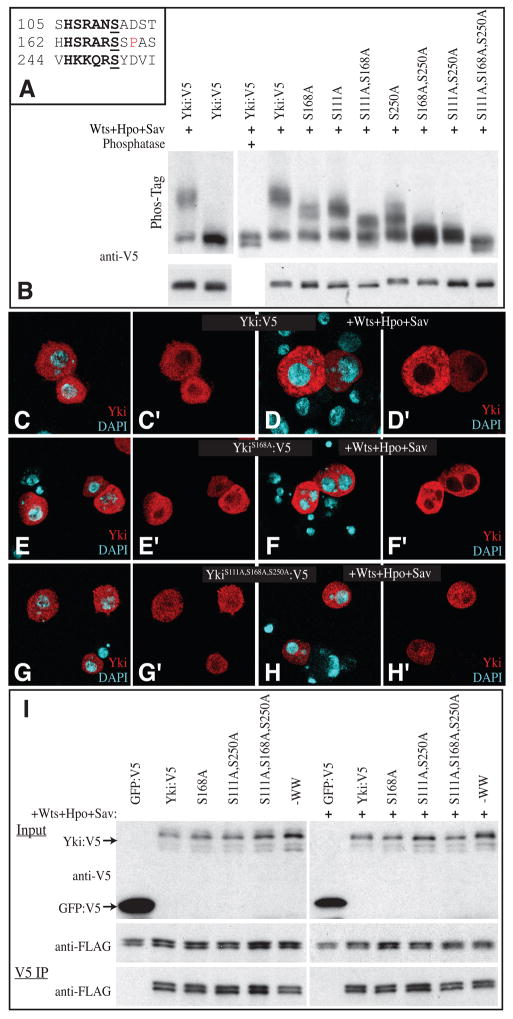
Drosophila Yki contains three potential Wts/Lats phosphorylation sites, at amino acids 111, 168 and 250 (Fig. 1A)(Hao et al., 2008; Zhao et al., 2007). Phosphorylation of Ser168 has been established (Dong et al., 2007; Oh and Irvine, 2008). To determine whether Ser111 and Ser250 are phosphorylated, Ser to Ala mutations were introduced, and the resulting Yki isoforms were expressed in cultured Drosophila S2 cells. Their mobility was then compared on Phos-tag gels, which incorporate a phosphate-binding compound that retards the mobility of phosphorylated proteins in proportion to their degree of phosphorylation (Kinoshita et al., 2006; Oh and Irvine, 2008). In the presence of exogenous Wts, together with its activators Hpo and Sav, Yki is phosphorylated. This can be visualized by a decrease in the mobility of an epitope-tagged form of Yki, Yki:V5, as compared to phosphatase treated Yki:V5, or Yki:V5 without exogenous, activated Wts (Fig. 1B). Single Ser to Ala mutations at consensus Wts phosphorylation sites (S111A, S168A and S250A) each resulted in less mobility shift compared to wild-type Yki:V5, although their mobilities were still retarded compared to phosphatase treated Yki:V5 (Fig. 1B). Thus, Ser111, Ser168, and Ser250 are all Wts sites.
Figure 1. Phosphorylation of Yki Ser111 and Ser250 in S2 cells.
(A) Amino acid sequence surrounding the three consensus Wts phosphorylation sites within Yki. The consensus sequence motif (HX(R/H/K)XX(S/T)) is in bold, the phosphorylation site is underlined, and a Pro that forms part of a 14-3-3 binding motifis in red. (B) Western blots (anti-V5) on lysates of S2 cells co-transfected to express Yki:V5, or a phosphorylation site mutant isoform, and, where indicated, Wts, Hpo and Sav to promote Yki phosphorylation. Upper panels show Phos-Tag gels (50 μM Phos-Tag); lower panels show 4–15% gradient gels. Phosphorylation results in mobility shifts proportional to the extent of phosphorylation, which are reversed by phosphatase treatment. (C–H) S2 cells, stained for Yki (red) and nuclei (DAPI, cyan) in the presence (D, F and H) or absence (C, E and G) of exogenous Hpo, Sav and Wts. Panels marked ‘ show individual channels of the stain on the left. (C, D) Wild type Yki:V5; (E–F) YkiS168A:V5; (G–H) YkiS111A,S168A,S250A:V5. (I) Western blots (4–15% gel) of samples from S2 cells co-transfected to express the indicated proteins. Top (Yki:V5 and GFP:V5) and middle (Sd:FLAG) panels shows input, bottom panel (Sd:FLAG) shows blot on material precipitated by anti-V5 beads. Faint bands detected below Yki:V5 in top panel are the result of incomplete stripping-off of Sd:FLAG.
Each of the possible pair-wise combinations of Ser to Ala mutations was also examined, and they exhibited a reduced shift compared to the single mutants (Fig. 1B). The mobility of the S111A, S168A S250A triple mutant was not retarded at all by co-expression of Hpo, Sav and Wts. A fraction of triple mutant Yki was detected in a faster mobility band on Phos-tag gels, which is also detected upon phosphatase treatment of wild-type Yki. Although the basis for this isoform has not yet been identified, the observation that the mobility of the Yki:V5S111A,S168A,S250A triple mutant is unaffected by co-expression with activated Wts, or by phosphatase treatment, implies that Ser111, Ser168, and Ser250 are the only Wts phosphorylation sites in Yki.
Nuclear localization of Yki is prevented through phosphorylation of Ser168 and subsequent interaction with 14-3-3 proteins (Basu et al., 2003; Dong et al., 2007; Oh and Irvine, 2008; Zhao et al., 2007). In S2 cells, wild-type Yki:V5 is predominantly cytoplasmic, but faint staining is detectable in the nucleus (Fig. 1C). Co-transfection of Hpo, Sav and Wts eliminated detectable nuclear staining of Yki:V5 (Fig. 1D). Yki:V5S168A exhibited slightly increased nuclear staining in S2 cells compared to wild type Yki:V5 (Fig. 1E), but in the presence of activated Wts, nuclear localization of Yki:V5S168A was almost undetectable (Fig. 1F), consistent with the observation that the activity of Yki:V5S168A can be inhibited by over-expression of Hpo and Wts in vivo (Oh and Irvine, 2008). The Yki:V5S111A S168A S250A triple mutant appears equally distributed between the cytoplasm and the nucleus (Fig. 1G). In the presence of Hpo, Sav and Wts, substantial nuclear localization of Yki:V5S111A S168A S250A remained (Fig. 1H). Thus, Ser111 and Ser250 contribute to the regulation of Yki localization.
Two mechanisms that influence the nuclear localization of Yki/YAP have been described. One is the binding to 14-3-3 proteins, the other is interaction with Sd/TEAD, which promotes nuclear localization (Goulev et al., 2008; Wu et al., 2008; Zhang et al., 2008; Zhao et al., 2008). To investigate whether phosophorylation of Ser residues in Yki might decrease nuclear localization by inhibiting binding to Sd, we preformed co-immunopreciptation experiments, but mutation of Ser168 had no effect on Sd-Yki binding, either in the presence or in the absence of activated Wts (Fig. 1I). The Yki:V5S111A S168A S250A triple mutant and Yki:V5S111A S250A double mutant also exhibited similar binding to Sd, regardless of the expression of exogenous Wts (Fig. 1I).
Ser111 and Ser250 influence Yki activity in vivo
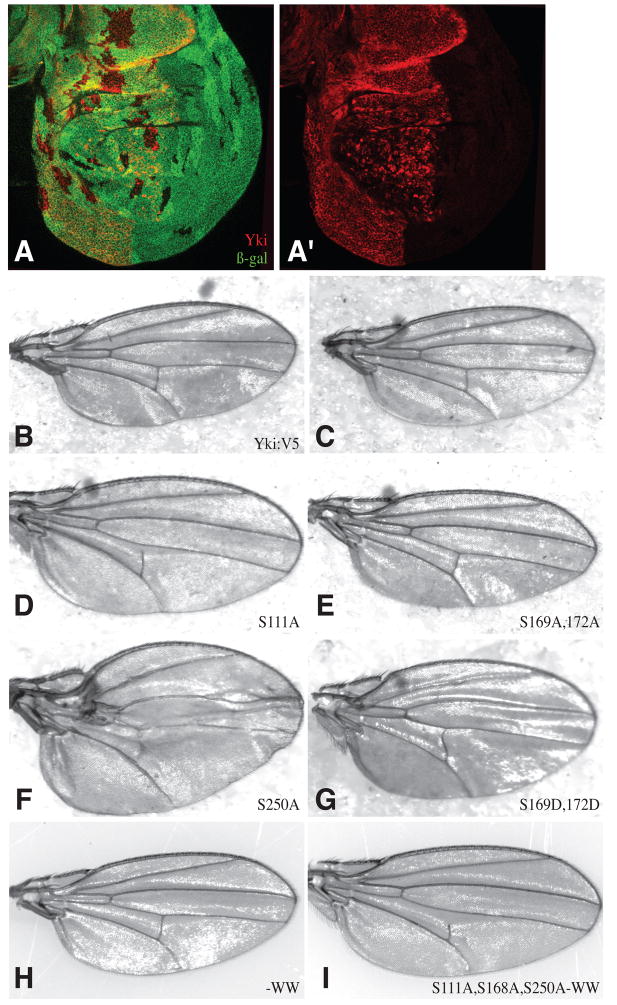
The influence of the S111A and S250A mutations on Yki in S2 cells suggested that Yki could be regulated via these sites in vivo. This was investigated by introducing mutant isoforms of Yki:V5 into a UAS-yki:V5 transgene. As different chromosomal insertions can result in different levels of expression, we used phiC31-mediated site-specific transformation to insert all of the UAS-yki:V5 transgenes into the same chromosomal location (attP2 at 68A) (Groth et al., 2004), such that their expression levels would be the same. The activity of wild-type UAS-yki:V5[attP68A] was confirmed by its ability to rescue the growth of ykiB5 null mutant clones in anterior cells when expressed under ci-Gal4 control (Fig. 2A), and to rescue ykiB5 mutant flies to adulthood when expressed ubiquitously under arm-Gal4 control.
Figure 2. Activity of Yki:V5 isoforms in the wing.
(A) Wing disc with ykiB5 mutant clones (marked by absence of lacZ, green) and homozygous wild-type clones (twin spots, bright green). In anterior cells, where Yki:V5 (red) is expressed under ci-Gal4 control these occur at similar size and frequency, but in posterior cells ykiB5 clones are rare and small. (B–G) Adult female wings from flies with sd-Gal4 driving expression of B) UAS-yki:V5, C) no UAS transgene, D) UAS-ykiS111A:V5, E) UAS-ykiS169A,S172A:V5, F) UAS-ykiS250A:V5 G) UAS-ykiS169DS172D:V5, H) UAS-yki-WW:V5 G) UAS-ykiS111A S168A S250A -WW:V5.
The activities of mutant forms of Yki were then compared by expressing them in eyes, using GMR-Gal4, or in wings, using sd-Gal4. Expression of wild-type Yki:V5 did not cause evident phenotypes (Figs 2B,3B), indicating that Yki expression, although above endogenous levels, was low enough to be repressed by endogenous upstream tumor suppressors. When Yki:V5 mutant proteins were expressed, overgrowth phenotypes and reduced viability were observed. The different isoforms could be arranged into a phenotypic series. Under GMR-Gal4 control, the relative phenotypic strength observed was wild type = S111A < S250A (increase in eye size) < S111A,S250A (greater increase in eye size, but eye mostly flat) < S168A (eye enlarged and folded due to extensive overgrowth) < S111A,S168A = S168A,S250A (eye greatly enlarged and folded, and viability reduced) < S111A, S168A,S250A (viability greatly reduced, eye enlarged and highly folded in surviving flies) (Fig. 3B-I). With sd-Gal4 the allelic series was wild type < S111A (slightly enlarged wings) < S250A (enlarged wings) < S111A,S250A (distorted wings and low viability) < S168A = S111A,S168A = S168A,S250A (extended third instar larval stage and eventual late larval lethality) < S111A, S168A,S250A (early larval lethality) (Fig. 2B–F and data not shown). The wing-specific driver vg-Gal4 was also lethal in combination with all of the Yki:V5 isoforms that included the S168A mutation.
Figure 3. Activity of Yki:V5 isoforms in the eye.
Adult female heads from flies with GMR-Gal4 and (A) no UAS transgene, B) UAS-yki:V5, C) _UAS_-ykiS111A:V5, D) _UAS_-ykiS250A:V5, E) _UAS_-ykiS111A S250A:V5, F) _UAS_-ykiS168A:V5, G) _UAS_-ykiS111A S168A:V5, H) _UAS_-ykiS168A S250A:V5, I) _UAS_-ykiS111A S168A S250A:V5, J) _UAS_-_yki:V5; tub_-Gal80 K) _UAS_-_ykiS168A:V5; tub_-Gal80 L) _UAS_-_ykiS111A S168A S250A:V5; tub_-Gal80, M) _UAS_-_YkiS111A:V5; UAS_-hpo, N) _UAS_-_ykiS250A:V5; UAS_-hpo, O) _UAS_-_ykiS111A S250A:V5; UAS_-hpo P) _UAS_-_ykiS111A S250A:V5; UAS_-_hpo, UAS_-wts Q) _UAS_-ykiS169A S172A:V5, R) _UAS_-ykiS169D S172D:V5, S) _UAS_-_ykiS169D S172D:V5; UAS_-hpo T) _UAS_-_ykiS169D S172D:V5; UAS_-_hpo, UAS_-wts U) _UAS_-_ykiS168A:V5; UAS_-_hpo UAS_-wts V) Side views comparing _UAS_-ykiS168A:V5 with _UAS_-_ykiS168A:V5; UAS_-_hpo UAS_-wts, W) _UAS_-_ykiS111A S168A S250A:V5; UAS_-_hpo UAS_-wts, X) _UAS_-ykiS111A,S168A,S250A -WW:V5.
Some of the mutants were only subtly different from each other, but we reasoned that over-expression might obscure differences in activity. Thus we reduced expression levels by co-expressing the Gal4 antagonist Gal80 (Lee and Luo, 2001). In animals containing a single copy of tub-Gal80 and two copies of GMR-Gal4, the S168A mutant exhibited only a subtle eye overgrowth (Fig 3K). However, S111A,S168A,S250A mutants exhibited obvious eye overgrowth (Fig. 3L). Thus, at these low expression levels, mutation of Ser168 alone only slightly enhances Yki activity. Moreover, at low levels of expression, mutation of Ser111 and Ser250 can make critical contributions to the regulation of Yki activity even when Ser168 is already mutant.
Yki Ser111 and Ser250 are sites of Warts regulation in vivo
To further explore the activities of Yki:V5 isoforms, they were co-expressed with Hpo, or Hpo and Wts together, under GMR-Gal4 control. This resulted in a phenotypic series in which wild type (pupal lethal with hpo or with hpo and wts) < S111A (very small eyes with hpo, lethal with hpo and wts) < S250A (small eyes with hpo, lethal with hpo and wts) < S111A,S250A (small eyes with hpo, very small eyes with hpo and wts) < S168A (greatly enlarged eyes with hpo, enlarged eyes with hpo and wts) < S111A,S168A = S168A,S250A (greatly enlarged eyes with hpo or with hpo and wts, but less so than in the absence of hpo and wts over-expression) < S111A, S168A,S250A (enlarged and highly folded eyes with hpo or hpo and wts, but viability increased compared to animals without hpo and wts over-expression) (Fig. 3M–W and data not shown). Thus, all three Wts phosphorylation sites can influence the sensitivity of Yki to Wts in vivo.
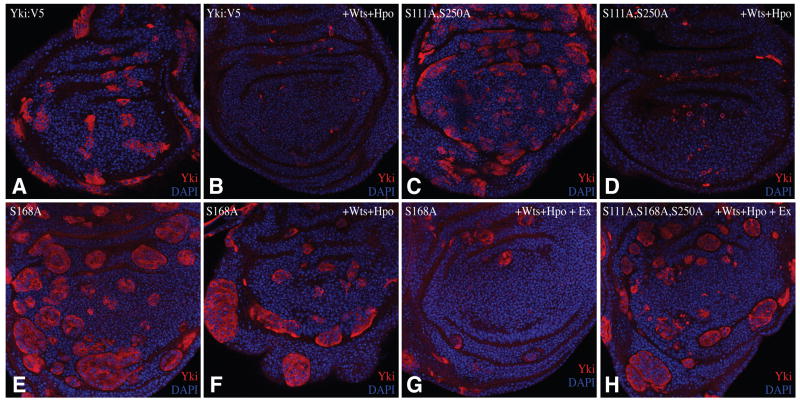
We observed previously that expression of Yki activated by mutation of Ser168 (Yki-S168A:GFP) under act-Gal4 control resulted in larger, rounder clones (Oh and Irvine, 2008). Co-over-expression of Hpo and Wts suppressed the growth of Yki-S168A:GFP-expressing clones. To examine whether this could be explained by phosphorylation of Ser111 and Ser250, Yki:V5 isoforms were expressed in clones of cells under act-Gal4 control in the presence or absence of Hpo and Wts. Yki:V5-expressing clones appeared to have wild-type size and shape (Fig. 4A), but Yki:V5S111A,S250 double mutant clones were rounder, consistent with the observation that mutation of these sites activates Yki (Fig. 4C). However, Yki:V5S111A,S250 clones didn’t survive well in the presence of Hpo and Wts (Fig. 4D), indicating that this double mutant can still be repressed by Wts. In contrast to our earlier results with Yki-S168A:GFP, when Yki:V5S168A was co-expressed with Hpo and Wts, large round clones were still observed (Fig. 4F). However, when another upstream tumor suppressor, Ex was also co-expressed with Hpo and Wts in Yki:V5S168A -expressing clones, the size and number of clones was decreased (Fig. 4G), indicating that the resistance of Yki:V5S168A to inactivation by upstream tumor suppressors can be overcome by increasing pathway activation. By contrast, cells expressing the Yki:V5S111A,S168A,S250A triple mutant made large round clones even in the presence of Ex, Hpo, and Wts (Fig. 4H). Thus, the Yki:V5S111A,S168A, S250A triple mutant is less sensitive to upstream tumor suppressors than Yki:V5S168A.
Figure 4. Influence of Wts on the growth of Yki:V5-expressing clones.
Wing imaginal discs with clones of cells expressing Yki:V5 isoforms under actin-Gal4 control, stained for Yki (red) and nuclei (DAPI, blue). A) UAS-yki:V5, (B) UAS-yki:V5; UAS-hpo UAS-wts, (C) UAS-ykiS111A S250A:V5, (D) UAS-ykiS111A S250A:V5; UAS-hpo, UAS-wts (E) UAS-ykiS168A:V5, (F) UAS-ykiS168A:V5; UAS-hpo UAS-wts (G) UAS-ykiS168A:V5; UAS-hpo UAS-wts UAS-ex, (H) UAS-ykiS111A S168A S250A:V5; UAS-hpo UAS-wts UAS-ex.
S111A and S250A mutations affect the nuclear localization of Yki in vivo
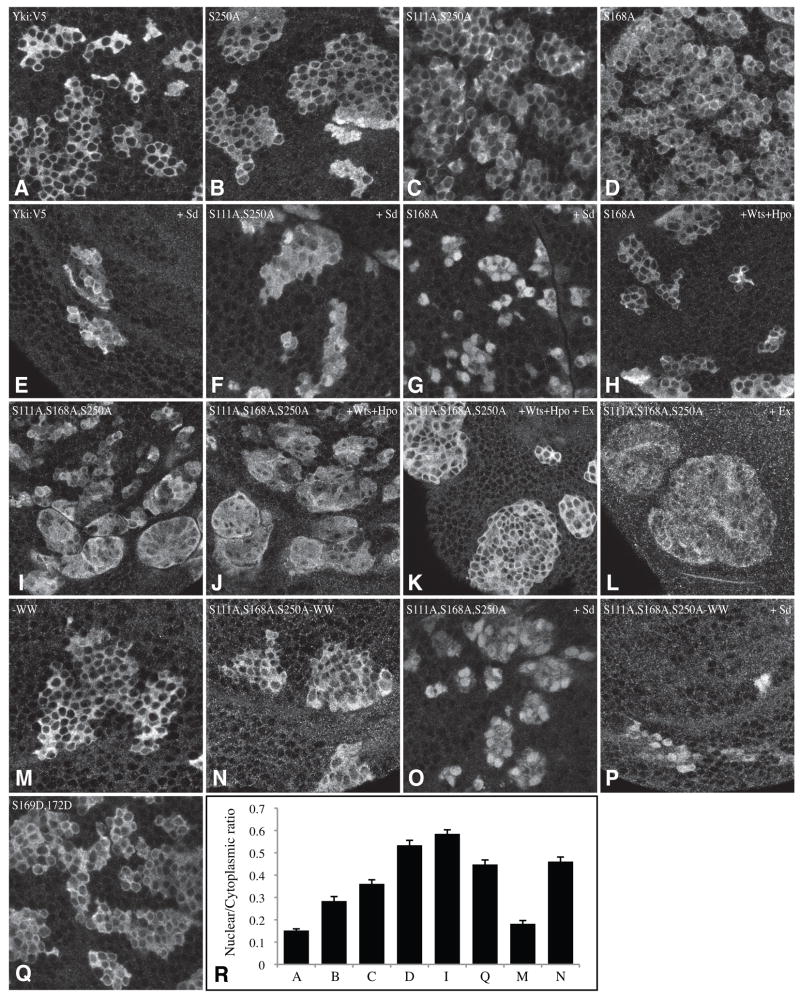
Previously, we observed that mutation of Ser168 resulted in a modest but consistent effect on the nuclear localization of Yki in imaginal disc cells (Oh and Irvine, 2008). The influence of Ser111 and Ser250 on Yki localization in imaginal discs was examined by expressing Yki:V5 mutant isoforms in clones. Yki:V5 and Yki:V5S111A were predominantly cytoplasmic (Fig. 5A and data not shown), with faint nuclear staining, similar to endogenous Yki or Yki:GFP (Oh and Irvine, 2008). Yki:V5S250A exhibited mildly enhanced nuclear localization (Fig. 5B), and the Yki:V5S111A,S250A double mutant exhibited still more nuclear localization (Fig. 5C), which suggests that its hyperactivity is caused by enhanced nuclear localization. Although the effect appears subtle, it was confirmed by quantitative image analysis (Fig. 5R). Enhanced nuclear localization of Yki:V5S111A,S250A was also evident when it was co-expressed with Sd (Fig. 5F). Nuclear localization of Yki:V5S168A was even greater than for Yki:V5S111A,S250A, both with and without Sd (Fig. 5D, G,R), indicating that Ser168 is the most important phosphorylation site for regulation of Yki localization.
Figure 5. Nuclear localization of Yki:V5 isoforms in vivo.
Close-ups of wing imaginal discs with clones of cells expressing Yki:V5 isoforms under actin-Gal4 control, stained for Yki (white). Not all nuclei are in the same focal plane, thus in some cases staining appears uniform because the optical section is above or below the nucleus for that cell. A) UAS-yki:V5, (B) UAS-ykiS250A:V5, (C) UAS-ykiS111A S250A:V5, (D) UAS-ykiS168A:V5, (E) UAS-yki:V5; UAS-sd, (F) UAS-ykiS111A S250A:V5; UAS-sd (G) UAS-ykiS168A:V5; UAS-sd (H) UAS-ykiS168A:V5; UAS-hpo UAS-wts (I) UAS-ykiS111A S168A S250A:V5, (J) UAS-ykiS111A S168A S250A:V5; UAS-hpo UAS-wts, (K) UAS-ykiS111A S168A S250A:V5; UAS-hpo UAS-wts UAS-ex, (L) UAS-ykiS111A S168A S250A:V5; UAS-ex, (M) UAS-yki-WW:V5, (N) UAS-ykiS111A S168A S250A -WW:V5 (O) UAS-ykiS111A S168A S250A:V5; UAS-sd, (P) UAS-ykiS111A S168A S250A -WW:V5; UAS-sd, (Q) UAS-ykiS169D S172D:V5. (R) Histogram showing nuclear/cytoplasmic ratio of fluorescent intensity (anti-Yki) in clones of cells expressing different UAS-Yki:V5 transgenes; the letters correspond to the genotypes as indicated by the figure and legend. Values depict the average of 30 measurements; error bars indicate s.e.m.
Even though the size and shape of Yki:V5S168A clones was not obviously affected by co-expression of Hpo and Wts, its nuclear localization was decreased (Fig. 5H). Thus, Yki:V5S168A expression is high enough that clone growth is not dramatically impaired by Hpo and Wts, but Yki:V5S168A protein is still affected. By contrast, Yki nuclear localization in clones expressing the Yki:V5S111A,S168A,S250A triple mutant with Hpo and Wts was similar to that in clones without Hpo and Wts (Fig. 5I,J). However, when Ex was also co-expressed together with Hpo and Wts, Yki:V5S111A,S168A, S250A nuclear localization appeared to decrease, although the clones still overgrow (Fig. 5K). Expression of Ex alone did not appear to affect Yki:V5S111A,S168A, S250A localization (Fig 5L). These results are consistent with the conclusion that the Yki:V5S111A,S168A,S250A triple mutant is less sensitive to the upstream tumor suppressors than Yki:V5S168A, and thus that phosphorylation at Ser111 and Ser250 by Wts can regulate Yki independently of Ser168. They further suggest that there might be an additional mechanism by which Ex, Hpo, and Wts can inhibit Yki. This additional mechanism doesn’t involve Yki phosphorylation, as it could affect the Yki triple mutant, yet expression of Ex alone or in combination with Wts and Hpo didn’t detectably affect Yki:V5S111A,S168A,S250A phosphorylation (Supplementary Fig. S1).
Phosphorylation of Ser111 and Ser250 influences phosphorylation of Ser168
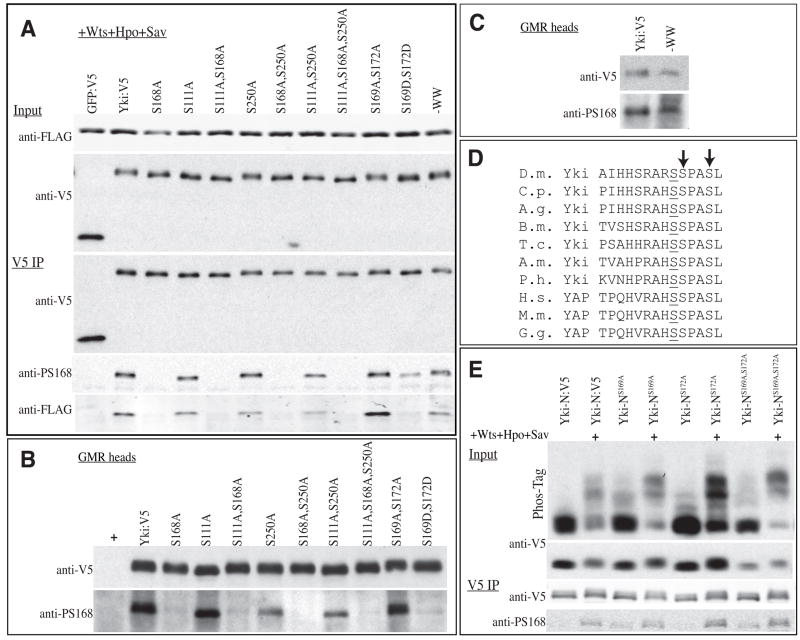
The detection of three Wts phosphorylation sites within Yki raises the question of whether or not they are each regulated independently. To investigate this, we employed a phospho-specific antibody that detects Ser168 phosphorylation (Dong et al., 2007). In S2 cells with exogenous Hpo, Sav and Wts, the level of Ser168 phosphorylation was slightly decreased for Yki:V5S111A, Yki:V5S250A, or Yki:V5S111A,S250A mutants compared to wild-type Yki:V5 (Fig. 6A). Consistent with this, the association between Yki:V5 isoforms and 14-3-3 proteins was also reduced (Fig. 6A). To characterize the influence of S111A and S250A mutations in vivo, we examined Ser168 phosphorylation in extracts of fly head from animals in which Yki mutant isoforms were expressed under GMR-Gal4 control. Decreased Ser168 phosphorylation was clearly observed in Yki:V5S111A,S250A and Yki:V5S250A mutants (Fig. 6B). The effect was even more obvious than in S2 cells, possibly due to limiting levels of endogenous upstream tumor suppressors. These results imply that Wts phosphorylation of Yki is cooperative. They also imply that the hyperactivity of S111A and S250A yki mutants in vivo might be partly due to deceased phosphorylation of Ser168. Nonetheless, the comparisons of S168A single mutants versus double and triple mutants indicate that phosphorylation of Ser111 and Ser250 can also influence Yki activity independently from their effect on Ser168.
Figure 6. Influence of other phosphorylation sites on Ser168 phosphorylation.
A) Western blots (4–15% gels) of samples from S2 cells co-transfected to express 14-3-3ε:FLAG, 14-3-3ζ:FLAG, Wts, Hpo, Sav, and GFP:V5 or Yki:V5 isoforms. Top two panels show (Input) show blots on cell lysate, using anti-FLAG (14-3-3) or anti-V5 (GFP or Yki) and bottom three panels (V5 IP) show blots on material precipitated by anti-V5 beads. 14-3-3ε andζ have similar mobilities and were not separated. (B,C) Western blots on lysates of adult heads from animals expressing UAS-yki:V5 isoforms under GMR-Gal4 control. Top and bottom panels show the same membrane, blotted with anti-V5 and anti-Yki-S168P, respectively. (D) Amino acid sequence alignment illustrating conservation of Ser residues at +1 and +4 positions (arrows) relative to Yki-Ser168. D.m.: Drosophila melanogaster, C.p.: Culex pipiens (mosquito), A.g.: Aedes aegypti (mosquito), B.m.: Bombyx mori (silkworm), T.c.: Tribolium castaneum (flour beetle), A.m.: Apis mellifera (honey bee), P.h.: Pediculus Humanus corporis (human body louse), H.s.: Homo sapiens, M.m.: Mus musculus, G.g.: Gallus gallus (chicken). (E) Western blots of samples from S2 cells co-transfected to express Yki-N (N-terminal 240 amino acids of Yki) isoforms, and, where indicated, Wts, Hpo, and Sav. Top two panels (Input) show blots on cell lysates, using anti-V5 (Yki), uppermost panel is a Phos-tag gel (25 μM Phos-tag) and below is a 4–15 % SDS-PAGE gel. Bottom two panels (V5 IP) show blots on material precipitated by anti-V5 beads, and run on 4–15% gels, using anti-V5 or anti-Yki-S168P, as indicated.
Role of the WW domains in Yki activity
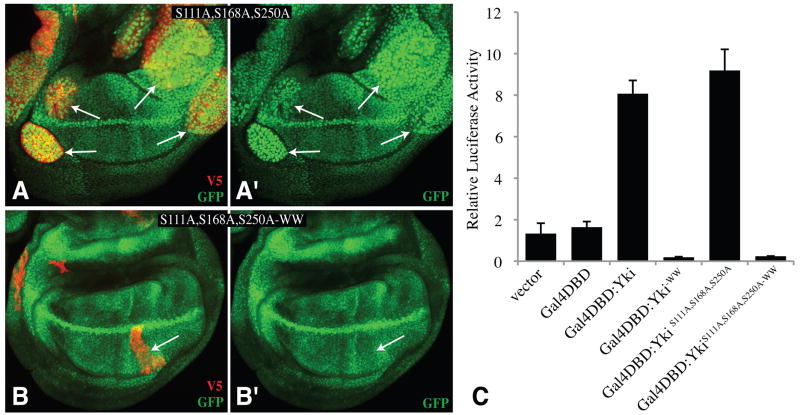
Yki was first identified as a Wts-binding protein, and it associates with Wts through a WW domain PPXY motif interaction (Huang et al., 2005). However, a fragment of Yki that lacks the WW domains (Yki-N) was still phosphorylated by Wts (Oh and Irvine, 2008). To evaluate the role of the WW domains, four point mutations, which abolish interaction with Wts (Huang et al., 2005), were introduced into Yki:V5 (Yki:V5-WW). Yki:V5-WW was still phosphorylated by Wts, both in S2 cells (Fig. 6A), and in vivo (Fig. 6C), and over-expression of Yki:V5-WW did not promote overgrowth (Fig. 2H), as would have been expected if phosphorylation were impaired. Instead, the WW domains are positively required for Yki activity, because Yki:V5-WW expressed under arm-Gal4 control could not rescue ykiB5 mutants, and when the WW mutations were introduced into the S111A,S168A,S250A triple mutant (Yki:V5S111A,S168A,S250A-WW), no overgrowth was induced by expression under GMR-Gal4 control (Fig. 3X), and only a very mild overgrowth was induced under sd-Gal4 control (Fig. 2I). The WW domains are not required for interaction with Sd (Fig. 1I). Indeed, when Yki:V5S111A,S168A,S250A-WW was co-expressed with Sd, it was predominantly nuclear (Fig. 5P), but inactive, as clones were irregular and small. The inactivity of Yki isoforms with WW domain mutations was also evidenced by the inability of Yki:V5S111A,S168A,S250A-WW to promote the transcription of a Yki-responsive reporter construct (Zhang et al., 2008) in imaginal discs (Fig. 7B). Moreover, mutation of the WW domains rendered Yki inactive in a Sd-independent cell-based transcriptional assay involving fusion of Yki to the DNA binding domain of Gal4 (Huang et al., 2005) (Fig. 7C). These observations suggest that the WW domains are required for interaction with an unidentified nuclear co-factor that contributes to the activation of downstream genes.
Figure 7. Requirement for WW domains for Yki activity.
A,B) Wing imaginal discs expressing the Diap1-GFP3.5 reporter gene (Zhang et al., 2008)(green) and with clones of cells expressing YkiS111A S168A S250A:V5 (A) or YkiS111A S168A S250A-WW:V5 (B), marked by expression of V5 (red). Panels marked prime show only the GFP chennel; arrows point to examples of clones. (C) Histogram showing the relative transcriptional activity of a UAS-luciferase reporter in S2 cells co-transfected to express the Gal4 DNA-binding domain (Gal4DBD) fused to the indicated Yki proteins.
Neighboring Ser residues influence Ser168 phosphorylation
While examining potential phosphorylation sites within Yki, we discovered that the presence of Ser residues at +1 and +4 positions relative to Ser168 (e.g., Ser169 and Ser172 of Drosophila Yki) is conserved among Yki and YAP proteins (Fig. 6D). These Ser residues do not conform to consensus Wts phosphorylation sites, and the lack of Wts-mediated phosphorylation in the Yki:V5S111A,S168A,S250A mutant implies that they are not Wts sites. However, because of their proximity to Ser168, and the possibility that they might be phosphorylated by other kinases, we examined their influence on Ser168 phosphorylation. To facilitate specific examination of Ser168, we first focused on Yki-N, as it has better resolution on Phos-tag gels than full length Yki, and only includes 2 of the three Wts sites. Yki-N:V5, with S169A, S172A or S169A S172A mutations was transfected into S2 cells in the presence and absence of additional Wts, Sav and Hpo. Interestingly, even without exogenous Wts, Sav and Hpo, a small fraction of the Yki-N-S169A, S172A double or single mutant proteins exhibited a mobility shift on Phos-tag gels (Fig. 6E), implying that Yki-N-S169A, S172A is a better substrate for phosphorylation by endogenous kinases in S2 cells than is wild-type Yki-N. Increased phosphorylation was also detected in the presence of Wts, Hpo, and Sav, and was also detected using anti-phospho-Ser168 antisera (Fig. 6E).
To further investigate this, we introduced phosphomimetic mutations (Ser to Asp) at Ser169 and 172. In S2 cells transfected with Wts, Sav, Hpo, and full length Yki:V5, phosphorylation at Ser168 was decreased in a Yki:V5S169D,S172D mutant (Fig. 6A). Increased phosphorylation of Yki:V5S169A,S172A was not obvious, but because this assay relies on detecting a stronger band, as opposed to a novel band, it is not as sensitive as the Phos-tag gel assay. Co-IP experiments suggested that 14-3-3 proteins had increased affinity for Yki:V5S169A,S172A, and decreased affinity for Yki:V5S169D,S172D, consistent with phosphorylation changes at Ser168 (Fig. 6A).
To evaluate the activities of Yki:V5S169A,S172A and Yki:V5S169D,S172D in vivo, we generated transgenic flies, using the attP2(68A) insertion site. Similar to wild type, Yki:V5S169A,S172A didn’t exhibit phenotypes when expressed under sd-Gal4 or GMR-Gal4 control, and resulted in irregular shaped clones with almost no detectible nuclear localization (Figs 2E,3Q, and data not shown). Yki:V5S169A,S172A had some activity, as it rescued the growth of ykiB5 mutant clones when expressed under ci-Gal4 control (not shown). However, Yki:V5S169A,S172A expressed under arm-Gal4 control wasn’t able to rescue the lethality of ykiB5 mutant flies. Moreover, co-expression of Yki:V5S169A,S172A with UAS-hpo in eyes at relatively low levels (under GMR-Gal4 control, but at 18 °C, which lowers expression levels) results in lethality, whereas flies expressing wild-type Yki:V5 survived. These results suggest that Yki:V5S169A,S172A is less active than wild-type Yki:V5. By contrast, Yki:V5S169D,S172D exhibited mild overgrowths when expressed under sd-Gal4 or GMR-Gal4 control (Fig. 2G, 3R), partial resistance to Hpo over-expression (Fig. 3S,T), and increased nuclear localization (Fig. 5Q,R). The severity of the overgrowth phenotypes placed it in between the Yki:V5S111A and Yki:V5S250A mutants, implying that phosphorylation of Ser168 is reduced, but not abolished. Direct examination using phospho-specific antibody revealed that phosphorylation of Ser168 is reduced on Yki:V5S169D,S172D in vivo (Fig. 6B). Thus, it is likely that if phosphorylation of Ser169 and 172 occurred, it would reduce Ser168 phosphorylation, thereby promoting Yki activity.
DISCUSSION
Prior studies identified multiple sites of Wts/Lats phosphorylation on Yki and YAP. Most attention has focused on a conserved site at Yki-Ser127/YAP-Ser168, which, when phosphorylated, forms a binding site for 14-3-3 proteins (Dong et al., 2007; Oh and Irvine, 2008; Zhao et al., 2007). However, regulation of Yki/YAP by Wts/Lats cannot be fully accounted for by Ser127/YAP-Ser168 phosphorylation (Hao et al., 2008; Oh and Irvine, 2008; Zhao et al., 2007). Mutation of the five consensus Lats phosphorylation sites within YAP had stronger effects on YAP localization and transcriptional activity in cultured mammalian cells than mutation of Ser127 alone (Hao et al., 2008; Zhao et al., 2007), but the respective contributions of the different sites were not analyzed in detail. The results described here extend our understanding of Yki regulation in Drosophila by biochemically identifying all of the Wts phosphorylations sites within Yki, characterizing the respective contributions of each of these sites to Yki regulation, and providing insights into the mechanism by which phosphorylation sites regulate Yki activity. In addition, we have determined that the critical role of the WW domains in Yki is not to promote Yki phosphorylation, but rather to bind another factor that is positively required for Yki activity. A number of transcription factors contain PPXY motifs, including some that interact with YAP in mammalian cells, but further studies will be required to identify the relevant Drosophila WW-domain interacting Yki partner(s).
Taking all of the observations on Wts site mutants together, a consistent allelic series emerged in which the relative activity of different Yki isoforms was wild type < S111A < S250A < S111A,S250A < S168A < S111A,S168A = S168A,S250A< S111A,S168A,S250A. Phosphorylation of Ser111 and Ser250 clearly influence Wts activity, since mutation of Ser111 and Ser250, alone or in combination, hyperactivates Yki and makes Yki partially resistant to over-expression and activation of Wts, both when Ser168 is wild-type and when it is not. At the same time, the different Wts phosphorylation sites don’t contribute equally – Ser 168 has the greatest influence. The observations that the double mutant combinations have stronger phenotypes than single mutant combinations, and that the triple mutant combination has the strongest phenotype, indicate that the three sites act additively.
All three sites influence the subcellular localization of Yki. Since either S111A, S250A double mutants, or S168A mutants, are predominantly cytoplasmic when there is sufficient active Wts, these sites can act independently to retain Yki in the cytoplasm. The amino acid sequence around Ser168 conforms to a consensus 14-3-3 binding site (RXXSpXP), and binding between Yki and 14-3-3 proteins that depends on phospho-Ser168 has been established (Dong et al., 2007; Oh and Irvine, 2008). The Wts phosphorylation sites at Ser111 and Ser250 don’t conform to consensus 14-3-3 binding sites. A few exceptional cases of 14-3-3 binding to divergent sequences have been reported (reviewed in Fu et al., 2000), but the requirement for Ser168 in 14-3-3 binding experiments (Dong et al., 2007; Oh and Irvine, 2008) implies that Ser111 and Ser250 do not mediate binding to 14-3-3 proteins. Thus, it is possible that phosphorylated Ser111 and Ser250 bind to some other cytoplasmic anchoring protein. Alternatively, it might be that Ser111 and Ser250 bind to 14-3-3 proteins, but with too low an affinity to be detected by co-immunoprecipitation experiments.
We also observed an unexpected influence of Ser250 and Ser111 on phosphorylation of Ser168. This suggests a model in which phosphorylation occurs in a complex that includes Yki, scaffolding proteins (e.g. 14-3-3), and Wts, such that binding to the scaffold enhances further phosphorylation of Yki by Wts. This observation has implications for Yki/YAP dependent oncogenesis, as it provides an additional mechanism by which mutations at secondary sites could influence Yki/YAP activity, and implies that the effects of such mutations could be detectable through an influence on Yki-Ser168/YAP-Ser127 phosphorylation. Nonetheless, our studies indicate that Ser250 and Ser111 can also act independently to influence Yki. It is also worth emphasizing that the level of phosphorylation at Ser168 is higher in S111A, S250A double mutants than in S169D, S172D double mutants, but S111A, S250A mutants have more severe overgrowth phenotypes. This further supports the conclusion that Ser111 and Ser250 can regulate Yki independently from their effect on Ser168 phosphorylation.
Our results emphasize that both the levels of Yki/YAP expression, and its phosphorylation state, are critical. When wild-type Yki is over-expressed, unless this over-expression is at very high levels, endogeneous tumor suppressors can keep it in check. Conversely, most assays for oncogenic mutations in Yki/YAP have involved high level over-expression, but when an activating mutation was present (Ser168A) and expression was kept low by Gal80 co-expression, then the effects were mild; strong overgrowth required either additional mutations or higher level expression. In this regard, it is interesting that over-expression of YAP and/or amplification of the YAP locus has been identified in many cancers (Steinhardt et al., 2008; Zender et al., 2006; Zeng and Hong, 2008). Thus, over-expression of YAP appears to act in concert with decreased phosphorylation of YAP (whether through mutations in YAP itself, or upstream tumor suppressors) to effect oncogenic transformation.
Finally, our results have raised the possibility of additional regulatory inputs into Yki/YAP phosphorylation. The presence of Ser residues at +1 and +4 positions relative to Yki-Ser168/YAP-Ser127 is highly conserved. Moreover, mutation of this site to a non-phosphorylatable amino acid was associated with a decrease in Yki activity, whereas mutation to a phosphomimetic amino acid was associated with Yki hyperactivity and decreased Ser168 phosphorylation. While confirmation of the existence and importance of phosphorylation at these sites awaits the actual identification of relevant kinases, the discovery of these sites emphasizes that there may be additional regulatory inputs into Yki/YAP regulation, with implications for growth control both during normal development and oncogenesis.
Supplementary Material
supplementary
Acknowledgments
We thank P. Beachy, D. Pan, J. Jiang, G. Halder and the Bloomington Drosophila Stock Center for antibodies, plasmids, and Drosophila stocks, and C. Rauskolb for comments on the manuscript. This research was supported by the Howard Hughes Medical Institute and NIH postdoctoral fellowship 1F32GM079817 to H.O.
Footnotes
MATERIALS AND METHODS are in Supplemental Material
References
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–47. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–41. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–82. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor Suppressor LATS1 Is a Negative Regulator of Oncogene YAP. J Biol Chem. 2008;283:5496–509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5:749–57. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J. 2004;381:329–42. doi: 10.1042/BJ20031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–8. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–38. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008 doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Cagan RL. Drosophila models for cancer research. Curr Opin Genet Dev. 2006;16:10–6. doi: 10.1016/j.gde.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–98. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–92. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–87. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary