Cumulative Deficits Better Characterize Susceptibility to Death in the Elderly than Phenotypic Frailty: Lessons from the Cardiovascular Health Study (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 29.
Abstract
OBJECTIVES:
To compare how well frailty measures based on a phenotypic frailty approach proposed in the Cardiovascular Health Study (CHS) and a cumulative deficits approach predict mortality.
DESIGN:
Cohort study.
SETTING:
The main cohort of the Cardiovascular Health Study (CHS).
PARTICIPANTS:
4721 individuals.
MEASUREMENTS:
A phenotypic frailty index (PFI) was defined in the same way as proposed in the CHS: assessing weight loss, exhaustion, low physical activity, slowness, and poor grip strength. A cumulative deficit index (DI) was defined based on 48 elderly deficits (signs, symptoms, impairments, diseases) included in the index, with equal weights.
RESULTS:
Of the 1,073 frailest individuals with the lowest survival, the PFI, categorized as proposed in the CHS into robust, prefrail, and frail categories, underestimated the risk of death for 720 persons, whereas the DI categorized into the same three frailty categories underestimated the mortality risk for 134 persons. The higher power of the DI for discriminating frail individuals in their susceptibility to death also followed from comparison of quasi-instantaneous values of both indices. The three-level DI identified 219 individuals as frail of 361 individuals identified as frail according to the three-level PFI.
CONCLUSIONS:
The DI can more precisely evaluate chances of death because it assesses a broader spectrum of disorders than the PFI. Both indices appear to be frailty related. Integration of both approaches is highly promising for increasing the precision of discrimination of the risk of death and especially for identification of the most vulnerable elderly people.
Keywords: Frailty, mortality, aging and well-being, health, survival
INTRODUCTION
Frailty appears to be an important aspect of human senescence. Exploring the frailty phenomenon might provide further insights on the aging-associated processes and survival [1-6]. Frailty is typically viewed as a physiological state that results from general decline of organism's reserves and deregulation of multiple physiologic systems. Frail individuals are believed to have increased non-specific vulnerability and are more susceptible to various adverse health outcomes including death, disability, and hospitalization [7-12]. The operational definition of frailty, nevertheless, remains controversial [13-16]. A variety of approaches have been suggested to identify frail individuals [17]. Few of them have been directly tested [18-21].
One wide-spread approach, proposed by Fried and colleagues [12], is to distinguish phenotypic frailty as a clinical syndrome, i.e. a set of signs and symptoms that tend to occur together thus characterizing a specific medical condition. The phenotypic frailty definition rests on selected indicators of physical frailty, i.e., unintentional weight loss, exhaustion, weakened grip strength, slow walking, and low physical activity. It is believed that the physical frailty appears due to physiological aging (basic cause) and a disease (serving as a risk factor) and results in inability to cope with everyday stresses of life and, thus, in increased vulnerability to adverse health outcomes [12,14,16].
Another wide-spread approach, proposed by Rockwood and Mitnitski and colleagues [22,23], rests on the proposition that frailty is a non-specific multifactorial state which is better characterized by the quantity rather than quality of health/well-being disorders (called deficits) accumulated by individuals during their life course (e.g., signs, symptoms, impairments, abnormal lab tests, diseases). This approach was validated in different populations and ethnic and cultural groups [2,8,24]. According to the latter approach, frailty also reflects the impact of physiological aging and results in increased vulnerability to adverse health outcomes including death. Diseases (or, more generally, deficits), however, are considered as non-specific and equally weighted markers of frailty rather than its risk factors. To better understand the rationale behind this (at a first glance oversimplified) approach, let us assume that the impact of physiological aging/senescence on the risk of death overcomes the impact of a particular disease or disorder that might be the case at advanced ages. Indeed, while the total and some cause-specific (particularly acute) mortality risks continue to increase among the oldest old, the relative risks of deaths due to particular causes (e.g., fractures, heart disease, cancer, etc.) seem to decline (see, e.g., [25-28]). That is, the aging-associated increase in mortality may happen regardless of specificity of health disorders yet be accompanied by increasing number of deficits (of diverse nature) in individuals.
Recently, these two approaches to operationalize frailty were tested directly using data from the Canadian Study of Health and Aging [19,21]. These studies showed that phenotypic frailty discriminates broader levels of risks of adverse health outcomes than the accumulated deficits approach. The risk of mortality for the very elderly individuals can be significantly characterized by the accumulated deficits approach while the phenotypic frailty measure might be non-significant [21]. Phenotypic frailty can be, however, readily operationalized for clinical purposes, while the cumulative deficits approach requires clinical translation. The basic limitations of those studies were that: i) they were focused on the database which did not replicate entirely the same measures as those used by Fried and colleagues for their frailty definition, and ii) they did not quantify predictive power of adverse health outcomes of these approaches.
This study addresses both of these issues. Specifically, it is focused on the original definition of the phenotypic frailty, as was introduced by Fried and colleagues [12], and employs the same dataset; the main cohort of the Cardiovascular Health Study (CHS). A direct quantitative comparison of both approaches is performed in order to elucidate which of the two approaches is better suited for predicting mortality outcomes in the CHS dataset.
METHODS
The CHS is a population-based, longitudinal study of risk factors for the development and progression of heart disease and stroke in the Medicare-eligible older individuals aged 65+ years at enrollment [29]. The main cohort of 5201 study participants was examined annually from 1989 through 1999. Deaths were ascertained through surveillance and at semi-annual contacts [12]. Components of the annual examinations included physical function (disability, upper extremity score, grip strength), medical history (e.g., vision and hearing problems, heart problems, hypertension, diabetes), neurological history, behaviors (e.g., cigarette, alcohol), physical exercise, cognition, depression, prescription medication use, electrocardiograms, physiological markers (blood pressure, cholesterol), etc. Focus of this study is on the baseline examination of the main cohort of the CHS and on the participants' survival.
Phenotypic frailty (PF) and the phenotypic frailty index (PFI)
The PFI was defined using five criteria for weight loss, exhaustion, low activity, slowness, and grip strength following Fried and colleagues [12]. It was argued [12] that, to properly evaluate frailty, individuals with a history of certain conditions should be excluded from the analysis. Consequently, to follow this logic, we excluded individuals with stroke (N=196), those who used non-tricyclic (N=46) or tri- and tetra-cyclic (N=142) antidepressants, and those with Mini-Mental scores below 18 (N=37) at baseline examination as well as those who did not provide this information (N=12). Excluding also individuals who did not authorize data release (N=76), a total sample comprises 4721 individuals. Note that in [12] individuals with history of Parkinson's disease and those who were taking Sinemet or Aricept were also excluded. Those persons in this analysis were not excluded since this information was not available in the public release file of the CHS data.
To ensure that the results of the analyses are not sensitive to exclusions of individuals with the selected health conditions, the analyses reported below were replicated on the full main CHS cohort sample, excluding only individuals without permission to release the data (N=5125). The results of this analysis did not alter the estimates for the adjusted cohort sample. Thus, the results reported below concern analyses of the adjusted sample (N=4721).
Table 1 shows percentage of individuals with given PF components and the number of positive criteria for the PF. The original measure of the PF is defined in [12] on the basis of counts of the PF components with positive criteria for frailty. This definition will be re-examined in Section “Results”. For later convenience, the phenotypic frailty index (PFI) is defined using a count of the PF components and rescaling it to the unit interval (i.e., 0→0, 1→0.2, …, 5→1).
Table 1.
Prevalence of components of phenotypic frailty in the main cohort (N=4721) of the Cardiovascular Health Study
| Frequency of phenotypic frailty components | % (% of missing values) |
|---|---|
| Weight loss | 4.5 (6.5) |
| Exhaustion | 16.1 (0.2) |
| Low physical activity | 19.7 (0.3) |
| Slow walk | 28.8 (1.3) |
| Grip strength | 21.7 (7.2) |
| The number of positive criteria for the phenotypic frailty | % |
| 0 | 42.5 |
| 1 | 33.4 |
| 2 | 16.5 |
| 3 | 6.1 |
| 4 | 1.4 |
| 5 | 0.1 |
Since there is relatively large number of missing values for two of five PF components, a test of whether missing information could alter the results was performed. To do this the same methodology as that used to define the index of cumulative deficits (DI) below was, first, considered. Specifically, the PFI is defined as
number of the positive criterianumber of the positive criteria+number of the negative criteria.
This approach is equivalent to the main definition above, except missing answers for the denominator were excluded by counting only non-missing criteria. Second, analyses were performed for the sub-sample of the selected sample explicitly excluding all individuals for whom information on any PF component was missing. Finally, the results obtained by using sample means as imputed values for missing observations were tested. In all these analyses, similar results were obtained and, thus, we use only the main definition of the PFI considering individuals with 3+ non-missing PF components, i.e., considering situations as in [12]. Accordingly, the PFI ranges between 0 and 1 with 0.2 increments.
Index of cumulative deficits (DI)
The conceptual framework behind the DI can be summarized in a simplified scheme in which the individual's vulnerability state can be characterized by a proportion of failed units out of a large number, N, of such units (subsystems). The failure of each unit is associated with a “deficit”. The proportion of deficits accumulated by age x characterizes individual's health/well-being status and affects chances of occurring further health-related events and mortality risk. Note that the data often do not allow for observing failures of the all N units. Therefore, an empirical estimate of this proportion in a given individual, i.e., the DI(x), can be calculated by selecting a set of M units out of a list with N such units, summing the number of failed units from the selected set M up to age x, m(x), and calculating an empirical estimate as _DI(x)_=m(x)/M. Prior studies suggest that the properties of the DI are weakly sensitive to the choice of a subset M [30]. Therefore, following these arguments, the DI is defined as a count of the number of deficits divided by the total number of all potential deficits considered for a person [3,22-24]. Consequently, it theoretically ranges between 0 and 1. Since large number of deficits is considered, this index is quasi-continuous.
To construct the DI, sets of 48, 52, and 60 deficits were considered. Since the results were qualitatively similar, for the final presentation a set of 48 deficits was retained. They are: pulmonary diseases; nervous/emotional disorder; high blood pressure; hearing problems; vision problems; heart disease; diabetes; arthritis; cancer; difficulty walking; feeling about life; life satisfaction; people to talk when lonely; walking for exercise; household chores; mowing lawn; raking lawn; gardening; exercise cycle; dancing; calisthenics exercises; pulmonary embolus; sleep on 2+ pillows to help breathe; awakened by trouble breathing; swelling of feet/ankles; pain in leg; pneumonia; asthma; cough; short of breath; palpitations; dizziness; fatigue; weakness; nausea; indigestion; diarrhea; groggy; trouble falling asleep; walking 1/2 mile, 10 steps; difficulties lifting, reaching out, gripping; bleeding; problems staying; hypotension, and major ECG abnormality.
Analysis
Relative risks of death were calculated using the Cox regression model in which time in the survey was used as a time scale. The CHS data also provide an indicator of vital status. First, the analysis was performed to verify how predictive of death each of the PFI components was within short (3 and 4 years) and longer (7 and 11 years) follow-up periods. Then, comparative analyses of the predictive power of the DI and PFI were performed using Cox regression that included each of the indices separately (“univariate”) and both of them together (“multivariate”). Next, a composite measure constructed on the basis of these indices (as defined in Section “Results”) was explored. To balance the follow-up period and the number of deceased persons, a 4-year follow-up was selected for the latter two analyses. The models in all analyses were adjusted for sex and age.
RESULTS
Fried and colleagues defined three frailty phenotypes: robust (no positive criteria for frailty), pre-frail (1-2 positive criteria), and frail (3+ positive criteria) arguing that groups “… with three components positive for frailty had significantly worse survival than those with two components, or the “no frailty” groups…” To check whether these arguments hold, a Cox regression analysis of the predictive power of the PFI was performed. This analysis shows that, indeed, three components of the PFI predict death significantly better than either one or two for the short follow-up periods (Table 2). Moreover, the risk for the three PFI components increases nonlinearly (e.g., the increase is more than two-fold for three components and about 1.4-fold for two components for the short follow-up) suggesting that 3+ positive components can capture a clinical syndrome of frailty. The predictive power, however, decreases for longer follow-ups (that is in agreement with frailty conceptualization when frail individuals die sooner). Therefore, three groups of individuals corresponding to the original PF categorization were selected, i.e., robust (PFI=0), pre-frail (0 < PFI ≤ 0.4), and frail (PFI>0.4). Further analyses were focused on shorter (4 years) follow-up periods. Since the DI is quasi-continuous, appropriate stratification for the DI can also be performed.
Table 2.
Relative Risk of Death According to the Number of Phenotypic Frailty Components
| Follow-up period, yrs | The number of phenotypic frailty components | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 3 | 1.93 (1.38, 2.69) | 2.71 (1.88, 3.89) | 5.78 (3.90, 8.56) | 6.15 (3.31, 11.4) | -- |
| 4 | 1.89 (1.45, 2.46) | 2.52 (1.88, 3.37) | 4.59 (3.30, 6.39) | 5.63 (3.39, 9.37) | 12.9 (4.01, 41.5) |
| 7 | 1.53 (1.29, 1.81) | 2.14 (1.77, 2.57) | 2.95 (2.34, 3.72) | 3.92 (2.73, 5.64) | 4.63 (1.47, 14.6) |
| 11 | 1.52 (1.33, 1.73) | 1.96 (1.69, 2.28) | 2.74 (2.27, 3.31) | 3.47 (2.55, 4.74) | 2.42 (0.77, 7.57) |
To directly compare how predictive of death the PFI and DI are, the two strategies are used. One is suggested by the nature of the DI. Specifically, the PFI is considered as a quasi-continuous (not ordinal) measure in the regression analyses. The other is suggested by the PFI categorization, i.e., the DI can be similarly categorized according to different levels of risks.
Since the PFI is rescaled to the unit interval, its effect can be directly compared to that due to the DI following the first strategy. For this analysis, both indices were expressed in percentages to calculate risks attributable to a 1% increase in the respective index. Each of the indices predicts the risk of death quite well. For the 4-year follow-up, the Relative Risk (RR) for the DI is RRDI=1.049; 95% Confidence Interval [CI]=1.040-1.057 and for the PFI RRPFI=1.022; CI=1.018-1.026 when considering them in separate analyses. Comparative analysis (i.e., when both indices are included into the same regression model) shows that the DI significantly better predicts death than the PFI, i.e., RRDI=1.035; CI=1.026-1.045 vs. RRPFI=1.014; CI=1.009-1.019. The same conclusion follows considering integrated risks attributable to each index. Specifically, while theoretically the DI ranges from 0 to 100%, its actual maximum (excluding outliers) in these data is about 70% (which seems to be sample independent [4,31]). Then, approximating the integrated risk for a 70% increment for the DI and for a full 100% increment for the PFI by evaluation of the product of 1%-risks, the former is obtained to be _e_70×(1.035−1) / _e_100×(1.014−1) ≈ 2.9 times larger than the latter. Direct evaluation of the hazards rates for a 70% increment for the DI and for a full 100% increment for the PFI in the alternative Cox regression analysis (i.e., considering the DI and PFI as variables with 70% and 100% increments, respectively) provides far more convincing results: RRDI=30.9; CI=11.9-80.4 vs. RRPFI=4.25; CI=2.59-6.98.
For the second strategy, the DI has to be categorized into three levels in a similar way as was the PFI. Since such a categorization is not unique, the following strategy is adopted. A preliminary categorization is performed arbitrarily. Then it was refined in order to have the same estimates of the relative risks when the Cox regression includes both the 3-level PFI (PFI3) and 3-level DI (DI3). This procedure yields the estimates shown in Table 3 (see column “multivariate”). Table 3 (column “univariate”) shows also the respective risks in the univariate analysis for comparison. The DI categorization under these conditions is: robust (0≤ DI ≤ 0.2), pre-frail (0.2 < _DI_ ≤ 0.35), and frail (_DI_ > 0.35). According to this categorization and the death risk, the DI identifies 1331 robust, 2451 pre-frail, and 939 frail individuals while the PFI identifies 2008 robust, 2352 pre-frail, and 361 frail individuals.
Table 3.
Relative Risk of Death According to the Three-level Deficit Index (DI3) and Three-Level Phenotypic Frailty Index (PFI3)
| Measure | Condition | Univariate | Multivariate |
|---|---|---|---|
| PFI3 | pre-frail | 2.08 (1.63, 2.66) | 1.67 (1.29, 2.15) |
| frail | 4.81 (3.53, 6.55) | 3.00 (2.15, 4.19) | |
| DI3 | pre-frail | 1.94 (1.45, 2.61) | 1.66 (1.23, 2.25) |
| frail | 4.45 (3.26, 6.08) | 3.07 (2.20, 4.28) |
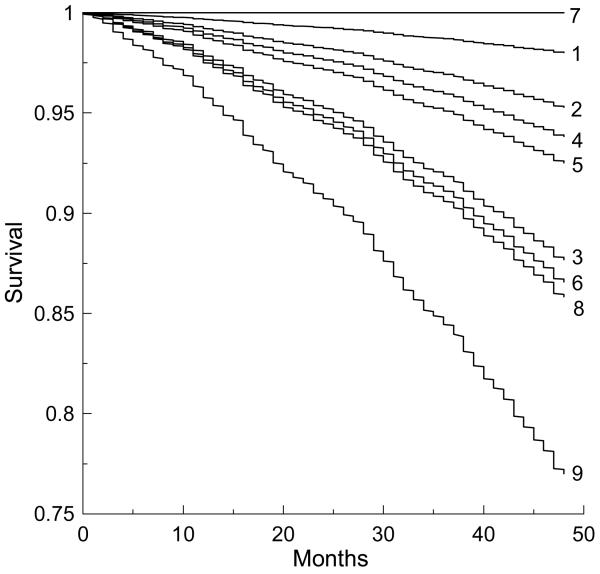
Stratifying the sample by categories of the PFI3 and DI3, 9 sub-groups were selected (Table 4). Figure 1 shows survival functions smoothed for each sub-group by the Cox regression. Individuals who were recognized as frail by both the phenotypic frailty and deficit definitions (Table 4; N=219) have the lowest survival prospects and die faster than those in the other sub-groups. For sub-groups 3, 6, and 8, survival is nearly the same. Sub-group 8 is recognized as frail by the PFI and as pre-frail by the DI (Table 4; N=134). Individuals in this sub-group, however, have the same risk of dying as in groups 3 (robust by the PFI; N=135) and 6 (pre-frail by the PFI; N=585). Thus, the PFI underestimates risks of mortality for 585+135=720 individuals, while the DI underestimates the risk for 134 persons. The PFI also recognizes 8 persons (sub-group 7) as frail, while all of them stay alive during at least 4 years and are recognized as robust by the DI.
Table 4.
Cross-tabulation of the total number of individuals in each sub-group defined according to the same-risk 3-level categorization of the Deficit Index and Phenotypic Frailty measure (see Table 3) along with the number of individuals dying and surviving within 4-year follow-up period.
| Phenotypic Frailty categories | Factor | Deficit Index categories | ||
|---|---|---|---|---|
| Robust | Pre-frail | Frail | ||
| Sub-group | 1 | 2 | 3 | |
| Robust | Alive | 838 | 961 | 117 |
| Dead | 21 | 53 | 18 | |
| Total | 859 | 1014 | 135 | |
| Sub-group | 4 | 5 | 6 | |
| Pre-frail | Alive | 427 | 1178 | 506 |
| Dead | 37 | 125 | 79 | |
| Total | 464 | 1303 | 585 | |
| Sub-group | 7 | 8 | 9 | |
| Frail | Alive | 8 | 107 | 158 |
| Dead | 0 | 27 | 61 | |
| Total | 8 | 134 | 219 |
Figure 1.
Survival curves smoothed by Cox regression for each of the 9 selected sub-groups defined on the basis of categorization of the phenotypic frailty index (PFI) and deficit index (DI) into 3 categories (see Table 4) as robust, pre-frail, and frail: 1) PFI3robust and DI3robust; 2) PFI3robust and DI3pre-frail; 3) PFI3robust and DI3frail; 4) PFI3pre-frail and DI3robust; 5) PFI3pre-frail and DI3pre-frail; 6) PFI3pre-frail and DI3frail; 7) PFI3frail and DI3robust; 8) PFI3frail and DI3pre-frail; and 9) PFI3frail and DI3frail.
DISCUSSION AND CONCLUSIONS
The phenotypic frailty and cumulative deficits approaches to characterize frailty were evaluated to elucidate which of them can better predict death of the elderly individuals. The first approach is based on the Fried's and colleagues' original definition of the phenotypic frailty [12]. The second approach is based on the index of cumulative deficits [3-6,22-24]. Since the analyses were performed using the CHS dataset, which was originally used by Fried and colleagues, the definition of the phenotypic frailty used in this analysis is exactly the same as proposed in [12].
The PFI has clear advantages for clinical operationalization, since only five substantive characteristics for each person are considered. This is also a weak point of this measure since this considerably restricts its flexibility. Specifically, current analyses show that for the proposed scale of robust, pre-frail, and frail phenotypes [12], the PFI underestimates the chances of death for 720 persons, while the DI does so for 134 persons under the same categorization (Table 4). Obviously, the DI can be categorized more finely to more precisely evaluate chances of death. The lower power of the PFI to discriminate frail individuals susceptible to death follows also from comparing the quasi-instantaneous values of the DI and PFI.
The deficit index identifies 219 individuals as frail out of 361 individuals recognized as frail by the phenotypic frailty definition (Table 4). This fact along with possible connection of the PFI with frailty syndrome (see Table 2) indicates that the DI is also frailty-related.
Figure 1 suggests that integration of both approaches is highly promising for increasing precision of the risk discrimination, especially among the most vulnerable part of the elderly. This means that from the health and well-being history of an individual, the survival chances of elderly individuals can be evaluated more precisely by using both a measure of health/well-being and a more specific measure. This statement seems to be intuitively clear, especially for clinicians, but it has not been formally stated and demonstrated. The DI gives a reasonable alternative for operationalizing this intuitive understanding by providing an appropriate measure of health/well-being and linking it to the aging-associated processes in an organism [3,5,24]. Possible disadvantage of the DI associated with problems with its clinical translation is mitigated by wide-spread informational technologies used in clinical practice. From these, the whole-life health and well-being history of an individual can be readily made available to the clinicians. Given also existing attempts to elaborate such surrogate measures of health and well-being for certain types of patients (e.g., health-related quality of life for cancer patients [32]), it seems that standardization of such cumulative measures becomes an emerging and realistic issue.
ACKNOWLEDGEMENTS
Funding sources: The research reported in this paper was supported by the grants 1R01 AG028259, 1R01-AG-027019 and 5P01-AG-008761 from the National Institute on Aging.
Sponsor's Role: Sponsor institution (NIA) did not played a direct role in the study design, conducts, management, data analysis, review, or authorization for submission.
Footnotes
Financial Disclosure: The research reported in this paper was supported by the grants 1R01 AG028259, 1R01-AG-027019 and 5P01-AG-008761 from the National Institute on Aging (NIA).
Conflict of Interest Disclosures: Each co-author asserts no proprietary interest in the results and no financial conflict of interest as shown in the table.
| Elements of Financial/Personal Conflicts | Author 1 | Author 2 | Author 3 | Etc. | |||
|---|---|---|---|---|---|---|---|
| AMK | SVU | IVK | KGA, KL, AIY | ||||
| Yes | No | Yes | No | Yes | No | Yes | No |
| Employment or Affiliation | X | X | X | X | |||
| Grants/Funds | X | X | X | X | |||
| Honoraria | X | X | X | X | |||
| Speaker Forum | X | X | X | X | |||
| Consultant | X | X | X | X | |||
| Stocks | X | X | X | X | |||
| Royalties | X | X | X | X | |||
| Expert Testimony | X | X | X | X | |||
| Board Member | X | X | X | X | |||
| Patents | X | X | X | X | |||
| Personal Relationship | X | X | X | X |
REFERENCES
- 1.Mitnitski A, Graham J, Mogilner A, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 3.Kulminski A, Yashin A, Ukraintseva S, Akushevich I, Arbeev K, Land K, et al. Accumulation of health disorders as a systemic measure of aging: Findings from the NLTCS data. Mech Ageing Dev. 2006;127:840–848. doi: 10.1016/j.mad.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007;55:935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Health decline, aging and mortality: how are they related? Biogerontology. 2007 doi: 10.1007/s10522-006-9073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity. Rejuvenation Res. 2007;10:75–86. doi: 10.1089/rej.2006.0500. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Guralnik JM, Cavazzini C, Bandinelli S, Lauretani F, Bartali B, et al. The frailty syndrome: a critical issue in geriatric oncology. Crit Rev Oncol Hematol. 2003;46:127–137. doi: 10.1016/s1040-8428(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 8.Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 9.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 11.Woo J, Goggins W, Sham A, Ho SC. Public health significance of the frailty index. Disabil Rehabil. 2006;28:515–521. doi: 10.1080/09638280500215867. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AL. Just what defines frailty? J Am Geriatr Soc. 2005;53:2229–2230. doi: 10.1111/j.1532-5415.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 15.Lally F, Crome P. Understanding frailty. Postgrad Med J. 2007;83:16–20. doi: 10.1136/pgmj.2006.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levers MJ, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs. 2006;56:282–291. doi: 10.1111/j.1365-2648.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 20.Chin APMJ, Dekker JM, Feskens EJ, Schouten EG, Kromhout D. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol. 1999;52:1015–1021. doi: 10.1016/s0895-4356(99)00077-3. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood K. Abeysundera MJ, Mitnitski A: How should we grade frailty in nursing home patients? J Am Med Dir Assoc. 2007;8:595–603. doi: 10.1016/j.jamda.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Mitnitski A, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59:M627–632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 23.Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev. 2004;125:517–519. doi: 10.1016/j.mad.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, et al. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: Results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007;128:250–258. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 26.Forsen L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 27.Richmond J, Aharonoff GB, Zuckerman JD, Koval KJ. Mortality risk after hip fracture. J Orthop Trauma. 2003;17:53–56. doi: 10.1097/00005131-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Horiuchi S, Wilmoth JR. Age patterns of the life table aging rate for major causes of death in Japan, 1951-1990. J Gerontol A Biol Sci Med Sci. 1997;52:B67–77. doi: 10.1093/gerona/52a.1.b67. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 30.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–496. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]