Folate receptor alpha as a tumor target in epithelial ovarian cancer (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 9.
Published in final edited form as: Gynecol Oncol. 2008 Jan 28;108(3):619–626. doi: 10.1016/j.ygyno.2007.11.020
Abstract
Objectives
Folate receptor α (FRα) is a folate-binding protein overexpressed on ovarian and several other epithelial malignancies that can be used as a target for imaging and therapeutic strategies. The goal of this study is to improve historical data that lack specific information about FRα expression in rare histological subtypes, primary disease versus metastatic foci, and recurrent disease.
Methods
FRα expression was analyzed by immunohistochemistry on 186 primary and 27 recurrent ovarian tumors, including 24 pairs of samples obtained from the same individuals at diagnosis and at secondary debulking surgery. For 20 of the 186 primaries, simultaneous metastatic foci were also analyzed. FRα staining was analyzed in light of disease morphology, stage, grade, debulking status, and time from diagnosis to recurrence and death.
Results
FRα expression was apparent in 134 of 186 (72%) primary and 22 of 27 (81.5%) recurrent ovarian tumors. In 21 of 24 (87.5%) matched specimens, recurrent tumors reflected the FRα status detected at diagnosis. Metastatic foci were similar to primary tumors in FRα staining. FRα status was not associated with time to recurrence or overall survival in either univariate or multivariable analyses.
Conclusion
FRα expression occurs frequently, especially in the common high-grade, high-stage serous tumors that are most likely to recur. New findings from this study show that FRα expression is maintained on metastatic foci and recurrent tumors, suggesting that novel folate-targeted therapies may hold promise for the majority of women with either newly diagnosed or recurrent ovarian cancer.
Keywords: Folate receptor, Ovarian cancer
Introduction
The folate receptor alpha (FRα) is a glycosylphosphatidyl-inositol-linked protein that is overexpressed in several epithelial malignancies, including ovarian, renal, lung, and breast cancers [1]. While the function of FRα in tumors is unclear, in the kidney it serves as a high-affinity salvage receptor that retrieves folate from the filtrate and returns it via transcytosis to the blood [2]; in the brain, it likely concentrates folate in cerebrospinal fluid [3]. Expression of FRα in normal tissues is restricted to the apical surfaces of polarized epithelial cells [4], where it is not exposed to the blood stream. Unlike the more ubiquitously expressed reduced folate carrier and proton-coupled folate transporter that regulate folate homeostasis, FRα allows internalization of folic acid that has been conjugated to low molecular weight compounds, proteins, or nanoparticles [5]. This property has implications for targeting of chemotherapeutic drugs, cytotoxic viruses, or imaging agents to FRα-expressing cells.
FRα is an attractive candidate for targeted biologic therapy of ovarian cancer [6,7]. It is reported to be expressed in the majority of non-mucinous epithelial ovarian tumors at levels 10- to 100-fold higher than its normal expression in the kidney and on lung and breast epithelial cells [8]. In addition, FRα is a tumor antigen, with 70% of women with ovarian or breast cancer showing measurable immune responses against this protein [9]. The tumor specificity and high levels of FRα expression and the potential to boost immunity to tumors with FRα-specific approaches have generated significant enthusiasm for testing strategies targeting FRα in ovarian cancer patients. For example, MORAb-003, a humanized, high-affinity monoclonal antibody against FRα based on the murine LK26 clone, is currently undergoing phase II testing in ovarian cancer patients after showing cell-mediated cytotoxicity, complement-dependent killing, and non-immune mediated, FRα-dependent inhibition of growth under folate-limiting conditions [10]. What is unknown in attempting to apply novel FRα-based therapeutics is the FRα-expression status of tumors with more rare histologies, the stability of expression of FRα across the multiple sites of disease typically present at diagnosis in ovarian cancer and whether FRα expression is lost or maintained with disease recurrence. In addition, although one investigative team has stated that FRα overexpression is an indicator of platinum resistance in ovarian cancer, the study was small and performed in the pre-taxane era [11].
We performed this study to define the extent of FRα expression in ovarian cancers of different histologies, grades, and stages and to determine whether FRα status was associated with time to recurrence and overall survival. In addition, we examined multiple metastatic sites from a subset of patients with advanced disease at diagnosis as well as matched samples from patients at diagnosis and subsequent recurrence.
Methods
Tissues studied
Ovarian cancer specimens from women who had surgery at Mayo Clinic Rochester between June 15, 1991 and June 15, 2005 were eligible for inclusion in the study. Invasive tumors encompassing serous, endometrioid, clear cell, mucinous, or mixed morphologies were selected prior to retrieval of any clinical information. Borderline tumors and non-epithelial malignancies were excluded. The specimens chosen for the study were similar to all available patients in this time period in terms of patient age, stage of disease, and grade. There was a difference between the sample set and all available samples in the distribution of tumor morphologies. This was caused by oversampling the rare morphologies, which resulted in ∼56% of the primary tumors studied (compared to 70% of all eligible epithelial malignancies) having serous morphology. The demographic characteristics of patients represented in this study are shown in Table 1. A total of 186 cancers from women with newly diagnosed ovarian cancer and 27 samples of recurrent disease comprise the main sample. In addition, FRα expression was analyzed in concomitant primary versus metastatic sites from the initial surgery in a subset of 20 patients with stage IIIC or IV disease from the group of 186 primary samples. To examine FRα expression on metastatic foci, paraffin-embedded tumors from the following locations were selected as available: fallopian tube, pelvic sidewall, abdominal wall, omentum, small and large intestine, appendix, spleen, liver, diaphragm, and various lymph nodes. Finally, 24 patients (again, representing a subset of the 186 primary and 27 recurrent samples) had subsequent secondary debulking surgery from which tissue samples were available, allowing analysis of matched pairs of primary tumors and recurrent disease following chemotherapy. Patients provided informed consent based on institutional policy at the time of their cancer diagnosis. This retrospective, minimal risk study was reviewed and approved by the Mayo Clinic Institutional Review Board.
Table 1.
Patient and tumor characteristics
| All tumors, n | Primary tumors, n (%) | Recurrent tumors, n | |
|---|---|---|---|
| Tumor histology | |||
| Serous | 126 | 104 (55.9) | 22 |
| Endometrioid | 41 | 39 (21.0) | 2 |
| Clear cell | 32 | 30 (16.1) | 2 |
| Mucinous | 9 | 9 (4.8) | 0 |
| Mixed | 5 | 4 (2.2) | 1 |
| Total: | 213 | 186 | 27 |
| Grade | |||
| 1 | 14 | 14 (7.5) | 0 |
| 2 | 12 | 11 (5.9) | 1 |
| 3 | 110 | 96 (51.6) | 14 |
| 4 | 77 | 65 (34.9) | 12 |
| FIGO stage | |||
| Stage I | 34 (18.3) | ||
| Stage II | 8 (4.3) | ||
| Stage III | 115 (61.8) | ||
| Stage IV | 29 (15.6) | ||
| Debulking status, n (%) | |||
| Optimal | 159 (85.5) | ||
| Sub-optimal | 26 (14.0) | ||
| Undocumented | 1 (0.5) | ||
| Follow-up time | |||
| Time of follow-up (months), median (minimum, maximum) | 43.7 (0.2, 178) | ||
| Time to recurrence (years), median (95% CI a) | 2.0 (1.5, 2.6) | ||
| Time to death (years), median (95% CI) | 4.5 (3.6, 6.2) | ||
| Recurrence status, n (%) | |||
| No recurrence | 66 (35.5) | ||
| Recurrence | 117 (62.9) | ||
| Unknown | 3 (1.6) | ||
| Vital status, n (%) | |||
| Alive | 64 (34.4) | ||
| Deceased | 122 (65.6) |
Collection of histologic and clinical data
Hematoxylin and eosin-stained slides from the initial surgery were reviewed by a gynecologic pathologist (GLK) to confirm disease morphology and grade. Tumors were graded 1–4, representing well-differentiated, moderately differentiated, poorly differentiated, or undifferentiated, respectively. Pathology reports and medical records were reviewed to confirm disease staging, which was defined using FIGO guidelines [12]. The clinical course of the disease was followed for each patient using the medical record. Endpoints represent dates of recurrence, death or dates of last contact.
Sample preparation
Samples were examined for FRα expression by assessing triplicate 0.6 mm cores of formalin-fixed, paraffin-embedded tumors in tissue microarrays (TMAs) or slides made from a full paraffin block. After a gynecologic pathologist selected appropriate regions of each tumor for core removal, TMAs were manually constructed as described [13].
Antibody
Tissues were stained with FBP343, a monoclonal IgG1 antibody derived by immunization with human FRα purified from the KB nasopharyngeal carcinoma cell line [14]. This clone was selected by radioimmunoassay for the ability to bind 125I-labeled folic acid complexed to purified folate receptor and subsequently characterized using Western blot, immunohistochemistry, and flow cytometry.
Immunohistochemistry
Five-micron sections were cut and placed on positively charged slides. After rehydration, tissues were subjected to antigen retrieval and blocking of endogenous peroxidases prior to staining with 3.6 μg/ml FBP343 or a non-specific isotype matched antibody as a negative control for 30 min. An additional negative control was liver tissue on each TMA. Positive controls were ovarian cancer specimens (not included in this study) that had previously been identified as expressing FRα using a polyclonal antibody [15]. After washing the slides, signals were detected using the mouse MACH3 system (Biocare Medical, Walnut Creek, CA). Slides were counterstained with Modified Schmidt's Hematoxylin and permanently mounted.
Image capture and analysis
Slides were archived using digital imaging performed using a Bliss “Virtual Microscopy” microscope and computer system (Bacus Laboratories, Lombard, IL). The staining intensity (strong, moderate, weak, or negative) (Fig. 1) and proportion of FRα-positive cells among the malignant cells were scored independently on the digital images by two observers who were blinded to all clinical outcome data. For 10 of the 212 samples (4.7%), divergent scores were obtained. These were reconciled by jointly reviewing the cores in question and coming to a consensus. The proportion of cells positive for FRα expression was categorized into quartiles: >75% positive, 51–75% positive, 26–50% positive, and <25% positive. Analysis was performed on three cores or slides for the majority (56%) of patients; 4–12 samples were available for 20% and <3 samples for 24% of the patients. Tumor samples were defined as negative when there was no evidence of FRα staining on any examined sample from a given individual. If staining was observed, the patient was classified as having a FRα-positive tumor.
Fig. 1.
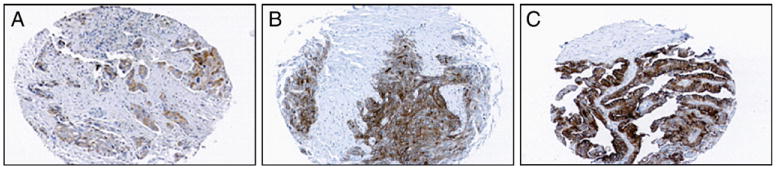
Weak, moderate, and strong FRα expression. Shown are representative cores of ovarian cancer tissues on TMAs that were scored with weak (A), moderate (B), and strong (C) FRα expression after immunohistochemistry using FBP343 antibody. Note that the tumor stroma is negative and malignant epithelial cells are largely positive.
Statistical analysis
Student's _t_-test for age and Chi-square or Fisher's Exact tests for categorical data were used to compare demographic parameters in FRα-positive and FRα-negative tumors. Kaplan–Meier analysis was used to determine whether survival differed between patients with FRα-positive and FRα-negative tumors or different characteristics at diagnosis such as disease stage, grade, tumor morphology, or optimal debulking status (defined as no tumor nodule >1 cm remaining). Univariate and multivariable Cox proportional hazards modeling was used to evaluate whether individual or multiple factors including FRα status were correlated with time to recurrence or survival. Statistical tests were two sided using a significance level of 5%.
Results
Patient and tumor characteristics
The median time from diagnosis to date of last contact or death for the 186 patients with newly diagnosed disease was 43.7 months (range of 0.2 to 178 months), with 122 women in the study being deceased and 64 alive at last follow-up. 159 patients (85.5%) had optimal debulking surgery; 26 patients (14%) had unresected tumor larger than 1 cm and 1 patient's debulking status could not be determined. Chemotherapy was given to 159 patients (85.5%); 18 patients (14 with stage I disease) received no chemotherapy (9.7%) and treatment for 9 patients (4.8%) was unknown. Of the 159 patients who received chemotherapy, 74.8% received a platinum agent in combination with a taxane. The remaining patients, generally those diagnosed before mid-1994, received cyclophosphamide and a platinum agent. The primary tumors analyzed in this sample set are representative of the typical clinical presentation of epithelial ovarian cancer in the distribution of the stage at diagnosis, grade, and patient age [16,17,12] (Tables 1 and 2); more patients with non-serous tumors were included than would be expected in the population.
Table 2.
Distribution of FRα expression in primary epithelial ovarian tumors (_n_=186)
| FRα+, _n_=134 | FRα−, n =52 | p values | |
|---|---|---|---|
| Age, mean (SD) | 62.0 (11.8) | 61.3 (14.6) | 0.546 |
| Tumor histology, n (%) | <0.001 a | ||
| Serous | 85 (81.7) | 19 (18.3) | |
| Endometrioid | 26 (66.7) | 13 (33.3) | |
| Clear cell | 19 (63.3) | 11 (36.7) | |
| Mucinous | 2 (22.2) | 7 (77.8) | |
| Mixed | 2 (50.0) | 2 (50.0) | |
| FIGO Stage, n (%) | 0.203 b | ||
| Stage I: 34 | 21 (61.8) | 13 (38.2) | |
| Stage II: 8 | 6 (75.0) | 2 (25.0) | |
| Stage III: 115 | 85 (73.9) | 30 (26.1) | |
| Stage IV: 29 | 22 (75.9) | 7 (24.1) | |
| Grade, n (%) | 0.004 c | ||
| 1: 14 | 7 (50.0) | 7 (50.0) | |
| 2: 11 | 5 (45.5) | 6 (54.5) | |
| 3: 96 | 69 (71.9) | 27 (28.1) | |
| 4: 65 | 53 (81.5) | 12 (18.5) |
Folate receptor expression in primary cancers
Overall, 72% (134 of 186) of patients with newly diagnosed epithelial ovarian cancer had tumors that were positive for FRα expression (Table 2). Stromal cells were clearly FRα-negative (Fig. 1). Epithelial cells were uniformly negative in 52 (28%) patients. Of the positively staining tumors, 123 samples demonstrated moderate or strong FRα expression and only 11 (8.2%) had uniformly weak staining tumors. Scoring extent of positive cells, 80 patients (59.7%) had >75% of the cells positive, 36 patients (26.9%) had between 51% and 75% of the epithelial cells positive, 10 patients (7.5%) had between 26% and 50% of the epithelial cells positive, and 8 patients (6%) had no more than 25% of the epithelial cells positive. Because of the low numbers of available tumors with less common morphologies, we compared serous tumors to all other morphologies combined, a design with 85% power to detect a difference of 20% in the proportion of patients with FRα-positive tumors. Serous tumors were more likely to be FRα-positive than all other morphologies combined (81.7% versus 59.8% positive, respectively, p<0.001), with 66.7% of endometrioid (26 of 39) and 63.3% of clear cell (19 of 30) tumors and only two of nine mucinous tumors expressing FRα. Similarly, high-grade tumors (75.8% positive) were more likely than low-grade tumors (48% positive) to express FRα (_p_=0.004). Tumors from patients with advanced stage disease were slightly more likely to express FRα (_p_=0.203) (Table 2).
Primary versus simultaneous metastatic foci
In considering FRα as a therapeutic target, we examined its expression on primary tumors and concomitant metastatic foci. Thus, twenty patients with stage IIIC or IV disease were selected for additional study. Three to twelve different tumor sites, all obtained at initial surgery, were scored from each of these patients. In this sample, 18 of the 20 patients had tumors that expressed FRα (see Table 3 for tumor morphology). FRα staining was evident in 109 of the 110 sections examined for these 18 patients. Only one of these patients, with a clear cell tumor, had discrepant results. Specifically, of the three blocks available from her disease, there was strong staining in two sites, the ovary and the right para-aortic lymph node, but no staining in a biopsy of malignant material on the right pelvic peritoneum. The other two patients in this subset of 20 individuals had tumors that were uniformly negative for FRα expression, with 13 of 13 slides showing no evidence of staining.
Table 3.
Tumor morphologies of the samples used in the two subset analyses
| Primary and synchronous metastatic foci | ||||
|---|---|---|---|---|
| Histology | FRα+, n (%) | FRα−, n (%) | ||
| Serous | 12 (60) | 0 | ||
| Endometrioid | 1 (5) | 1 (5) | ||
| Clear cell | 3 (15) | 0 | ||
| Mucinous | 1 (5) | 1 (5) | ||
| Mixed | 1 (5) | 0 | ||
| Total | 18 (90) | 2 (10) | ||
| Matched primary and recurrent disease | ||||
| Histology | FRα status at diagnosis/recurrence, n (%) | |||
| +/+ | −/− | +/− | −/+ | |
| Serous | 15 (62.5) | 3 (12.5) | 1 (4.2) | 1 (4.2) |
| Endometrioid | 1 (4.2) | 0 | 0 | 0 |
| Clear cell | 1 (4.2) | 1 (4.2) | 0 | 0 |
| Mixed | 0 | 0 | 0 | 1 (4.2) |
| Total | 17 (70.8) | 4 (16.7) | 1 (4.2) | 2 (8.3) |
FR expression in primary versus recurrent disease
To determine whether FRα expression changes after exposure to chemotherapy, we analyzed matched samples from the 24 patients in the sample set of 186 women who had multiple surgeries at Mayo Clinic. The median time between surgeries was 23 months (range 6–83 months). Therefore, this subset of patients with recurrent disease included both individuals with longer disease-free periods, good performance status, and localized disease [18,19] and those with bowel obstructions or pain requiring palliative procedures. Again, most samples expressed abundant FRα (Fig. 2), with 18 of 24 (75%) primary and 19 of 24 (79.2%) recurrent samples being positive. 17 patients (70.8%) had tumors that expressed FRα throughout the course of disease (see Table 3 for tumor histology). Four patients (16.7%) had tumors that did not express FRα at either diagnosis or recurrence. Interestingly, 2 patients who had tumors that were negative for FRα expression at diagnosis showed strong staining for FRα at recurrence, including one patient who had two subsequent debulking surgeries, both of which yielded tumors that were strongly positive. Only one patient with an initially FRα-positive tumor at diagnosis lost FRα expression at recurrence.
Fig. 2.
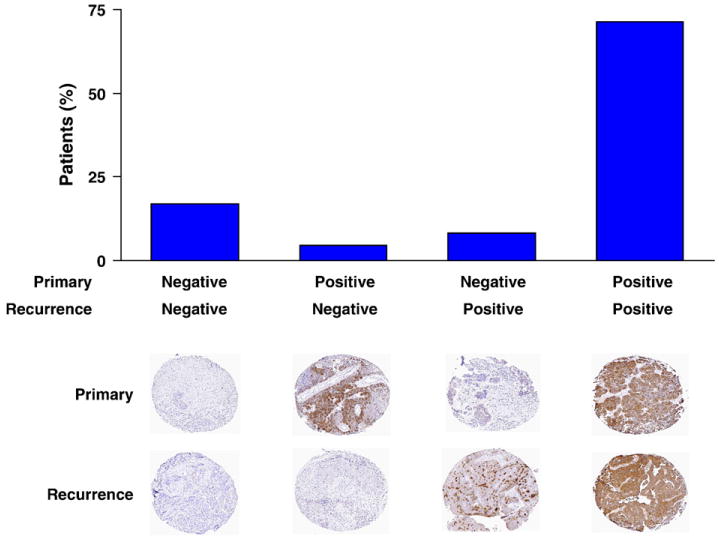
FRα expression in primary and recurrent samples. The frequency of FRα expression in matched tumor samples from an initial diagnostic surgery and subsequent secondary debulking surgery for disease recurrence is shown. Examples of four patients representing each of the expression patterns are shown.
Patient outcomes
Disease recurrence was experienced by 117 patients (62.9%), while 66 patients (35.5%) remain free of disease and 3 individuals are lost to follow-up. Univariate analysis showed that advanced disease stage, serous morphology (compared to all other morphologies combined), high-grade, and sub-optimal debulking were all associated with a higher risk of disease recurrence. FRα status was not associated with time to recurrence as a univariate variable or after adjusting for disease stage, morphology, grade, and debulking status (Table 4). Similarly, when patients with fewer than 50% of malignant cells positive for FRα expression were grouped with the FRα-negative samples in order to assess the effect of the highest levels of FRα expression, no association could be found between FRα expression and time to recurrence as a univariate variable or after adjusting as above (data not shown). A similar analysis was performed for overall survival (Table 4). Again, advanced stage, serous morphology, high-grade, and sub-optimal debulking were all significantly associated with an increased risk of death as univariate variables. The improved prognosis conferred by having a tumor of non-serous morphology appears to be driven by the endometrioid tumors. When assessing only tumor morphology, serous tumors have significantly worse prognosis than endometrioid tumors [HR 2.23 (95% CI 1.31, 3.80), _p_=0.003]. This is consistent with the effect of serous versus endometrioid tumor morphology on outcome in a study of stage III patients [20]. However, this is not maintained after adjusting for stage, grade, FRα expression, and debulking status [HR 1.23 (95% CI 0.70, 2.15), _p_=0.47]. FRα status did not impact survival as a univariate variable or in multivariable analyses. Kaplan–Meier plots showed no statistically significant differences based on FRα status, although there was a modest trend towards earlier recurrence with FRα-positive tumors that was not reflected in the survival data (Fig. 3).
Table 4.
Effect of patient characteristics on time to disease recurrence and overall survival
| Time to recurrence | ||||
|---|---|---|---|---|
| Univariate | Multivariable | |||
| Hazard ratio(95% CI) | p value | Hazard ratio(95% CI) | p value | |
| Advanced stage (III–IV) | 6.84 (3.55–13.2) | <0.001 | 4.94 (2.49–9.81) | <0.001 |
| High grade (3–4) | 4.28 (1.87–9.77) | <0.001 | 2.17 (0.91–5.21) | 0.082 |
| Serous morphology | 2.63 (1.76–3.93) | <0.001 | 1.48 (0.96–2.28) | 0.079 |
| Sub-optimal debulking | 2.48 (1.50–4.10) | <0.001 | 1.63 (0.97–2.73) | 0.064 |
| FRα+ | 1.21 (0.79–1.84) | 0.38 | 0.90 (0.58–1.39) | 0.63 |
| Time to death | ||||
| Univariate | Multivariable | |||
| Hazard ratio(95% CI) | p value | Hazard ratio(95% CI) | p value | |
| Advanced stage (III–IV) | 2.96 (1.79–4.90) | <0.001 | 2.26 (1.31–3.90) | 0.003 |
| High grade (3–4) | 3.42 (1.59–7.35) | 0.002 | 2.77 (1.24–6.19) | 0.013 |
| Serous morphology | 1.74 (1.20–2.52) | 0.004 | 1.01 (0.67–1.53) | 0.96 |
| Sub-optimal debulking | 2.52 (1.56–4.08) | <0.001 | 2.13 (1.29–3.50) | 0.003 |
| FRα+ | 1.02 (0.68–1.52) | 0.92 | 0.86 (0.57–1.31) | 0.49 |
Fig. 3.
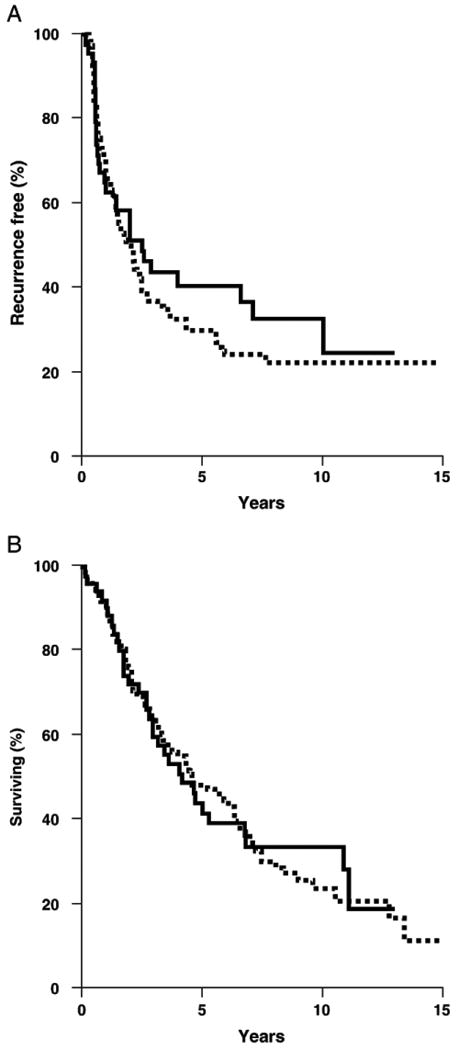
Kaplan–Meier analyses of recurrence-free and overall survival. Recurrence-free survival (A) and overall survival (B) in patients with FRα-positive (thick line) and FRα-negative (hatched line) ovarian tumors.
Discussion
We analyzed FRα expression in a cohort of women with ovarian cancer who were treated at our institution with rigorous surgical debulking followed by chemotherapy when indicated by their disease stage and debulking status. We examined the extent of FRα expression in 186 primary and 27 recurrent ovarian cancers, including matched samples from 24 patients who had primary and secondary debulking surgeries at our institution. In addition, we examined primary disease and concomitant metastatic disease in a subset of 20 patients. We show that 134 of 186 (72%) primary tumors and 22 of 27 (81.5%) recurrent tumors were positive for FRα expression, that synchronous metastatic disease has similar characteristics to the primary tumor, and that FRα expression is commonly maintained in recurrent disease. These observations support the use of FRα-targeted strategies in most women with both newly diagnosed and recurrent ovarian cancer. The data also suggest that immunohistochemical analysis of FR status at initial biopsy can guide the decision whether to use an FRα-targeted therapy upon recurrence.
We also analyzed whether FRα expression was associated with outcome. While FRα staining was more prevalent in women with aggressive disease (high-grade, serous morphology), we did not detect an association between FRα expression and overall survival. This is similar to the findings of Toffoli et al., who analyzed the survival of 99 ovarian cancer patients who had been treated with a platinum agent between 1990 and 1995 [11]. Given the common expression of FRα on aggressive ovarian cancers (i.e. only 20 high-stage, high-grade serous tumors lacked FRα expression in this study), very large sample sizes will be required to accrue sufficient numbers of patients with FRα-negative tumors for statistically meaningful comparisons. This makes analysis of the impact of FRα expression in ovarian cancer rather more difficult than in breast and uterine cancers; in these diseases, with strong FRα expression in a smaller proportion of patients, FRα expression is a negative prognostic factor [21,22].
The Toffoli study concluded that high FRα expression was a predictor of cisplatin resistance in a subset of 58 women with >2 cm residual disease after surgery. Our analysis showed no interaction of FRα staining and debulking status. Moreover, we saw no relationship between FRα expression and time to disease recurrence, a commonly accepted surrogate for responsiveness to initial chemotherapy [12,17,16].
While the function of FRα in cancers is not fully understood, folates are critical metabolites for nucleotide synthesis and methylation reactions, so it is logical to propose that rapidly dividing cells may realize a growth advantage when this folate transporter is upregulated. Several lines of evidence support this idea. Specifically, overexpression of FRα in osteosarcoma [23] or NIH/3T3 [24] cells allows increased growth in folate-limited medium and formation of larger NIH/3T3 tumors in vivo [25]. Down-regulating FRα using antisense strategies decreased the proliferation rate of breast cancer cell lines and increased their sensitivity to doxorubicin [26], and intracellular anti-FRα antibodies reduced the ability of ovarian cancer cell lines to proliferate and to grow in soft agar [27]. In human disease, it is plausible that the tumor microenvironment creates local folate-deficient areas in which FRα-positive cells could have a growth advantage.
Regardless of the physiological role for FRα in malignant tumors, its tumor-specific overexpression and ability to internalize folate-conjugates suggest that the majority of women with epithelial ovarian cancer may benefit from strategies targeting FRα. These strategies include use of anti-FRα antibodies [10] or folic acid-conjugates to localize drugs [28–30], imaging compounds [31–33], T cells [34,35], nanoparticles [36], or oncolytic viruses [37] to FRα-expressing tumors. Besides these efforts, we have recently shown that FRα may have potential as a target for immunotherapeutic approaches in ovarian cancer. FRα is a tumor-associated antigen that induces detectable immune responses in 70% of patients with breast and ovarian cancer [9]. The presence of endogenous immune reactivity raises the possibility that the immune response could be further enhanced by vaccines targeting the FRα.
Other groups have reported using PCR or radioligand binding assays to analyze FRα expression in ovarian cancer cell lines [38,39] or small numbers of ovarian tumor specimens [40,41,8]. The strengths of our study include the use of a large cohort of 186 patients with newly diagnosed, previously untreated ovarian cancer. Unlike a previous report addressing the association of FRα expression and outcome [11], the majority of these patients received optimal debulking surgery and combination chemotherapy that included a taxane. Another strength is the extensive follow-up available and complete clinical annotation of these specimens due to the retrospective nature of the study. We utilized formalin-fixed, paraffin-embedded tissues archived through the Mayo Clinic Tissue Registry, which has systematically preserved surgical specimens since the early 1900s. Immunohistochemistry was chosen because it is efficient for large numbers of samples (especially when tissue microarrays are utilized), allows examination of malignant epithelial cells without microdissection, and avoids specificity concerns in radioligand binding assays such as non-malignant cells (for example, activated macrophages that would also bind radiolabeled folic acid) contributing to the signal. Nevertheless, studies assessing FRα expression by other methodologies such as direct protein measurement, including quantitative detection of shed FRα in serum, may have different results. A limitation to the study is that FRα expression occurs frequently, especially in the common high-grade, high-stage serous tumors that are most likely to recur. In addition, because the less common morphologies that ultimately showed less FRα expression were oversampled, this retrospective study cannot be used to establish the prevalence of FRα+ tumors in ovarian cancer patients; in other words, our observed proportion of FRα-positive tumors of 72% is likely an underestimate of true prevalence. Prospective studies of FRα expression and its association with outcome would be required to confirm these results. It is clear, however, that the small number of ovarian cancer patients with tumors that do not express this tumor marker makes it difficult to assess FRα expression as an independent factor potentially influencing outcome.
In summary, the common expression of FRα on primary and synchronous metastatic disease as well as on recurrent disease suggests that FRα-based therapeutic strategies may be helpful for most women with ovarian cancer, whether newly diagnosed with disseminated disease or experiencing disease recurrence. A number of strategies to take advantage of this observation are being pursued, with several folate-targeted drugs currently undergoing testing in clinical trials.
Acknowledgments
We gratefully acknowledge Dr. Wilbur L. Franklin for the gift of the FBP343 monoclonal antibody, Linda Murphy for expert technical assistance with immunohistochemistry, Darren Riehle for tissue imaging, Karin Goodman for clinical follow-up, Dr. William Cliby for helpful discussions, and Vicki Shea for assistance with the manuscript. This work was supported by the Minnesota Ovarian Cancer Alliance, the CanCurables Foundation, the Paul Calabresi Program in Clinical–Translational Research at Mayo Clinic (CA80628) (KRK), and the Mayo Clinic Cancer Center Women's Cancer Program (LCH).
References
- 1.Elnakat H, Ratnam M. Role of folate receptor genes in reproduction and related cancers. Front Biosci. 2006;11:506–19. doi: 10.2741/1815. [DOI] [PubMed] [Google Scholar]
- 2.Sandoval RM, Kennedy MD, Low PS, Molitoris BA. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol. 2004;287:C517–26. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- 3.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56:1085–97. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401. [PubMed] [Google Scholar]
- 5.Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci U S A. 1991;88:5572–6. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–52. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 7.Reddy JA, Allagadda VM, Leamon CP. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr Pharm Biotechnol. 2005;6:131–50. doi: 10.2174/1389201053642376. [DOI] [PubMed] [Google Scholar]
- 8.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–93. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Knutson KL, Krco C, Goodman K, et al. T cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J Clin Oncol. 2006;24:4254–61. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- 10.Ebel W, Routhier EL, Foley B, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- 11.Toffoli G, Russo A, Gallo A, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer. 1998;79:121–6. doi: 10.1002/(sici)1097-0215(19980417)79:2<121::aid-ijc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Ozols RF, Rubin SC, Thomas GM, Robboy SJ. Epithelial ovarian cancer. In: Hoskins WJ, Perez CA, Young RC, Barakat RR, Markman M, Randall ME, editors. Principles and practice of gynecologic oncology. 4th. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 895–987. [Google Scholar]
- 13.Chien J, Aletti G, Baldi A, et al. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest. 2006;116:1994–2004. doi: 10.1172/JCI27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin WA, Waintrub M, Edwards D, et al. New anti-lung-cancer antibody cluster 12 reacts with human folate receptors present on adenocarcinoma. Int J Cancer Suppl. 1994;8:89–95. doi: 10.1002/ijc.2910570719. [DOI] [PubMed] [Google Scholar]
- 15.Kelemen LE, Sellers TA, Keeney GL, Lingle WL. Multivitamin and alcohol intake and folate receptor alpha expression in ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2168–72. doi: 10.1158/1055-9965.EPI-05-0260. [DOI] [PubMed] [Google Scholar]
- 16.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 17.Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–70. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Montes TP, Bristow RE. Secondary cytoreduction for patients with recurrent ovarian cancer. Curr Oncol Rep. 2005;7:451–8. doi: 10.1007/s11912-005-0010-4. [DOI] [PubMed] [Google Scholar]
- 19.Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933–9. doi: 10.1002/cncr.21845. [DOI] [PubMed] [Google Scholar]
- 20.Winter WE, III, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann LC, Keeney GL, Lingle WL, et al. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–42. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 22.Allard JE, Risinger JI, Morrison C, et al. Overexpression of folate binding protein is associated with shortened progression-free survival in uterine adenocarcinomas. Gynecol Oncol. 2007;107(1):52–7. doi: 10.1016/j.ygyno.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Yang R, Kolb EA, Qin J, et al. The folate receptor alpha is frequently overexpressed in osteosarcoma samples and plays a role in the uptake of the physiologic substrate 5-methyltetrahydrofolate. Clin Cancer Res. 2007;13:2557–67. doi: 10.1158/1078-0432.CCR-06-1343. [DOI] [PubMed] [Google Scholar]
- 24.Bottero F, Tomassetti A, Canevari S, Miotti S, Menard S, Colnaghi MI. Gene transfection and expression of the ovarian carcinoma marker folate binding protein on NIH/3T3 cells increases cell growth in vitro and in vivo. Cancer Res. 1993;53:5791–6. [PubMed] [Google Scholar]
- 25.Bottero F, Tomassetti A, Canevari S, Miotti S, Menard S, Colnaghi MI. Gene transfection and expression of the ovarian carcinoma marker folate binding protein on NIH/3T3 cells increases cell growth in vitro and in vivo. Cancer Res. 1993;53:5791–6. [PubMed] [Google Scholar]
- 26.Jhaveri MS, Rait AS, Chung KN, Trepel JB, Chang EH. Antisense oligonucleotides targeted to the human alpha folate receptor inhibit breast cancer cell growth and sensitize the cells to doxorubicin treatment. Mol Cancer Ther. 2004;3:1505–12. [PubMed] [Google Scholar]
- 27.Figini M, Ferri R, Mezzanzanica D, et al. Reversion of transformed phenotype in ovarian cancer cells by intracellular expression of anti folate receptor antibodies. Gene Ther. 2003;10:1018–25. doi: 10.1038/sj.gt.3301962. [DOI] [PubMed] [Google Scholar]
- 28.Leamon CP, Reddy JA, Vlahov IR, et al. Comparative preclinical activity of the folate-targeted Vinca alkaloid conjugates EC140 and EC145. Int J Cancer. 2007 doi: 10.1002/ijc.22853. [DOI] [PubMed] [Google Scholar]
- 29.Reddy JA, Westrick E, Vlahov I, Howard SJ, Santhapuram HK, Leamon CP. Folate receptor specific anti-tumor activity of folate–mitomycin conjugates. Cancer Chemother Pharmacol. 2006;58:229–36. doi: 10.1007/s00280-005-0151-z. [DOI] [PubMed] [Google Scholar]
- 30.Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005;94:2135–46. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 31.Muller C, Hohn A, Schubiger PA, Schibli R. Preclinical evaluation of novel organometallic 99mTc-folate and 99mTc-pteroate radiotracers for folate receptor-positive tumour targeting. Eur J Nucl Med Mol Imaging. 2006;33:1007–16. doi: 10.1007/s00259-006-0111-9. [DOI] [PubMed] [Google Scholar]
- 32.Okarvi SM, Jammaz IA. Preparation and in vitro and in vivo evaluation of technetium-99m-labeled folate and methotrexate conjugates as tumor imaging agents. Cancer Biother Radiopharm. 2006;21:49–60. doi: 10.1089/cbr.2006.21.49. [DOI] [PubMed] [Google Scholar]
- 33.Siegel BA, Dehdashti F, Mutch DG, et al. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical results. J Nucl Med. 2003;44:700–7. [PubMed] [Google Scholar]
- 34.Roy EJ, Gawlick U, Orr BA, Kranz DM. Folate-mediated targeting of T cells to tumors. Adv Drug Deliv Rev. 2004;56:1219–31. doi: 10.1016/j.addr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Kranz DM, Patrick TA, Brigle KE, Spinella MJ, Roy EJ. Conjugates of folate and anti-T-cell-receptor antibodies specifically target folate-receptor-positive tumor cells for lysis. Proc Natl Acad Sci U S A. 1995;92:9057–61. doi: 10.1073/pnas.92.20.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. Synthesis and grafting of thioctic acid–PEG–folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem. 2006;17:603–9. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa K, Nakamura T, Harvey M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–8. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 38.Campbell IG, Jones TA, Foulkes WD, Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991;51:5329–38. [PubMed] [Google Scholar]
- 39.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications Cancer. 1994;73:2432–43. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Holm J, Hansen SI, Hoier-Madsen M, Helkjaer PE, Bzorek M. Folate receptor in malignant effusions of ovarian carcinoma. Apmis. 1995;103:663–70. doi: 10.1111/j.1699-0463.1995.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 41.Holm J, Hansen SI, Hoier-Madsen M, Birn H, Helkjaer PE. High-affinity folate receptor in human ovary, serous ovarian adenocarcinoma, and ascites: radioligand binding mechanism, molecular size, ionic properties, hydrophobic domain, and immunoreactivity. Arch Biochem Biophys. 1999;366:183–91. doi: 10.1006/abbi.1999.1188. [DOI] [PubMed] [Google Scholar]