The role of aneuploidy in promoting and suppressing tumors (original) (raw)
Abstract
Impaired mitotic checkpoint signaling can both promote and suppress tumors. The mitotic checkpoint targets Cdc20, the specificity factor of the ubiquitin ligase that promotes anaphase by targeting cyclin B and securin for destruction. In this issue, Li et al. (2009. J. Cell Biol. doi:10.1083/jcb.200904020) use gene replacement to produce mice expressing a Cdc20 mutant that cannot be inhibited by the mitotic checkpoint. In addition to the expected aneuploidy, these animals have a high tumor incidence that is likely caused by persistent aneuploidy coupled with nonmitotic functions of mutant Cdc20.
The mitotic checkpoint (also known as the spindle assembly checkpoint) is the major cell cycle control mechanism during mitosis and acts to prevent chromosome missegregation and its accompanying aneuploidy (Fig. 1 A). The signaling pathway is partially established; kinetochores on unattached chromosomes generate an inhibitor that binds to Cdc20, the substrate specificity factor of the anaphase-promoting complex (APC), an E3 ubiquitin ligase. Cdc20 activates recognition by APC of substrates whose ubiquitination is required for chromosome segregation during anaphase, including cyclin B and securin. The mitotic checkpoint proteins Mad2, BubR1, and a complex containing both, have all been shown to bind directly to Cdc20, thereby inhibiting APC-mediated ubiquitination of cyclin B and securin and blocking anaphase initiation. Six mouse models expressing reduced levels of mitotic checkpoint proteins (Mad1, Mad2, Bub1, BubR1, Bub3, and centromere protein E [CENP-E]), as well as mice overexpressing Mad2, have previously been shown to produce elevated rates of chromosome missegregation (Dobles et al., 2000; Babu et al., 2003; Baker et al., 2004; Iwanaga et al., 2007; Jeganathan et al., 2007; Perera et al., 2007; Sotillo et al., 2007; Weaver et al., 2007). However, these animals exhibit high variability in spontaneous tumor development. In this framework, Li et al. (see p. 983 of this issue) now report that mice expressing a dominant mutant in Cdc20, the factor whose activity is targeted by the mitotic checkpoint signaling pathway, exhibit aneuploidy and a remarkably high tumor incidence.
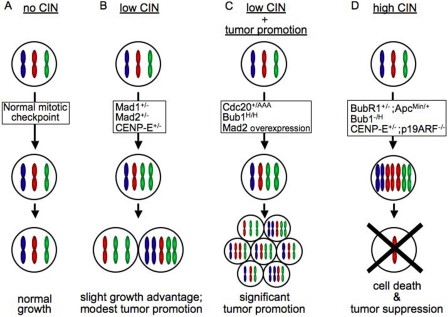
Figure 1.
The consequences of CIN. Low rates of CIN promote tumors, but high rates of CIN cause cell death and tumor suppression. Divisions in a hypothetical cell with three chromosomes. (A) Normal cells do not exhibit CIN and produce genetically identical progeny. (B) Animals heterozygous for the mitotic checkpoint components Mad1, Mad2, or CENP-E missegregate one or a few chromosomes per division (low CIN) and exhibit a modest tumor phenotype. Approximately 20–25% of these animals develop late onset spontaneous tumors. (C) Coupling a low rate of CIN with another tumor-promoting activity, such as a reduced rate of cell death, results in a higher rate of tumor formation with a shorter latency. (D) High rates of CIN lead to massive chromosome missegregation and cell death from loss of both copies of ≥1 essential chromosome.
Li et al. (2009) designed a Cdc20 mutant that would not interact with Mad2 by mutating to alanine two charged residues and a proline in the Mad2-binding site. Interestingly, this mutant (Cdc20AAA) also displays significantly reduced binding to both of its sites on BubR1, so much so that overexpression of BubR1 cannot rescue the mitotic checkpoint defect in Cdc20AAA/AAA cells. Thus, Cdc20AAA cannot be inhibited by the mitotic checkpoint. As predicted, its presence results in aneuploidy from chromosomal instability (CIN), the recurrent missegregation of chromosomes during multiple divisions. Mice expressing the Cdc20AAA mutant develop tumors rapidly, with 50% of mice developing tumors, some palpable as early as 7 mo.
Earlier analyses of mice with reduced levels of mitotic checkpoint components have come to divergent outcomes vis a vis aneuploidy driving tumorigenesis (Fig. 1). Reduced levels of Bub3 (Baker et al., 2006) and BubR1 (Baker et al., 2004) do not produce an increase in spontaneous tumors, but about a quarter of mice with half the normal levels of Mad1 (Iwanaga et al., 2007), Mad2 (Michel et al., 2001), or CENP-E (Weaver et al., 2007) develop spontaneous tumors late in life (≥18 mo of age; Fig. 1 B). A higher proportion (∼50%) of mice expressing reduced levels of Bub1 (Jeganathan et al., 2007) or high levels of Mad2 (Sotillo et al., 2007) develop tumors, many with a short latency (Fig. 1 C).
Although it was initially tempting to attribute the differing tumor incidence in mice with weakened mitotic checkpoint signaling to disparate levels of aneuploidy, the combined evidence does not support this conclusion. The percentage of aneuploid cells in fibroblasts and/or splenocytes in these models is similar, whereas tumorigenesis is not. Therefore, what seems most plausible is that aneuploidy drives a high incidence of tumorigenesis only if the reduction or overexpression of specific mitotic checkpoint components produces other defects that predispose them to tumors, such as an increase in DNA damage, chromosomal rearrangements, and/or a decreased incidence of cell death (Fig. 1 C). There is persuasive evidence underlying this conclusion. Roles outside mitosis are known or implicated for several mitotic checkpoint components. Mad1 is involved in nuclear import, Bub3 participates in transcriptional repression, and BubR1 functions in cell death, the DNA damage response, aging, and megakaryopoiesis (Weaver and Cleveland, 2006). Genetic evidence has indicated that budding yeast homologues of Mad2, BubR1, and Bub3 participate in gross chromosomal rearrangements (Myung et al., 2004). Consistent with this yeast evidence, Mad2 overexpression in mouse cells drives development of numerous chromosomal breaks, gaps, and rearrangements (Sotillo et al., 2007), causing DNA damage and the potential for oncogenic fusion proteins. The role of Bub1 in preventing cell death (Baek et al., 2005; Perera and Freire, 2005; Jeganathan et al., 2007) is likely to predispose Bub1 hypomorphic mice to tumorigenesis. Similarly, Mad2 has been reported as a negative regulator of DNA damage repair (Fung et al., 2008), predicting that Mad2-overexpressing mice have increased DNA damage, although this was not directly examined (Sotillo et al., 2007). Relevant to the Li et al. (2009) study, Cdc20 has been reported to act as a transcriptional repressor in human cells (Yoon et al., 2004). It has also been proposed to have roles in the DNA damage checkpoint (Lim and Surana, 1996) and in precipitating premature entry into mitosis (Clarke et al., 2003). On balance, considering the wealth of mouse models with mitotic checkpoint deficiencies, the existing evidence favors an interpretation in which the nonmitotic roles of Cdc20 have a key influence on the tumor phenotype of Cdc20+/AAA mice.
The only mitotic checkpoint component for which no role outside of mitosis has been suggested is CENP-E. Unlike the other checkpoint components, which are present throughout interphase and in noncycling cells, CENP-E is tightly cell cycle regulated in all reported contexts. It accumulates, like the mitotic cyclins, just before mitosis and is quantitatively degraded at the end of mitosis. Its absence outside mitosis as well as failure to identify additional roles for CENP-E in DNA damage or chromosomal rearrangements (Weaver et al., 2007) argue that the most plausible scenario is that CENP-E functions exclusively in chromosome segregation. Thus, it is likely that the modest increase in spontaneous tumorigenesis from reduced levels of CENP-E represents the true contribution of whole chromosomal aneuploidy in driving tumorigenesis.
An increase in whole chromosomal aneuploidy does promote tumors in some contexts, presumably the result of an imbalance of gene expression (Duesberg et al., 2006), gain of oncogenes, and/or loss of tumor suppressors. However, aneuploidy does not always promote tumorigenesis, and aneuploid animals are largely indistinguishable from wild-type animals for most of their lifespan. Most intriguingly, aneuploidy can suppress tumors in certain contexts. Reduction of BubR1 suppresses tumor formation in the small intestine of mice expressing the multiple intestinal neoplasia allele of the Apc tumor suppressor (Rao et al., 2005). Further reduction of Bub1 (to 20% of the wild-type level) causes a decreased incidence of spontaneous liver tumors (Jeganathan et al., 2007). Heterozygous deletion of CENP-E suppresses tumors in three distinct contexts: spontaneous liver tumors, tumors caused by the carcinogen DMBA (9,10-dimethyl-1,2-benzanthracene), and tumors caused by loss of the tumor suppressor p19ARF (Weaver et al., 2007). Thus, three independent groups, using three distinct methods of inducing aneuploidy, have shown that aneuploidy can suppress tumors in five contexts (Fig. 1 D).
Aneuploidy-mediated tumor suppression is likely the result of high rates of CIN that cause loss of both copies of one or more essential chromosomes. High rates of CIN caused by complete depletion of Mad2 or BubR1 have been shown to cause rapid death in tumor cells (Kops et al., 2004; Michel et al., 2004). Cell death can be prevented by inhibiting cytokinesis, which is fully consistent with death caused by loss of ≥1 essential chromosome (Fig. 1 D; Kops et al., 2004). In addition, aneuploidy is growth suppressive in previously diploid human tumor cells (Thompson and Compton, 2008) and in otherwise normal mouse cells triploid for chromosomes 1, 13, 16, or 19 (Williams et al., 2008).
A consistent theme is that missegregation of one or a few chromosomes per division (low CIN) promotes tumorigenesis (Fig. 1 B), whereas missegregation of a larger number of chromosomes per division (high CIN) drives cell death and tumor suppression (Fig. 1 D). If this is so, what about “highly aneuploid” tumor cells in which the chromosome number is significantly >46? The current data support that acquisition of these highly abnormal chromosome contents occurs slowly over numerous cell divisions involving more subtle changes, which permits the cells to make any compensatory changes in gene expression necessary for survival.
Lastly, in human tumors, aneuploidy often occurs in cells that contain additional genomic changes that are likely to influence the effect of an increased rate of CIN. The interplay between aneuploidy and specific additional defects is now one of the key unresolved questions that will be needed to predict, in a given context, whether increasing the rate of CIN will promote tumors, suppress tumors, or neither.
References
- Babu J.R., Jeganathan K.B., Baker D.J., Wu X., Kang-Decker N., van Deursen J.M. 2003. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation.J. Cell Biol. 160:341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K.H., Shin H.J., Jeong S.J., Park J.W., McKeon F., Lee C.W., Kim C.M. 2005. Caspases-dependent cleavage of mitotic checkpoint proteins in response to microtubule inhibitor.Oncol. Res. 15:161–168 [DOI] [PubMed] [Google Scholar]
- Baker D.J., Jeganathan K.B., Cameron J.D., Thompson M., Juneja S., Kopecka A., Kumar R., Jenkins R.B., de Groen P.C., Roche P., van Deursen J.M. 2004. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice.Nat. Genet. 36:744–749 [DOI] [PubMed] [Google Scholar]
- Baker D.J., Jeganathan K.B., Malureanu L., Perez-Terzic C., Terzic A., van Deursen J.M. 2006. Early aging–associated phenotypes in Bub3/Rae1 haploinsufficient mice.J. Cell Biol. 172:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D.J., Segal M., Andrews C.A., Rudyak S.G., Jensen S., Smith K., Reed S.I. 2003. S-phase checkpoint controls mitosis via an APC-independent Cdc20p function.Nat. Cell Biol. 5:928–935 [DOI] [PubMed] [Google Scholar]
- Dobles M., Liberal V., Scott M.L., Benezra R., Sorger P.K. 2000. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2.Cell. 101:635–645 [DOI] [PubMed] [Google Scholar]
- Duesberg P., Li R., Fabarius A., Hehlmann R. 2006. Aneuploidy and cancer: from correlation to causation.Contrib. Microbiol. 13:16–44 [DOI] [PubMed] [Google Scholar]
- Fung M.K., Han H.Y., Leung S.C., Cheung H.W., Cheung A.L., Wong Y.C., Ling M.T., Wang X. 2008. MAD2 interacts with DNA repair proteins and negatively regulates DNA damage repair.J. Mol. Biol. 381:24–34 [DOI] [PubMed] [Google Scholar]
- Iwanaga Y., Chi Y.H., Miyazato A., Sheleg S., Haller K., Peloponese J.M., Jr., Li Y., Ward J.M., Benezra R., Jeang K.T. 2007. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice.Cancer Res. 67:160–166 [DOI] [PubMed] [Google Scholar]
- Jeganathan K., Malureanu L., Baker D.J., Abraham S.C., van Deursen J.M. 2007. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis.J. Cell Biol. 179:255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G.J., Foltz D.R., Cleveland D.W. 2004. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint.Proc. Natl. Acad. Sci. USA. 101:8699–8704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Fang X., Wei Z., York J.P., Zhang P. 2009. Loss of spindle assembly checkpoint–mediated inhibition of Cdc20 promotes tumorigenesis in mice.J. Cell Biol. 185:983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.H., Surana U. 1996. Cdc20, a beta-transducin homologue, links RAD9-mediated G2/M checkpoint control to mitosis in Saccharomyces cerevisiae.Mol. Gen. Genet. 253:138–148 [DOI] [PubMed] [Google Scholar]
- Michel L.S., Liberal V., Chatterjee A., Kirchwegger R., Pasche B., Gerald W., Dobles M., Sorger P.K., Murty V.V., Benezra R. 2001. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells.Nature. 409:355–359 [DOI] [PubMed] [Google Scholar]
- Michel L., Diaz-Rodriguez E., Narayan G., Hernando E., Murty V.V., Benezra R. 2004. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells.Proc. Natl. Acad. Sci. USA. 101:4459–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K., Smith S., Kolodner R.D. 2004. Mitotic checkpoint function in the formation of gross chromosomal rearrangements in Saccharomyces cerevisiae.Proc. Natl. Acad. Sci. USA. 101:15980–15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera D., Freire R. 2005. Human spindle checkpoint kinase Bub1 is cleaved during apoptosis.Cell Death Differ. 12:827–830 [DOI] [PubMed] [Google Scholar]
- Perera D., Tilston V., Hopwood J.A., Barchi M., Boot-Handford R.P., Taylor S.S. 2007. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint.Dev. Cell. 13:566–579 [DOI] [PubMed] [Google Scholar]
- Rao C.V., Yang Y.M., Swamy M.V., Liu T., Fang Y., Mahmood R., Jhanwar-Uniyal M., Dai W. 2005. Colonic tumorigenesis in BubR1+/−ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability.Proc. Natl. Acad. Sci. USA. 102:4365–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R., Hernando E., Diaz-Rodriguez E., Teruya-Feldstein J., Cordon-Cardo C., Lowe S.W., Benezra R. 2007. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice.Cancer Cell. 11:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S.L., Compton D.A. 2008. Examining the link between chromosomal instability and aneuploidy in human cells.J. Cell Biol. 180:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver B.A., Cleveland D.W. 2006. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 18:658–667 [DOI] [PubMed] [Google Scholar]
- Weaver B.A., Silk A.D., Montagna C., Verdier-Pinard P., Cleveland D.W. 2007. Aneuploidy acts both oncogenically and as a tumor suppressor.Cancer Cell. 11:25–36 [DOI] [PubMed] [Google Scholar]
- Williams B.R., Prabhu V.R., Hunter K.E., Glazier C.M., Whittaker C.A., Housman D.E., Amon A. 2008. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells.Science. 322:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.M., Baek K.H., Jeong S.J., Shin H.J., Ha G.H., Jeon A.H., Hwang S.G., Chun J.S., Lee C.W. 2004. WD repeat-containing mitotic checkpoint proteins act as transcriptional repressors during interphase.FEBS Lett. 575:23–29 [DOI] [PubMed] [Google Scholar]