Alpha Interferon Induces Long-Lasting Refractoriness of JAK-STAT Signaling in the Mouse Liver through Induction of USP18/UBP43 (original) (raw)
Abstract
Recombinant alpha interferon (IFN-α) is used for the treatment of viral hepatitis and some forms of cancer. During these therapies IFN-α is injected once daily or every second day for several months. Recently, the long-acting pegylated IFN-α (pegIFN-α) has replaced standard IFN-α in therapies of chronic hepatitis C because it is more effective, supposedly by inducing a long-lasting activation of IFN signaling pathways. IFN signaling in cultured cells, however, becomes refractory within hours, and little is known about the pharmacodynamic effects of continuously high IFN-α serum concentrations. To investigate the behavior of the IFN system in vivo, we repeatedly injected mice with IFN-α and analyzed its effects in the liver. Within hours after the first injection, IFN-α signaling became refractory to further stimulation. The negative regulator SOCS1 was rapidly upregulated and likely responsible for early termination of IFN-α signaling. For long-lasting refractoriness, neither SOCS1 nor SOCS3 were instrumental. Instead, we identified the inhibitor USP18/UBP43 as the key mediator. Our results indicate that the current therapeutic practice using long-lasting pegIFN-α is not well adapted to the intrinsic properties of the IFN system. Targeting USP18 expression may allow to exploit the full therapeutic potential of recombinant IFN-α.
Since their discovery in 1957, type I interferons (IFNs) have become valuable and widely used clinical drugs (13, 20). Alpha IFN (IFN-α) is used for the treatment of chronic hepatitis B and hepatitis C and of some forms of cancer, whereas IFN-β is effective for treating multiple sclerosis. With an estimated 3% of the global population affected, chronic hepatitis C (CHC) represents a major health concern since it can lead to liver cirrhosis and hepatocellular carcinoma (27). Treatment of CHC with recombinant IFN-α-2a or IFN-α-2b injected three times a week achieved sustained virological response (SVR) in 15 to 20% of patients. A major improvement of the SVR to 35 to 40% was observed by the addition of the broad-spectrum antiviral agent ribavirin (39). The introduction of a pegylated, long-acting form of IFN-α-2 (pegIFN-α-2) further increased the SVR to 50 to 55% of patients (12, 37). The reasons for the improved efficacy of pegIFN-α are not known, but it is assumed that the constant high serum concentrations achieved with pegIFN-α provide for uninterrupted antiviral activity through a permanent stimulation of the IFN signaling pathways, whereas the serum concentrations of standard IFN-α (with an elimination half-life of 4 to 10 h) decline below pharmacologically active levels in the second half of each 48 h dosing interval (4, 54). There is, however, no experimental evidence for the hypothesis that continuously high IFN-α concentrations achieve a better activation of the IFN-α-induced antiviral effector systems. In fact, there is evidence against this hypothesis provided by cell culture experiments.
It has been known for many years that cultured cells become refractory to IFN within hours and remain unresponsive for up to 3 days (26). Maximal activation of the IFN signaling pathways is observed within the first 2 h of IFN treatment. Continuous exposure to IFN results in a “desensitization” characterized by a return to pretreatment levels of IFN-stimulated gene (ISG) transcription. Moreover, during the 48 to 72 h after the initial IFN-α stimulation of the cells, any further IFN treatment fails to reinduce the transcription of ISGs. At present, it is not known, whether refractoriness also occurs during IFN therapies in patients. Such knowledge would be important for a rational design of IFN therapies. Dosing intervals shorter than the period of refractoriness would strongly reduce the efficacy of the administered IFN. Likewise, the use of modified IFN-α with a prolonged serum half-life such as pegIFN-α with the aim to achieve constant (peg)IFN-α serum concentrations would not increase efficacy either, if the target cells remain unresponsive during most of the dosing interval. Admittedly, the clinical experience showing an improved therapeutic response rate with pegIFN-α argues against the occurrence of desensitization in patients. To investigate whether the liver becomes refractory to IFN-α in vivo, we analyzed IFN-α signaling in mice repeatedly injected with IFN-α.
Type I IFNs (IFN-α and IFN-β) exert their effects through the JAK-STAT signaling pathway (9). Upon binding of IFN-α to its cell surface receptor (IFNAR), the receptor-associated tyrosine kinases JAK1 and TYK2 become activated and phosphorylate tyrosines on the cytoplasmic tails of IFNAR chains 1 and 2. The phosphorylated receptors provide specific docking sites for STAT1, -2, and -3. STATs are activated at the receptor-kinase complex by tyrosine phosphorylation (17, 50). Activated STATs dissociate from the receptor and translocate to the nucleus, where they act as transcription factors binding to specific regions in the promoters of ISGs (19). In response to IFN-α, STAT1-STAT2 heterodimers combine with IFN regulatory factor 9 (IRF9) to form the transcription complex ISGF3, which binds to IFN-stimulated response elements (ISREs) within the promoters of ISGs (15). IFN-α also activates homo- and heterodimers of STAT1 and STAT3, which bind to gamma-activated sequence (GAS) response elements (8).
The activation of the JAK-STAT pathway is tightly controlled by several negative regulatory mechanisms. SOCS1 and SOCS3 prevent STAT activation by inhibiting JAKs (25). Further downstream, the protein inhibitor of activated STAT 1 binds to hypomethylated STAT dimers and inhibits STAT-DNA interaction (30, 40, 49). STATs are deactivated by the nuclear phosphatase TC-PTP, followed by nuclear export (53). Recently, ubiquitin-specific peptidase 18 (USP18/UBP43) has been described as negative regulator in type I IFN signaling. USP18/UBP43 was originally identified as a protease cleaving ubiquitinlike modifier ISG15 from target proteins but was recently found to play a negative regulatory role independently of its ISG15-deconjugating ability (31, 33). By the use of molecular, biochemical, and genetic approaches, Malakhova et al. demonstrated that UBP43 specifically binds to the IFNAR2 receptor subunit and inhibits the activity of receptor-associated JAK1 by blocking the interaction between JAK1 and the IFN receptor (35). UBP43-deficient mice show a severe phenotype characterized by brain cell injury, poly(I-C) hypersensitivity, and premature death (36, 46). Interestingly, they are resistant to otherwise fatal cerebral infections with lymphocytic choriomeningitis virus and vesicular stomatitis virus (45) and have substantially lower hepatitis B virus DNA levels in a mouse model of acute hepatitis B (22). Importantly, USP18/UBP43 is elevated in livers of future nonresponders to pegIFN-α therapy (5, 47). Moreover, USP18/UBP43 silencing in cells with a replicating chimeric HCV genome results in deregulation of STAT1 signaling and potentiation of IFN′s ability to inhibit HCV-RNA replication (43).
To investigate the sensitivity of the liver during prolonged exposure to therapeutic concentrations of IFN-α, we treated mice repeatedly with subcutaneous (s.c.) injections of IFN-α and investigated IFN-α signaling in liver extracts. We report here that liver cells in vivo become refractory within hours after the first injection of IFN-α. The negative regulators of IFN-α signaling, SOCS, were found to be responsible for the early inhibition of STAT phosphorylation within the first 2 to 4 h but not for the long-term refractoriness. Rather, a long-lasting upregulation of USP18/UBP43 was found to cause unresponsiveness to prolonged IFN-α exposure. In the absence of USP18/UBP43, even a strong upregulation of SOCS1 did not prevent activation of STAT1 and STAT2.
Taken together, our results demonstrate a refractoriness of IFN-α signaling in vivo, and indicate that USP18/UBP43 plays a crucial role in the long-term desensitization of this signal transduction pathway in the mouse liver. Our findings have implications for the treatment of patients with CHC. Strategies aimed at restoring sensitivity to IFN-α, by targeting the upregulation of USP18/UBP43 in liver cells, could increase the efficacy of IFN-α therapies.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from BRL (Biological Research Laboratories, Füllinsdorf, Switzerland), interleukin-10 (IL-10)-deficient mice and Alb-Cre [strain B6.Cg-Tg(Alb-cre)21Mgn/J] transgenic mice were obtained from Jackson Laboratory, Bar Harbor, ME. STAT3 lox/lox mice and SOCS3 lox/lox mice were described previously (2, 6). STAT3 lox/lox and SOCS3 lox/lox mice were crossed to Alb-Cre transgenic mice to generate AlbCre+STAT3 lox/lox and AlbCre+ SOCS3 lox/lox conditional knockout (KO) mice, respectively. All transgenic mice were viable and fertile. _AlbCre_− STAT3 lox/lox and _AlbCre_− SOCS3 lox/lox littermates were used as negative controls in the experiments. The generation of _SOCS1_−/− _IFN_-γ−/− and _IFN_-γ−/− mice was described previously (1, 7, 52), as was the generation of _UBP43_−/− mice on a FVB background (23, 46). Genotyping for the Cre transgene was performed by PCR using the nucleotides Cre-1 (5′-CACCATTGCCCCTGTTTCACTATC-3′) and Cre-2 (5′-GCCAGGCGTTTTCTGAGCATAC-3′). Genotyping for the IL-10-deficient mice was performed by PCR using the nucleotides IL-10-1 (5′-GCCTTCAGTATAAAAGGGGGA CC-3′), IL-10-2 (5′-GTGGGTGCAGTTATTGTC TTCCCG-3′), and IL-10-Neo (5′-AATCCATCTTGTTCAATGGCCGATC-3′). STAT3 lox/lox genotyping was performed by using the primers APRF 11 Up (5′-CACCAACAC ATGCTATTTGTAGG-3′), APRF 11 Down (5′-CCTGTCTCTGACAGGCCATC-3′), and APRF 14 Down (5′-GCAGCAGAATACTCTACAGCTC-3′). SOCS3 lox/lox genotyping was performed with SR221 (5′-GAGTTTTCTCTGGGCGTCCTCCTAG-3′) and SR222 (5′-TGGTACTCGCTTTTGGAGCTGAA-3′). The animals were maintained on a 12-h day and 12-h night schedule with ad libitum access to food and drinking water. Mice were bred in a specific-pathogen-free environment. Procedures with the animals were conducted with the approval of the Animal Care Committee of the Canton Basel-Stadt, Switzerland. All _UBP43_−/− animals used in the studies were handled in accordance with guidelines of The Scripps Research Institute, and the procedures were approved by the Institutional Animal Care and Use Committee of the institute.
Six- to eight-week-old male animals were used for all experiments. The animals were anesthetized with isofluorane before blood was dawn from the tail vessels. The animals were euthanized by CO2 narcosis. The resected liver lobes were immediately frozen in liquid nitrogen and kept at −70°C until further processing; one lobe of liver was frozen in TRIzol for RNA isolation. The s.c. injections with phosphate-buffered saline (PBS) or mIFN-α were performed between 8:00 a.m. and 5:00 p.m. Recombinant mIFN-α was purchased from CalBiochem (Juro Supply GmbH, Switzerland). Pegylated human IFN-α-2b was provided by Essex Chemie AG, Lucerne, Switzerland. PBS was obtained from the University Hospital Basel. Mouse IL-10 monoclonal antibody was from Pierce (Perbio Science Switzerland SA, Lausanne, Switzerland) and was injected intraperitoneally at a dose of 100 μg 30 min prior to the mIFN-α injections.
ELISA.
To isolate serum from mIFN-α- or PBS-injected C57/BL6 mice, 20 to 30 μl of blood from mouse tail was collected at different time points, kept for 10 min at room temperature and for 30 min at 4°C, and then centrifuged at 2,500 × g for 20 min at 4°C. The supernatant was again spun at 1,500 × g for 10 min at 4°C. For measurement of mIFN-α, the serum was diluted 1:100 in dilution buffer, and an enzyme-linked immunosorbent assay (ELISA) was performed using a mouse interferon ELISA kit (Pierce/Perbio Science Switzerland SA, Lausanne, Switzerland) according to the manufacturer's instructions.
To measure the mouse IL-10 level, the serum was diluted 1:4 in dilution buffer and ELISA was performed by using a Quantakine mouse IL-10 immunoassay (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions.
Protein preparation and Western blot analysis.
Portions (30 to 50 mg) of liver tissue were homogenized in a buffer containing 100 mM NaCl, 50 mM Tris (pH 7.5), 1 mM EDTA, 0.1% TX-100, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM vanadate, and 1× protease inhibitor cocktail tablets (Roche Diagnostics GmbH, Mannheim, Germany). Samples were kept at 4°C for 30 min and centrifuged for 5 min at 15,000 rpm at 4°C. The protein concentration was determined by using a Lowry protein assay (Bio-Rad Laboratories AG, Reinach, Switzerland).
Then, 10 to 20 μg of total protein from mouse liver lysates was loaded for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Schleicher & Schuell, Switzerland). The membranes were blocked in 3% bovine serum albumin-3% milk-0.1% Triton X-100 for 1 h, washed with Tris-buffered saline-Tween 20, and incubated with the primary antibody overnight at 4°C.
Proteins were detected with primary antibody specific to phospho-STAT1 (Tyr 701) (catalog no. 9171) and phospho-STAT3 (Tyr 705) (catalog no. 9131; Cell Signaling, Bioconcept, Allschwil, Switzerland) and phospho-STAT2 (Tyr 689) (catalog no. 07-224; Upstate, Lake Placid, NY). STAT1 p84/p91 (sc-346), STAT2 (sc-950), and STAT3 (sc-482) were purchased from Santa Cruz (LabForce AG, Nunningen, Switzerland). Mouse monoclonal STAT1-Ab (carboxy terminus; catalog no. 610186) was from Transduction Laboratories, BD Biosciences, Pharmingen. Anti-SOCS-1 (ab3691) was purchased from Abcam, Cambridge, United Kingdom. Anti-β-actin was from Sigma-Aldrich Chemie GmbH, Steinheim, Germany. Blot-FastStain was obtained from Geno Technology, Inc. (Cell Concepts GmbH, Umkirch, Germany).
After three washes with Tris-buffered saline-Tween 20, the membranes were incubated with anti-rabbit antibody-horseradish peroxidase conjugate and anti-mouse antibody-horseradish peroxidase conjugate obtained from Cell Signaling (Bioconcept, Allschwil, Switzerland), and signals were detected with SuperSignal West Pico chemiluminescent substrate (Pierce/Perbio Science Switzerland SA). Alternatively, signals were detected by using an Odyssey infrared imaging system from Li-Cor after incubation with infrared fluorescent secondary goat anti-mouse (IRDye 680) or anti-rabbit (IRDye 800) antibodies (both from Li-Cor Biosciences) for 1 h at room temperature. The infrared image was obtained in a single scan, and the signal was quantified by using the integrated intensity.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts from 150 to 200 mg of liver tissue were prepared as previously described (16). Nuclear extract (5 to 10 ng of protein sample) aliquots (2 μl) were incubated with a 32P-radiolabeled mutated serum-inducible element oligonucleotide designated m67-hSIE with the sequence 5′-CATTTCCCGTAATCAT-3′ for STAT1 and STAT3 or the ISRE probe derived from the IFN-stimulated gene 15 promoter (ISRE-015) with the sequence 5′-GAAAGGGAAACCGAACTGAAGC-3′ (18). For supershift experiments, 1 μl of antibody specific for STAT1, STAT2, or STAT3 was added to the gel shift incubation reactions.
The samples were loaded on a 5% nondenaturing polyacrylamide gel, and electrophoresis was performed for 4 h at 400 V at 4°C. The gel was dried and visualized by autoradiography.
RNA isolation and Northern blot analysis.
RNA was purified using TRIzol (TriReagent) provided by the Molecular Research Center, Inc. (Lucerna Chem AG, Lucerne, Switzerland). RNA was divided into aliquots and stored at −75°C. The denatured RNA was separated on a 1.2% agarose formaldehyde MOPS gel and transferred to a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech Europe GmbH, Dübendorf, Switzerland) by capillary diffusion using 20× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membranes were hybridized to 32P-labeled SOCS1 and SOCS3 probes at 65°C for overnight in a Quickhyb oven (Stratagene Europe, Switzerland) and washed twice with 2× SSC-0.2% SDS at 42°C for 15 min and then twice with 0.2× SSC-0.1% SDS at 42°C for 10 min. The results were visualized by autoradiography.
Real-time quantitative reverse transcription-PCR (RT-PCR).
RNA was purified from frozen liver tissue (<20 mg) with NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. RNA was divided into aliquots and stored at −75°C. RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Promega Biosciences, Inc., Wallisellen, Switzerland) in the presence of random hexamers (Promega) and deoxynucleoside triphosphate. The reaction mixture was incubated for 5 min at 70°C and then for 1 h at 37°C. The reaction was stopped by heating at 95°C for 5 min. SYBR-PCR was performed based on SYBR green fluorescence (SYBR green PCR master mix; Applied Biosystems, Foster City, CA). The Δ_CT_ value was derived by subtracting the threshold cycle (CT) value for mouse ribosomal protein L19 (mRPL19), which served as an internal control, from the CT values for SOCS1, SOCS3, protein kinase R (PKR), and USP18, respectively. The primers were designed across exon-intron junctions to prevent influence from genomic DNA amplification. The primers were 5′-ATCCGCAAGCCTGTGACTGT-3′ and 5′-TCGGGCCAGGGTGTTTTT-3′ for mRPL19 and 5′-GTGGTTGTGGAGGGTGAGATG-3′ and 5′-GGGATGAGGTCTCCAGCCA-3′ for mSOCS1. The primers were 5′-CCTTTCTTATCCGCGACAGC-3′ and 5′-CGCTCAACG TGAAGAAGTGG-3′ for mSOCS3. Primers were 5′-CGTGCTTGAGAGGGTCATTTG-3′ and 5′-GGTCCGGAGTCCACAACTTC-3′ for mUSP18 and 5′-AAGAGCCCGCCGAAAACT-3′ and 5′-AGCCACTGAATGTAGATGTGACAAC-3′ for mPKR.
All reactions were run in duplicate by using an ABI 7000 sequence detection system (Applied Biosystems). The mRNA expression level between T0 and T_n_ was expressed as an n_-fold increase according to the formula 2Δ_CT(T0)−Δ_CT_(T_n_). The mRNA expression levels of the transcripts were calculated relative to mRPL19 from the Δ_CT_ values using the formula 2−Δ_CT_.
RESULTS
Pharmacokinetics of mouse IFN-α.
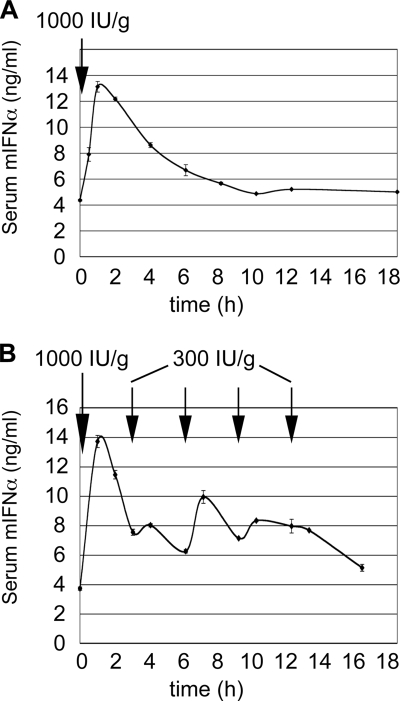
We first studied the pharmacokinetics of s.c. administered mIFN-α by measuring the mIFN-α serum concentration in mice at different time points after injection (Fig. 1). A single dose of 1,000 IU/g of body weight resulted in a fast increase of mIFN-α from basal 4.3 ± 0.07 (mean ± the standard error of the mean [SEM]) to 13.1 ± 0.41 ng/ml within 60 min, followed by a decline to pretreatment levels 8 h after the injection (Fig. 1A). The serum mIFN-α half-life was estimated as 4 to 5 h. To achieve constantly elevated serum mIFN-α concentrations like those obtained with human pegIFN-α, we used a priming dose of 1,000 IU/g, followed by repeated injections of 300 IU/g, and this approach led to IFN-α serum concentrations oscillating between 6 and 14 ng/ml for up to 16 h (Fig. 1B).
FIG. 1.
Pharmacokinetics of mIFN-α injection into mice. (A) Serum concentrations of mIFN-α after s.c. injection of a single dose of 1,000 IU/g. The profile was obtained from two C57/BL6 mice receiving mIFN-α by s.c. injection. The peak of serum concentration was reached 1 h after the injection. After 8 h, the concentration of mIFN-α returned to pretreatment levels. The error bars represent the SEM. (B) Serum concentrations of mIFN-α after multiple injections. Two C57/BL6 mice were injected s.c. with 1,000 IU of mIFN-α/g as a priming dose and four times with 300 IU/g as a maintenance doses every 3 h. Sera were collected immediately before each injection and one h after each injection. The black arrows indicate time points of mIFN-α injections. The error bars represent the SEM.
IFN-α signaling in the mouse liver.
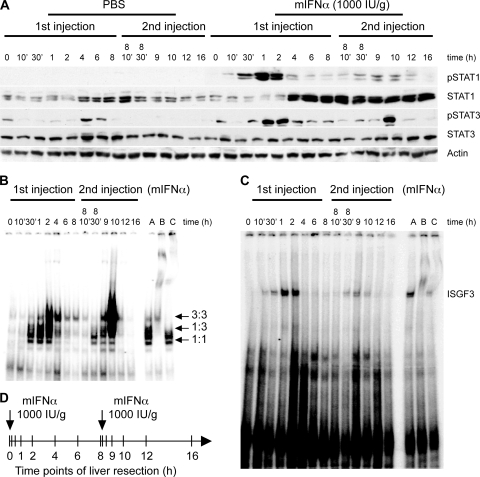
To study the kinetics of IFN-α induced activation of the JAK-STAT pathway, we sacrificed mice at different time points after mIFN-α injections and analyzed the activation of pathway components in extracts of resected livers. The first applied mIFN-α injection scheme was based on the use of two doses of 1,000 IU/g given at an 8-h interval (Fig. 2D). In this setting, the second injection was given at the time point when serum mIFN-α returned to baseline levels (see Fig. 1A); this approach simulates the clinical setting of treatment of patients with CHC with standard IFN-α, where IFN-α serum concentrations decline below pharmacologically active levels in the second half of each 48-h dosing interval (4, 54). Analysis of the response to mIFN-α revealed a strong phosphorylation of STAT1 within 30 min after the first injection (Fig. 2A). STAT1 activation reached its maximum after 1 to 2 h and then declined within 4 h. The second injection at 8 h induced very little STAT1 phosphorylation, although the amount of STAT1 in the liver was strongly and persistently elevated in response to the first mIFN-α injection (Fig. 2A). Moreover, in an independent long-term experiment with seven injections given every 8 h, we did not observe restoration of IFN-α sensitivity for up to 48 h (data not shown). To control for circadian variations and stress, mice were injected at the same time points with PBS (Fig. 2A). Although there was some variation in STAT1 expression during the experiment, the levels were much lower than those induced by mIFN-α. As expected, PBS did not induce STAT1 phosphorylation. Treatment with mIFN-α also resulted in STAT3 phosphorylation in liver cells. The maximal activation occurred at 1 to 2 h and, in contrast to STAT1, the activation pattern was similar after the second injection. There was no upregulation of STAT3 expression after mIFN-α treatment (Fig. 2A).
FIG. 2.
IFN-α induces refractoriness of JAK-STAT signaling in the mouse liver. (A) C57/BL6 mice were treated with two injections of mIFN-α (1,000 IU/ml) or PBS, according to the time course indicated in panel D. IFN-α-induced tyrosine phosphorylation of STAT1 and STAT3 was assessed by immunoblotting of liver whole-cell extracts. Blots were stripped and reprobed for total STAT1 and STAT3 and for actin as a loading control. (B) DNA binding of STAT1 and STAT3 homo- and heterodimers upon stimulation with mIFN-α. Nuclear extracts of liver cells obtained from two-dose mIFN-α injected mice were analyzed in EMSAs with the SIE-m67 oligonucleotide probe. Nuclear extract from mouse liver 1 h after mIFN-α stimulation was used as a positive control (lane A). Antisera specific to STAT1 (lane B) and STAT3 (lane C) were used to shift the STAT1 homodimers, STAT3 homodimers, and STAT1-STAT3 heterodimers (all marked with arrows). (C) DNA binding of ISGF3 in liver cells after two-dose mIFN-α treatment. Liver nuclear extracts of C57/BL6 mice were analyzed in EMSAs using ISRE oligonucleotide probes. Nuclear extract from mouse liver 1 h after mIFN-α stimulation was used as a positive control (lane A), and shifts of the ISGF3 band were performed by using antiserum specific for STAT1 (lane B) and STAT2 (lane C). (D) Schematic diagram showing the time course of the experiment with two injections of mIFN-α. The interval between the injections is 8 h and the vertical bars in the diagram indicate time points of animal sacrifice and liver resection.
These results were confirmed by EMSA. Phosphorylated STATs form dimers that can translocate into the nucleus and bind specific response elements such as the GAS element. The first injection of mIFN-α induced a strong STAT1 homodimer gel shift using the m67-SIE oligonucleotide (a GAS element), whereas the second injection had little effect (Fig. 2B). STAT3 homodimers were strongly induced after both the first and the second injections. IFN-α also induces ISGF3, a heterotrimeric transcription factor composed of activated STAT1 and STAT2, and IRF9. Similarly to STAT1 homodimer formation, ISGF3 activity was induced only after the first, but not after the second injection of mIFN-α (Fig. 2C). Taken together, these data indicate that IFN-α induced JAK-STAT signaling in the mouse liver is transient and, furthermore, refractory to a second mIFN-α dose applied 8 h after the first dose, at a time point when serum concentrations of mIFN-α have returned to pretreatment levels.
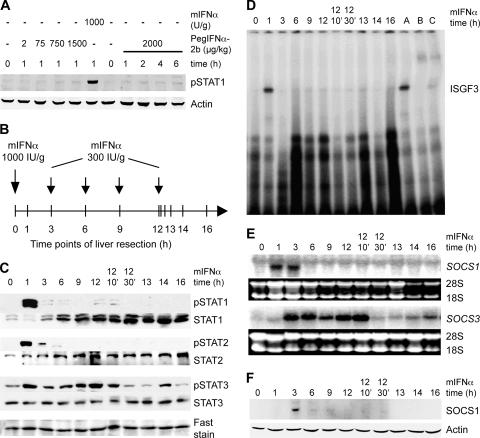
We hypothesized that this long-lasting refractoriness of the signal transduction pathway could explain a limited success of anti-HCV therapies where standard IFN-α injected 48 h after the previous dose encountered still refractory signaling components. Since pegIFN-αs with their long half-life are therapeutically more potent, we next analyzed whether the continuous presence of high serum concentrations of IFN-α prevented the induction of refractoriness. Pegylated mIFN-α is not available, but since human pegIFN-α-2b was reported to exert antiviral effects in SCID mice infected with Modoc virus (a flavivirus) (28), we investigated whether human pegIFN-α induces STAT1 phosphorylation in the mouse liver. We injected mice s.c. with pegIFN-α-2b in doses ranging from 2 to 2,000 μg/kg and found no STAT1 activation within 6 h of treatment (Fig. 3A). Because human pegIFN-α did not stimulate JAK-STAT signaling in the mouse liver, we applied the second mIFN-α injection scheme, in which mice were injected with 1,000 IU of mIFN-α/g as a priming dose, followed by four injections of 300 IU of mIFN-α/g as maintenance doses (Fig. 3B). With this regimen, steadily elevated mIFN-α concentrations were maintained for up to 16 h mimicking the pharmacokinetics of pegIFN-α in patients (Fig. 1B). Mice were sacrificed at different time points (Fig. 3B), and IFN-α signaling was analyzed with Western blots and EMSAs. There was strong but transient phosphorylation of STAT1 and STAT2 1 h after initial injection of mIFN-α, but subsequent injections failed to induce further STAT1 phosphorylation, although STAT1 expression was highly upregulated (Fig. 3C). Accordingly, the ISGF3 gel shift signal was only detectable at 1 h after the initial injection (Fig. 3D). In contrast to STAT1 and STAT2, activation of STAT3 was prolonged in the continuous presence of mIFN-α (Fig. 3C). In conclusion, IFN-α treatment of mice induced a strong initial activation of STAT1 and STAT2, followed by a rapid inhibition of signaling and a persistent refractoriness also in the continuous presence of IFN-α.
FIG. 3.
Refractoriness of JAK-STAT signaling in the presence of continuously elevated mIFNα. (A) C57/BL6 mice were treated with human pegIFN-α-2b s.c. for 1 h at increasing doses (lanes 1 to 5) and for up to 6 h with 2,000 μg/kg (body weight) (lanes 7 to 11). Injection of mIFN-α served as positive control (lane 6). (B) Schematic diagram showing the time course of the multiple injection experiment. Mice were injected with 1,000 IU of mIFN-α/g as a priming dose, followed by four injections of 300 IU of mIFN-α/g as maintenance doses. The vertical bars in the diagram indicate the time points of animal sacrifice and liver resection. (C) The phosphorylation of STAT1, STAT2, and STAT3 was assessed by Western blotting with extracts of mouse liver after mIFN-α treatment. Blots were stripped and re blotted for total STAT1, STAT2, and STAT3 and stained with Blot-FastStain as a loading control. (D) DNA binding of ISGF3 in liver cells after continuous mIFN-α treatment. Liver nuclear extracts of C57/BL6 mice were analyzed in EMSAs using ISRE oligonucleotide probes. Extract from mouse liver 1 h after mIFN-α stimulation was used as a positive control (lane A), and shifts of the ISGF3 band were performed by using antibody specific for STAT1 (lane B) and STAT2 (lane C). (E) SOCS1 and SOCS3 mRNA expression level in the continuous presence of mIFN-α. Total RNA from mIFN-α-injected mice was prepared and subjected to Northern blot analysis for SOCS1 and SOCS3 mRNA expression. Equal loading of the gel was verified by ethidium bromide staining and comparing the intensities of the 28S rRNA and 18S rRNA bands. (F) SOCS1 protein expression was determined by Western blotting with whole-cell extracts of mouse liver after mIFN-α treatment.
Negative regulation of IFN-α signaling by SOCS1 and SOCS3 in the mouse liver.
Within hours, IFN-α induces the transcriptional upregulation of SOCS1 and SOCS3, two negative regulators of the JAK-STAT pathway that are instrumental for the termination of STAT phosphorylation at the receptor-kinase complex (25). We therefore tested whether the long-term refractoriness of the IFN signal transduction system in mouse liver is due to a continuous high-level expression of SOCS proteins. SOCS1 mRNA was detectable at 1 and 3 h, but not during later time points (Fig. 3E) despite the continuously high serum concentrations of mIFN-α. SOCS1 protein was upregulated at 3 h but was barely detectable at later time points (Fig. 3F). Induction of SOCS3 showed a different pattern. In the continuous presence of high mIFN-α levels, SOCS3 mRNA expression was induced after 3 h and remained substantially elevated for an extended period of time (Fig. 3E). The observed SOCS3 upregulation could be caused by the prolonged STAT3 activation, because STAT3 is a transcriptional inducer of the SOCS3 gene (3). Since SOCS3 is known to inhibit IFN-α-induced STAT1 phosphorylation (51), the prolonged in vivo refractoriness of the IFN system might be caused by the observed SOCS3 induction.
Role of IL-10, STAT3, SOCS3, and SOCS1 in the long-term refractoriness of IFN-α signal transduction.
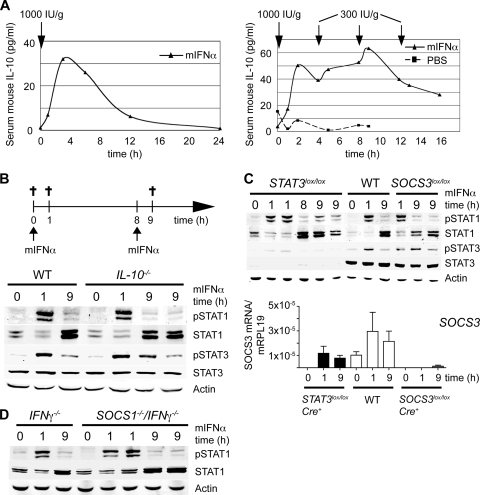
Because signaling through the IFN-α receptor-kinase complex is inhibited by SOCS3, the signals that maintain high STAT3 phosphorylation and SOCS3 expression cannot be transmitted through the IFN receptor but rather have to be derived from a cytokine receptor that is independent of SOCS3. IL-10 is an attractive candidate, because it is a strong activator of STAT3 and inducer of SOCS3 and, importantly, the IL-10 receptor is not inhibited by SOCS3 (55). Furthermore, IL-10 inhibits expression of IFN-α induced genes by suppressing STAT1 phosphorylation in monocytes (21) and attenuates IFN-α-induced STAT1 phosphorylation in the mouse liver (48). We therefore measured mouse IL-10 serum levels upon a single injection or multiple injections of mIFN-α and indeed found strong induction of IL-10 (Fig. 4A). After a single mIFN-α injection, the IL-10 serum concentrations were transiently elevated (Fig. 4A, left panel), but in the setting of multiple injections with the resulting constantly elevated serum IFN-α concentration, the IL-10 levels remained high (Fig. 4A, right panel). This indicates that the IFN-α induced pathways that lead to elevated serum IL-10 do not become refractory.
FIG. 4.
IL-10, STAT3, SOCS3, and SOCS1 are not responsible for refractoriness of IFN-α-induced JAK-STAT signaling. (A) In the left panel, injection of a single s.c. dose of mIFN-α (1,000 IU/g) induces a transient increase in IL-10 serum concentrations. The level of mouse IL-10 as determined by ELISA is shown. In the right panel, repeated injections of 300 IU of mIFN-α/g every 4 h after an initial priming dose of 1,000 IU/g induce constantly high mouse IL-10 serum concentrations. During mIFN-α maintenance doses, the concentration of IL-10 stays at levels of ∼40 pg/ml (black triangles). As a negative control, the level of serum IL-10 was determined in a mouse multiply injected with PBS (see the graph with black squares). (B) STAT1 activation in response to two injections of mIFN-α in IL-10-deficient mice. The upper panel shows a schematic representation of the experimental design with two mIFN-α injections performed 8 h apart. Animals were sacrificed at time points 0 (control) and 1 h and 9 h (1 h after the second injection). Immunoblotting for pSTAT1/STAT1, pSTAT3/STAT3, and actin is shown for C57BL/6 WT mice and for _IL-10_−/− animals. (C) The upper panel shows results for STAT activation in response to two injections of mIFN-α in mice with liver-specific KO of STAT3 or SOCS3. Immunoblotting for pSTAT1/STAT1, pSTAT3/STAT3, and actin is shown for WT C57BL/6 mice and for SOCS3 lox/lox Cre+ and STAT3 lox/lox Cre+ animals. The lower panel presents the results of quantitative RT-PCR analysis of SOCS3 mRNA expression in the livers of WT, SOCS3 lox/lox Cre+, and STAT3 lox/lox Cre+ animals at time points 0, 1 h, and 9 h (1 h after the second injection). The data are plotted as the amount of SOCS3 mRNA relative to mRPL19 mRNA and are means (with the SEM) of two animals per time point for the KO mice and four animals per time point for the WT mice. (D) STAT activation in response to two injections of mIFN-α in mice deficient for SOCS1. Immunoblotting for pSTAT1/STAT1 and actin is shown for _IFN_-γ−/− mice and for _IFN_-γ−/− _SOCS1_−/− mice.
To clarify the role of IL-10 in the observed refractoriness of IFN-α signaling, we used IL-10-deficient mice and injected them with two doses of mIFN-α given 8 h apart (Fig. 4B, upper panel). STAT1 activation in these mice was assessed 1 h after the first and the second injections (Fig. 4B, lower panel). While the first mIFN-α injection induced a strong phospho-STAT1 signal after 1 h, the second injection 8 h later had little effect on STAT1 phosphorylation (time point 9 h) both in the wild-type (WT) and the IL-10-deficient mice. These results were confirmed in WT mice injected with neutralizing IL-10 antibody 30 min prior to mIFN-α injections (data not shown). This indicates that refractoriness of IFN-α signaling is not IL-10 dependent. Of note, there was no decrease of STAT3 phosphorylation in the IL-10-deficient mice compared to the WT mice. We conclude that the elevated serum IL-10 concentrations induced by mIFN-α injections are not required for the prolonged activation of STAT3 and do not cause refractoriness.
To test whether the activation of STAT3 or the upregulation of SOCS3 are required for the induction of the refractory state, we used hepatocyte-specific STAT3- and SOCS3-deficient mice. Toward this aim, we crossed STAT3 lox/lox mice (2) and SOCS3 lox/lox mice (6) with albumin-Cre transgenic mice (42). The IFN-α signal transduction pathway was assessed in these mice by using the two-dose mIFN-α injection setting (Fig. 4B, upper panel). Neither the deletion of STAT3 nor the deletion of SOCS3 could restore responsiveness to the second injection of mIFN-α (Fig. 4C), arguing against a substantial role of STAT3 and SOCS3 as mediators of IFN-α refractoriness.
SOCS1 mRNA and protein levels were elevated only at early time points of the 16-h kinetic analysis (Fig. 3B, E, and F); it was therefore unlikely that this negative regulator mediates long-term refractoriness. We nevertheless wanted to directly test if long-term refractoriness is mediated by SOCS1. Mice deficient in SOCS1 are not viable due to hypersensitivity to IFN-γ (52). We therefore analyzed the role of SOCS1 in SOCS1_−/−/IFN_-γ−/− mice (and _IFN_-γ−/− mice as controls) using the two-dose mIFN-α injection setting (Fig. 4B, upper panel). _IFN_-γ−/− mice showed similar pSTAT1 signaling in response to mIFN-α if compared to WT mice and, as expected, the deletion of SOCS1 did not prevent refractoriness in response to a second injection of mIFN-α (Fig. 4D).
Prolonged upregulation of USP18/UBP43 is responsible for IFN-α refractoriness.
Recently, USP18/UBP43 emerged as an important negative regulator in type I IFN signaling (31, 33, 35, 43). We therefore measured USP18 mRNA levels in livers of WT, IL-10-deficient, and hepatocyte-specific STAT3- and SOCS3-deficient mice after two injections of mIFN-α. In all of these mouse strains, USP18 mRNA was strongly upregulated 1 h after the first injection and also 1 h after the second injection (time point 9 h) (Fig. 5A). In the setting of repeated injections (Fig. 3B), leading to constantly elevated serum mIFN-α concentrations, USP18 mRNA was upregulated 1 h after the first injection and remained induced more than fivefold at all later time points for up to 16 h (data not shown). This expression profile suggests an involvement of USP18/UBP43 in the induction and maintenance of a refractory state of the IFN-α signaling pathway in the liver.
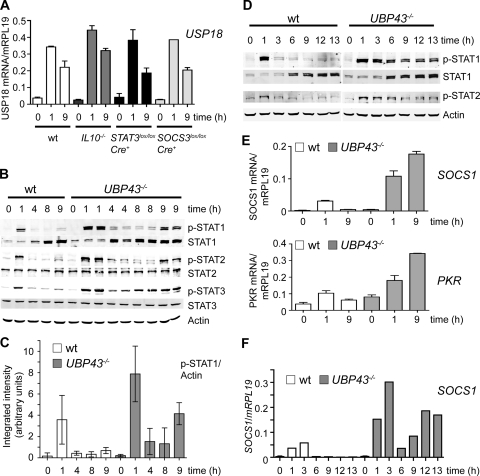
FIG. 5.
USP18/UBP43-deficient mice remain responsive to IFN-α. (A) Quantitative RT-PCR analysis of USP18 mRNA expression in response to the first and second injections of mIFN-α. RNA was isolated from the livers of WT, _IL-10_−/−, SOCS3 lox/lox Cre+, and STAT3 lox/lox Cre+ animals at time points 0, 1 h, and 9 h (1 h after the second injection). The data are plotted as the amount of USP18 mRNA relative to mRPL19 and are means (with the SEM) of two animals per time point for the KO mice and four animals per time point for the WT mice. (B) Activation of the JAK-STAT signaling pathway in response to the first and second injection of mIFN-α in WT and USP18/UBP43-deficient mice. Phosphorylation of STAT1, STAT2 (upper band), and STAT3 was assessed by Western blotting with extracts of mouse liver from WT and _USP18/UBP43_−/− mice. Blots were stripped and reblotted for total STAT1, STAT2, and STAT3 and for actin as a loading control. (C) Bar diagram of phospho-STAT1 signals in WT and USP18/UBP43-deficient mice in response to the first and second injections of mIFN-α. Phospho-STAT1 and actin signals from three mice per time point were quantified by using an Odyssey infrared imaging system. The integrated intensities of phospho-STAT1 signals were divided by the actin values. The y axis displays arbitrary units. Shown are mean values and the 95% confidence intervals. (D) STAT activation in response to continuous presence of mIFN-α in WT and USP18/UBP43-deficient mice. Phosphorylation of STAT1 and STAT2 (upper band) was assessed by Western blotting with extracts of mouse liver from WT and _USP18/UBP43_−/− mice. Blots were stripped and reblotted for total STAT1 and for actin as loading control. (E) Quantitative RT-PCR analysis of SOCS1 and PKR mRNA expression in response to the first and the second injections of mIFN-α in WT and in USP18/UBP43-deficient mice. Two mice were sacrificed per time point. Shown are mean values (with the SEM) of the ratio of SOCS1 and PKR mRNA over mRLP19 mRNA. (F) Quantitative RT-PCR analysis of SOCS1 mRNA expression in response to continuous stimulation with mIFN-α in WT mice and in USP18/UBP43-deficient mice.
We therefore assessed IFN-α signaling in livers of USP18/UBP43-deficient mice (36, 46). In the two-dose injection setting, _UBP43_−/− mice showed a 2.2-fold stronger STAT1 phosphorylation after the first injection of mIFN-α than WT controls (Fig. 5B and C). This finding is consistent with previous in vitro findings in mouse embryonic fibroblasts lacking UBP43 and in human hepatoma Huh7.5 cells where silencing of USP18/UBP43 leads to prolonged responsiveness to IFN-α and enhanced antiviral efficacy against HCV (35, 43). Importantly, _UBP43_−/− mice were responsive also to the second injection of mIFN-α and showed significant increase of pSTAT1 signals at 9 h if compared to the 8-h time point (Fig. 5B and C). The pSTAT1 signals were increased at all time points in _UBP43_−/− mice, again supporting the important negative regulatory role of UBP43 in IFN-α signaling. Of note, the pSTAT1 signal at 9 h was not as strong as at 1 h. We conclude that UBP43 is not the only mediator of long-term refractoriness. However, UBP43 is a very important component of long-term refractoriness, because the second dose of mIFN-α cannot only induce a pSTAT1 signal that is significantly stronger than at time point 8 h, but is as strong as the pSTAT1 signal observed after a first injection in a WT mouse (Fig. 5B and C; compare the time point 1 h in the WT mice to the time point 9 h in the _UBP43_−/− mice). Supporting the role of UBP43 in long-term refractoriness, we observed a persistent phosphorylation of STAT1 and STAT2 for up to 13 h in _UBP43_−/− mice that were repeatedly injected with mIFN-α (Fig. 5D; see Fig. 3B for experimental setup). We conclude that IFN-α refractoriness is associated with the presence of USP18/UBP43 in the liver and that absence of USP18/UBP43 allows for a substantial stimulatory effect of the second mIFN-α dose, as well as of maintenance doses in the setting of repeated injections.
To investigate whether maintained phosphorylation of STATs in _UBP43_−/− mice is reflected by maintenance of IFN-α target gene induction, we measured SOCS1 and PKR expression in WT and USP18/UBP43-deficient mice. While in WT mice SOCS1 and PKR mRNA were induced only in response to the first injection, _UBP43_−/− mice were hyper-responsive at 1 h and, at 9 h, when SOCS1 and PKR mRNA were no longer inducible in WT mice, their levels even further increased in _UBP43_−/− mice (Fig. 5E). Remarkably, despite these high SOCS1 mRNA levels at 9 h, all IFN-α stimulated STATs showed a strong phosphorylation (Fig. 5B). Similarly, continuous stimulation with mIFN-α resulted in continuously elevated mRNA levels of SOCS1 in _UBP43_−/− mice (Fig. 5F). We therefore conclude that in the absence of USP18/UBP43, SOCS1 cannot inhibit IFN-α-induced phosphorylation and activation of STAT1, STAT2, and STAT3. This provides a strong argument for the importance of USP18/UBP43 as negative regulator of IFN responses in the liver.
DISCUSSION
Desensitization of the IFN signal transduction pathways during prolonged exposure of cultured cells to IFN-α has been described more than 20 years ago (26), but very little was known regarding whether and to what extent IFN refractoriness occurs in animals and humans. Infections that activate the endogenous type I IFN system usually last for several days and weeks and can even last for years, such as chronic viral hepatitis. Intuitively, one would assume that the IFN system remains responsive and effective, at least in all those situations in which the infection is being cleared. In the present study we present strong evidence that the IFN-α signaling pathways in mouse liver become unresponsive within hours after the first application of mIFN-α, indicating that desensitization may also occur upon clinical use of IFN and negatively influence the therapeutic outcome.
Refractoriness was observed in mice that received multiple injections of mIFN-α and had sustained serum IFN-α levels between 6 and 14 ng/ml, i.e., concentrations that induce a strong STAT1 activation before the initiation of the refractory state (see 30-min time points in Fig. 1A and 2A). The repeated injection scheme was applied to mimic the constant high pegIFN-α serum levels observed in CHC patients, because pegylated mouse IFN-α is not available and human pegIFN-α does not activate the JAK-STAT pathway in mouse liver (Fig. 3A). We cannot formally prove that pegylated mouse IFN-α would induce a refractory state of the IFN signaling pathway. However, pegIFN-α binds to the same receptor and uses the same signaling pathway as unmodified IFN-α and is therefore very likely to also induce the same negative regulators. Refractoriness was also observed in mice that received two mIFN-α doses, i.e., a second injection 8 h after the initial dose, and thus at a time when mIFN-α serum concentrations returned to pretreatment levels. The refractory state was characterized by an almost complete inhibition of tyrosine phosphorylation of STAT1 and STAT2. The residual STAT1 and STAT2 activation documented by the faint phospho-STAT1 and -STAT2 signals detected in Western blots was not sufficient to induce target genes, such as SOCS1 and PKR. One possible explanation may be provided by the IFN-α-induced increase of total STAT1 and, to a lesser extent, STAT2 protein amounts, which further reduced the ratio of phosphorylated to unphosphorylated STATs. The induction pattern of SOCS1 mRNA with a peak at 1 to 3 h is consistent with its well-known role in the early negative-feedback regulation of IFN-α signaling. Since SOCS1 is not expressed to any detectable degree at later time points (Fig. 3E and F), its involvement in the long-lasting inhibition of STAT1 and STAT2 phosphorylation is unlikely and, indeed, SOCS1-deficient mice exhibit refractoriness to a second dose of mIFN-α (Fig. 4D).
STAT3 can be activated by IFN-α to form transcriptionally active homodimers or STAT1-STAT3 heterodimers (14). Interestingly, STAT3 showed an activation pattern that differed from STAT1 and STAT2. STAT3 was maximally phosphorylated after 1 h and remained activated at most time points during the course of the multiple injection experiment (Fig. 3C). Accordingly, expression of SOCS3, a known target gene of STAT3, was also upregulated during the entire experiment (Fig. 3E). Assuming that SOCS3 inhibits IFN-α signaling in the mouse liver, as has been reported in cultured cells (51), the continuous activation of STAT3 cannot be due to IFN-α-induced signals. Among other cytokines known to stimulate STAT3 activation, IL-10 was an attractive candidate, particularly because its receptor-kinase complex is not inhibited by SOCS3 (55). IFN-α not only exerts direct antiviral effects against HCV but also plays an important immunomodulatory role in chronic HCV infection. IL-10 as an immunosuppressive cytokine is potentially implicated in the treatment outcome in CHC. For instance, IL-10 production was substantially increased in PBMCs from CHC patients obtained 12 h after the first injection of IFN-α-2 when in vitro stimulated with lipopolysaccharide or the HCV protein NS3 (32). Blocking of the IL-10 receptor, in turn, was shown to generate favorable T-helper cell responses in vitro in PBMCs originating from CHC patients (44). Interestingly, baseline IL-10 levels were significantly increased in patients with CHC and no response to IFN-based treatment compared to responders and healthy control subjects (38). Likewise, production of IL-10 during LCMV infection in mice was associated with viral persistence, and blockade of the IL-10 receptor resulted in viral clearance (11). Our novel finding of high IL-10 levels in mouse sera in response to repeated mIFN-α injections was therefore a very promising candidate mechanism for explaining refractoriness of IFN-α signal transduction. However, we found induction of a refractory state in IL-10-deficient mice, indicating that IL-10 is not responsible for IFN-α refractoriness (Fig. 4B). Likewise, mice with liver-specific deficiency in STAT3 and SOCS3 were refractory to prolonged mIFN-α stimulation (Fig. 4C), a finding that further argues against an important role of the STAT3-SOCS3 axis in the induction of IFN-α refractoriness.
USP18/UBP43 was originally identified as a protease cleaving ubiquitinlike modifier ISG15 from target proteins. ISG15 is an ubiquitinlike protein that conjugates to numerous proteins in cells treated with IFN-α. The negative regulatory role of UBP43 in IFN-α signaling was initially thought to be mediated through its ISG15-deconjugating ability (31, 33). However, ablation of ISG15 did not reverse the IFN-hypersensitive phenotype of _UBP43_−/− mice (24, 41). Moreover, IFN-α-induced STAT1 phosphorylation and ISG induction were inhibited by an active site cysteine mutant (c61s) of UBP43, UBP43C61S, which is no longer enzymatically active (35). Indeed, USP18/UBP43 blocks JAK1 phosphorylation through a specific interaction with the IFNAR2 subunit of the receptor and thereby attenuates IFN signaling independent of its isopeptidase activity toward ISG15 (35). USP18/UBP43 is induced by IFN-α (10, 34) and provides a negative-feedback loop that restricts IFN-α signals. In the liver, USP18/UBP43 shows a low constitutive expression (31), and we found a strong upregulation of USP18 mRNA after treating mice with s.c. injections of mIFN-α (Fig. 5A). In contrast to SOCS1 with its transient upregulation in response to the first injection of mIFN-α (Fig. 3E), USP18/UBP43 was highly induced also 1 h after a second injection of mIFN-α (Fig. 5A) and remained fivefold increased for up to 48 h (data not shown). Since the apparent half-life of USP18 mRNA is 3 to 4 h (29), this prolonged upregulation of USP18/UBP43 requires continuous transcriptional activation of its gene, possibly sufficiently induced by the very weak STAT1 activity observed after a second injection of mIFN-α (Fig. 2A). This would implicate that the USP18 gene promoter is more sensitive to STAT1 stimulation than promoters of other ISGs, i.e., of SOCS1 (Fig. 5E). Whatever the mechanism that maintains its prolonged upregulation, UBP43 is clearly important for the induction of IFN-α refractoriness, since USP18/UBP43-deficient mice remain sensitive to continuous stimulation with mIFN-α (Fig. 5B to F). It is interesting in this context that USP18 mRNA expression, but not SOCS1 expression, is increased in the livers of “preactivated” future nonresponders to pegIFN-α treatment (5, 47). USP18/UBP43 therefore is of special interest not only as predictor of treatment outcome but may also be a potentially critical determinant of responses to pegIFN-α in patients with CHC.
USP18/UBP43 restricts the IFN-β-induced upregulation of more than 700 genes, among them SOCS1 (56). Silencing of USP18 in Huh7.5 cells leads to increased cellular protein ISGylation in response to IFN-α and a general enhancement of ISG expression (43). Indeed, SOCS1 was highly expressed in the liver of _UBP43_−/− mice injected with mIFN-α (Fig. 5E and F). Interestingly, in _UBP43_−/− mice SOCS1 expression was further increased after the second injection of mIFN-α. Despite the very high expression of SOCS1 at 9 h, the second injection of mIFN-α induced a strong phosphorylation of STAT1 in _UBP43_−/− mice (Fig. 5B and E). Similarly, SOCS1 mRNA was highly increased in _UBP43_−/− mice during the entire 13 h of the experiment with repeated mIFN-α injections, while at the same time STAT1 phosphorylation was strong (Fig. 5D and F). These results provide genetic evidence that for a complete inhibition of IFN-α-induced STAT phosphorylation, SOCS1 requires the presence of USP18/UBP43.
Our results have potentially important consequences for the treatment of patients with chronic viral hepatitis with recombinant IFN-α. If we assume that also the human liver becomes refractory to IFN-α within hours after the first administration of recombinant IFN-α and that liver cells remain unresponsive to further IFN-α stimulation for an unknown time, then the current practice of injecting pegIFN-α with its very long half-life would lack a pharmacodynamic rational. The pegIFN-α effect on its prime target cells, the HCV-infected hepatocytes, may be restricted to the early phase of the dosing interval, whereas the prolonged pegIFN-α presence could have unwanted secondary effects in other organ systems such as the central nervous system, the skin, the muscles and the joints. Although the mechanisms underlying the increased efficacy of pegIFN-α compared to standard IFN-α remain unsolved, it is conceivable that the continuously high serum IFN-α concentrations obtained with pegIFN-α lead to an activation of IFN-α signal transduction as soon as hepatocytes recover from their refractory state. Ideally, choosing a dosing interval for standard IFN-α that would avoid the refractory period of hepatocytes and result in a maximal restimulation of the IFN system might represent a cost-effective strategy and also reduce toxic side effects of (peg)IFN-α therapies. The results presented here should therefore motivate an in-depth analysis of the pharmacodynamic effects of the current pegIFN-α treatments in the livers of patients with CHC.
Acknowledgments
This study was supported by Swiss National Science foundation grant 320000-116106, Swiss Cancer League grant KLS-01832-02-2006, Krebsliga Basel grant 8/05, and National Institutes of Health grant HL091549 (d.-E.Z.), as well as by a grant from the Roche Research Foundation (M.S.-F.).
Footnotes
▿
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Alexander, W. S., R. Starr, J. E. Fenner, C. L. Scott, E. Handman, N. S. Sprigg, J. E. Corbin, A. L. Cornish, R. Darwiche, C. M. Owczarek, T. W. Kay, N. A. Nicola, P. J. Hertzog, D. Metcalf, and D. J. Hilton. 1999. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98597-608. [DOI] [PubMed] [Google Scholar]
- 2.Alonzi, T., D. Maritano, B. Gorgoni, G. Rizzuto, C. Libert, and V. Poli. 2001. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation in the liver. Mol. Cell. Biol. 211621-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auernhammer, C. J., C. Bousquet, and S. Melmed. 1999. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc. Natl. Acad. Sci. USA 966964-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouki, F. M., F. R. Witter, D. E. Griffin, P. I. Nadler, A. Woods, D. L. Wood, and P. S. Lietman. 1987. Time course of interferon levels, antiviral state, 2′,5′-oligoadenylate synthetase and side effects in healthy men. J. Interferon Res. 729-39. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., I. Borozan, J. Feld, J. Sun, L. L. Tannis, C. Coltescu, J. Heathcote, A. M. Edwards, and I. D. McGilvray. 2005. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology 1281437-1444. [DOI] [PubMed] [Google Scholar]
- 6.Croker, B. A., D. L. Krebs, J. G. Zhang, S. Wormald, T. A. Willson, E. G. Stanley, L. Robb, C. J. Greenhalgh, I. Forster, B. E. Clausen, N. A. Nicola, D. Metcalf, D. J. Hilton, A. W. Roberts, and W. S. Alexander. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 4540-545. [DOI] [PubMed] [Google Scholar]
- 7.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 2591739-1742. [DOI] [PubMed] [Google Scholar]
- 8.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 2771630-1635. [DOI] [PubMed] [Google Scholar]
- 9.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 2641415-1421. [DOI] [PubMed] [Google Scholar]
- 10.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 9515623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejrnaes, M., C. M. Filippi, M. M. Martinic, E. M. Ling, L. M. Togher, S. Crotty, and M. G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2032461-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347975-982. [DOI] [PubMed] [Google Scholar]
- 13.Friedman, R. M. 2008. Clinical uses of interferons. Br. J. Clin. Pharmacol. 65158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim, M. H. 2000. Intracellular signalling and antiviral effects of interferons. Dig. Liver Dis. 32257-263. [DOI] [PubMed] [Google Scholar]
- 15.Heim, M. H. 1999. The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus. J. Recept. Signal Transduct. Res. 1975-120. [DOI] [PubMed] [Google Scholar]
- 16.Heim, M. H., G. Gamboni, C. Beglinger, and K. Gyr. 1997. Specific activation of AP-1 but not Stat3 in regenerating liver in mice. Eur. J. Clin. Investig. 27948-955. [DOI] [PubMed] [Google Scholar]
- 17.Heim, M. H., I. M. Kerr, G. R. Stark, and J. E. Darnell, Jr. 1995. Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science 2671347-1349. [DOI] [PubMed] [Google Scholar]
- 18.Heim, M. H., D. Moradpour, and H. E. Blum. 1999. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J. Virol. 738469-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horvath, C. M. 2000. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem. Sci. 25496-502. [DOI] [PubMed] [Google Scholar]
- 20.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. London B Biol. Sci. 147258-267.13465720 [Google Scholar]
- 21.Ito, S., P. Ansari, M. Sakatsume, H. Dickensheets, N. Vazquez, R. P. Donnelly, A. C. Larner, and D. S. Finbloom. 1999. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 931456-1463. [PubMed] [Google Scholar]
- 22.Kim, J. H., J. K. Luo, and D. E. Zhang. 2008. The level of hepatitis B virus replication is not affected by protein ISG15 modification but is reduced by inhibition of UBP43 (USP18) expression. J. Immunol. 1816467-6472. [DOI] [PubMed] [Google Scholar]
- 23.Kim, K. I., M. Yan, O. Malakhova, J. K. Luo, M. F. Shen, W. Zou, J. C. de la Torre, and D. E. Zhang. 2006. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol. Cell. Biol. 26472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knobeloch, K. P., O. Utermohlen, A. Kisser, M. Prinz, and I. Horak. 2005. Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol. Cell. Biol. 2511030-11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs, D. L., and D. J. Hilton. 2001. SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19378-387. [DOI] [PubMed] [Google Scholar]
- 26.Larner, A. C., A. Chaudhuri, and J. E. Darnell, Jr. 1986. Transcriptional induction by interferon. New protein(s) determine the extent and length of the induction. J. Biol. Chem. 261453-459. [PubMed] [Google Scholar]
- 27.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 34541-52. [DOI] [PubMed] [Google Scholar]
- 28.Leyssen, P., C. Drosten, M. Paning, N. Charlier, J. Paeshuyse, E. De Clercq, and J. Neyts. 2003. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob. Agents Chemother. 47777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, X. L., J. A. Blackford, C. S. Judge, M. Liu, W. Xiao, D. V. Kalvakolanu, and B. A. Hassel. 2000. RNase-l-dependent destabilization of interferon-induced mRNAs. A role for the 2-5A system in attenuation of the interferon response. J. Biol. Chem. 2758880-8888. [DOI] [PubMed] [Google Scholar]
- 30.Liu, B., J. Liao, X. Rao, S. A. Kushner, C. D. Chung, D. D. Chang, and K. Shuai. 1998. Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. USA 9510626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, L. Q., R. Ilaria, Jr., P. D. Kingsley, A. Iwama, R. A. van Etten, J. Palis, and D. E. Zhang. 1999. A novel ubiquitin-specific protease, UBP43, cloned from leukemia fusion protein AML1-ETO-expressing mice, functions in hematopoietic cell differentiation. Mol. Cell. Biol. 193029-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luik, A., S. Knapp, M. Thursz, H. C. Thomas, and J. F. Schlaak. 2004. Autoregulatory role of interleukin-10 in hepatitis C patients treated with IFN-alpha. J. Interferon Cytokine Res. 24585-593. [DOI] [PubMed] [Google Scholar]
- 33.Malakhov, M. P., O. A. Malakhova, K. I. Kim, K. J. Ritchie, and D. E. Zhang. 2002. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 2779976-9981. [DOI] [PubMed] [Google Scholar]
- 34.Malakhova, O., M. Malakhov, C. Hetherington, and D. E. Zhang. 2002. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J. Biol. Chem. 27714703-14711. [DOI] [PubMed] [Google Scholar]
- 35.Malakhova, O. A., K. I. Kim, J. K. Luo, W. Zou, K. G. Kumar, S. Y. Fuchs, K. Shuai, and D. E. Zhang. 2006. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 252358-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malakhova, O. A., M. Yan, M. P. Malakhov, Y. Yuan, K. J. Ritchie, K. I. Kim, L. F. Peterson, K. Shuai, and D. E. Zhang. 2003. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 17455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358958-965. [DOI] [PubMed] [Google Scholar]
- 38.Marin-Serrano, E., C. Rodriguez-Ramos, F. Diaz, L. Martin-Herrera, and J. A. Giron-Gonzalez. 2006. Modulation of the anti-inflammatory interleukin 10 and of proapoptotic IL-18 in patients with chronic hepatitis C treated with interferon alpha and ribavirin. J. Viral Hepat. 13230-234. [DOI] [PubMed] [Google Scholar]
- 39.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 3391485-1492. [DOI] [PubMed] [Google Scholar]
- 40.Mowen, K. A., J. Tang, W. Zhu, B. T. Schurter, K. Shuai, H. R. Herschman, and M. David. 2001. Arginine methylation of STAT1 modulates IFNα/β-induced transcription. Cell 104731-741. [DOI] [PubMed] [Google Scholar]
- 41.Osiak, A., O. Utermohlen, S. Niendorf, I. Horak, and K. P. Knobeloch. 2005. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol. Cell. Biol. 256338-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postic, C., M. Shiota, K. D. Niswender, T. L. Jetton, Y. Chen, J. M. Moates, K. D. Shelton, J. Lindner, A. D. Cherrington, and M. A. Magnuson. 1999. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knockouts using Cre recombinase. J. Biol. Chem. 274305-315. [DOI] [PubMed] [Google Scholar]
- 43.Randall, G., L. Chen, M. Panis, A. K. Fischer, B. D. Lindenbach, J. Sun, J. Heathcote, C. M. Rice, A. M. Edwards, and I. D. McGilvray. 2006. Silencing of USP18 potentiates the antiviral activity of interferon against hepatitis C virus infection. Gastroenterology 1311584-1591. [DOI] [PubMed] [Google Scholar]
- 44.Rigopoulou, E. I., W. G. Abbott, P. Haigh, and N. V. Naoumov. 2005. Blocking of interleukin-10 receptor: a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin. Immunol. 11757-64. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie, K. J., C. S. Hahn, K. I. Kim, M. Yan, D. Rosario, L. Li, J. C. de la Torre, and D. E. Zhang. 2004. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 101374-1378. [DOI] [PubMed] [Google Scholar]
- 46.Ritchie, K. J., M. P. Malakhov, C. J. Hetherington, L. Zhou, M. T. Little, O. A. Malakhova, J. C. Sipe, S. H. Orkin, and D. E. Zhang. 2002. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 162207-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarasin-Filipowicz, M., E. J. Oakeley, F. H. Duong, V. Christen, L. Terracciano, W. Filipowicz, and M. H. Heim. 2008. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl. Acad. Sci. USA 1057034-7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen, X., F. Hong, V. A. Nguyen, and B. Gao. 2000. IL-10 attenuates IFN-α-activated STAT1 in the liver: involvement of SOCS2 and SOCS3. FEBS Lett. 480132-136. [DOI] [PubMed] [Google Scholar]
- 49.Shuai, K. 2000. Modulation of STAT signaling by STAT-interacting proteins. Oncogene 192638-2644. [DOI] [PubMed] [Google Scholar]
- 50.Shuai, K., G. R. Stark, I. M. Kerr, and J. E. Darnell, Jr. 1993. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 2611744-1746. [DOI] [PubMed] [Google Scholar]
- 51.Song, M. M., and K. Shuai. 1998. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 27335056-35062. [DOI] [PubMed] [Google Scholar]
- 52.Starr, R., D. Metcalf, A. G. Elefanty, M. Brysha, T. A. Willson, N. A. Nicola, D. J. Hilton, and W. S. Alexander. 1998. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA 9514395-14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ten Hoeve, J., M. de Jesus Ibarra-Sanchez, Y. Fu, W. Zhu, M. Tremblay, M. David, and K. Shuai. 2002. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell. Biol. 225662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wills, R. J. 1990. Clinical pharmacokinetics of interferons. Clin. Pharmacokinet. 19390-399. [DOI] [PubMed] [Google Scholar]
- 55.Yasukawa, H., M. Ohishi, H. Mori, M. Murakami, T. Chinen, D. Aki, T. Hanada, K. Takeda, S. Akira, M. Hoshijima, T. Hirano, K. R. Chien, and A. Yoshimura. 2003. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 4551-556. [DOI] [PubMed] [Google Scholar]
- 56.Zou, W., J. H. Kim, A. Handidu, X. Li, K. I. Kim, M. Yan, J. Li, and D. E. Zhang. 2007. Microarray analysis reveals that type I interferon strongly increases the expression of immune-response related genes in Ubp43 (Usp18) deficient macrophages. Biochem. Biophys. Res. Commun. 356193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]