A Review of the Preclinical and Clinical Evidence for Protein Kinase C as a Target for Drug Development for Bipolar Disorder (original) (raw)
. Author manuscript; available in PMC: 2009 Aug 15.
Published in final edited form as: Curr Psychiatry Rep. 2008 Dec;10(6):510–519. doi: 10.1007/s11920-008-0081-7
Abstract
In this article, we review preclinical studies investigating the role of protein kinase C (PKC) in the role of mania and effective antimanic agents. We then discuss clinical studies conducted with tamoxifen, a relatively selective PKC inhibitor in acute bipolar mania. We conclude that PKC in an important target --arguably the first mechanistically distinct drug target for bipolar disorder. PKC holds considerable promise as a novel target for the development of a new line of treatments for bipolar disorder.
Keywords: antimanic, bipolar depression, protein kinase C, mania, tamoxifen, treatment
Introduction
Although depression dominates the course of bipolar disorder (1), mania is the defining characteristic of the disease (2). Mania is characterized by changes in energy, behavior, and cognition and frequently manifests itself with the symptoms of increased energy, extreme irritability, increased goal directed activity, decreased need to sleep, distractibility, flight of ideas, intrusiveness, and increased pleasure seeking activities with potential serious and painful consequences (2). Coincidentally, mania also conveys increased risk for abusing alcohol and illicit substances (3), which in itself significantly contributes to a negative outcome (4). The consequences of mania are devastating and include financial, occupational, familial, and societal problems (2). As described above, the course of bipolar disorder consists predominantly of lingering residual depressive symptoms or recurrent depressive episodes intermixed with hypomanic or manic episodes (1). Cycling into depression from mania is not an uncommon occurrence (5) and some have argued that aggressively treating mania and preventing recurrent manic episodes would lessen the burden of depression in part by minimizing the risk of post-manic depressive cycling.
Although several medications are approved for the treatment of mania, it is clear that many patients do not have an optimal response to them (6). In addition, intolerance in the form of metabolic disturbances and weight gain is another problem that is frequently cited as a common complication by patients, thus limiting treatment options for some (7). Although controlling relapses is the most important aspect of treating bipolar disorder, if we are to develop novel improved treatments we may elect to first target the acute phase of the illness (manic, mixed, depressive episodes) before proceeding to the maintenance phase. With the exception of lithium, available antimanic medications consist of either antipsychotic or anticonvulsant drugs (8). Besides these classes of drugs, there have been no mechanistically distinct drugs developed to treat bipolar disorder. The lack of novel treatments for bipolar disorder is undoubtedly due in part to the complexity of studying this illness (e.g. difficulty in recruiting patients, natural course of the disease, placebo effects, and high rates of drop-out). More significantly, this lack results from our incomplete understanding of the key cellular and molecular underpinnings of the disease or of its effective treatments. This paper reviews a new, mechanistically distinct drug target that appears promising for the development of a new line of treatments for bipolar disorder: protein kinase C (PKC).
The identification of PKC as a promising target for bipolar disorder is the result of over a decade of work consisting of molecular pharmacology studies, animal studies, human assay studies, and proof-of-concept studies. To our knowledge, PKC is the first mechanistically distinct target identified for the treatment of bipolar disorder.
Protein Kinase C (PKC)
PKC consists of a family of structurally related isozymes (chemically distinct forms of an enzyme that perform the same biochemical function) with a widely varied distribution throughout the body. It is implicated in several immune, pulmonary, infectious, and cardiovascular system diseases. The brain has been implicated as well. It is possible the PKC isozyme selectivity for different organ systems might explain the frequent medical comorbidity seen in patients with mood disorders.
In the brain, PKC has a role in regulating pre- and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release (Figure 1). Crucially, it acts as a hub or primary coordinator of intracellular signaling resulting from external activation of cells via different neurotransmitter systems (9). Another important function of PKC is in modulating long-term changes in gene expression and neuronal plasticity. Recent work found that PKC signaling pathways regulate the morphology of dendritic spines in cultured neuronal synaptic preparations (10, 11).
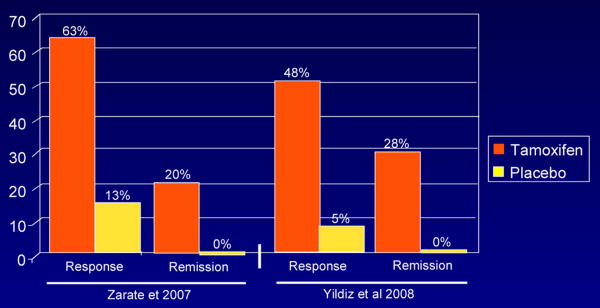
Figure 1. Response and Remission Rate.
Response: 50% decrease in YMRS from baseline; Remission: *YMRS ≤ 7 (Zarate et al 2007); **YMRS ≤ 12 (Yildiz et al 2008)
Earlier studies in humans identified changes in PKC in patients with bipolar disorder. PKC activity and PKC translocation in response to serotonin was found to change in platelets of patients with bipolar disorder; these ratios were elevated in manic patients and subsequently decreased with lithium treatment (12). Postmortem tissue studies of patients with bipolar disorder also found increased activation of PKC activity and translocation (13), thus supporting the previous finding.
Molecular pharmacology studies have found that the effective antimanic agents lithium and valproate both target PKC even though these drugs possess a distinct chemical structure (14). Such studies were paramount in helping us understand the relevance of PKC in mania (i.e., the brain regions relevant to mood effects and isozymes involved) and its role in antimanic treatment. For example, chronic treatment with lithium in rats leads to decreases in PKC stimulation-induced release with phorbol esters in important regions and components of circuits implicated in mood disturbances (i.e., hypothalamus, cortex, and hippocampus). The PKC isozymes α and ε significantly decreased following chronic treatment with lithium in both frontal cortex and hippocampus. The antimanic agent valproate also produced isozyme-specific decreases in PKC α and ε (reviewed in (14)). Target isozymes has importance as it could lead to more specific therapeutic effects and possibly minimize the occurrence of unnecessary adverse events.
To understand the functional consequences of activating and inhibiting the PKC pathway, a series of animal studies was conducted. It was hypothesized that activating PKC—for example with stimulants or cocaine—would mimic manic symptoms. In contrast, inhibition of PKC with lithium and valproate (PKC inhibitors as discussed above) in paradigms where PKC had been activated with stimulant drugs would reverse the “manic symptoms” elicited in rodents. Indeed, it was demonstrated that amphetamine elicited manic-like behaviors in rodents (reviewed in (15, 16) by activating PKC and phosphorylation of GAP-43 (implicated in neurotransmitter release) (17-19). Subsequently, lithium, valproate, and tamoxifen (also a PKC inhibitor) were found to significantly reduce amphetamine-induced hyperactivity in a large open field test and normalize amphetamine-induced increased visits to the center of an open field in rodents; this test is used as an animal model of mania representing risk-taking behavior (15). Other evidence from animal models includes the ability of PKC inhibitors to reduce aggressive behaviors and hedonic drive (20); these phenotypes in animals purportedly represent the counterpart symptoms in patients with mania.
In summary, the preclinical evidence reviewed to this point suggests that pharmacologic-induced activation of PKC in animals results in many behavioral changes seen in mania, such as hyperactivity, risk-taking behavior, and increased hedonic drive. In stark contrast, the inhibition of this target reverses these same behavioral changes in a manner similar to existing antimanic agents.
Clearly, the preclinical and human assay studies summarized above suggested the involvement of PKC in mania but required confirmation by proof-of-concept studies. In the late 1990s, drugs with PKC inhibiting properties were sought out. At the time, required criteria for drug testing in humans included: 1) the ability to inhibit PKC in brain regions implicated in mood disorders; 2) efficacy in more than one animal model of mania (i.e., ability to reverse manic-like behaviors); 3) evidence of blood brain penetrance; and 4) good tolerability. No selective PKC inhibitors were then available to test in mania; the most acceptable candidate with PKC inhibitory properties meeting the above-specified criteria was tamoxifen. Tamoxifen, a synthetic antiestrogen widely used in the treatment of breast cancer has an acceptable side effect profile over the short-term (21), and readily crosses the blood-brain barrier. The typical dose of tamoxifen used in cancer trials is 20 mg/day. Although tamoxifen also has antiestrogen properties and is thus not selective for PKC, it was the most acceptable candidate drug then available to test the relevance of PKC inhibition in antimanic effects.
Tamoxifen
This section reviews the use of the fairly selective PKC inhibitor tamoxifen in the treatment of bipolar disorder. To date, several studies have been conducted with the goal of testing whether PKC inhibition brings about antimanic effects. The clinical characteristics, study characteristics, and results of these studies are summarized in Tables 1, 2, and 3.
Table 1. Clinical Characteristics.
| Bebchuck et al. 2000 | Kulkarni et al. 2006 | Zarate et al. 2007 | Yildiz et al. 2008 | |
|---|---|---|---|---|
| Male, % | 5 (71) | 0 (0) | 14 (88) | 32 (48) |
| Psychotic features, % | Not specified | Yes, proportion not specified | 8 (50) | 44 (67) |
| Mixed state, % | 1 (14) | No, only manic episode studied | 5 (28) | 2 (3) |
| Rapid cycler, % | 1 (14) | Not specified | 6 (33) | Yes, proportion not specified but observed in logistic regression model |
| Lifetime diagnosis of substance abuse, % | Not specified | Not specified | 9 (56) | Not specified |
| Previous medication trials | Not specified | Not specified | Many prior trials | Many prior trials |
Table 2. Study Characteristics.
| Bebchuck et al. 2000 | Kulkarni et al. 2006 | Zarate et al. 2007 | Yildiz et al. 2008 | |
|---|---|---|---|---|
| Study population | Bipolar I manic with or without psychotic features; women and men (18-44 yrs) | Bipolar hypomanic and manic with or without psychotic features; women only (23-48 yrs) | Bipolar I manic or mixed with or without psychotic features; women and men (18-65 yrs) | Bipolar I manic or mixed with or without psychotic features; women and men (18-60 yrs) |
| Inpatient/outpatient status | Inpatient or outpatient | Not specified | Inpatient | Inpatient but could transition to outpatient if CGI-M was 3 or less with a 50% decrease in YMRS scores from baseline |
| Exclusion criteria | Diagnostic criteria for any other psychiatric disorder, including substance abuse/dependence; history of coagulopathies; hypersensitivity to tamoxifen | Men; exclusion criteria not specified | Substance abuse or dependence within 30 days of screen; QTC >450 ms at screen, being or planning to become pregnant or breastfeeding, history of coagulopathy, deep vein thrombosis, or pulmonary embolism; presence of retinal pathology; unstable medical illnesses; comorbid Axis I anxiety disorder diagnoses were permitted; currently significant suicidal or homicidal ideation or plans | Being or planning to become pregnant or breastfeeding, history of coagulopathy, deep vein thrombosis, or pulmonary embolism, known hypersensitivity to tamoxifen, presence of any substance abuse at screening or substance dependence within 2 month; DSM-IV diagnosis of schizophrenia, dementia, delirium, seizure disorder, obsessive-compulsive disorder, or major and clinically unstable cardiac, hepatic, or renal disease; currently significant suicidal or homicidal ideation or plans |
| Study design | Single-blind (raters) uncontrolled, add-on (n=2) or monotherapy (n=5) | DB, PC, 3-arm (Tamoxifen, MPA, Placebo), add-on to lithium (0.8-1.0 mmol/l) and/or valproate (valproic acid level not specified); washout: none | DB, PC, 2-arm, monotherapy; washout 48 hours | DB, PC, 2-arm, monotherapy; washout: 24 hours |
| Sample size, n | 7 | 13 (Tam 5, MPA 5, PBO 4) | 16 (Tam 8, PBO 8) | 66 (Tam 35, PBO 31) |
| Duration of study | Range 4-15 days, mean 8.4 days | 4 weeks | 3 weeks | 3 weeks |
| Completion rates | Not specified | Not specified | 56% | 76% |
| Dose of tamoxifen | 80 mg/day (given twice daily); final Tam dose was 80 mg n=2, 60 mg n=3, 40 mg n=1, and 20 mg n=1; mean daily dose 57 mg | 40 mg/day | 20-140 mg/day (mean daily dose=110 mg) | 80 mg (given twice daily; mean daily dose not specified) |
| Dose titration of tamoxifen | Started at 10 mg/day and “rapidly titrated” (up to a maximal daily dose of 80 mg/day) | None | Starting dose of Tam was 20 mg/day; dose increments of 20 mg/day until response or side effects up to 140 mg/day permitted | Starting dose of Tam was 20 mg which a day; dose adjusted by 10 mg to achieve 80 mg/day (give twice daily) |
| Concomitant medication use | Paroxetine n=1; Fluphenazine, lithium carbonate, benztropine, and lorazepam n=1 | Not specified | Lorazepam up to 2 mg/day for the first 10 days | Lorazepam up to 5 mg/24 hours; use of lorazepam was avoided after 12 days whenever possible |
| Severity inclusion criteria | YMRS ≥ 14 | CARS-M ≥ 15 | YMRS ≥ 14 | YMRS ≥ 20 |
| Diagnostic instrument used | SCID | SCID | SCID | |
| Outcome measures | CARS-M, YMRS, HAMD | CARS-M, PANSS | YMRS, MADRS, PANSS | YMRS, CGI-M, MADRS, 17-HAMD, PANSS |
| Frequency of outcome measures obtained | Every 3 to 7 days | Baseline and Days 7, 14, 21, and 28 | Baseline and Days 1-7, 14, and 218 | Baseline and Days 7, 14, and 21 |
| Baseline CARS-M | Tam: 28.4 | Tam: 25.2PBO: 24.3 | … | … |
| Baseline YMRS total | Tam: 31.1 | … | Tam: 30.3PBO: 24.3 | Tam: 38.6PBO: 37.2 |
| Baseline MADRS total | … | … | Tam: 20.1PBO: 12.3 | Tam: 7.5PBO: 6.2 |
| Baseline PANSS total | … | Tam: 77.4PBO: 78.8 | Tam: 82.1PBO: 57.5 | Tam: 68.2PBO: 66.5 |
Table 3. Study Results.
| Bebchuck et al. 2000 | Kulkarni et al. 2006 | Zarate et al. 2007 | Yildiz et al. 2008 | |
|---|---|---|---|---|
| Mean change in CARS-M from baseline | Tam: -11.8 | Tam: -22.2PBO: -8.5 | … | … |
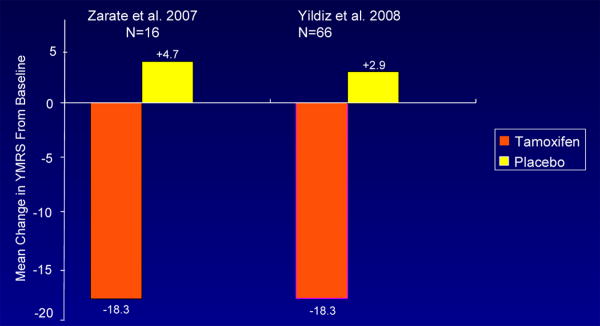
| Mean change in YMRS from baseline | Tam:-10.2 | … | Tam: -18.3PBO: +4.7 | Tam: -18.3PBO: +2.9 |
| Time to improvement in manic symptoms with Tam | Not specified | Not specified | 5 days | 7 days |
| Response (≥ 50% decrease in YMRS from baseline), % | Tam: 71NNT: Not specified | Not specifiedNNT: Not specified | Tam: 63PBO: 13NNT: Not specified | Tam: 48PBO: 5NNT: 2.30 |
| Remission at endpoint, % | Tam: 43** | Not specifiedNNT: Not specified | Tam: 20%*PBO: 0%NNT: Not specifiedEffect size between Tam and PBO: d= 1.08, 95% CI 0.45-1.71) at 21 days and d=0.59, 95% CI 0.17-1.00) at day 5 for Tam | Tam: 28%**PBO: 0%NNT: 3.63 |
| CGI-M | … | … | … | Tam: significant change from baseline |
| PANSS | … | Tam: significant change | Tam: no change | Tam: significant change |
| MADRS/HAMD | Tam: no significant change | … | Tam: no significant change | Tam: no significant change |
| Sub-item improvement in YMRS | Not specified | … | Tam: significant improvement in elevated mood, increased motor activity or energy, increased sexual interest, sleep, speech, and appearance | Tam: significant improvement in all 11 sub-items of the YMRS |
| Adverse events | Mild flushing n=1, increase in depression rating scores n=1 | Not specified | Loss of appetite significantly different than PBO | No significant differences from PBO; Tam: headache n=2, worsening acne n=2, dry skin n=1, flushing n=1, loss of appetite n=1; serious adverse events included suicide attempts (Tam n=1, PBO n=1) |
| Biological measure obtained | Women in the Tam group showed a significant increase in estradiol during the study while the other groups showed decreases | … | … | … |
Bebchuck and colleagues (22) conducted the first study in mania in 2000. The goals of this pilot study were to obtain preliminary evidence on the safety and efficacy of tamoxifen in mania in an uncontrolled study and to determine the feasibility of conducting a controlled study. Seven subjects ages 18-65 years (5 males and 2 females) with a diagnosis of bipolar mania and a Young Mania Rating Scale (YMRS; (23)) score of ≥14 received tamoxifen in a single-blind study in doses of 80 mg/day (given twice daily; tamoxifen was started at 20 mg day) for up to 15 days. Subjects received tamoxifen for a mean of eight days. Staff administered ratings every three to seven days blind to treatment with tamoxifen. A significant decrease in YMRS scores (clinical improvement) from baseline was noted; the mean decrease was 10.3 points (p=<.05). Seventy-one percent of the subjects met response criteria (50% decrease in the YMRS score from baseline). There were no significant changes in the depression rating scale scores from baseline although one subject showed an increase in the same scale. The mean daily dose was 57.1 mg, and the doses used by participants are summarized in Table 3. Tamoxifen was reported to be well tolerated.
The next study by Kulkarni and colleagues (2006) was the first controlled study of tamoxifen in mania, and involved only women (24). The pilot study examined how much of the antimanic effects of tamoxifen were due to PKC inhibition (vs its antiestrogen effects). For that reason, tamoxifen was compared to medroxyprogesterone acetate. This trial was a four-week, three-arm, double-blind, placebo-controlled, add-on study involving 13 women where tamoxifen (n=5) was compared to medroxyprogesterone acetate (n=4) and placebo (n=4). All patients were receiving concomitant treatment that consisted of either lithium (0.8-1.0 mmol/l) and/or valproate. Subjects in the tamoxifen group had a significantly greater decrease in symptoms of manic and positive symptoms of psychosis compared to the placebo group. No depression ratings were obtained and there were no reports of tamoxifen-induced depression. A significant increase in estradiol levels but no significant change in progesterone levels was reported in the tamoxifen group. This latter finding of hormonal level changes with tamoxifen does address the question of how much of tamoxifen's antimanic effects are due to hormonal modulation, although it does not fully answer it. This trial showed that both tamoxifen and medroxyprogesterone acetate had antimanic effects compared to placebo, but the small sample size and add-on design limit the conclusions that can be drawn.
The next two studies to investigate this issue—by Zarate and colleagues (2007) and Yildiz and colleagues (2008)— were 3-week monotherapy studies (25, 26). The main difference was that the study by Zarate and colleagues used much higher doses of up to 140 mg/day and obtained daily ratings for the first week, thus permitting the assessment of early antimanic effects. In the study by Yildiz and colleagues, tamoxifen was dosed up to 80 mg/day and weekly ratings were obtained. That study, however, was much larger than the one by Zarate and colleagues, and powered to be able to discern the effects of tamoxifen on other symptom domains (e.g., psychosis and depression) and to perform multivariate regression models of response.
Both studies found that the fairly selective PKC inhibitor tamoxifen resulted in significant antimanic effects. The studies by Kulkarni and colleagues and Yildiz and colleagues also found improvement in the psychotic symptoms associated with mania, while the study by Zarate and colleagues did not; however, this could be due to the sample size of the study. No study conducted to date has found an improvement or worsening of depressive symptoms associated with the short-term treatment of mania with tamoxifen. Both monotherapy trials with tamoxifen found a worsening of YMRS scores in the placebo group (25, 26). Most mania trials have found that, in general, manic patients on placebo have an improvement in manic symptoms over the course of several weeks. For the implications of these findings, see the accompanying editorial by Dr. Mauricio Tohen. He hypothesizes that differences in methodology, severity of the population under study, and single-site status could in part explain the differences found in the two studies reviewed here compared to previous ones (27).
The two controlled monotherapy studies permit the opportunity to discern the relative contribution of the PKC inhibitory effects of tamoxifen as an antimanic agent. Because the study by Kulkarni and colleagues was an add-on to lithium and/or valproate, it cannot be discerned from that study the observed antimanic effects were due to PKC inhibition, as both lithium and valproate are also PKC inhibitors.
In contrast, the study by Zarate and colleagues (2007) was a three-week, pilot, double-blind, placebo-controlled trial with tamoxifen monotherapy in patients with bipolar mania that sought to determine tamoxifen's antimanic efficacy (26). Participants were men and women 18 to 65 years old who were inpatients with a current diagnosis of bipolar disorder, current episode manic or mixed, with or without psychotic features, as diagnosed by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID). Inclusion severity criteria required a score of ≥14 on the YMRS at screening and at randomization, and a history of prior treatment with one or more antimanic agents at some point during the course of their illness (i.e., valproate, lithium, carbamazepine, or an antipsychotic). After a 48-hour washout period, subjects were randomized to flexible dosing of either tamoxifen (20-140 mg/day) or placebo. The dose of tamoxifen was initiated at 20 mg/day and could be increased by the same amount on a daily basis until response was obtained or intolerable side effects occurred. The dose titration and maximum dose allowed in this study was higher than in the other studies with tamoxifen, which allowed up to 80 mg/day.
In the study by Zarate and colleagues, the concomitant use of lorazepam (up to 2 mg/day) was allowed for the first 10 days of the double-blind phase. As mentioned, in contrast to the other studies reviewed here, daily ratings were obtained for the first week, and weekly ratings thereafter to assess early antimanic effects with tamoxifen. Traditional studies have used weekly ratings, although and some recent trials have introduced the four-day time point in addition to the seven-day one (28).
In the same study, eight subjects received tamoxifen, and eight received placebo (14 men and 2 women; mean age=35.4 ±7.8). More than one half had a history of any substance abuse or dependence, approximately two-thirds were in a manic episode, and one half had psychotic features. Most of the subjects had had significant prior treatment history with antimanic or mood stabilizers.
A significant difference between placebo and tamoxifen was found at 21 days; the antimanic effects of tamoxifen appeared as early as day five. The mean change in the different outcome scales is summarized in Table 3. At endpoint, YMRS scores on placebo changed by 4.7 points and by -18.3 points on tamoxifen. In terms of response (50% decrease in the YMRS score from baseline) and remission (≤7 YMRS at endpoint) rates, on tamoxifen 63% patients met response criteria and 20% met remission criteria. In contrast, in the placebo group only 13% of subjects met response criteria and 0% met remission criteria. The response and remission rates and changes in YMRS from baseline between the two studies are contrasted in Figure 1 and 2. The placebo response rates in these two studies (13% and 5%, respectively) is substantially lower than what has been reported in a meta-analysis of controlled treatment studies in acute mania which averaged 31.2% (29).
Figure 2. YMRS: Change From Baseline.
Tamoxifen was associated with improvements in the sub-items of elevated mood, increased motor activity or energy, decreased sleep, increased speech, increased sexual interest, and appearance. No beneficial effects were noted on depressive and psychotic symptoms scores, but this could be due to insufficient power to find differences on these other scales. However, no changes in depression ratings were found with the Montgomery-Asberg Depression Rating Scale (MADRS) or HAM-D in the larger study by Yildiz and colleagues either. No patient discontinued treatment because of an adverse event, and only loss of appetite was significantly more common with tamoxifen than with placebo.
Our placebo-controlled monotherapy trial with tamoxifen, a PKC inhibitor confirmed the initial hypothesis investigated by Bebchuk et al. (2000) (22) and the body of work suggesting that directly inhibiting PKC would improve manic symptoms. However, it is important to emphasize that this study was a pilot study, and thus required additional evidence to support the antimanic effects of tamoxifen.
That evidence was recently provided by the well-powered study by Yildiz and colleagues (2008) that investigated tamoxifen's antimanic properties (25). In this study, 66 patients with bipolar disorder I (48% male) aged 18 to 60 years, were randomized to tamoxifen (20-80 mg/day, given twice daily) or placebo for 21 days. Three percent were currently in a manic or mixed state, 67% had psychotic features, and all had a baseline YMRS score ≥ 20. Ratings were obtained on days seven, 14, and 21. Oral lorazepam up to 5 mg/24 was permitted during the study as clinically indicated. However, its use was avoided after the initial 12 days whenever possible, and it was not given within 12 hours of scheduled ratings. Intent-to-treat analysis with YMRS and Clinical Global Impressions-Mania (CGI-M) showed greater improvements with tamoxifen than placebo. The mean decreases in scores on the YMRS and CGI-M were 5.8 and 0.73 points per week, respectively, compared with mean increases of 1.5 and 0.10 points per week, respectively, with placebo. Tamoxifen was superior to placebo on all 11 YMRS sub-items. Significant changes were also found on the Positive and Negative Syndrome Scale (PANSS) Total and Positive subscale scores. Kulkarni and colleagues also reported this finding (24).
No significant improvements were seen in 17-item Hamilton Depression Rating Scale (HAMD-17) and MADRS scores. This absence of improvement or worsening of depressive symptoms in mania was a consistent finding in the two monotherapy studies. However, it is important to mention that the severity of depressive symptoms was fairly low in study by Yildiz and colleagues (MADRS: tamoxifen 7.5, placebo 6.2), and that may have affected the ability to discriminate changes in depressive symptoms after treatment with tamoxifen. In the study by Zarate and colleagues, depressive symptoms were somewhat higher than in the study by Yildiz and colleagues (MADRS: tamoxifen 20.1, placebo 12.3), but the study's small sample size may have limited its ability to detect changes in depression ratings. Nevertheless, it appears that over the short-term (three weeks) tamoxifen has no great propensity to induce depressive symptoms, at least in manic subjects.
Rates of response (≥ 50% decrease in YMRS from baseline) in the study by Yildiz and colleagues were 48% (14/29) with tamoxifen vs. 5% (1/21) with placebo. Remission (YMRS ≤12) rates were 28% with tamoxifen vs 0% with placebo (Table 3, Figure 2). Overall, tamoxifen was well tolerated.
Regarding side effects, all the studies reviewed above found that tamoxifen treatment in individuals with mania was well tolerated over the short-term (within four weeks). The only study that found a significant difference in side effects with tamoxifen compared to placebo was the study by Zarate and colleagues (2007), who found that loss of appetite was more common (the study by Yildiz and colleagues (2008) reported this adverse event in only one subject). It is possible that the higher doses used in the former study (140 mg/day vs. 80 mg/day) may explain this difference. The mechanism for this event is currently unknown but has been hypothesized to result from increased malonyl-COA in the hypothalamus and inhibition of fatty acid synthase expression specifically in the ventromedial nucleus of the hypothalamus (30).
With regards to depression, the short-term studies reviewed here indicated no significant worsening of depression over the short-term. Whether depressive symptoms occur more frequently when tamoxifen is used for long periods of time or in patients who have a liability for depression remains to be determined. A recent case report in a patient with bipolar disorder and breast cancer has suggested that tamoxifen caused stabilization of hypomania, but at the expense of a depressive state (31).
Conclusions
In summary, preclinical and clinical evidence supports further study with PKC inhibitors in bipolar disorder. Future studies will need to assess PKC isozyme selectivity, brain penetrance, short and long-term tolerability, and safety. Selective PKC inhibitors are presently in Phase I-III of development to treat a variety of conditions (see (32)) and are possible candidates to test in bipolar disorder.
Although the results with tamoxifen as an antimanic agent are encouraging, they are preliminary and would need to be confirmed in larger studies. In addition, because tamoxifen has antiestrogen effects it cannot be entirely concluded that PKC inhibition is responsible for its therapeutic effects. Whether tamoxifen will ultimately have clinical utility will need to be further explored, and its antiestrogen effects will certainly be an important consideration in premenopausal women, especially if used long-term. It also remains to be determined whether tamoxifen will have depressogenic effects, especially if used for any considerable period of time. Finally, it is important to emphasize that tamoxifen is not FDA-approved for the treatment of bipolar disorder. The proof-of-concept studies reviewed here are based on small sample sizes, and additional study of this drug is necessary in larger controlled trials before it can be generalized to current clinical practice.
Acknowledgments
This study was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health, and Department of Health & Human Services. Ioline Henter provided invaluable editorial assistance.
References
• Of importance
••Of major importance
- 1.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–7. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2007 [Google Scholar]
- 3.Cerullo MA, Strakowski SM. The prevalence and significance of substance use disorders in bipolar type I and II disorder. Subst Abuse Treat Prev Policy. 2007;2:29. doi: 10.1186/1747-597X-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fossey MD, Otto MW, Yates WR, Wisniewski SR, Gyulai L, Allen MH, Miklowitz DJ, Coon KA, Ostacher MJ, Neel JL, Thase ME, Sachs GS, Weiss RD. Validity of the distinction between primary and secondary substance use disorder in patients with bipolar disorder: data from the first 1000 STEP-BD participants. Am J Addict. 2006;15(2):138–43. doi: 10.1080/10550490500528423. [DOI] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, Tohen M, Fletcher K. Cycling into depression from a first episode of mania: a case-comparison study. Am J Psychiatry. 2001;158(9):1524–6. doi: 10.1176/appi.ajp.158.9.1524. [DOI] [PubMed] [Google Scholar]
- 6.Sachs GS, Gardner-Schuster EE. Adjunctive treatment of acute mania: a clinical overview. Acta Psychiatr Scand Suppl. 2007;(434):27–34. doi: 10.1111/j.1600-0447.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 7.Fagiolini A, Chengappa KN. Weight gain and metabolic issues of medicines used for bipolar disorder. Curr Psychiatry Rep. 2007;9(6):521–8. doi: 10.1007/s11920-007-0071-1. [DOI] [PubMed] [Google Scholar]
- 8.Manji HK, Zarate CA. Molecular and cellular mechanisms underlying mood stabilization in bipolar disorder: implications for the development of improved therapeutics. Mol Psychiatry. 2002;7 1:S1–7. doi: 10.1038/sj.mp.4001068. [DOI] [PubMed] [Google Scholar]
- 9.Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry. 1999;46(10):1328–51. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]; This paper reviews the preclinical evidence of PKC as a drug target for bipolar disorder treatments
- 10.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48(1):77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Craske ML, Fivaz M, Batada NN, Meyer T. Spines and neurite branches function as geometric attractors that enhance protein kinase C action. J Cell Biol. 2005;170(7):1147–58. doi: 10.1083/jcb.200503118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry. 1993;33(7):520–5. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biol Psychiatry. 1996;40(7):568–75. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- 14.Manji HK, Bebchuk JM, Moore GJ, Glitz D, Hasanat KA, Chen G. Modulation of CNS signal transduction pathways and gene expression by mood-stabilizing agents: therapeutic implications. J Clin Psychiatry. 1999;60 2:27–39. discussion 40-1, 113-6. [PubMed] [Google Scholar]
- 15.Einat H, Yuan P, Szabo ST, Dogra S, Manji HK. Protein kinase C inhibition by tamoxifen antagonizes manic-like behavior in rats: implications for the development of novel therapeutics for bipolar disorder. Neuropsychobiology. 2007;55(34):123–31. doi: 10.1159/000106054. [DOI] [PubMed] [Google Scholar]
- 16.Harrison-Read PE. Models of mania and antimanic drug actions: progressing the endophenotype approach. J Psychopharmacol. 2008 doi: 10.1177/0269881108089840. [DOI] [PubMed] [Google Scholar]
- 17.Giambalvo CT. Protein kinase C and dopamine transport--2. Effects of amphetamine in vitro. Neuropharmacology. 1992;31(12):1211–22. doi: 10.1016/0028-3908(92)90049-u. [DOI] [PubMed] [Google Scholar]
- 18.Giambalvo CT. Protein kinase C and dopamine transport--1. Effects of amphetamine in vivo. Neuropharmacology. 1992;31(12):1201–10. doi: 10.1016/0028-3908(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 19.Gnegy ME, Hong P, Ferrell ST. Phosphorylation of neuromodulin in rat striatum after acute and repeated, intermittent amphetamine. Brain Res Mol Brain Res. 1993;20(4):289–98. doi: 10.1016/0169-328x(93)90055-t. [DOI] [PubMed] [Google Scholar]
- 20.Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31(6):825–31. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan VC. Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Res Treat. 1994;31(1):41–52. doi: 10.1007/BF00689675. [DOI] [PubMed] [Google Scholar]
- 22.Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry. 2000;57(1):95–7. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- 23.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni J, Garland KA, Scaffidi A, Headey B, Anderson R, de Castella A, Fitzgerald P, Davis SR. A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology. 2006;31(4):543–7. doi: 10.1016/j.psyneuen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- ••25.Yildiz A, Guleryuz S, Ankerst DP, Ongur D, Renshaw PF. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65(3):255–63. doi: 10.1001/archgenpsychiatry.2007.43. [DOI] [PubMed] [Google Scholar]; This is the largest controlled study conducted with tamoxifen in acute bipolar mania
- ••26.Zarate CA, Jr, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA, Manji HK. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561–70. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]; This is a pilot controlled study supporting the results of an earlier study by our group
- 27.Tohen M. Clinical trials in bipolar mania: implications in study design and drug development. Arch Gen Psychiatry. 2008;65(3):252–3. doi: 10.1001/archgenpsychiatry.2007.44. [DOI] [PubMed] [Google Scholar]
- 28.Keck PE, Jr, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, Ingenito G. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160(9):1651–8. doi: 10.1176/appi.ajp.160.9.1651. [DOI] [PubMed] [Google Scholar]
- 29.Sysko R, Walsh BT. A systematic review of placebo response in studies of bipolar mania. J Clin Psychiatry. 2007;68(8):1213–7. doi: 10.4088/jcp.v68n0807. [DOI] [PubMed] [Google Scholar]
- 30.Lopez M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S, Blount M, Vazquez MJ, Finer N, Powles TJ, O'Rahilly S, Saha AK, Dieguez C, Vidal-Puig AJ. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55(5):1327–36. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- 31.Palmer JT, Payne JL. Stabilization of hypomania following initiation of tamoxifen. Am J Psychiatry. 2008;165(5):650–1. doi: 10.1176/appi.ajp.2007.07071165. [DOI] [PubMed] [Google Scholar]
- 32.Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59(11):1006–20. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]