Long-Term Trends in the Incidence of Heart Failure after Myocardial Infarction (original) (raw)
. Author manuscript; available in PMC: 2009 Nov 11.
Abstract
Background
Although mortality after myocardial infarction (MI) declined in the United States in recent decades, there are few community-based investigations of the long-term trends in incidence of heart failure post-MI and their results appear to be conflicting.
Methods and Results
We evaluated 676 Framingham Heart Study participants between ages 45-85 years (mean age 67 years, 34% women) who developed a first MI between 1970 and 1999. We assessed the incidence rates of heart failure and death without heart failure in each of three decades (1970-79, 1980-89, 1990-99). We estimated the multivariable-adjusted risk of events in the latter two decades, with the period 1970-79 serving as referent. The 30-day incidence of heart failure post-MI rose from 10% in 1970-79 to 23.1% in 1990-99 (p for trend 0.003), whereas 30-day mortality post-MI declined from 12.2% (1970-79) to 4.1% (1990-99). The 5-year incidence of heart failure post-MI rose from 27.6% in 1970-79 to 31.8% in 1990-99 (p for trend 0.02), whereas 5-year mortality post-MI declined from 41.1 (1970-79) to 17.3% (1990-99). In multivariable analyses, compared to the period 1970-79, we observed higher 30-day (risk ratio [RR] 2.05, 95% confidence interval [CI] 1.25-3.36) and 5-year risks of heart failure (RR 1.74, 95% CI 1.07-2.84) in the decade 1990-1999. These trends were accompanied by lower 30-day (RR 0.21, 95% CI 0.09-0.47) and 5-year mortality (risk ratio 0.31, 95% CI 0.18-0.54) in 1990-99.
Conclusions
In our community-based sample, we observed an increase in incidence of heart failure in recent decades paralleling decrease in mortality post-MI.
Keywords: Heart failure, myocardial infarction, trends
Introduction
Myocardial infarction (MI) is a leading cause of morbidity and mortality in the United States.1 Major advances in treatment over the last 4 decades have translated into considerable decline in mortality rates post-MI.2,3 Heart failure is a common complication of MI,1 with the estimated incidence varying from 10-40%.4 Post-MI heart failure is associated with a markedly elevated risk of death,5 with an estimated median survival of about 4 years.6
Given the burden posed by heart failure post-MI, it is important to understand the long-term trends in this condition. However, relatively few population-based studies have evaluated the long-term trends in the incidence of post-MI heart failure. Furthermore, investigators examining the data from two major epidemiological studies on such trends reported apparently conflicting results. Investigators from the Rochester Epidemiological Project reported a 28% decline in the incidence of heart failure post-MI between 1979 and 1994 and concluded that there is a declining trend.7 Whereas an initial report from the Worcester Heart Attack Study noted a modest decline,8 a more recent report highlighted an upward trend in incidence of heart failure post-MI between 1975 and 2001 in adjusted analyses.9 An earlier report from the Framingham Heart Study that evaluated trends in incidence of heart failure after a Q-wave MI (during the time period between 1950 and 1989) demonstrated no long term change in incidence.10
Divergent longitudinal trends in factors influencing heart failure post-MI may have contributed to the inconsistent results in the literature noted above. Thus, improved survival after an MI (due to reperfusion therapy) could lead to an increased pool of ‘high-risk’ patients who are more susceptible to heart failure.11 Yet, major therapeutic advances (such as use of angiotensin converting enzyme inhibitors12 and angiotensin receptor blockers13 post-MI) may have reduced the occurrence of left ventricular dysfunction and stemmed the susceptibility to heart failure. A potential limitation of the aforementioned investigations is a lack of analyses that explored concurrently, the incidence of heart failure and mortality post-MI without heart failure, as competing events. Such an analytical strategy could elucidate the relative contributions of the divergent trends noted above to the incidence of heart failure post-MI.
We hypothesized that the incidence of heart failure post-MI may have increased in recent times due to a lower mortality associated with the condition. We tested this hypothesis by evaluating trends in the incidence of heart failure and death after a first MI in Framingham Heart Study participants over the time period 1970-1999.
Methods
Study Sample and Design
The design and characteristics of the Original14 and the Offspring cohorts 15 of the Framingham Heart Study have been detailed elsewhere. Briefly, 5209 participants (original cohort) were enrolled in 1948 and have been evaluated approximately every two years. The members of the Framingham offspring cohort, comprising of 5124 individuals (children of the original cohort and their spouses) were enrolled in 1971 and have been evaluated approximately every four years. Participants from both cohorts who attended routine examinations between the years 1970 and 1999 constituted the sampling frame for this study. All participants provided written informed consent and the study was approved by the Institutional Review Board of Boston Medical Center.
Participants aged 45-85 years, who experienced a first MI between the years 1970 and 1999 were eligible for the present investigation (N=715). After excluding participants who had a history of heart failure prior to the index MI (n=39), 676 individuals remained eligible for this investigation. We grouped participants with a first MI according to their decade of onset of the event, i.e., 1970-79, 1980-89; 1990-99. We chose the three decades of interest to capture the pre-thrombolysis, thrombolysis and percutaneous coronary intervention eras in the management of MI. We could not extend observations to the most recent decade (2000 onwards) because participants with an MI in this decade would not have a follow-up comparable to that for the previous decades. Also, we wanted to minimize ascertainment bias in the diagnosis of MI and heart failure as a result of increased use of highly sensitive biomarkers of acute myocardial necrosis (e.g. cardiac troponins) or neurohormonal activation (e.g. b-type natriuretic peptide), respectively, in the post-2000 time period.
It is important to note that only the MI event should have occurred in a decade of interest for the participant to be grouped under that decade. Thus, a participant who developed a first MI in 1978 and then developed heart failure in 1981 would be classified in the decade 1970-79.
Ascertainment of Endpoints
All Framingham participants are under surveillance for risk of cardiovascular events (including heart failure) and death, which are identified from data collected at each Framingham examination, and from hospitalization records and physician office visits. An endpoint review committee consisting of three physicians reviews all records and adjudicates occurrence of events. Criteria for these events have been published previously.16 Briefly, MI was considered to have occurred when participants demonstrated two out of three criteria: new diagnostic Q-waves on electrocardiograms, prolonged ischemic chest discomfort, and elevation of circulating cardiac enzymes suggesting myocardial necrosis.
The Framingham criteria for heart failure17 were used to adjudicate episodes of heart failure post-MI. Briefly, a diagnosis of heart failure requires presence of 2 major or of 1 major and 2 minor criteria. The major criteria include a history of paroxysmal nocturnal dyspnea or orthopnea, presence of jugular venous distention, hepatojugular reflux, pulmonary rales, presence of third heart sound, increasing radiographic cardiomegaly, X-ray evidence of acute pulmonary edema, presence of a third heart sound, and evidence of weight loss >4.5 kg during the first 5 days of treatment for suspected heart failure. The minor criteria include history of a nocturnal cough, dyspnea on ordinary exertion, presence of bilateral ankle edema, hepatomegaly, heart rate more than 120 beats per minute and X-ray evidence of bilateral pleural effusions and/or pulmonary vascular congestion. Major or minor criteria were attributed to heart failure only when there was no alternative explanation for the symptoms or signs (such as due to other medical conditions like cirrhosis, renal failure, or chronic pulmonary disease).
Statistical Analyses
First, participants with a first MI were classified into three groups based on the decade of incidence, i.e., 1970-1979, 1980-1989 and 1990-1999. Next, we evaluated the incidence of heart failure or death (free of heart failure) on follow-up during the 30-day and the 5-year time periods post-MI. Mortality free of heart failure was evaluated as opposed to total mortality because mortality in individuals with both MI and heart failure may be attributable to heart failure, and we intended to assess mortality as a competing event to the incidence of heart failure: i.e. people who die free of heart failure ‘escape’ heart failure. Third, we performed multivariable analyses comparing the incidences of heart failure and death (separate analyses for each outcome) over the 30-day and 5-year follow-up period (separate analyses for each follow-up period) after MI occurrence in each of the 3 decades, with the decade 1970-79 serving as the referent group. Fourth, we repeated analyses evaluating the 5-year incidence of heart failure and death among participants with an MI who survived beyond 30 days, i.e., these analyses paralleled earlier analyses but excluded people who died or developed heart failure within 30 days. Fifth, we performed additional analyses that evaluated incidence of heart failure or death at time points between 30 days and 5 years, i.e., at 6 months, 1 year and 2 years post-MI.
Poisson regression was used for modeling events during the 30-day period after MI, whereas Cox proportional hazards regression was used for incidence of events over the 5-year period after MI. All regression models adjusted for the following covariates (obtained from the Heart Study examination preceding the incident MI event): age, sex, body mass index, smoking status, systolic blood pressure, hypertension treatment, diabetes and total cholesterol. Given the changes in the ascertainment of MI across the decades18, we conducted additional analysis adjusting for the proportion of MIs diagnosed without diagnostic ECG changes (i.e., on the basis of cardiac biomarkers and clinical history without diagnostic Q waves) in each decade.
All statistical analyses were performed using SAS software Version 8.0 (SAS Institute, Cary, NC) and a p value of <0.05 was used to denote statistical significance.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The study sample consisted of 676 participants with a first occurrence of MI over the 3 decades of observation. The baseline characteristics of study participants are shown in Table 1 according to the decade of MI incidence (data for covariates being obtained from the examination preceding the onset of MI). Participants with a first MI in the most recent decade were older, more likely to be women, have diabetes and to be on antihypertensive agents, but had lower serum cholesterol concentrations and smoking rates. A larger proportion of MIs in the 1990s were non-Q MIs (Table 1).
Table 1. Baseline characteristics by decade of incidence of MI.
| Decade of MI Incidence | |||
|---|---|---|---|
| Clinical Characteristic | 1970-79(N=230) | 1980-89(N=251) | 1990-99(N=195) |
| Age-years, mean (SD) | 63.6 (10.1) | 66.9 (10.2) | 69.9 (10.9) |
| Women, % | 30.9% | 31.5% | 38.5% |
| Body mass index-kg/m2, mean (SD) | 27.2 (4.43) | 27.0 (4.31) | 28.1 (4.66) |
| Systolic Blood Pressure-mm Hg, mean (SD) | 145.2 (22.2) | 139.5 (20.5) | 142.7 (21.4) |
| Diastolic BP-mm Hg, mean (SD) | 83.8(11.8) | 80.8(10.4) | 77.9(11.7) |
| Hypertension treatment, (%) | 29.3% | 36.5% | 44.6% |
| Total Cholesterol-mg/dl, mean (SD) | 239.7 (42.4) | 235.3 (44.3) | 216.5 (41.7) |
| Diabetes mellitus, % | 11.5% | 17.5% | 21.5% |
| Smoking, % | 48.2% | 38.5% | 29.5% |
| Q-wave MI, % | 75.2 | 71.7 | 47.7 |
Trends in the Incidence of Heart Failure and Death (Table 2)
Table 2. Age- sex-adjusted Event rates after a MI and adjusted relative risk of events across decades.
| 1970-79 | 1980-89 | 1990-99 | p for trend | ||
|---|---|---|---|---|---|
| A. 30-day events | |||||
| CHF | No. of events/ no. at risk (%) | 23/230 (10.0) | 36/251 (14.3) | 45/195 (23.1) | 0.003 |
| Event Rate (95% CI)* | 11.8 (7.5-18.6) | 14.6(10.1-21.2) | 19.2 (13.6-27.0) | ||
| Risk Ratio (95% CI) | Referent | 1.33 (0.80-2.22) | 2.05 (1.25-3.36) | ||
| Death without CHF | No. of events/ no. at risk (%) | 28/230 (12.2) | 19/251 (7.6) | 8/195 (4.1) | <0.0001 |
| Event Rate (95% CI)* | 15.0 (9.2-24.5) | 7.6 (4.4-13.2) | 3.4 (1.6-7.1) | ||
| Risk Ratio (95% CI) | Referent | 0.51 (0.29-0.90) | 0.21 (0.09-0.47) | ||
| CHF or death | No. of events/ no. at risk (%) | 51/230 (22.2) | 55/251 (21.9) | 53/195 (27.2) | 0.79 |
| Event Rate (95% CI)* | 26.6 (19.8-35.7) | 22.2 (16.8-29.5) | 22.4 (16.9-29.8) | ||
| Risk Ratio (95% CI) | Referent | 0.85 (0.61-1.19) | 0.95 (0.67-1.34) | ||
| B. 5-year events (including 30 day events) | |||||
| CHF | No. of events/ no. at risk (%) | 45/230 (19.6) | 54/251 (21.5) | 66/195 (33.9) | 0.02 |
| Event Rate (95% CI)* | 27.6 (18.8-35.0) | 24.6 (17.3-30.9) | 31.9 (23.5-39.0) | ||
| Hazards Ratio (95% CI) | Referent | 1.05 (0.68-1.63) | 1.74 (1.07-2.84) | ||
| Death without CHF | No. of events/ no. at risk (%) | 66/230 (28.7) | 47/251 (18.7) | 26/195 (13.3) | <0.0001 |
| Event Rate (95% CI)* | 41.1 (30.8-48.9) | 23.9 (16.2-30.4) | 17.3 (10.1-23.7) | ||
| Hazards Ratio (95% CI) | Referent | 0.48 (0.32-0.73) | 0.31 (0.18-0.54) | ||
| CHF or death | No. of events/ no. at risk (%) | 111/230 (48.3) | 101/251 (40.2) | 92/195 (47.2) | 0.25 |
| Event Rate (95% CI)* | 54.0 (45.6-60.3) | 40.7 (33.3-46.9) | 41.6 (33.8-48.2) | ||
| Hazards Ratio (95% CI) | Referent | 0.72 (0.53-0.97) | 0.83 (0.58-1.8) | ||
| C. 5 year events in people surviving 30 days | |||||
| CHF | No. of events/ no. at risk (%) | 22/179 (12.3) | 18/196 (9.2) | 21/142 (14.8) | 0.99 |
| Event Rate (95% CI)* | 17.0 (8.1-24.6) | 10.6 (4.6-16.0) | 14.6 (6.9-21.5) | ||
| Hazards Ratio, 95% CI | Referent | 0.69 (0.34-1.39) | 1.02 (0.47-2.21) | ||
| Death without CHF | No. of events/ no. at risk (%) | 38/179 (21.2) | 28/196 (14.3) | 18/142 (12.7) | 0.01 |
| Event Rate (95% CI)* | 28.3 (17.7-36.6) | 14.9 (8.4-20.7) | 12.2 (5.8-17.9) | ||
| Hazards Ratio (95% CI) | Referent | 0.53 (0.31-0.90) | 0.43 (0.21-0.87) | ||
| CHF or death | No. of events/ no. at risk (%) | 60/179 (33.5) | 46/196 (23.5) | 39/142 (27.5) | 0.06 |
| Event Rate (95% CI)* | 38.6 (28.5-46.4) | 23.3 (16.0-29.7) | 24.2 (16.0-31.2) | ||
| Hazards Ratio (95% CI) | Referent | 0.59 (0.39-0.91) | 0.64 (0.38-1.07) |
Heart failure post-MI occurred in 165 (24.4%) participants with MI, whereas 139 (20.6%) participants died without heart failure over the 3 decades of observation. Table 2 displays the age- and sex-adjusted rates of incident events (heart failure and death free of heart failure) in each decade separately for the initial 30-days post-MI and for the 5-year period after the MI.
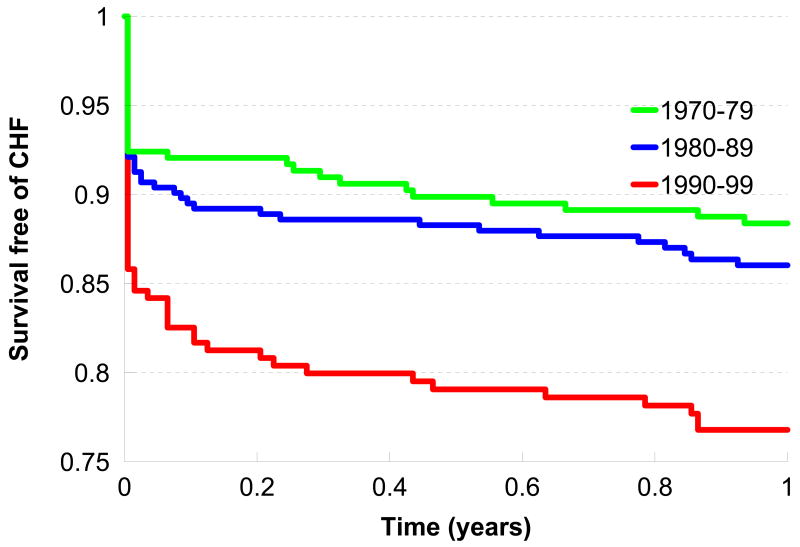
Figure 1 displays the age- and sex-adjusted survival free of heart failure during the initial period after an MI in the 3 decades. The incidence of heart failure during the 30 days post-MI was higher in the decades 1980-89 and 1990-99 compared to 1979-79 (p for trend =0.003). In contrast, the incidence of death without heart failure during the 30 days post-MI was lower in these decades (1980-89 and 1990-99) compared to the period 1970-79 (p for trend <0.0001). The multivariable-adjusted hazards ratio for heart failure post-MI during the 30-day period was about 2-fold higher in the 1990s compared to participants who developed MI in the 1970s. In contrast, the adjusted hazard of death without heart failure was 50% lower in the 1980s and 80% lower in the 1990s compared to the referent decade of 1970s. When the composite outcome of ‘death or heart failure’ post-MI was modeled, there were no statistically significant differences in incidence across the 3 decades (Table 2).
Figure 1. Trends in survival free of heart failure in the 1-year post-MI period by decade of incident MI.
Age and sex-adjusted survival free of heart failure post-MI in the 3 decades of interest during the year after the incident MI.
For the 5-year post-MI time period (including the 30-day post-MI period), the incidence of heart failure post-MI rose in the 1990s compared to the 1970s (p for trend =0.02), whereas the incidence of death without heart failure declined over this time period (p for trend <0.0001). In multivariable models, the risk of new-onset heart failure was about 75% higher in the 1990s relative to the 1970s. In comparison, adjusted risk of death free of heart failure declined by 50 (1980s) to 70% (1990s) over this time period (Table 2). The trend in the incidence of the composite outcome ‘death or heart failure’ post-MI was not statistically significant.
For participants who survived beyond 30 days post-MI without heart failure, there was no statistically significant difference in the incidence of heart failure over a 5-year period, although the incidence of death without heart failure was lower in the 1980s and 1990s compared to the 70's (p-value for trend = 0.01). The incidence of the composite outcome of ‘death or heart failure’ post-MI declined by about 36%, a finding that was of borderline statistical significance. The test for a statistical interaction between the decade of MI incidence and the timing of post-MI heart failure (dichotomized at 30 days) was highly significant (p<0.001), confirming that the effect of decade of MI incidence on the incidence of heart failure post-MI diminished beyond the 30-day period post-MI.
In additional analyses adjusting for the proportion of MIs ascertained without diagnostic ECG changes in each decade, the observed trends in post-MI HF incidence across decades remained robust.
The Appendix Table presents data on incidence of heart failure and death at 6 months, 1 year and 2 years post-MI. These data suggest that the higher incidence of heart failure observed at 30 days in recent decades was maintained in analyses of heart failure and death post-MI at these time points as well.
Appendix Table. Age- sex-adjusted Event rates after a MI and adjusted relative risk of events across decades at 6, 12 and 24 months of follow-up.
| 1970-79 | 1980-89 | 1990-99 | p for trend | ||
|---|---|---|---|---|---|
| A. 6 month events (including 30 day events) | |||||
| CHF | No. of events/ no. at risk (%) | 26/230 (11.3) | 40/251 (15.9) | 51/195 (26.2) | 0.006 |
| Event Rate (95% CI)* | 13.8 (8.1–19.0) | 16.9 (10.9-22.2) | 22.5 (15.5-28.7) | ||
| Risk Ratio (95% CI) | Referent | 1.33 (0.77-2.29) | 2.27 (1.25-4.13) | ||
| Death without CHF | No. of events/ no. at risk (%) | 40/230 (17.4) | 26/251 (10.4) | 13/195 (6.7) | <0.0001 |
| Event Rate (95% CI)* | 24.7 (15.2-32.4) | 12.7 (6.8-18.0) | 7.4 (2.8-11.6) | ||
| Risk Ratio (95% CI) | Referent | 0.45 (0.26-0.78) | 0.23 (0.11-0.50) | ||
| CHF or death | No. of events/ no. at risk (%) | 66/230 (28.7) | 66/251 (26.3) | 64/195 (32.8) | 0.96 |
| Event Rate (95% CI)* | 33.5 (25.4-40.3) | 26.6 (19.9-32.6) | 27.5 (20.6-33.5) | ||
| Risk Ratio (95% CI) | Referent | 0.79 (0.54-1.15) | 0.93 (0.60-1.43) | ||
| B. 1 year events (including 30 day events) | |||||
| CHF | No. of events/ no. at risk (%) | 30/230 (13.0) | 42/251 (16.7) | 52/195 (26.7) | 0.01 |
| Event Rate (95% CI)* | 16.5 (10.1-22.3) | 17.9 (11.8-23.4) | 22.9 (16.0-29.0) | ||
| Hazards Ratio (95% CI) | Referent | 1.22 (0.72-2.03) | 2.06 (1.16-3.64) | ||
| Death without CHF | No. of events/ no. at risk (%) | 42/230 (18.3) | 31/251 (12.4) | 13/195 (6.7) | <0.0001 |
| Event Rate (95% CI)* | 25.9 (16.4-33.7) | 15.3 (8.8-21.1) | 7.4 (2.9-11.5) | ||
| Hazards Ratio (95% CI) | Referent | 0.51 (0.30-0.85) | 0.22 (0.10-0.46) | ||
| CHF or death | No. of events/ no. at risk (%) | 72/230 (31.3) | 73/251 (29.1) | 65/195 (33.3) | 0.49 |
| Event Rate (95% CI)* | 36.3 (28.1-43.1) | 29.5 (22.5-35.6) | 27.9 (21.1-33.8) | ||
| Hazards Ratio (95% CI) | Referent | 0.79 (0.56-1.14) | 0.87 (0.57-1.33) | ||
| C. 2 year events (including 30 day events) | |||||
| CHF | No. of events/ no. at risk (%) | 33/230 (14.6) | 45/251 (17.9) | 56/195 (28.7) | 0.006 |
| Event Rate (95% CI)* | 18.7 (11.7-24.8) | 19.5 (13.1-25.2) | 25.1 (17.8-31.5) | ||
| Hazards Ratio, 95% CI | Referent | 1.23 (0.75-2.02) | 2.16 (1.24-3.75) | ||
| Death without CHF | No. of events/ no. at risk (%) | 50/230 (21.7) | 36/251 (14.3) | 17/195 (8.7) | <0.0001 |
| Event Rate (95% CI)* | 30.5 (20.5-38.4) | 17.7 (10.8-23.8) | 10.3 (4.8-15.3) | ||
| Hazards Ratio (95% CI) | Referent | 0.53 (0.33-0.86) | 0.27 (0.14-0.54) | ||
| CHF or death | No. of events/ no. at risk (%) | 83/230 (36.1) | 81/251 (32.3) | 73/195 (37.4) | 0.68 |
| Event Rate (95% CI)* | 41.4 (33.0-48.2) | 32.7 (25.5-38.9) | 31.9 (24.7-38.2) | ||
| Hazards Ratio (95% CI) | Referent | 0.81 (0.58-1.14) | 0.93 (0.62-1.39) |
Discussion
Principal findings
Our principal findings are twofold. First, participants with a first MI had a decreasing trend for mortality free of heart failure between 1970 and 1999, and a concomitant increasing trend for the incidence of heart failure. These trends were evident for both the 30-day post-MI period and the 5-year post MI period. Additional analyses suggested that these trends were not influenced by the increasing trend for ascertainment of MI based on biomarkers (and the resultant potential change in case mix of MI). Also, we consistently used the same set of criteria for the ascertainment of HF across the decades. However, trends in the ascertainment of HF based on a greater performance of imaging tests and/or a greater diagnostic suspicion in more recent decades may have contributed to our finding of a greater incidence of heart failure in the 1990s.19 Second, in the participants who survived beyond the 30-day post MI period without heart failure, we did not observe any temporal trends in the incidence of heart failure post-MI. These data suggest that the rising trend in incidence of heart failure post-MI was largely driven by the trend for increased incidence noted for the 30-day post-MI period in recent decades. It is conceivable that our observations are consistent with the well acknowledged lower mortality and better myocardial salvage of individuals with an MI in the 1990s.20 Survivors of MI have residual myocardial damage and a higher risk of developing heart failure.21 Improved survival in recent decades may have contributed to an increase in the pool of people at risk for developing heart failure, thereby explaining the increasing trend in the incidence of heart failure post-MI from 1970-1999; such individuals may have experienced higher mortality rates in the earlier decades.
Of note, a previous Framingham report that evaluated trends in the incidence of heart failure (of any etiology) in the time period 1950-1999, demonstrated that incidence is stable in men but may be decreasing in women.22 In the present report, we demonstrate an increasing trend in post-MI heart failure over the time period from 1970-1999. It may be important to analyze trends in incidence of heart failure due to specific etiologies because temporal patterns may vary based on the etiology of heart failure. For instance, it is conceivable that heart failure on the basis of hypertension may have declined in more recent decades due to better control of high blood pressure, thereby explaining the decline in overall incidence of heart failure in women (in whom the contribution of high blood pressure is greater relative to that of MI23).
Trends in heart failure post-MI Incidence: Comparison with Previous Literature
The results of our investigation vary from that of some other reports in the published literature. It is important to note that the differences in the case mix in the study samples (inclusion of first versus recurrent MI; incident versus prevalent heart failure), varying durations of follow-up (in-hospital versus short- and long-term follow-up after MI), and distinctions in the time periods of observation (inclusion of the early and late 1990s versus analysis of data from the early 1990s) may have contributed to the apparently dissimilar findings across these studies, as detailed below.
An investigation of Olmsted county population by Hellerman et al 7 utilizing a study sample and design similar to ours (prospective cohort with incident MIs, no prevalent heart failure, and mean follow-up of 7.6 years), reported a 28% decline in incidence of post-MI heart failure between 1979 and 1994. A major difference between this report and ours is the time period under study (1979-94 versus 1970-99). As noted by Goldberg et al9 the use of primary percutaneous intervention (PCI) as a treatment for MI became more common in the late 1990s. It is possible that increased survival of “sicker” patients with MI because of the efficacy of PCI led to an accrual of more susceptible people in the latter half of the decade of 1990, explaining why we may have observed an increasing trend in post-MI heart failure by studying people up to 1999.
Several reports from the Worcester Heart Attack Study (WHAS), a longitudinal community-based surveillance study, also have evaluated temporal trends in the incidence of heart failure post-MI. These reports were based on abstraction of hospitalization records of patients with MI in the Worcester Metropolitan area in different time periods, focused on incidence of HF during the initial hospital stay, evaluated all hospitalized MIs (both first and recurrent), and included patients with or without prevalent heart failure. In contrast, our investigation evaluated both short- and long-term incidence (both during and beyond the initial hospital stay) of HF after a first MI. An initial analysis of 20 years of data (up to the year 1995) by Spencer et al8 from WHAS showed an inconsistent trend for heart failure incidence. The 25-year analysis (up to year 2001) reported by Goldberg et al9 showed unadjusted heart failure incidence rates that were similar across the time periods evaluated. However, after statistical adjustment for age, sex, prevalent coronary artery disease, MI order and type, the investigators observed an increasing trend in heart failure incidence in recent decades. As noted above, it is possible that the latter findings are consistent with our observations because of the similarity in the time periods studied.
Strengths and Limitations
The present study extends prior observations in several respects: we evaluated both in-hospital heart failure and events after the index MI over a period of 5 years. Furthermore, we evaluated concomitantly trends in both incidence of heart failure and death (free of heart failure) to assess how changing case-fatality rates may influence incidence of post-MI heart failure.
Nevertheless, several limitations of our investigation must be noted. We did not model temporal trends in use of specific treatments (such as use of angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), aldosterone antagonists or beta-blockers) or revascularization procedures for participants with a MI. It is more likely that higher proportions of participants in the latter decades received these therapies, than participants in the decade of 1970-7920. However, treatments that improve post-MI survival have also been shown to decrease incidence of heart failure. Another limitation of our study is the lack of information on the type of heart failure (systolic versus diastolic), or occurrence of LV systolic dysfunction post-MI. Lastly, our sample is predominantly white of European ancestry and caution needs to be exercised in generalizing these results to other ethnicities.
Conclusions
Our longitudinal observations on a large community-based sample demonstrate reciprocal trends of a decrease in mortality post-MI accompanied by an increase in incidence of heart failure in more recent years (1990s) relative to the 1970s. Greater salvage of high-risk MI patients in recent time periods may contribute to these trends.
Acknowledgments
None
Funding sources: This work was supported by the NIH/NHLBI Contract No. N01-HC-25195, and 2K24HL4334 (Dr. Vasan).
Footnotes
Disclosures: None.
Clinical Summary: Mortality from myocardial infarction (MI) has decreased in recent decades. However, few community-based epidemiological investigations have addressed the long-term trends in the incidence of heart failure post- MI. We evaluated trends in the incidence of heart failure post-MI in the time period 1970 to 1999 in the Framingham Heart Study cohort. We related the decade of MI incidence to the occurrence of heart failure in the early (within 30 days of MI) and late post MI periods (after 30 day and up to 5 years) and to the incidence of death free of heart failure. We observed a striking increase in the incidence of heart failure post-MI in the decade 1990-1999 (compared to the decade 1970-1979) accompanied by a decrease in the incidence of death without heart failure post-MI over the same time period. We conclude that the increase in heart failure incidence post-MI in recent decades was mostly explained by increases in the early post-MI period, in part due to a major decrease in mortality during this period in recent decades. Our data are consistent with the notion that a greater salvage of high-risk patients in recent time periods may have contributed to the observed trends in post-MI heart failure.
Reference List
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 4.Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006;97:13F–25F. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 6.Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7:119–125. doi: 10.1016/j.ejheart.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, Gersh BJ, Redfield MM, Rodeheffer RJ, Yawn BP, Roger VL. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157:1101–1107. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 8.Spencer FA, Meyer TE, Goldberg RJ, Yarzebski J, Hatton M, Lessard D, Gore JM. Twenty year trends (1975-1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34:1378–1387. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RJ, Spencer FA, Yarzebski J, Lessard D, Gore JM, Alpert JS, Dalen JE. A 25-year perspective into the changing landscape of patients hospitalized with acute myocardial infarction (the Worcester Heart Attack Study) Am J Cardiol. 2004;94:1373–1378. doi: 10.1016/j.amjcard.2004.07.142. [DOI] [PubMed] [Google Scholar]
- 10.Guidry UC, Evans JC, Larson MG, Wilson PW, Murabito JM, Levy D. Temporal trends in event rates after Q-wave myocardial infarction: the Framingham Heart Study. Circulation. 1999;100:2054–2059. doi: 10.1161/01.cir.100.20.2054. [DOI] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 12.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de WF, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 14.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Wolf PA, Garrison RJ, editors. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements. Section 34. The Framingham Heart Study: 30 Year Follow-Up. National Institute of Health; Bethesda, MD: 1987. NIH publication 87-2703. [Google Scholar]
- 17.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 18.Roger VL. Epidemiology of myocardial infarction. Med Clin North Am. 2007;91:537–552. doi: 10.1016/j.mcna.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Jimenez F, Goraya TY, Hellermann JP, Jacobsen SJ, Reeder GS, Weston SA, Roger VL. Measurement of ejection fraction after myocardial infarction in the population. Chest. 2004;125:397–403. doi: 10.1378/chest.125.2.397. [DOI] [PubMed] [Google Scholar]
- 20.Rogers WJ, Canto JG, Lambrew CT, Tiefenbrunn AJ, Kinkaid B, Shoultz DA, Frederick PD, Every N. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000;36:2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 21.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 22.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 23.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]