Glucocorticoid receptors modulate mitochondrial function: A novel mechanism for neuroprotection (original) (raw)
Abstract
It has become increasingly clear that glucocorticoid (GC) signaling not only comprises classic nuclear receptor binding—that is, glucocorticoid receptors (GRs) to their response element in the nucleus—but also involves rapid, non-genomic efforts to regulate signaling cascades and other cell functions in the cytoplasm as well as other cell organelles. In a recent study, we found that GRs form a complex with B-cell lymphoma 2 (Bcl-2), trans- locate to mitochondria in response to corticosterone (CORT), and modulate mitochondrial calcium and oxidation in an inverted U-shaped manner. It is also well established that steroid and thyroid hormone receptors regulate mitochondrial function to protect cells against various challenges and modulate synaptic plasticity. Here, we explore how such work reveals a fundamental mechanism whereby GCs regulate mitochondrial functions, and provides a mechanistic basis for therapeutic methods to rescue mitochondrial dysfunction during chronic stress or related psychiatric and neurodegenerative disorders.
Key words: glucocorticoid, nuclear receptor, mitochondria, Bcl-2, steroid, plasticity, mood disorders, neuroprotection
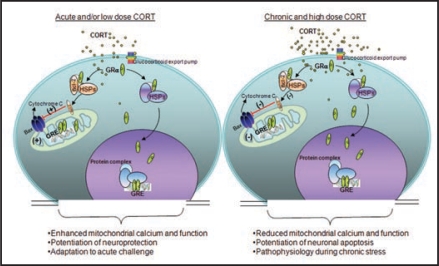
Glucocorticoids (GCs) act through their nuclear receptors in the brain to influence behavior and physiology.1,2 In addition to their well-known genomic effects on gene transcription, GCs also exert rapid, non-genomic effects on neurons in the brain.3 Accumulating data have also shown that certain receptors traditionally considered to be nuclear receptors, particularly glucocorticoid receptors (GRs) translocate into mitochondria and modulate mitochondrial gene expression.4–6 However, the molecular mechanism of this regulation remains unclear. Recently, we found that in rat brain cells treated with corticosterone (CORT), GRs latched onto B-cell lymphoma 2 (Bcl-2), a protein that affects cytochrome C and calcium release from mitochondria. This GR/Bcl-2 complex moves into mitochondria and regulates mitochondrial functions in an inverted “U”-shaped manner (Fig. 1). Specifically, short-term exposure to CORT enhanced mitochondrial functions, while high doses or long-term treatment with CORT decreased levels of GRs and Bcl-2 in mitochondria (Fig. 1). Similar results occur in rats exposed to chronic CORT.7
Figure 1.
Biphasic effect of Glucocorticoids (GCs) in regulating mitochondrial function. GCs are secreted by adrenal glands in a circadian and stress-related fashion. GCs readily penetrate the cell membrane and interact with cytoplasmic glucocorticoid receptors (GRs). GRs travel to the nucleus to regulate gene expression by binding to glucocorticoid response element (GRE). Here, GRs formed a complex with the anti-apoptotic protein B-cell-lymphoma 2 (Bcl-2) in response to corticosterone (CORT) treatment, and translocated with Bcl-2 into mitochondria after acute treatment with low or high doses of CORT in primary cortical neurons; they also upregulated mitochondrial calcium levels, membrane potential and oxidation. However, after long-term (three days) treatment, high, but not low, CORT decreased GR and Bcl-2 levels in mitochondria. In addition, three independent measures of mitochondrial function—mitochondrial calcium holding capacity, mitochondrial oxidation and membrane potential—were also regulated by long-term CORT treatment in an inverted “U”-shape. Bcl-2 was able to inhibit the formation of Bax-containing pores on the mitochondrial outer membrane and reduced the release of calcium and cytochrome C from the mitochondria. This regulation of mitochondrial function by CORT correlated with neuroprotection; that is, treatment with low doses of CORT demonstrated a neuroprotective effect, whereas treatment with high doses of CORT enhanced kainic acid (KA)-induced toxicity of cortical neurons.
Such work suggests that, under physiological conditions, GCs enhance mitochondrial functions to provide cells with more energy for coping with and adapting to acute challenges. However, chronic stress may lead to chronically elevated levels of GCs, which in turn may reduce cell functioning via the interaction between GRs/Bcl-2 and mitochondria (Fig. 1). The decrease in proper cell function may contribute to the pathophysiology of several stress-related mental disorders, including major depressive disorder (MDD), and post-traumatic stress disorder (PTSD).
How Does the GR Protein Complex Translocate to the Mitochondria?
Previous studies have shown that GRs form protein complexes with heat shock protein 70/90 (HSP70/90) and Bcl-2-associated athanogene (Bag-1) in response to GC treatment.8–10 It is also well established that the proteins targeting mitochondria associate with chaperones that help in their mitochondrial translocation. One of the major chaperones in this category is HSP70.11,12 Based on the signal information in the precursor protein, it could be targeted to any of the four locations: the mitochondrial outer membrane, the mitochondrial inner membrane, the intermembrane space, or the mitochondrial matrix.13 Furthermore, Bcl-2 is one of the tail-anchored molecules localized at the outer membrane of mitochondria.14 In contrast, GRs travel to the matrix of the mitochondria and modulate mitochondria-coded protein expression.15 It is possible that the GR/Bcl-2 complex shares the machinery for mitochondrial protein translocation by binding to HSP70/90 chaperone proteins, in much the same manner that estrogen receptors (ERs) can.16
Bag-1, which binds to Bcl-2, is also a GR chaperone protein. Bag-1 attenuates nuclear translocation of GR, activates the extracellular receptor kinase (ERK) pathway, and potentiates the anti-apoptotic function of Bcl-2.10,17 In addition, Bag-1 transgenic mice showed less anxious-like behavior on the elevated plus maze test and more resilience in recovering from learned helplessness behavior and amphetamine-induced manic-like behaviors.18 Because Bag-1 binds to both Bcl-2 and GRs, its role in GR/Bcl-2 complex translocation to mitochondria becomes a key issue.
Why Are Bcl-2 Family Genes a Key Modulator for Mitochondrial Function and Neuroprotection in Response to GCs?
Bcl-2 family proteins play a key role in apoptosis because of their ability to regulate the integrity of the mitochondrial outer membrane. Proteins such as Bcl-2 and Bcl-xL prevent the release of apoptogenic proteins from mitochondria and therefore protect against outer membrane permeabilization. Pro-apoptotic Bcl-2 family members—such as Bax and Bak—induce outer membrane permeabilization and cause the release of pro-apoptotic factors from mitochondria.19,20 The Bcl-2 proteins act either alone or together with other proteins like Bax to regulate the permeability of the outer membrane.21 Our recent findings indicate that GRs form a complex with Bcl-2 in response to CORT treatment and, furthermore, translocate with Bcl-2 into mitochondria. This may be a critical step in blocking pore formation in the mitochondrial outer membrane. Therefore, calcium and cytochrome C release from the mitochondria would be attenuated (Fig. 1).
Additional findings have shown that Bcl-2 overexpression leads to enhanced mitochondrial calcium levels.22 Notably, increased calcium levels operate mitochondrial metabolic checkpoints. Specifically, the aspartate/glutamate metabolite carriers are activated by calcium; in turn, recombinant expression of wild-type aspartate/glutamate metabolite carriers enhances adenosine triphosphate (ATP) production in response to cell stimulation.23
Is the Hormone Receptor Protein Complex a Common Mechanism for Regulating Mitochondrial Function?
GRs, ERs, androgen receptors (ARs), progesterone receptors (PRs) and thyroid hormones (TRs) have been detected in the mitochondria of various cell types by western blotting, immunofluorescence labeling, confocal microscopy and immunogold electron microscopy.24 Steroid and thyroid hormones regulate energy production. Thus, both steroid and thyroid receptors modulate the expression of nuclear-encoded genes and mitochondrial-encoded genes involved in oxidative phosphorylation.13,24 Recent studies also indicate that, via genomic and non-genomic mechanisms, these hormone receptors play a role in neuroprotection and apoptosis.13,24 In this context, our recent finding that the GR/Bcl-2 complex translocates into mitochondria to regulate mitochondrial function provides a novel mechanism for understanding GR-mediated neuroprotection.
Conclusion and Future Directions
The regulation of neuronal mitochondrial function by steroids is directly linked to neuroprotection and synaptic plasticity.1,25–27 Furthermore, the central role that mitochondria play in neurodegeneration and psychiatric disorders has become apparent over the last decade as the molecular mechanisms that influence both neuronal death and neuroplasticity have been increasingly elucidated.28 Accumulating evidence regarding mitochondrial translocation and the function of traditional “nuclear receptors” has revealed interesting and novel mechanisms in mitochondria that are regulated by steroids—particularly GCs—but also estradiol.2,16 However, the study of steroid hormone receptor regulation of mitochondrial function is still in its infancy. Future research will be needed to address several unanswered questions. For instance, what is the protective molecule that enhances GR/Bcl-2 trafficking to mitochondria in response to GCs? Is translocation of Bcl-2 family genes to mitochondria necessary for the modulation of mitochondrial functions by GCs? Do other traditional nuclear receptors form protein complexes with the Bcl-2 family of genes? The new knowledge gained from such studies will be instrumental in understanding the protection or dysfunction that steroid hormone receptors may exert via the regulation of mitochondrial function, and their role in the etiopathophysiology of stress-related psychiatric diseases such as MDD or PTSD.
Acknowledgements
We acknowledge the support of the Intramural Research Program of the NIMH. Ioline Henter provided outstanding editorial assistance.
Footnotes
References
- 1.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croxtall JD, van Hal PT, Choudhury Q, Gilroy DW, Flower RJ. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br J Pharmacol. 2002;135:511–519. doi: 10.1038/sj.bjp.0704474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sionov RV, Kfir S, Zafrir E, Cohen O, Zilberman Y, Yefenof E. Glucocorticoid-induced apoptosis revisited: a novel role for glucocorticoid receptor translocation to the mitochondria. Cell Cycle. 2006;5:1017–1026. doi: 10.4161/cc.5.10.2738. [DOI] [PubMed] [Google Scholar]
- 5.Koufali MM, Moutsatsou P, Sekeris CE, Breen KC. The dynamic localization of the glucocorticoid receptor in rat C6 glioma cell mitochondria. Mol Cell Endocrinol. 2003;209:51–60. doi: 10.1016/j.mce.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Moutsatsou P, Psarra AM, Tsiapara A, Paraskevakou H, Davaris P, Sekeris CE. Localization of the glucocorticoid receptor in rat brain mitochondria. Arch Biochem Biophys. 2001;386:69–78. doi: 10.1006/abbi.2000.2162. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filipovic D, Gavrilovic L, Dronjak S, Radojcic MB. Brain glucocorticoid receptor and heat shock protein 70 levels in rats exposed to acute, chronic or combined stress. Neuropsychobiology. 2005;51:107–114. doi: 10.1159/000084168. [DOI] [PubMed] [Google Scholar]
- 9.Pratt WB, Morishima Y, Murphy M, Harrell M. Chaperoning of glucocorticoid receptors. Handb Exp Pharmacol. 2006:111–138. doi: 10.1007/3-540-29717-0_5. [DOI] [PubMed] [Google Scholar]
- 10.Cato AC, Mink S. BAG-1 family of cochaperones in the modulation of nuclear receptor action. J Steroid Biochem Mol Biol. 2001;78:379–388. doi: 10.1016/s0960-0760(01)00114-5. [DOI] [PubMed] [Google Scholar]
- 11.Cvoro A, Dundjerski J, Trajkovic D, Matic G. Association of the rat liver glucocorticoid receptor with Hsp90 and Hsp70 upon whole body hyperthermic stress. J Steroid Biochem Mol Biol. 1998;67:319–325. doi: 10.1016/s0960-0760(98)00103-4. [DOI] [PubMed] [Google Scholar]
- 12.Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem. 1997;272:21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Sharma S, Kim J, Ferrante RJ, Ryu H. Mitochondrial nuclear receptors and transcription factors: who's minding the cell? J Neurosci Res. 2008;86:961–971. doi: 10.1002/jnr.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wattenberg BW, Clark D, Brock S. An artificial mitochondrial tail signal/anchor sequence confirms a requirement for moderate hydrophobicity for targeting. Biosci Rep. 2007;27:385–401. doi: 10.1007/s10540-007-9061-0. [DOI] [PubMed] [Google Scholar]
- 15.Demonacos C, Djordjevic-Markovic R, Tsawdaroglou N, Sekeris CE. The mitochondrion as a primary site of action of glucocorticoids: the interaction of the glucocorticoid receptor with mitochondrial DNA sequences showing partial similarity to the nuclear glucocorticoid responsive elements. J Steroid Biochem Mol Biol. 1995;55:43–55. doi: 10.1016/0960-0760(95)00159-w. [DOI] [PubMed] [Google Scholar]
- 16.Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Nat Acad Sci USA. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb RA. Role of mitochondria in apoptosis. Crit Rev Eukaryot Gene Expr. 2000;10:231–239. doi: 10.1615/critreveukargeneexpr.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 20.Daniel PT, Schulze-Osthoff K, Belka C, Guner D. Guardians of cell death: the Bcl-2 family proteins. Essays Biochem. 2003;39:73–88. doi: 10.1042/bse0390073. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong JS. Mitochondria: a target for cancer therapy. Br J Pharmacol. 2006;147:239–248. doi: 10.1038/sj.bjp.0706556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy AN. Ca2+-mediated mitochondrial dysfunction and the protective effects of Bcl-2. Ann N Y Acad Sci. 1999;893:19–32. doi: 10.1111/j.1749-6632.1999.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 23.Lasorsa FM, Pinton P, Palmieri L, Fiermonte G, Rizzuto R, Palmieri F. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 24.Psarra AM, Sekeris CE. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life. 2008;60:210–223. doi: 10.1002/iub.37. [DOI] [PubMed] [Google Scholar]
- 25.Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmann RF, Schloesser RJ, Gould TD, Manji HK. Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol Neurobiol. 2005;32:173–202. doi: 10.1385/MN:32:2:173. [DOI] [PubMed] [Google Scholar]