Amygdala volume in Major Depressive Disorder: A meta-analysis of magnetic resonance imaging studies (original) (raw)
. Author manuscript; available in PMC: 2009 Sep 8.
Published in final edited form as: Mol Psychiatry. 2008 May 27;13(11):993–1000. doi: 10.1038/mp.2008.57
Abstract
Major Depressive Disorder has been associated with volumetric abnormality in the amygdala. In this meta-analysis we examine results from magnetic resonance imaging volumetry studies of the amygdala in depression in order to assess both the nature of the relationship between depression and amygdala volume as well as the influence of extra-experimental factors that may account for significant variability in reported findings. We searched PubMed and ISI Web of Knowledge databases for articles published from 1985 to 2008 that used the wildcard terms “Depress*” and “Amygdal*” in the title, keywords, or abstract. From the 13 studies that met inclusion criteria for our meta-analysis, we calculated aggregate effect size and heterogeneity estimates from amygdala volumetric data; we then used meta-regression to determine whether variability in specific extra-experimental factors accounted for variability in findings. The lack of a reliable difference in amygdala volume between depressed and never-depressed individuals was accounted for by a positive correlation between amygdala volume differences and the proportion of medicated depressed persons in study samples: whereas the aggregate effect size calculated from studies that included only medicated individuals indicated that amygdala volume was significantly increased in depressed relative to healthy persons, studies with only unmedicated depressed individuals showed a reliable decrease in amygdala volume in depression. These findings are consistent with a formulation in which an antidepressant-mediated increase in levels of brain derived neurotrophic factor promotes neurogenesis and protects against glucocorticoid toxicity in the amygdala in medicated but not in unmedicated depression.
Keywords: major depressive disorder, SSRI, SNRI, MRI volumetry, antidepressant, neurogenesis, BDNF, glucocorticoid, neurotoxicity
Introduction
Major Depressive Disorder (MDD) is characterized by a constellation of emotional and behavioral symptoms, and requires sustained sad mood or significantly diminished enjoyment of daily activities to yield a DSM-IV diagnosis of depression (1). Given that depression is, in important ways, a disorder of affective expression, experience, and regulation, attempts to understand the neural substrates of this disorder have focused primarily on structures implicated in the experience of emotion and the processing of affective information and stimuli (e.g., 2, 3). Although anomalies in the structure and function of a number of limbic and paralimbic structures have been found to be associated with depression — most reliably the dorsolateral and ventral prefrontal cortex, hippocampus, and amygdala — the amygdala, a structure crucial to the perception of and memory for emotional material (e.g., 4, 5–7), has, arguably, been the focus of the most extensive theoretical and empirical examination.
Studies using functional neuroimaging have found both elevated baseline activity in the amygdala (e.g., 8) and heightened amygdala responsivity to affective stimuli (e.g., 9) in depressed, compared with nondepressed, participants. Moreover, within depressed samples, elevated baseline amygdala activity has been found to be associated with symptom severity (8, 10) and, further, to return to normal levels following successful pharmacotherapy (8).
In several structural neuroimaging studies, investigators have examined differences between depressed and nondepressed individuals in amygdala volume. These studies have yielded variable findings, with some reporting that depressed participants were characterized by smaller amygdala volume than were nondepressed participants (3, 11–15), some finding greater amygdala volume in depressed than in nondepressed individuals (16–19), and others finding no difference in amygdala volume between depressed and nondepressed persons (17, 20, 21). It is not surprising, therefore, that a recent meta-analysis of six studies reported no aggregate difference in amygdala volume associated with depression (22).
The inconsistent results of these studies suggest that there is not a reliable association between depressive illness and amygdala volume. It is important to note, however, that the findings of these studies have not been examined systematically as an aggregate and, further, that there are several factors that distinguish studies of amygdala volume in depression that could account for the discrepant results obtained in these investigations. Indeed, some of these factors have been found in other studies to be associated with variation in volume in structures comprising the medial temporal lobes, including the use of antidepressant medication (23, 24), the presence of chronic stress (25, 26), and the age (27) and gender composition of samples studied (28, 29).
We conducted a meta-analysis of amygdala volume in depression to examine two questions: (1) whether there are reliable differences between depressed and healthy individuals in amygdala volume when the full body of available empirical work is considered systematically; and (2) whether there is significant heterogeneity in reported between-group amygdala volume differences and, if so, what factors account for this variability. Given the inconsistent findings in this literature, we predicted that there would not be a reliable difference between depressed and nondepressed individuals in amygdala volume, but that there would be significant heterogeneity across studies. Moreover, because depressed and nondepressed individuals in these studies differed with respect to antidepressant use and chronicity, or recurrence, of depressive illness, but not typically with respect to age and gender composition, we predicted further that variation in antidepressant use and chronicity of depressive illness, but not in age and gender composition, would predict significant variation in amygdala volume differences. More specifically, given evidence that antidepressant medication supports neurogenesis (24, 30) we predicted that average amygdala volume in depressed, relative to healthy, samples would increase with the proportion of medicated individuals in depressed samples. In addition, and consistent with studies showing volumetric decrease in the hippocampus associated with recurrence of depression (14, 22, 31), we predicted that amygdala volume in these samples would decrease relative to controls as a function of increasing chronicity of depression.
Methods
We used ISI Web of Knowledge (http://portal.isiknowledge.com) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) electronic databases to conduct our literature search. We conducted a subject search for articles published from 1985 to 2008 that used the wildcard terms “Depress*” and “Amygdal*” in either the title or abstract. Next, one of the authors (JPH) scanned the titles and, if necessary, the abstracts of the studies containing these search terms in order to identify those that conducted MRI-based volumetry of the amygdala with depressed participants. Several inclusion and exclusion criteria were applied by two of the authors (IHG and JPH) to the resultant set of studies. These criteria were: 1) diagnosis of depression was made using DSM-III, DSM-IV, or ICD-10 criteria; 2) individuals with current drug and/or alcohol dependency or neurological disorder were excluded from the study; 3) participants diagnosed with bipolar disorder were not included in the depressed group; 4) there was no report of unusual etiology of depressive illness in depressed samples; 5) the amygdala was defined and measured independent of the hippocampus; 6) the average estimated amygdala volume for both depressed and control groups was reported; 7) the average age of participants included in the study was between 20 and 60; and 8) depressed and control groups were equated for age and gender composition. Fourteen of the 1503 studies identified by the literature search met these criteria and were included in the meta-analysis presented here. Two of these 14 studies used overlapping participant samples: one was an assessment of volumetric abnormalities in the amygdala in recurrent depression (17), and the other was a one-year follow up to that study (32). In order to not violate assumptions of independence that underlie the statistical tests used in our meta-analysis, only data from the initial study (17) were analyzed, yielding a final sample of 13 studies.
Meta-analyses were conducted using a random-effects model (33) that weighted the contribution of each study inversely with the standard deviation of its mean in calculating a mean population effect size estimate, Cohen’s d (34). We followed this by conducting a Q test in order to determine whether the degree of heterogeneity among studies exceeded chance levels. We then attempted to account for any sources of significant heterogeneity in results across studies by using a multi-level model approach to meta-analysis (35), also known as meta-regression. This statistical approach permitted an assessment of the independent impact of various study characteristics that might explain differences in the effect sizes obtained. In this approach, as in the random effects meta-analysis, studies were weighted by their precision (36). We used a restricted maximum likelihood estimation method to predict variation in effect size among studies with the following four factors: age (average age of the sample); gender (percent of female participants in each study); chronicity (the mean number of depressive episodes reported by MDD participants); and medication (percent of depressed participants in each study who were taking antidepressant drugs). All analyses were conducted with programs available in STATA (37). The criterion level for statistical significance of each of the analyses conducted here was set at p = .01.
Results
Table 1 presents participant information and results for the 13 studies that met criteria for inclusion in the meta-analysis presented here. An initial random effects meta-analysis yielded a weighted mean effect across all 13 studies of d = −.208 that did not differ significantly from an effect size of zero (z = − 0.722, p = .47). A test for heterogeneity of the effects across studies, however, yielded a highly significant result (Q = 117.98, p ≪ .01), indicating that the variation in volume differences across the 13 studies exceeds chance levels. We then examined whether variation in the characteristics of studies could explain this heterogeneity. This analysis, conducted with those studies that included all four variables of interest in our meta-regression — age, chronicity, gender and medication — indicated that only medication explained a significant amount of the variation in amygdala volume difference across studies. Specifically, as the proportion of depressed individuals taking antidepressant medication increased across studies, so did amygdala volume in the depressed relative to the control participants. Regression fit coefficients and their corresponding z-scores and probabilities under the null hypothesis for each of the four variables of interest are presented in Table 2.
Table 1.
Participant Information and Results from MRI Studies of Amygdala Volume in Major Depression
| Study | n | Avg. MDD Age | % Female | % MDDs on Antidepressant Meds | Avg. Number of Episodes | Avg. MDD Bilateral Volume (SD) in mm3 | Avg. CTRL Bilateral Volume (SD) in mm3 | Avg. Volume Difference (mm3) | Additional information |
|---|---|---|---|---|---|---|---|---|---|
| Hastings, Parsey, Oquendo, Arango and Mann (12) | 18 MDD18 CTRL | 40 | 55% | 0% | 4.7 | 2865 (280) | 3563 (282) | −698* | Right amygdala significantly smaller and left marginally smaller (p = .08) in MDD. Effect driven by female participants.. |
| von Gunten, Fox, Cipolotti and Ron (15) | 14 MDD14 CTRL | 57 | 57% | 79% | NA | 3247 (271) | 3758 (245) | −511* | Left amygdala significantly smaller and right marginally smaller in MDD. |
| Caetano et al. (11) | 31 MDD31 CTRL | 38 | 77% | 0% | 5 | 3870 (430) | 4200 (385) | −330* | Both currently and remitted depressed included. Left amygdala marginally smaller in MDD. |
| Hickie et al. (13) | 45 MDD16 CTRL | 52 | 66% | 64% | 6.9 | 3100 (699) | 3400 (500) | −300* | Total amygdala volume reported; significaantly smaller in MDD. |
| Monkul et al. (20) | 10 MDD17 CTRL | 36 | 100% | 0% | 3.9 | 3810 (445) | 4070 (410) | −260 | Neither left nor right amygdala volumes differed significantly between groups. |
| Sheline, Sanghavi, Mintun and Gado (14) | 24 MDD24 CTRL | 53 | 100% | 67% | 4.8 | 3273 (207) | 3437 (178) | −164* | Volume of core nuclei but not whole amygdala was significantly decreased in MDD, bilaterally. |
| Sheline, Gado and Price (3) | 20 MDD20 CTRL | 54 | 100% | 70% | NA | 3374 (307) | 3534 (296) | −160* | Volume of core nuclei but not whole amygdala was significantly decreased in MDD, bilaterally. |
| Frodl et al. (17) | 27 MDD27 CTRL | 49 | 48% | 100% | NA | 3542 (242) | 3556 (280) | −14 | Neither left nor right amygdala volumes differed significantly between groups. |
| Munn et al. (21) | 26 MDD18 CTRL | 21 | 100% | 8% | NA | 3617 (225) | 3572 (221) | 45 | Neither left nor right amygdala volumes differed significantly between groups. |
| Lange and Irle (18) | 17 MDD17 CTRL | 34 | 100% | 100% | 2 | 2550 (260) | 2260 (185) | 290* | Left and right amygdala volume together, but not individually, was significantly greater in |
| Weniger, Lange & Irle (19) | 21 MDD23 CTRL | 34 | 100% | 100% | 1.4 | 2600 (300) | 2300 (200) | 300* | Both left and right amygdala significantly larger in MDD. |
| Frodl et al. (17) | 30 MDD27 CTRL | 40 | 56% | 100% | 1 | 3895 (277) | 3591 (286) | 304* | Left amygdala significantly larger in MDD. |
| Bremner et al. (16) | 16 MDD16 CTRL | 43 | 38% | 100% | 2 | 3351 (474) | 2682 (449) | 669* | Left and right amygdalae marginally larger in MDD. |
Table 2.
Results of a Meta-Regression of Amygdala Volume Differences Against Percentage of Female Participants, Proportion of Medicated Depressed, Age of Sample, and Number of Episodes for MDD Study Participants.
| Regression Coefficient | SE | z | p | |
|---|---|---|---|---|
| Medication | 2.47 | 0.92 | 2.67 | < 0.01 |
| Chronicity | 0.03 | 0.27 | 0.09 | 0.93 |
| Age | −0.07 | 0.06 | −1.17 | 0.24 |
| Gender | −0.23 | 1.02 | −0.22 | 0.82 |
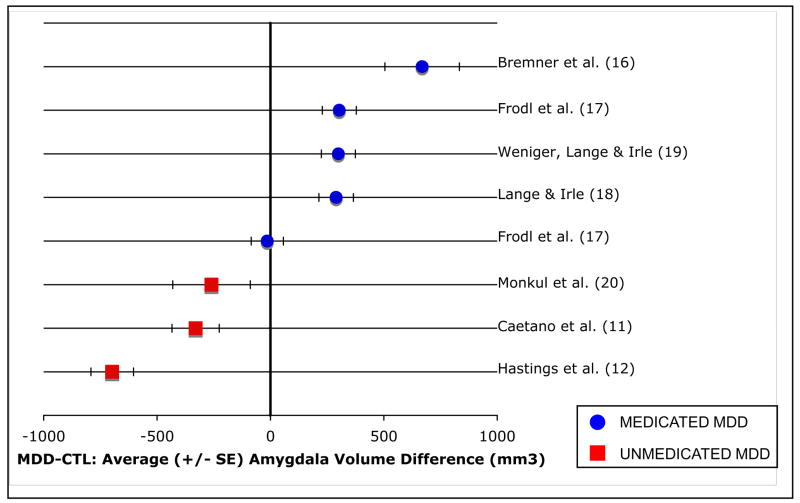
Given that we found the proportion of medicated depressed persons in a study to predict the magnitude of the difference in amygdala volume between depressed and control participants, we conducted a follow-up analysis to explore further how the inclusion of medicated participants in studies might affect conclusions drawn from the literature examining amygdala volume in depression. We calculated weighted mean effect sizes for two types of studies comparing amygdala volume in depressed and nondepressed samples: those using depressed samples composed entirely of unmedicated individuals and those using samples in which all of the depressed participants were medicated. These calculations indicate that whereas for studies that included only unmedicated depressed participants amygdala volume is significantly lower in depressed than in control groups (d = −1.238, z = −2.416, p = .01), studies in which all depressed participants were medicated show the opposite effect, with amygdala volume significantly increased in depressed relative to control participants (d = .938, z = 3.243, p < .01). These results are presented in Figure 1.
Figure 1.
Average (+/− SE) amygdala volume difference between depressed and nondepressed samples for studies in which all depressed participants were medicated and for studies using unmedicated depressed samples.
Discussion
The meta-analysis presented here showed that while there was no aggregate-level difference between depressed and nondepressed individuals in amygdala volume, there was significant inter-study variability in the difference between depressed and nondepressed groups in amygdala volume. Further, among four factors considered in studies of amygdala volume in depression – proportion of medicated depressed participants, chronicity of depressive illness, age, and gender composition of samples – the proportion of medicated depressed persons included in the studies predicted significant unique variation in group differences in amygdala volume. More specifically, the greater the proportion of medicated individuals in a depressed sample, the larger the difference between depressed and nondepressed participants in amygdala volume. Follow-up meta-analyses indicated that amygdala volume was significantly decreased in depression in studies that included unmedicated depressed participants, and significantly increased in depression when considering only studies with samples composed entirely of medicated depressed persons.
These results are important for several reasons. First, they are consistent with an account of the inconsistencies in the literature relating depression to amygdala volume in which confounding of depression with antidepressant usage biases estimates of amygdala volume in depression. Second, these findings support the formulation that depression, as an unmedicated disorder, is associated with decreased amygdala volume. Finally, these results provide evidence that antidepressant pharmacotherapy can facilitate neuro- or gliogenesis in the human amygdala.
Our finding that unmedicated depression is associated with decreased amygdala volume is consistent with a literature demonstrating decreased hippocampal volume in depression (14, 22, 31, 38). Stress-induced glucocorticoid excitotoxicity, which has been postulated to underlie hippocampal atrophy in psychiatric illness (e.g., 39), stands as a potential moderator of amygdalar volume loss in depression. This is a reasonable hypothesis given that the amygdala, like the hippocampus, is dense with glucocorticoid receptors (e.g., 40). However, if cumulative effects of glucocorticoid exposure account for volumetric decrease of the amygdala in depression then we would also expect a negative correlation between amygdala volume and recurrence or chronicity of depression, as has been shown in studies of hippocampal volume and depression (14, 22, 31, 38). It is noteworthy, then, that we did not find that chronicity of depression predicted variability in group differences in amygdala volume across studies. One likely reason for this is that, perhaps not unexpectedly, in the studies included in the present meta-analysis, there was a near-significant negative correlation between chronicity of depression and proportion of medicated depressed participants in a given sample [_r_(7) = −.63, p = .07]. After this association was accounted for in our meta-regression, the medication variable continued to predict significant variation in differences in amygdala volume, while the chronicity variable did not. Excluding the medication factor in a meta-regression (i.e., including only age, gender, and chronicity variables), we found chronicity, but not age or gender, to account for significant variation in group differences in average amygdala volume (regression coefficient = −.67, p < .01). It will be important in future research to distinguish and separate medication status and chronicity of depression in order to assess the independent contribution of these of these factors and their interaction in affecting amygdala volume in depression.
Our finding that amygdala volume is significantly increased in samples of medicated depressed participants is consistent with, and extends, a growing body of evidence that antidepressant treatment facilitates growth of new neurons and glia. In studies with both rodents (24, 30) and nonhuman primates (41), administration of antidepressant medication has been found to lead to neurogenesis in the hippocampus. Adding to this, there is evidence that neurogenesis also takes place in the amygdala (42–44), and that gliogenesis in the amygdala can occur following induced electroconvulsive seizures (45), a common and effective intervention for intractable depression. It is possible that increased production of brain-derived neurotrophic factor (BDNF), which has been demonstrated to facilitate neurogenesis and protect against excitotoxicity in both the hippocampus (46–48) and striatum (49, 50), mediates the increase in amygdala volume in samples of medicated depressed individuals. This is a viable hypothesis given both that administration of antidepressant medication has been found to increase BDNF expression in the hippocampi of rodents (51) and humans (52), and that BDNF and its receptor, tyrosine kinase B, are present in the amygdala and are centrally involved in amygdala-dependent learning (53, 54).
One variable that was not investigated in the present meta-analysis was the way in which, across the various studies, the amygdala was defined for purposes of volume estimation. This is an important variable to consider in light of work by Sheline et al. (3) showing a significant decrease in core amygdala nuclei (basal, accessory basal, and lateral) but not in total amygdala volume in depression. While we were careful to include only studies in which the amygdala was defined independent of the hippocampus, the definitions of amygdala volume reported in the larger literature were highly variable and not easily quantifiable and were, thus, not included in our meta-analysis. Future meta-analyses that will be able to incorporate a larger number of studies will be important in examining the impact of variation in amygdala boundary definitions on estimated amygdala volumetric differences between depressed and healthy individuals.
The present findings raise several questions to be addressed in future research. While investigators have found smaller amygdala volume in depression to be associated with increased responsivity to affective stimuli (55), the functional implications of increases in amygdala volume in depression – potentially instigated by antidepressant treatment – have not been adequately explored. It is likely that the cellular composition of any increase in amygdala volume will determine the nature of its functional consequences. Work by Wennstrom, Hellsten, and Tingstrom (45), for example, suggests that generation of oligodendrocytes in the amygdala is responsible for the antidepressant-related volumetric increase in this structure. These investigators found that inducing electroconvulsive seizures in rats facilitated generation of amygdalar oligodendrocytes, cells shown both to be reduced in the amygdala of depressed individuals (56) and to be implicated in uptake of extracellular glutamate (57), a neurotransmitter which, when present in excess in the extracellular environment, promotes excitotoxic neural degeneration (39). It will be important in future research to examine whether pharmacological antidepressant therapy similarly influences oligodendrocyte proliferation in humans.
To the extent that the current findings show that a volumetric decrease in the amygdala is associated with unmedicated depression, they also raise questions concerning how this volumetric decrease relates to depressive pathology. Does abnormally low amygdala volume precede and function as a potential risk factor for depression, or might it be a symptom or consequence of a depressive episode? Research with never-depressed populations at high risk for depression, and with identical twins discordant for depressive illness, will be instrumental in addressing these issues concerning the temporal and causal relation between amygdala abnormality and depression.
Acknowledgments
We thank Rebecca Cooney and Victoria Thornton for their contributions to this manuscript. The authors had full access to all of the meta-analytic data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Preparation of this manuscript was funded by NIMH Grants MH059259 and MH074849 awarded to Ian H. Gotlib and NIH Grant MH079651 awarded to J. Paul Hamilton.
Contributor Information
J. Paul Hamilton, Department of Psychology, Stanford University, Stanford, CA 94305, USA
Matthias Siemer, Department of Psychology, University of Miami, Coral Gables, FL 33146, USA
Ian H. Gotlib, Department of Psychology, Stanford University, Stanford, CA 94305, USA
References
- 1.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 2.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: A potential predictor of treatment response. Neuroreport. 1997 Mar;8(4):1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 3.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998 Jun;9(9):2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 4.Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning & Memory. 1997 Sep–Oct;4(3):291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 5.Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. Journal of Cognitive Neuroscience. 2004 Apr;16(3):453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- 6.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The Amygdala and Emotional Memory. Nature. 1995 Sep;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 7.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996 Oct;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 8.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology. 2002 Dec;12(6):527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry. doi: 10.1016/j.biopsych.2007.12.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A Functional Anatomical Study of Unipolar Depression. Journal of Neuroscience. 1992 Sep;12(9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Research-Neuroimaging. 2004 Dec;132(2):141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004 May;29(5):952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 13.Hickie IB, Naismith SL, Ward PB, Scott EM, Mitchell PB, Schofield PR, et al. Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. Journal of Affective Disorders. 2007 Feb;98(1–2):137–142. doi: 10.1016/j.jad.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999 Jun;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. Journal of Neuropsychiatry and Clinical Neurosciences. 2000 Fal;12(4):493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- 16.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. American Journal of Psychiatry. 2000 Jan;157(1):115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 17.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry. 2003 Feb;53(4):338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 18.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychological Medicine. 2004 Aug;34(6):1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 19.Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. Journal of Affective Disorders. 2006 Aug;94(1–3):219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda ALT, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Molecular Psychiatry. 2007 Apr;12(4):360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 21.Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, et al. Amygdala volume analysis in female twins with major depression. Biological Psychiatry. 2007;62(5):415–422. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. American Journal of Psychiatry. 2004 Apr;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 23.Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biological Psychiatry. 2006 Jun;59(11):1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. Journal of Neuroscience. 2000 Dec;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disorders. 2002 Apr;4(2):117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky RM. Why stress is bad for your brain. Science. 1996 Aug;273(5276):749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 27.Bertoni MA, Sclavi NE, Sauer HJ. Volumetry of the hippocampus and amygdala with magnetic resonance imaging. International Journal of Neuroradiology. 1998 Jul–Aug;4(4):291–295. [Google Scholar]
- 28.Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. Journal of Comparative Neurology. 2007 Apr;501(6):904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- 29.van Elst LT, Woermann F, Lemieux L, Trimble MR. Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neuroscience Letters. 2000 Mar;281(2–3):103–106. doi: 10.1016/s0304-3940(00)00815-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang J-W, David DJ, Monckton JE, Battaglia F, Hen R. Chronic Fluoxetine Stimulates Maturation and Synaptic Plasticity of Adult-Born Hippocampal Granule Cells. J Neurosci. 2008 February 6;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. American Journal of Psychiatry. 2004 Nov;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 32.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. Journal of Clinical Psychiatry. 2004 Apr;65(4):492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 33.Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; San Diego: 1985. [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 35.Hox JJ. Multilevel analysis. Techniques and applications. Erlbaum; Mahwah, NJ: 2002. [Google Scholar]
- 36.Hox JJ, Kreft IGG. Multilevel Analysis-Methods. Sociological Methods & Research. 1994 Feb;22(3):283–299. [Google Scholar]
- 37.Sharp SJ. Meta-analysis regression. Stata Technical Bulletin. 1998;42:16–22. [Google Scholar]
- 38.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003 Aug;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 39.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000 Oct;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 40.Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136(1):289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 41.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. Journal of Neuroscience. 2007 May;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proceedings of the National Academy of Sciences of the United States of America. 2002 Aug;99(17):11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. Journal of Neurobiology. 2002;51(2):115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 44.Fowler CD, Liu Y, Ouimet C, Wang Z. Mating and social isolation differentially affect adult neurogenesis in the female prairie vole. Hormones and Behavior. 2001;39(4):331. [Google Scholar]
- 45.Wennstrom M, Hellsten J, Tingstrom A. Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat amygdala. Biological Psychiatry. 2004 Mar;55(5):464–471. doi: 10.1016/j.biopsych.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experimental Neurology. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Willis L, Hunter C, Quintero EM, Granholm AC. Neurogenesis is increased in hippocampal grafts that are treated with BDNF. Experimental Neurology. 2002;175(2):437–437. [Google Scholar]
- 48.Khaspekov LG, Verca MSB, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. European Journal of Neuroscience. 2004;19(7):1691–1698. doi: 10.1111/j.1460-9568.2004.03285.x. [DOI] [PubMed] [Google Scholar]
- 49.Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brainderived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132(3):767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Navarro E, Alberch J, Neveu I, Arenas E. Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype and prevent degenerative changes in striatal projection neurons after excitotoxicity in vivo. Neuroscience. 1999;91(4):1257–1264. doi: 10.1016/s0306-4522(98)00723-4. [DOI] [PubMed] [Google Scholar]
- 51.Nibuya M, Morinobu S, Duman RS. Regulation of Bdnf and Trkb Messenger-Rna in Rat-Brain by Chronic Electroconvulsive Seizure and Antidepressant Drug Treatments. Journal of Neuroscience. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry. 2001;50(4):260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 53.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature Neuroscience. 2006;9(7):870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. Journal of Neuroscience. 2004;24(20):4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals - An fMRI investigation. Amygdala in Brain Function: Bacic and Clinical Approaches. 2003:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biological Psychiatry. 2004 Mar;55(6):563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Damelio F, Eng LF, Gibbs MA. Glutamine-Synthetase Immunoreactivity Is Present in Oligodendroglia of Various Regions of the Central-Nervous-System. Glia. 1990;3(5):335–341. doi: 10.1002/glia.440030504. [DOI] [PubMed] [Google Scholar]