Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease (original) (raw)
Abstract
Adiponectin (APN) is an adipocyte-derived factor that exists at high concentrations in serum and has anti-inflammatory and systemic vascular-protective properties. In this study, we investigated the role of APN in pulmonary vascular homeostasis. We found that APN localizes to the luminal side of blood vessels in lung and acts in vitro to block TNF-α-induced E-selectin upregulation in pulmonary artery endothelial cells. Targeted deletion of the APN gene in mice leads to a vascular phenotype in lung characterized by E-selectin upregulation and age-dependent increases in perivascular inflammatory cell infiltration and pulmonary arterial pressures. Taken together, these findings demonstrate an important role for APN in lung vascular homeostasis and suggest that APN-deficient states may contribute to the pathogenesis of inflammatory pulmonary vascular disease and to the development of pulmonary hypertension.
Keywords: adipose tissue, inflammation, obesity
pulmonary arterial hypertension (PAH) is a clinical condition associated with sustained elevations in pulmonary arterial pressure, eventually leading to right ventricular failure and death. PAH can develop as a primary disorder but often occurs in association with other conditions such as congenital heart disease and liver disease as well as with various inflammatory conditions including connective tissue disease and HIV infection (3).
Adiponectin (APN) is an adipocyte-derived protein that is abundantly present in plasma (5, 17, 23). Circulating APN levels are high in lean, healthy individuals, but levels are reduced in individuals with increased body mass index, insulin resistance, and various cardiovascular disease states (1). Interest in APN relates to its ability to regulate diverse biological processes in a wide range of tissues in experimental models (4, 13, 18, 19, 22). APN was initially recognized as an insulin-sensitizing agent; however, recent findings support a role in vascular homeostasis and inflammation (7, 17, 18). For example, APN has been shown to activate nitric oxide synthase (eNOS) and upregulate prostaglandin I2 synthase production and downregulate expression of select adhesion molecules in endothelial cells (12–15). Furthermore, APN appears important in the regulation of systemic vascular tone as APN −/− mice are more susceptible to salt-sensitive hypertension, and APN supplementation leads to a sustained decrease in blood pressure in these mice (13). Recent reports also demonstrate that APN protects tissues against ischemic injury. For example, APN −/− mice are more susceptible to ischemia-induced myocardial and cerebral injury, and overexpression of APN reduces tissue damage in these models (12, 14, 20). The protective affects of APN are mediated, at least in part, by its ability to block inflammation and stimulate nitric oxide production via an AMPK-dependent pathway (20).
To date, studies investigating the role of APN in vascular homeostasis have largely been restricted to the systemic circulation; however, two recent reports indicate that APN-deficient mice develop an abnormal pulmonary vascular phenotype when placed in chronic hypoxic conditions or exposed to chronic allergic airway inflammation (9, 11). In this study, we show that APN protein localizes throughout the pulmonary vascular endothelium and that deficiency in APN leads to upregulation of E-selectin in lung. Moreover, APN −/− mice develop a spontaneous pulmonary vascular phenotype that is characterized by an age-dependent increase in perivascular inflammatory cell infiltration and elevated pulmonary artery pressures. Together, these observations provide further support for the role of APN as a regulator of pulmonary vascular homeostasis and suggest for the first time that chronically low levels of APN may be associated with development of pulmonary vascular disease.
METHODS
Mice.
APN −/− mice were generated by targeted gene disruption as previously described (20). All studies were performed using male mice. Age-matched wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Mice were maintained in 12:12-h light-dark cycle and given food and water ad libitum. Euthanasia was performed by isoflurane anesthesia followed by cervical dislocation. Heart, body weight, and tibial length were recorded in all mice (Table 1). All animal studies were conducted according to protocols approved by the National Institutes of Health and the Boston University Institutional Animal Care and Use Committee.
Table 1.
Morphometric data obtained from WT and ADP-deficient mice at 1 yr of age
| WT at 1 yr | APN −/− at 1 yr | |
|---|---|---|
| Body wt, g | 37.5±1.5 | 47.7±1.5† |
| TL, mm | 17.7±0.1 | 17.5±0.3 |
| Whole heart weight, mg | 142.6±4.6 | 164.5±4.4† |
| LV + SW, mg | 115.6±3.3 | 131.5±3.8† |
| RV weight, mg | 26.46±1.5 | 33.6±1.05† |
| RV/LV + SW | 0.23±0.009 | 0.26±0.009* |
| LV + SW/TL, mg/mm | 6.18±0.17 | 7.5±0.18† |
| RV/TL, mg/mm | 1.40±0.03 | 1.85±0.11† |
Lung fixation.
Lung fixation was performed after flushing the vasculature with saline. A blunt 22-gauge needle was inserted into the trachea, and perfused lungs were inflated using 4% paraformaldehyde. Tissue was dissected en block from the thoracic cavity and fixed overnight at 4°C. Lung tissue was dehydrated in graded alcohols and embedded in paraffin blocks. Five-micrometer tissue sections were cut and placed on charged slides for immunohistochemistry staining.
Histochemistry.
Tissue sections were deparaffinized and rehydrated through graded alcohol to water. Immunofluorescent staining for E-selectin and α-smooth muscle actin was performed immediately after quenching tissue sections with sodium borohydride for 20 min and blocking with 1% goat serum. E-selectin antibody staining was performed using a rat polyclonal antibody directed against mouse E-selectin (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:25. The primary anti-α-smooth muscle actin MAb was obtained from Sigma-Aldrich (St. Louis, MO) and was already FITC conjugated. Secondary staining for E-selectin was performed using a biotin-conjugated goat anti-rat antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1:100 followed by a streptavidin-FITC (BD Pharmingen, San Jose, CA) step at a dilution of 1:100. In parallel, isotype control staining was performed. On completion of staining, slides were washed in PBS and counterstained using the nuclear dye 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich).
APN and CD45 staining were performed using diaminobenzidine (DAB) substrate detection methods. Slides were initially quenched with 3% H2O2 and blocked with 1% goat serum. Staining for APN was performed using a monoclonal rat anti-mouse APN (R&D Systems, Minneapolis, MN) at a dilution of 1:400. Staining for CD45 was performed using a monoclonal rat anti-mouse CD45 (BD Biosciences, San Jose, CA) at a dilution of 1:40. Secondary staining was performed using a biotin-conjugated goat anti-rat antibody (Vector Laboratories) at a dilution of 1:100. Slides were then treated with ABC reagent (Vector Laboratories) and developed using DAB for 1–3 min (Vector Laboratories). On completion of staining, slides were washed in PBS and counterstained.
Morphometry and quantitative assessment of vascular inflammation.
Perivascular inflammation was assessed in tissue sections after staining for the pan-hematopoietic marker CD45. Images were captured at ×40 using a Zeiss N HBO100 microscope (Thornwood, NY) fitted with an AxioCam MR CCD digital camera. A total of 80–100 blood vessels ranging from 150 to 300 μm were analyzed using AxioVision 3.1 software. The number of perivascular CD45-positive inflammatory cells was compared in age-matched WT and APN −/− mice. Perivascular inflammatory cells were defined as the number of CD45-expressing cells present outside the vascular lumen but in juxtaposition to the blood vessel wall.
Smooth muscle thickness was measured in fully muscularized blood vessels captured in cross-section after staining for α-smooth muscle actin. The thickness of the smooth muscle layer (the transverse diameter) was assessed at 2 sites along the blood vessel, and ≥50 blood vessels of similar size (150–200 μm) were analyzed per group.
Cell culture.
Human pulmonary artery endothelial cells (HPAEC) were grown to confluence in EBM-2 media containing 10% FBS and growth supplements (Clonetics, San Diego, CA). All experiments were performed using cells at _passages 6_-10. E-selectin expression was determined in HPAEC pretreated with APN (10 μg/ml) or vehicle for 18 h followed by 6-h stimulation with TNF-α. E-selectin mRNA levels were quantified in RNA extracts. Recombinant human APN was obtained from BioVendor (Candler, NC). APN concentration in supernatant was measured by ELISA (R&D Systems).
Real-time PCR.
RNA was isolated from HPAEC. cDNA was generated from RNA extracts by using a reverse-transcription kit (Promega, Madison, WI). Quantitative expression of E-selectin was performed with 0.5 μg of starting total RNA. Values were calculated based on the change in cycle threshold (ΔΔCT) method. Human E-selectin levels were expressed relative to internal control gene 36B4 levels.
Western blot.
Lung tissue was homogenized with mortar and pestle and incubated in buffer containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 150 mM NaCl in the presence of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstatin A) for 30 min at 4°C. Lysates were sheared by passing through a 21-gauge needle before centrifugation. Protein concentration was measured in the supernatant using the Bradford assay. For analysis, 20 μg of protein was separated through a 10% SDS-polyacrylamide gel before electrophoretic transfer to a nitrocellulose membrane. Membranes were blocked with 2% BSA in TBS for 1 h. Primary antibody staining (E-selectin, dilution 1:2,500) was performed overnight in TBS with 0.1% Tween 20 (TBST) and 2% BSA. Mixture was incubated overnight at 4°C. Secondary antibody staining [anti-rabbit-horseradish peroxidase (HRP), dilution 1:6,000] was performed for 1 h at room temperature. Membranes were then washed three times with TBST. Chemiluminescence was performed with the Western Lightning detection reagent (PerkinElmer, Waltham, MA).
Hemodynamics.
One-year-old mice were anesthetized with isoflurane (1.5–2.5%). The right jugular vein and left carotid artery were isolated by standard cutdown approach. Hemodynamic measurements were obtained from the right ventricle, ascending aorta, and left ventricle using a 0.8-F catheter (Millar Instruments, Houston, TX). Measurements include heart rate, systolic blood pressure, diastolic blood pressure, mean arterial blood pressure, right and left ventricular end-diastolic pressure (LVEDP), maximum first derivative of right ventricular pressure (dP/d_t_), minimum dP/d_t_, and τ.
Echocardiography.
Transthoracic echocardiography was performed using a Vevo 770 High-Resolution Imaging System with 30-MHz RMV-707B scanning head (VisualSonics, Toronto, Ontario, Canada). Mice were anesthetized using 2% isoflurane, and heart rate was maintained at 400–500 beats/min on a thermal heated plate. Left ventricle dimension, percent fractional shortening, left ventricular mass index, anterior wall thickness, posterior wall thickness, and relative wall thickness were calculated as previously described (6). Right ventricular imaging was obtained by short-axis transverse view at the level of aortic valve, and right ventricle diameter was measured at end diastole. The peak velocity and acceleration time were measured by pulse-wave Doppler at the level of pulmonary artery root. Early filling wave (E), late filling wave (A), and the ratio of E to A was calculated using transmitral pulse-wave Doppler velocity. For measurement of transmitral flow velocity, heart rates were maintained at <400 beats/min to separate the E and A waves.
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was performed using two-tailed unpaired Student's _t_-test. Statistical significance was achieved when P < 0.05 at 95% confidence interval.
RESULTS
APN localizes to pulmonary vascular endothelium.
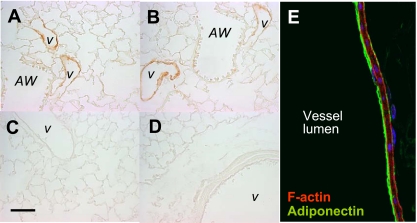
APN protein was examined in paraffin-embedded lungs sections using a polyclonal antibody directed against mouse APN. Findings demonstrate that APN was present at perivascular sites in lung (Fig. 1, A and B). As expected, APN staining was not detected in lungs of APN −/− mice (Fig. 1_C_) or in tissue sections stained with isotype control antibody (Fig. 1_D_). Confocal microscopy further localized APN protein expression to the luminal surface of the pulmonary vascular endothelium (Fig. 1_E_).
Fig. 1.
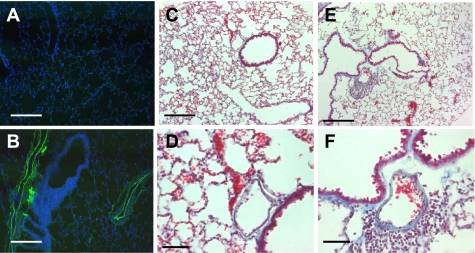
Adiponectin (APN) localizes to vascular sites in lung. A and B: representative sections showing that APN protein (brown stain) localizes to blood vessels (v) in lung. Staining is absent in proximal (AW) and distal airway structures. APN staining is not detected in lung sections of APN-deficient mice (C) and in wild-type (WT) lung sections stained with isotype control antibody (D). E: confocal microscopic analysis of APN protein localization was detected on the luminal side of blood vessels in lung where it colocalized with the vascular endothelium. APN (green) colocalizes to F-actin (red) on the endothelial side of the blood vessel wall. Scale bar = 200 μm.
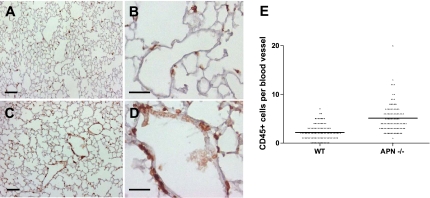
Based on the perivascular distribution of APN, we examined whether deficiency leads to a vascular phenotype in lung. Histological comparison of blood vessels in 3-mo-old WT and APN −/− mice revealed no significant structural differences; however, a mild increase in the number of perivascular inflammatory cells was visualized (Fig. 2). To quantify differences, WT and APN −/− lungs were immunostained with an antibody directed against the pan-hematopoietic marker CD45. Statistical analysis confirmed that perivascular inflammatory cell number was increased in lungs of APN −/− mice (Fig. 2_E_). Consistent with an inflammatory vascular phenotype, we found that E-selectin expression (Fig. 3) was increased, and wet-to-dry ratios (Supplemental Fig. S1 available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site) were slightly but significantly increased in lungs of APN −/− mice at this time point.
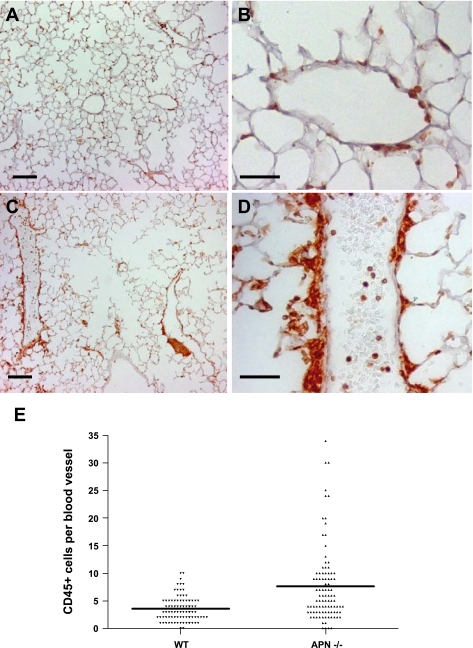
Fig. 2.
Perivascular inflammation is present in lungs of 3-mo-old APN-deficient mice. CD45 staining (brown) was performed in WT and APN-deficient lung sections. A and B: low- and high-power images of WT lungs, respectively. Scale bars = 200 μm. C and D: low- and high-power images of APN-deficient lung sections, respectively. Scale bars = 100 μm. E: bar graph showing the average number of perivascular CD45-positive cells per blood vessel (×40) in WT and APN-deficient lungs (*P < 0.05).
Fig. 3.
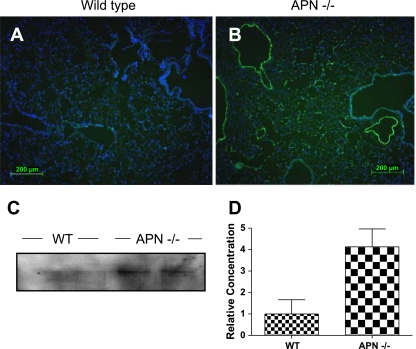
E-selectin is upregulated in pulmonary vascular endothelium of 3-mo-old APN-deficient mice. E-selectin staining is not detected by immunohistochemistry in lungs of WT mice (A), but staining is present throughout the endothelium of APN-deficient mice (B). Staining was not detected in APN-deficient sections stained with isotype control antibody (data not shown). C: Western blot showing E-selectin concentration in WT and APN-deficient lungs. D: bar graph showing relative E-selectin concentration when controlled for GAPDH.
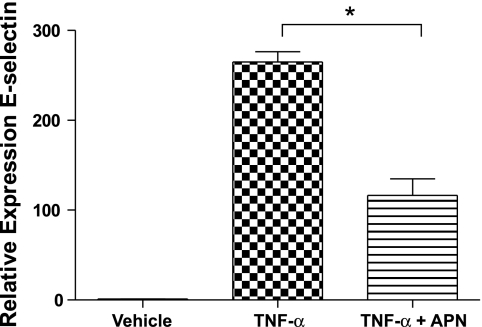
To corroborate the observation that APN regulates E-selectin expression in endothelial cells, HPAEC were cultured in presence or absence of physiological levels of APN protein (10 μg/ml) and stimulated with TNF-α (100 pg/ml) for 6 h. Of note, APN was not detected in media of HPAEC in standard culture conditions (data not shown). As expected, TNF-α stimulation led to robust upregulation of E-selectin transcript in HPAEC. Pretreatment with APN resulted in a marked reduction in TNF-α induced E-selectin expression (Fig. 4).
Fig. 4.
APN blocks TNF-α-induced E-selectin upregulation in human pulmonary artery endothelial cells (HPAEC). HPAEC were pretreated with APN for 18 h followed by stimulation with TNF-α for 6 h. Data are expressed as E-selectin mRNA levels relative to internal control gene 36B4 levels (n = 5; *P < 0.05).
APN deficiency leads to an age-dependent pulmonary vascular phenotype.
To determine whether prolonged deficiency in APN leads to more noticeable phenotype, detailed histological examination of the pulmonary vascular system was performed in 1-yr-old mice. Compared with young mice, a progressive inflammatory vascular phenotype was observed at 1 yr. As shown in Fig. 5, perivascular mononuclear cells were observed in WT and APN −/− lungs at 1 yr; however, inflammatory infiltrates were significantly more apparent in APN −/− mice. In addition, proteinaceous exudates were observed in the perivascular space of medium and large sized blood vessels in APN −/− mice, and E-selectin was noted to be upregulated (Fig. 6). Proteinaceous exudates and E-selectin expression were not detected in lungs of age-matched WT mice. Together, these findings indicate that prolonged APN deficiency leads to a progressive inflammatory vascular phenotype.
Fig. 5.
Perivascular inflammation increases with age. CD45 staining (brown) at 1 yr demonstrates the presence of perivascular inflammatory cell infiltrates in WT and APN-deficient lungs. Mild inflammation is observed in lungs of WT mice. A and B: low- and high-power images, respectively. Perivascular inflammation is readily apparent in lungs of APN-deficient mice. C and D: low- and high-power images, respectively. E: bar graph showing the average number of perivascular CD45-positive cells per blood vessel (×40) in WT and APN-deficient lungs (*P < 0.05). Scale bars: A and C, 200 μm; B and D, 100 μm.
Fig. 6.
Inflammatory vascular phenotype is present in lungs of 1-yr-old APN-deficient mice. E-selectin expression is detected in lungs of 1-yr-old APN-deficient mice (B) but not in lungs of age-matched WT mice (A). Perivascular inflammation and proteinaceous exudates are readily apparent in lungs of 1-yr-old APN-deficient mice (E, low-power; F, high-power view). Similar findings were not observed in lungs of 1-yr-old WT mice (C, low-power; D, high-power view). Scale bars: A, B, C, and E, 250 μm; D and F, 100 μm.
APN deficiency leads to an age-dependent increase in pulmonary artery pressures.
Given the link between pulmonary vascular inflammation and PAH in certain patient populations, we speculated whether APN deficiency might lead to increased pulmonary artery pressures. Noninvasive screening for elevated pulmonary artery pressures was performed by transthoracic echocardiography in age-matched WT and APN −/− mice. Consistent with the mild histological phenotype observed at 3 mo, no significant differences in right ventricular dimensions or pulmonary arterial hemodynamics were seen between WT and APN −/− mice at this time point (Supplemental Table S1). In contrast, right ventricle dimensions (Table 2) measured by transthoracic echocardiography were significantly increased in APN −/− mice (1.93 ± 0.02 vs. 2.28 ± 0.04 mm; P < 0.001) at 1 yr. Furthermore, invasive hemodynamic measurements demonstrated that right ventricular systolic pressure, right ventricular diastolic pressure, mean right ventricular pressure, and maximum dP/d_t_ were significantly increased in APN −/− mice at this time point (Table 3). These observations indicate that pulmonary artery pressure, as reflected by right ventricular dimensions and pressure, increases in the setting of chronic APN deficiency. In contrast, noninvasive and invasive measurements of left heart function (systemic blood pressure, cardiac output, and left ventricle function) were not significantly different in WT and APN −/− mice at 1 yr except for the observed increase in LVEDP in APN −/− mice.
Table 2.
Echocardiographic measurements in 1-yr WT and ADP-deficient mice
| WT at 1 yr | APN −/− at 1 yr | |
|---|---|---|
| No. of mice | 9 | 9 |
| RVDd, mm | 1.93±0.02 | 2.28±0.04† |
| LVDd, mm | 3.73±0.07 | 3.79±0.07 |
| PA ACT, ms | 22.4±0.7 | 19.4±0.8† |
| AWT, mm | 0.93±0.03 | 1.04±0.02† |
| PWT, mm | 0.90±0.03 | 1.09±0.03† |
| FS, % | 32.9±1.9 | 31.9±1.0 |
| RWT | 0.49±0.02 | 0.56±0.19 |
| TMF E/A | 1.66±0.11 | 1.69±0.07 |
| TDI E′/A′ | 1.29±0.13 | 0.94±0.12 |
| LVMI/TL | 10.5±0.3 | 11.9±0.3 |
Table 3.
Right and left heart catheterization performed in 1-yr WT and ADP-deficient mice
| Right Heart | Left Heart | |||
|---|---|---|---|---|
| WT | APN −/− | WT | APN −/− | |
| No. | 12 | 11 | 12 | 11 |
| Heart rate, beats/min | 518±7 | 525±9 | 499±9 | 502±5 |
| Systolic BP, mmHg | 21.5±0.7 | 27.0±0.9† | 104±2.0 | 109±2 |
| Diastolic BP, mmHg | 1.47±0.3 | 3.23±0.5* | 0.9±0.3 | 1.7±0.5 |
| EDP, mmHg | 3.1±0.7 | 6±0.8* | 4.8±0.6 | 7.3±0.8* |
| Mean, mmHg | 8.1±0.7 | 12.1±0.9† | 46±1.5 | 50±1.1 |
| Max dP/d_t_, mmHg/s | 943±97 | 1,273±114* | 7,774±142 | 7,652±179 |
| Min dP/d_t_, mmHg/s | −1,125±110 | −1,263±76 | −6,330±227 | −6,704±214 |
| τ | 0.02±0.008 | 0.03±0.04 | 0.01±0.001 | 0.01±0.001 |
DISCUSSION
The major finding of this study is that APN deficiency leads to an inflammatory vascular phenotype in lung. To our knowledge, this study is the first to indicate a direct role for APN in pulmonary vascular homeostasis and the first to suggest that prolonged APN-deficient states may contribute to the development of pulmonary vascular disease, including pulmonary hypertension. Although it has recently been reported that APN deficiency can give rise to pulmonary hypertension in conjunction with hypoxia and chronic airway inflammation, our study shows that APN deficiency per se is sufficient to produce pulmonary hypertension in aged mice. Furthermore, the PAH that develops in 1-yr-old APN-deficient mice may be a consequence of the perivascular inflammation that appears in young mice and progresses over time.
Our findings show that mononuclear cells accumulate around pulmonary blood vessels in APN −/− mice by 3 mo of age, and this phenotype is more notable when mice are 1 yr old. In addition, we demonstrate by invasive hemodynamic monitoring that pulmonary artery pressure is elevated in older APN −/− mice. It is well-recognized that there is a link between several inflammatory conditions, such as HIV infection and connective tissue disease, and PAH (2, 3, 24). Furthermore, inflammation is commonly observed in pathological specimens of patients with other forms of PAH. For example, lymphocytes, macrophages, mast cells, and antibody-complement deposits have been observed within plexiform lesions in patients with PAH (2, 24). Moreover, autoantibodies and elevated levels of various cytokines and chemokines have been observed in the plasma of patients with PAH (2, 3). However, despite evidence supporting an association between immune dysregulation and PAH, a causal association has yet to be established. Based on our findings, we speculate that APN may be a contributory molecule in the linkage between inflammation and PAH.
APN was first identified as an insulin-sensitizing agent but is now recognized for its anti-inflammatory activities (16–18, 22). For example, epidemiological studies show that serum APN levels are decreased in patients with chronic inflammatory conditions and that levels inversely correlate with circulating concentrations of proinflammatory markers like C-reactive protein (CRP), TNF-α, and IL-6 (17). Consistent with these observations, APN deficiency in mice is associated with greater elevations in inflammatory cytokines and autoantibody production in a model of autoimmune disease (22).
APN has anti-inflammatory actions on a variety of cell types. We and others have demonstrated that APN blocks LPS-induced TNF-α production in macrophages and downregulates NF-κB activation (4, 16, 20, 22). Furthermore, we (21) recently showed that APN acts in lung to suppress alveolar macrophage activation and that APN deficiency is associated with an emphysema-like phenotype. Emphysema develops in these mice, at least in part, from damage caused by excess alveolar macrophage proinflammatory cytokine production. Relevant to the current study, APN also suppresses inflammation in nonimmune cell types. For example, APN blocks agonist-induced IL-8 production in endothelial cells and downregulates reactive oxygen species production (8). The reported action of APN on endothelial cell types further supports the findings observed in this study.
Another finding of the current study is that APN localizes to the luminal side of blood vessels in lung and regulates E-selection expression in pulmonary vascular endothelial cells. These observations are consistent with published reports showing that APN acts on the systemic circulation to downregulate E-selectin expression and inhibit leukocyte-endothelial cell interactions (15, 19). E-selectin is normally expressed at very low levels in the pulmonary circulation but increases in response to lung injury (10). Furthermore, increased expression has been reported in the vascular endothelium of patients with PAH. Thus it is tempting to speculate that low APN levels may account for the observed increase in E-selectin expression in these patients.
Although our findings indicate that APN −/− mice develop PH, we recognize that some histological features were absent in this model. For example, pulmonary artery smooth muscle thickness was not significantly increased in 1-yr-old APN mice (Supplemental Fig. S2). We speculate that this relates to the fact that pulmonary artery pressures were only modestly elevated and that smooth muscle hyperplasia is not a prominent feature of murine models of PH. We acknowledge that histological evidence of smooth muscle hypertrophy might be present at later time points.
Although our findings suggest that chronic inflammation leads to the development of PAH, we recognize that other mechanisms may be contributing. For example, we have previously reported that APN −/− mice develop an emphysema-like phenotype. Thus it is possible that pulmonary artery hemodynamics could have been influenced by this phenotype. In addition, we observed an increase in LVEDP and left ventricular wall thickness in APN −/− mice. These findings suggest that diastolic left ventricular dysfunction may also spontaneously develop with age in APN −/− mice; however, hemodynamic (minimum dP/d_t_, τ) and echocardiogram (E/A) measurements do not support this alternative hypothesis.
In summary, our findings demonstrate a role for APN in lung vascular homeostasis and suggest that APN deficiency may directly contribute to the pathogenesis of inflammatory pulmonary vascular disease. We believe these data provide further rationale for investigating the role of APN in animal models of pulmonary hypertension and in patients with pulmonary vascular disease.
GRANTS
This work was supported by National Institutes of Health Grants K08-HL-077138, AG-15052, and HL-59215 and the Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension.
Supplementary Material
[Supplemental Figures and Tables]
REFERENCES
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 22: 358–363, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett 582: 1719–1724, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101: 10308–10313, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, Suzaki Y, Izawa Y, Fujimura M, Hashizume S, Kato M, Yagi S, Tamaki T, Kawano H, Matsumoto T, Azuma H, Kato S, Matsumoto T. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem 280: 29661–29666, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 43: 1318–1323, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res 97: 1245–1252, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin-deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. In press. [DOI] [PMC free article] [PubMed]
- 10.Müller AM, Cronen C, Müller KM, Kirkpatrick CJ. Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J Pathol 198: 270–275, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa Y, Kishida K, Kihara S, Funahashi T, Shimomura I. Adiponectin ameliorates hypoxia-induced pulmonary arterial remodeling. Biochem Biophys Res Commun 382: 183–188, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation 117: 216–223, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47: 1108–1116, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol 29: 3487–3499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 102: 1296–1301, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol 14: 561–566, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol 28: 1219–1221, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest 117: 1718–1726, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol 294: L1035–L1042, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117: 375–386, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol 440: 213–221, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Figures and Tables]