X-linked Inhibitor of Apoptosis Protein (XIAP) Regulates PTEN Ubiquitination, Content, and Compartmentalization (original) (raw)
Abstract
Apoptotic cell death plays a normal role in various physiological processes, and deregulated apoptosis is a hallmark of several diseases, including cancer. Cell fate is dictated by the balance between pro- and antiapoptotic factors. Akt is one of these antiapoptotic factors, which must be activated through phosphorylation. The phosphorylation of Akt has previously been shown to be promoted by X-linked inhibitor of apoptosis protein (XIAP), another antiapoptotic protein dictating the fate of normal and cancer cells. However, the underlying mechanisms are poorly understood. We have observed that XIAP associates with PTEN (phosphatase and tensin homolog deleted on chromosome ten), the best characterized negative regulator of Akt phosphorylation, in vitro and in vivo. XIAP knockdown reduces constitutive mono- and polyubiquitination of PTEN, increases PTEN protein levels, and prevents nuclear accumulation of PTEN. Overexpression of XIAP induces polyubiquitination of PTEN and proteasome-dependent decrease of PTEN protein levels. RNA interference experiments showed that XIAP-induced regulation of Akt phosphorylation is PTEN-dependent. Additional experiments confirmed that XIAP also regulates PTEN in vivo; primary mouse embryonic fibroblasts derived from XIAP−/− mice contain higher levels of PTEN protein, less mono- and polyubiquitinated PTEN, and less nuclear PTEN than primary mouse embryonic fibroblasts derived from XIAP+/+ mice. Finally, we found that XIAP can directly ubiquitinate PTEN in vitro. We thus propose that XIAP acts as an E3 ubiquitin ligase for PTEN and promotes Akt activity by regulating PTEN content and compartmentalization.
In normal and cancer cells, balance between survival and apoptosis is maintained by a complex network of proapoptotic and antiapoptotic factors. X-linked inhibitor of apoptosis protein (XIAP)3 and Akt are two antiapoptotic factors acting on distinct targets. Akt kinase inactivates various proapoptotic factors (1), whereas XIAP binds and sterically inhibits caspases (2). XIAP protein contains a RING domain with E3 ubiquitin ligase activity (3) and has been shown to ubiquitinate caspases to target them for proteasomal degradation (4, 5). XIAP is also thought to promote Akt activity; we have previously reported that overexpression of XIAP promotes Akt phosphorylation in normal and cancerous ovarian cells (6, 7). The mechanisms through which XIAP promotes Akt phosphorylation, however, have not been investigated.
Akt phosphorylation is positively regulated by phosphatidylinositol (PI) 3-phosphate kinase, which converts phosphatidylinositol 2-phosphate (PIP2) into PIP3, allowing recruitment and phosphorylation of Akt by PDK1 (8). PIP3-induced Akt phosphorylation, however, is antagonized by PTEN (phosphatase and tensin homolog deleted on chromosome ten), which converts PIP3 into PIP2 (9). PTEN content and activity are regulated transcriptionally (10) and post-translationally (11, 12). Similar to several other proteins, polyubiquitination of PTEN leads to proteasome-dependent degradation (12). Because XIAP has been shown to induce degradation of proapoptotic factors (4, 5), we hypothesized that XIAP promotes Akt phosphorylation by inducing PTEN degradation. Using in vitro and in vivo models, we have thus investigated whether XIAP regulates PTEN ubiquitination and content.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
Primary mouse embryonic fibroblasts (MEFs) derived from XIAP+/+ and XIAP−/− mice were kindly provided by Dr. Philip A. Barker (McGill University, Montreal, Quebec, Canada). Human embryonic kidney fibroblastic cells HEK-293-T were a generous gift from Dr. Lionel Berthoux (University of Quebec, Trois-Rivières, Quebec, Canada). Human breast adenocarcinoma cell line MCF-7 and human cervical carcinoma cell line HeLa were purchased from the ATCC. XIAP plasmid constructs were a kind gift from Dr. Robert G. Korneluk (University of Ottawa Eye Institute, Ottawa, Ontario, Canada).
Transfection with shRNAs and Constructs
Cells were seeded in 6-well plates at the required density to reach ∼75% confluency after 24 h. On the day of transfection, shRNAs (XIAP shRNA (5′-GCCACGCAGTCTACAAATTCT-3′) or control (scrambled) shRNA or PTEN shRNA (5′-GCAGCTAAAGGAAGTGAATCT-3′) or control (scrambled) shRNA) (all shRNAs inserted into pGeneClip (SABiosciences, Frederick, MD)) or XIAP constructs (Myc6-XIAP (WT), Myc6-XIAP-H467A (E3 ligase inactive mutant), or empty pcDNA3.1 vector (13)) were added to cells using a ratio of 3.6 μl of FuGENE:1.2 μg of DNA/well. Plates were incubated for 40 additional hours (total: 48 h) at 37 °C before cells were collected.
In Vitro Ubiquitination Assay
Recombinant human XIAP (730 ng) (or deionized water as a control) and PTEN (200 ng) proteins were mixed in a 6-μl final volume and incubated on ice for 1 h. The remaining components for the ubiquitination reaction were then added: 50 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 5 mm ATP, 2 μm ubiquitin aldehyde, 2 mm dithiothreitol, 15 mg of ubiquitin, 100 nm E1 enzyme, and 1 μm E2 enzyme (or deionized water as a control) (final reaction volume: 20 μl). Ubiquitination reaction was conducted for 1 h at 30 °C and stopped by adding 20 μl of 2× reducing sample buffer and by heating the samples for 5 min at 95 °C.
Please see the supplemental materials for immunofluorescence, Western blot, and RT-PCR procedures.
RESULTS
XIAP Decreases PTEN Content to Promote Akt Activity
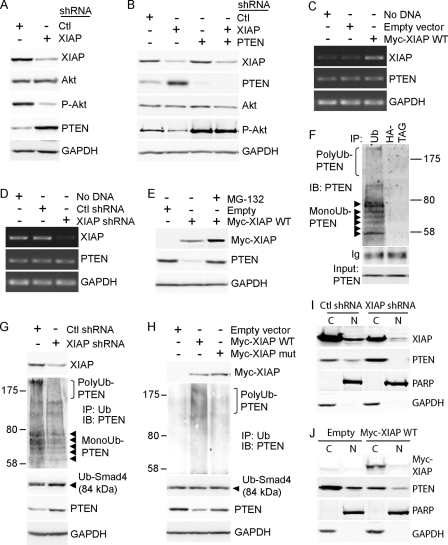
In accordance with a role for XIAP in promoting Akt activity, knockdown of XIAP using RNAi reduces the phosphorylation of Akt, as shown by a decrease of P-Akt levels when compared with total Akt levels (Fig. 1A). We have found that XIAP knockdown increased PTEN protein levels (Fig. 1A). Similar results were obtained using a small interfering RNA targeting a different sequence of XIAP (15) (supplemental Fig. S1_A_) and in other normal (HEK-293-T) or cancerous (HeLa) model cell lines (supplemental Fig. S1_B_). This indicates that XIAP down-regulates endogenous PTEN content, in various cell types. Akt phosphorylation is negatively regulated by PTEN (9); in accordance, knockdown of PTEN using RNAi increases Akt phosphorylation (Fig. 1B). We found that RNAi-induced silencing of PTEN prevented XIAP knockdown from reducing Akt phosphorylation (Fig. 1B), indicating that regulation of PTEN content is necessary for XIAP to regulate Akt phosphorylation.
FIGURE 1.
XIAP promotes Akt phosphorylation by inducing PTEN ubiquitination and proteasomal degradation. A, XIAP expression was silenced in MCF-7 cells using shRNA. 48 h after transfection, the impact on Akt content and phosphorylation and PTEN protein content was determined using Western blot. Ctl, control. B, XIAP and PTEN expression in MCF-7 cells was silenced alone or in combination for 48 h using shRNA, and the impact on Akt content and phosphorylation was determined using Western blot analysis. C and D, MCF-7 cells were transfected with a plasmid vector encoding Myc-tagged wild-type XIAP protein or with empty vector (C) or with XIAP shRNA or control (scrambled) shRNA (D), and the levels of PTEN transcripts were determined 48 h after transfection using reverse transcription-PCR. E, MCF-7 cells were pretreated or not with proteasome inhibitor MG-132 (10 μm, 1 h) and then transfected with a plasmid vector encoding Myc-tagged wild-type XIAP protein or with empty vector. The impact on PTEN protein levels was determined using Western blot. F–H, the presence of monoubiquitinated (MonoUb) PTEN (arrows) and polyubiquitinated (PolyUb) PTEN (brackets) was monitored in resting HEK-293-T cells (F) or following transfection with XIAP shRNA (G) or Myc-tagged WT or mutant XIAP proteins or empty vector (H) by immunoprecipitation (IP) using anti-ubiquitin antibody followed by Western blot (IB) analysis using anti-PTEN antibody. In G and H, monoubiquitination of Smad4 was monitored as a control. I and J, the impact of XIAP knockdown using shRNA (I) or delivery of plasmid vector encoding Myc-tagged WT XIAP protein or empty vector (J) on subcellular localization of PTEN in MCF-7 cells was determined using nuclear (N)/cytoplasmic (C) fractionation followed by Western blot analysis. Poly(ADP-ribose) polymerase-2 (PARP) and GAPDH were used as controls for the purity of nuclear and cytoplasmic fractions, respectively.
XIAP Regulates PTEN Ubiquitination
We observed that overexpression of XIAP (Fig. 1C) or silencing of XIAP using RNAi (Fig. 1D) had no impact on PTEN transcript levels, indicating that XIAP does not regulate PTEN content at the transcriptional level. However, pretreating the cells with proteasome inhibitor MG-132 prevented a decrease of PTEN protein levels following overexpression of XIAP (Fig. 1E), indicating that XIAP negatively regulates PTEN content in a proteasome-dependent manner. Proteasomal degradation of PTEN protein is induced by its polyubiquitination (16); we have thus investigated whether XIAP induces polyubiquitination of PTEN. Immunoprecipitation of ubiquitinated proteins using anti-ubiquitin antibody, followed by Western blot analysis using an anti-PTEN antibody, allows the visualization of mono- and polyubiquitination of PTEN protein in a given condition (Fig. 1F). Monoubiquitination of unrelated protein Smad4 (as described in Ref. 17) was monitored as a control. Using this approach, we found that although knockdown of XIAP in resting cells did not alter monoubiquitination of Smad4 (Fig. 1G), it reduced both monoubiquitination and polyubiquitination of PTEN (Fig. 1G). This indicates that XIAP constitutively regulates mono- and polyubiquitination of PTEN in resting cells. Reciprocally, vector-induced delivery of XIAP did not modify monoubiquitination of Smad4 but markedly increased polyubiquitination of PTEN (Fig. 1H), consistent with a proteasome-dependent decrease of PTEN protein levels following overexpression of XIAP (Fig. 1E).
XIAP Constitutively Promotes Nuclear Localization of PTEN
Because monoubiquitination of PTEN induces its nuclear import (11), we have investigated whether XIAP, which constitutively promotes monoubiquitination of PTEN (Fig. 1G), regulates subcellular localization of PTEN. We found that following XIAP knockdown, PTEN protein levels were increased in the cytosol but were decreased in the nucleus (Fig. 1I). These results indicate that XIAP constitutively promotes nuclear localization of PTEN. On the contrary, overexpression of XIAP decreased total PTEN content in both the cytosol and the nucleus, with no apparent change in PTEN localization (Fig. 1J). This result is consistent with the observation that overexpression of XIAP did not increase monoubiquitination of PTEN (Fig. 1H).
XIAP Associates with PTEN and Ubiquitinates PTEN in Vitro
Wang et al. (18) have shown that NEDD4.1 could act as an E3 ubiquitin ligase for PTEN. In our model cell lines, knockdown (supplemental Fig. S2_A_) or overexpression (supplemental Fig. S2_B_) of XIAP did not modify NEDD4 protein levels, and knockdown of NEDD4 before overexpression of XIAP did not prevent XIAP-induced reduction of PTEN protein levels (supplemental Fig. S2_C_). Altogether, these observations indicate that an E3 ligase different from NEDD4 mediates XIAP-induced ubiquitination of PTEN.
XIAP protein harbors a RING domain with E3 ubiquitin ligase activity (3) and has previously been shown to polyubiquitinate proapoptotic proteins to induce their proteasomal degradation (4, 5). We have thus investigated whether XIAP could act as an E3 ligase for PTEN. Supporting this idea, only wild-type XIAP protein and not E3 ligase-inactive mutant XIAP protein induced polyubiquitination of PTEN and reduction of PTEN levels (Fig. 1H), indicating that E3 ligase activity of XIAP is required for the induction of polyubiquitination and degradation of PTEN. We also found that PTEN co-immunoprecipitates with XIAP (Fig. 2A) and that XIAP co-immunoprecipitates with PTEN (Fig. 2B) in multiple cell types (supplemental Fig. S3), indicating that XIAP and PTEN proteins associate in resting cells. Moreover, ubiquitinated PTEN proteins co-immunoprecipitated with XIAP (Fig. 2A), even under highly stringent conditions where 2% SDS is added to the cell lysates before immunoprecipitation (19) (supplemental Fig. S4). Contrary to XIAP, NEDD4 did not co-immunoprecipitate with PTEN (supplemental Fig. S5_A_) or with XIAP (supplemental Fig. S5_B_), confirming that XIAP does not regulate PTEN ubiquitination by recruiting NEDD4 to a complex with PTEN. Finally, we have investigated whether XIAP could directly ubiquitinate PTEN, using in vitro ubiquitination assays. Commercially available recombinant PTEN protein that was used as a substrate already contained mono- and polyubiquitinated PTEN. In a first assay, recombinant XIAP and PTEN proteins were combined with the other reaction components except for E2 ubiquitin-conjugating enzyme, which was omitted in the control reaction, to allow specific monitoring of the ubiquitination of PTEN. The results showed increased levels of polyubiquitinated PTEN when E2 enzyme was present (Fig. 2C), showing that XIAP can ubiquitinate PTEN in vitro. To ensure that contaminating E3 ligase activity that could have been co-purified with PTEN was not already present in the recombinant PTEN protein mixture and responsible for the ubiquitination of PTEN (instead of XIAP), we also conducted ubiquitination assays comparing the ubiquitination of PTEN in the presence or in the absence of recombinant XIAP. The results showed increased polyubiquitination of PTEN in the reaction where XIAP was present (Fig. 2D). Altogether, these experiments indicate that XIAP can act as an E3 ubiquitin ligase for PTEN in vitro.
FIGURE 2.
XIAP associates with PTEN and ubiquitinates PTEN protein. A and E, XIAP was immunoprecipitated (IP) in HEK-293-T cells (A) or XIAP WT primary MEFs (E), and co-immunoprecipitation of PTEN was assessed using Western blot (IB). PolyUb, polyubiquitinated; MonoUb, monoubiquitinated. B and F, PTEN was immunoprecipitated in HEK-293-T cells (B) or XIAP WT primary MEFs (F), and co-immunoprecipitation of XIAP was assessed using Western blot. Control (Ctl) immunoprecipitation was performed using isotype-matched anti-HA tag antibody. C and D, recombinant PTEN protein (rhPTEN) was subjected to in vitro ubiquitination assay using recombinant XIAP protein (rhXIAP) as E3 ubiquitin ligase, in the presence or absence of E2 ubiquitin-conjugating enzyme (C) or in the presence or absence of XIAP protein (D). Following the assay, the reaction mixtures were subjected to Western blot analysis to monitor PTEN ubiquitination. low exp., low exposure; high exp., high exposure. E and F, XIAP (E) or PTEN (F) were immunoprecipitated from primary MEFs derived from control XIAP+/+ mice, and co-immunoprecipitation of PTEN (E) and XIAP (F) was determined using Western blot. Control immunoprecipitation was performed using isotype-matched anti-HA tag antibody. G, PTEN content and the extent of monoubiquitinated (arrows) and polyubiquitinated (brackets) PTEN protein were compared between primary MEFs derived from XIAP+/+ and XIAP−/− mice, using Western blot analysis (PTEN antibody). H, subcellular localization of PTEN was compared between primary MEFs derived from XIAP+/+ and XIAP−/− mice, using immunofluorescence. PTEN antibody was used (green); nuclei were counterstained with Hoechst dye 33258 (blue). Magnification: ×1000.
XIAP Associates with PTEN and Regulates PTEN Ubiquitination in Vivo
We have used primary MEFs freshly derived from genetically engineered XIAP knock-out (XIAP−/−) mice or from control (XIAP+/+) mice to investigate whether XIAP also regulates PTEN protein in vivo. In primary MEFs derived from control XIAP+/+ mice, PTEN co-immunoprecipitated with XIAP (Fig. 2E) and XIAP co-immunoprecipitated with PTEN (Fig. 2F), confirming that PTEN and XIAP proteins also associate in vivo. Ubiquitinated PTEN proteins also co-immunoprecipitated with XIAP (Fig. 2E), even under highly stringent conditions (supplemental Fig. S4), suggesting that XIAP can also ubiquitinate PTEN in vivo. Total PTEN levels were increased in primary XIAP−/− MEFs when compared with corresponding XIAP+/+ MEFs (Fig. 2G), supporting a role for XIAP in negatively regulating PTEN content in vivo. In agreement, less polyubiquitinated PTEN was present in XIAP−/− cells when compared with XIAP+/+ cells (Fig. 2G), showing that XIAP regulates PTEN ubiquitination in vivo. Finally, less monoubiquitinated PTEN was detected in XIAP−/− MEFs when compared with XIAP+/+ MEFs (Fig. 2G). In agreement, we found intense nuclear immunostaining for PTEN in XIAP+/+ primary MEFs, which was absent in XIAP−/− MEFs, where PTEN mainly localized to the cytoplasm (Fig. 2H). These results indicate that XIAP also regulates PTEN localization in vivo.
Physiological Stimulus Modifying XIAP Content Regulates PTEN Ubiquitination and Content
We have previously reported that TGF-β3, a cytokine widely expressed in normal and cancerous tissues, up-regulates XIAP and P-Akt levels in uterine cancer cells (15). Here, we have investigated whether up-regulation of XIAP by TGF-β3 modifies PTEN ubiquitination, content, and localization in these cells. Following treatment with TGF-β3, less non-ubiquitinated but more ubiquitinated PTEN proteins co-immunoprecipitated with XIAP (supplemental Fig. S6_A_). In agreement, TGF-β3-induced up-regulation of XIAP was accompanied by a reduction of total PTEN content (supplemental Fig. S6_B_). XIAP knockdown prevented TGF-β3-induced decrease of PTEN content (supplemental Fig. S6_C_), showing that up-regulation of XIAP is responsible for the decrease of PTEN levels following exposure to TGF-β3. Down-regulation of PTEN was observed in both the cytosol and the nucleus of treated cells (supplemental Fig. S6_D_), and immunofluorescence analysis showed no difference between the subcellular localization of PTEN following exposure to TGF-β3 (supplemental Fig. S6_E_). This is consistent with our observation that overexpression of XIAP mostly increases polyubiquitination of PTEN in vitro (Fig. 1H) and modifies PTEN content but not its localization (Fig. 1, I and J). It is noteworthy that exposure to a physiological stimulus rapidly increasing Akt phosphorylation without increasing XIAP levels, namely IGF-1, was not associated with a modification of PTEN content (supplemental Fig. S6_F_) or of the levels of ubiquitinated PTEN co-immunoprecipitating with XIAP (supplemental Fig. S6_G_). Altogether, these results indicate that physiological stimuli that modify XIAP levels result in altered PTEN ubiquitination and content.
DISCUSSION
The mechanisms and factors regulating PTEN ubiquitination only begin to be unraveled. We have found that XIAP regulates PTEN ubiquitination, content, and compartmentalization in vitro, and probably in vivo as it also occurs in primary cells freshly derived from mice. Previous studies support a role for XIAP in negatively regulating PTEN content in vitro and in vivo. Decrease of XIAP protein levels in cancer cells in response to the apoptosis-inducing agent mesalazine is accompanied, among others, by an increase of PTEN content (20), and exposure of mice to the apoptosis-inducing agent rosiglitazone modestly up-regulates PTEN protein levels in HCT116-XIAP+/+ cell-derived tumors but markedly increases PTEN content in HCT116-XIAP−/− cell-derived tumors (21). These studies further suggest that XIAP can regulate PTEN content in response to non-physiological stimuli, and in compliance with those studies, we show here that physiological stimuli can regulate XIAP-PTEN interaction. It is likely that only physiological stimuli modifying XIAP levels impact Akt phosphorylation through the XIAP-PTEN axis because in the case of rapid induction of Akt phosphorylation in response to IGF-1, which was not accompanied by increased XIAP levels, no changes of PTEN content were observed.
NEDD4.1 has already been identified as an E3 ubiquitin ligase(s) for PTEN (18); however, E3 ligases for PTEN different from NEDD4 could exist because PTEN ubiquitination has been reported to occur in the absence of NEDD4, in vitro and in vivo (22). Based on our findings that XIAP associates with PTEN as well as ubiquitinated PTEN proteins in vitro and in vivo and that XIAP can directly ubiquitinate PTEN in vitro, we propose that XIAP can act as an E3 ligase for PTEN. It is likely that multiple E3 ligases can act on PTEN, ensuring constant post-transcriptional control of its activity, depending on environmental conditions. We observed that although monoubiquitinated PTEN proteins co-immunoprecipitate with XIAP in resting cells and knockdown of XIAP decreases both monoubiquitination and polyubiquitination of PTEN, overexpression of XIAP or exposure to XIAP-inducing factors such as TGF-β3 only induces polyubiquitination of PTEN. It is therefore possible that XIAP preferentially mono- or polyubiquitinates PTEN depending on environmental conditions. Similarly, others have evidenced that different ubiquitin ligases could be responsible for mono- and polyubiquitination of another factor, Smad4, which translocates between the cytoplasm and the nucleus similar to PTEN (17). Thus, although we have gathered evidence demonstrating that NEDD4 was not involved in XIAP-induced ubiquitination of PTEN, regulation of PTEN stability and compartmentalization is probably not the sole responsibility of XIAP. In vivo, for example, mono- and polyubiquitination of PTEN can still be detected in XIAP KO mice. Because c-IAP1 and c-IAP2 are increased in XIAP KO cells (23) and because these enzymes have been shown to act as E3 ligases (24), cIAP-1 or cIAP-2 could possibly be involved in mono- and/or polyubiquitination PTEN.
Nuclear localization of PTEN has been shown to be altered in human tumors, including prostate cancers (25). By regulating PTEN compartmentalization, XIAP could thus be one of the factors influencing susceptibility to and/or progression of prostate cancer. Hwang et al. (23) observed no significant difference between XIAP WT and KO transgenic adenocarcinoma mouse prostate (TRAMP) mice for the onset and progression of prostate cancer; again, this could be due to a compensatory increase of c-IAP1 and c-IAP2 levels in XIAP KO cells (23). They did report, however, a trend toward more aggressive disease in XIAP-deficient mice (23), which is consistent with reduced nuclear localization of PTEN in the absence of XIAP.
The present study evidences a role for XIAP in down-regulating PTEN content and in promoting nuclear localization of PTEN. Because negative regulation of Akt phosphorylation only occurs when PTEN localizes to the cytoplasm (26), the ability of XIAP to promote nuclear import of PTEN likely constitutes an additional mechanism through which XIAP promotes Akt phosphorylation/activity. Considering the emerging roles that are ascribed to PTEN and Akt in cellular processes other than apoptosis/survival (such as DNA repair (27)), the discovery of a role for XIAP in regulating the PTEN-Akt axis broadens the view of regulatory functions that can be attributed to XIAP.
Supplementary Material
Supplemental Data
Acknowledgments
We thank K. Vincent and A.-M. Simard for technical assistance.
*
This work was supported by a research grant from the Canadian Institute of Health Research and a grant from Natural Sciences and Engineering Research Council of Canada.
1
A holder of postdoctoral fellowships from the Cancer Research Society and the Fonds de la recherche en santé du Québec.
3
The abbreviations used are:
XIAP
X-linked inhibitor of apoptosis protein
PTEN
phosphatase and tensin homolog deleted on chromosome ten
MEF
mouse embryonic fibroblast
E1
ubiquitin-activating enzyme
E2
ubiquitin-conjugating enzyme
E3
ubiquitin ligase
PI
phosphatidylinositol
PIP2
phosphatidylinositol 2-phosphate
PIP3
phosphatidylinositol 3-phosphate
HA
hemagglutinin
GAPDH
glyceraldehyde-3-phosphate dehydrogenase
TGF
transforming growth factor
P-Akt
phosphorylated Akt
shRNA
short hairpin RNA
RNAi
RNA interference
WT
wild type
KO
knockout.
REFERENCES
- 1.Cardone M. H., Roy N., Stennicke H. R., Salvesen G. S., Franke T. F., Stanbridge E., Frisch S., Reed J. C. (1998) Science 282, 1318–1321 [DOI] [PubMed] [Google Scholar]
- 2.Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. (1997) Nature 388, 300–304 [DOI] [PubMed] [Google Scholar]
- 3.Yang Y., Fang S., Jensen J. P., Weissman A. M., Ashwell J. D. (2000) Science 288, 874–877 [DOI] [PubMed] [Google Scholar]
- 4.Morizane Y., Honda R., Fukami K., Yasuda H. (2005) J. Biochem. 137, 125–132 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki Y., Nakabayashi Y., Takahashi R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 8662–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asselin E., Wang Y., Tsang B. K. (2001) Endocrinology 142, 2451–2457 [DOI] [PubMed] [Google Scholar]
- 7.Asselin E., Mills G. B., Tsang B. K. (2001) Cancer Res. 61, 1862–1868 [PubMed] [Google Scholar]
- 8.Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. (1995) Cell 81, 727–736 [DOI] [PubMed] [Google Scholar]
- 9.Stambolic V., Suzuki A., de la Pompa J. L., Brothers G. M., Mirtsos C., Sasaki T., Ruland J., Penninger J. M., Siderovski D. P., Mak T. W. (1998) Cell 95, 29–39 [DOI] [PubMed] [Google Scholar]
- 10.Whang Y. E., Wu X., Suzuki H., Reiter R. E., Tran C., Vessella R. L., Said J. W., Isaacs W. B., Sawyers C. L. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 5246–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trotman L. C., Wang X., Alimonti A., Chen Z., Teruya-Feldstein J., Yang H., Pavletich N. P., Carver B. S., Cordon-Cardo C., Erdjument-Bromage H., Tempst P., Chi S. G., Kim H. J., Misteli T., Jiang X., Pandolfi P. P. (2007) Cell 128, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W., Wang X., Zhang W., Reed W., Samet J. M., Whang Y. E., Ghio A. J. (2003) J. Biol. Chem. 278, 28258–28263 [DOI] [PubMed] [Google Scholar]
- 13.Arora V., Cheung H. H., Plenchette S., Micali O. C., Liston P., Korneluk R. G. (2007) J. Biol. Chem. 282, 26202–26209 [DOI] [PubMed] [Google Scholar]
- 14.Deleted in proof
- 15.Van Themsche C., Mathieu I., Parent S., Asselin E. (2007) J. Biol. Chem. 282, 4794–4802 [DOI] [PubMed] [Google Scholar]
- 16.Torres J., Pulido R. (2001) J. Biol. Chem. 276, 993–998 [DOI] [PubMed] [Google Scholar]
- 17.Dupont S., Mamidi A., Cordenonsi M., Montagner M., Zacchigna L., Adorno M., Martello G., Stinchfield M. J., Soligo S., Morsut L., Inui M., Moro S., Modena N., Argenton F., Newfeld S. J., Piccolo S. (2009) Cell. 136, 123–135 [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Trotman L. C., Koppie T., Alimonti A., Chen Z., Gao Z., Wang J., Erdjument-Bromage H., Tempst P., Cordon-Cardo C., Pandolfi P. P., Jiang X. (2007) Cell. 128, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher E., Enzler T., Matsuzawa A., Anzelon-Mills A., Otero D., Holzer R., Janssen E., Gao M., Karin M. (2007) Nat. Immunol. 8, 57–63 [DOI] [PubMed] [Google Scholar]
- 20.Schwab M., Reynders V., Loitsch S., Shastri Y. M., Steinhilber D., Schröder O., Stein J. (2008) Carcinogenesis 29, 1407–1414 [DOI] [PubMed] [Google Scholar]
- 21.Dai Y., Qiao L., Chan K. W., Zou B., Ma J., Lan H. Y., Gu Q., Li Z., Wang Y., Wong B. L., Wong B. C. (2008) Int. J. Cancer 122, 2858–2863 [DOI] [PubMed] [Google Scholar]
- 22.Fouladkou F., Landry T., Kawabe H., Neeb A., Lu C., Brose N., Stambolic V., Rotin D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8585–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang C., Oetjen K. A., Kosoff D., Wojno K. J., Albertelli M. A., Dunn R. L., Robins D. M., Cooney K. A., Duckett C. S. (2008) Cell Death. Differ. 15, 831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H., Joazeiro C. A., Bonfoco E., Kamada S., Leverson J. D., Hunter T. (2000) J. Biol. Chem. 275, 26661–26664 [DOI] [PubMed] [Google Scholar]
- 25.Song M. S., Salmena L., Carracedo A., Egia A., Lo-Coco F., Teruya-Feldstein J., Pandolfi P. P. (2008) Nature 455, 813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung J. H., Eng C. (2005) Cancer Res. 65, 8096–8100 [DOI] [PubMed] [Google Scholar]
- 27.Shen W. H., Balajee A. S., Wang J., Wu H., Eng C., Pandolfi P. P., Yin Y. (2007) Cell 128, 157–170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data