Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 15.
Abstract
Despite early benefits seen in cancer patients treated with anti-VEGF pathway targeted drugs, the clinical benefits obtained in terms of progression-free or overall survival have been more modest than expected. This is, at least in part, due to antiangiogenic drug resistance mechanisms that involve pathways mediated largely by the tumor, whether intrinsic or acquired in response to therapy, or by the host, which is either responding directly to therapy or indirectly to tumoral cues. The focus of this review is to distinguish, where possible, between such host and tumor-mediated pathways of resistance and discuss key challenges facing the preclinical/clinical development of antiangiogenic agents, including potential differences in drug efficacies when treating primary tumors or various stages of metastatic disease.
Background
The concept of targeting the tumor’s vasculature to block its growth has been validated clinically as an anti-cancer strategy with the approval of three targeted drugs that disrupt the vascular endothelial growth factor (VEGF)/VEGF receptor pathway 1. However, despite encouraging signs from some early preclinical studies that prolonged benefits would be seen in cancer patients, recent findings from the laboratory and clinic have uncovered several limitations to antiangiogenic therapy, posing future challenges for their expanding use. Currently approved antiangiogenic drugs include bevacizumab, the humanized monoclonal antibody to VEGF, as well as small molecule receptor tyrosine kinase inhibitors (RTKIs), such as sorafenib and sunitinib, which target VEGF and platelet-derived growth factor (PDGF) receptors (among a number of others). The VEGF RTKIs (approved thus far as single agents) and bevacizumab (approved for use only in combination with cytotoxic chemotherapy) can lead to disease stabilization and longer periods of progression free survival (PFS) or overall survival (OS) in many patients with metastatic disease, including colorectal carcinoma (CRC), metastatic breast carcinoma (MBC), non-small cell lung carcinomas (NSCLC), renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), gastrointestinal stromal tumors (GIST), and perhaps (though this has yet to be proven) in glioblastomas (GBM) (reviewed in 2). But tumors eventually become non-responsive, or do not respond at all — despite the presence of VEGF and VEGFR-2 - and PFS or OS in patients receiving antiangiogenic therapy has translated into benefits measured only in months, in most cases 3. Furthermore, in certain instances, increases in response rate and PFS does not always translate into increased OS for patients, as observed after bevacizumab treatment in RCC (as a single agent)4 or in MBC (in combination with a taxane chemotherapy) 5. It also remains unclear what role drug combinations play in the efficacy of VEGF pathway targeting (antiangiogenic) inhibitors and why, at least to date, bevacizumab has proved largely ineffective as a single agent while VEGF RTKIs, with one recent exception 6, have repeatedly failed in randomized phase III trials when used in combination with chemotherapy 7.
Thus there is a growing interest in understanding the mechanisms of resistance, whether intrinsic or acquired, after exposure to antiangiogenic drug treatment. Early indications are that these mechanisms may be highly diverse, perhaps in part due to the primary mode of action of such drugs, e.g. blocking ‘host’ tumor-supporting processes rather than blocking tumor growth directly. It is possible that resistance to antiangiogenic therapy may extend beyond classical drug resistance seen with traditional cytotoxic chemotherapy and radiation, or even molecular ‘tumor’ targeted therapy, which include rapid mutability and adaptability inherent to the tumor cells’ genetic instability (see review 8). Indeed an emerging question is whether the theoretical advantages of disrupting ‘host’ angiogenic processes, may be countered by significant disadvantages, including host-mediated resistance mechanisms involving the vascular microenvironment (perhaps largely independent of the tumor) as well as an altogether more disquieting possibility, namely, that antiangiogenic resistance may, in some instances, eventually increase or induce the invasive and metastatic potential of tumors as a result of therapy.
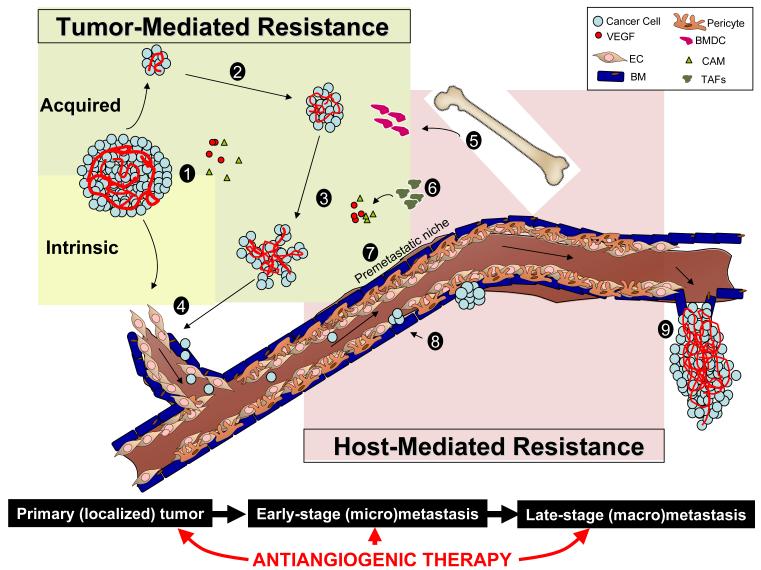
The focus of this review is to discuss two interrelated pathways. The first includes main pathways of resistance to antiangiogenic therapy, differentiating between those meditated by either the tumor itself or by the host (or both). The second pathway looks at disease progression from a localized primary tumor to established metastatic disease. It may be critical to consider both pathways simultaneously to understand and overcome some of these challenges facing antiangiogenic therapy, including mechanisms of drug resistance and how they may play a significant role in influencing tumor growth, for better or worse, at various stages of disease (Figure 1).
Figure 1. Mechanisms of resistance to antiangiogenic therapy may allow for differential efficacy in different stages of disease progression.
Tumor-mediated mechanisms, either intrinsic or acquired by primary or established metastatic tumors, allow for evasion of anti-VEGF pathway inhibition. These include: 1) tumoral upregulation of compensatory proangiogenic factors, 2) following initial tumor shrinkage, therapy-induced hypoxia mediates adaptation/evasion and tumor regrowth via rebound revascularization, vessel co-option, EMT, hypoxia, 3) Increased invasiveness, 4) Increased intravasive potential. Host-mediated mechanisms, either direct (in response to drug action) or indirect (in response to cues from the tumor), could include 5) BMDC mobilization, 6) microenvironment / stromal cell activation, 7) EC dysfunction/inflammation/ thrombosis, 8) Pericyte dysfunction. Steps 5-7 could similarly lead to upregulation of compensatory proangiogenic molecules. Taken together, antiangiogenic therapy may increase tumor extravasive ability (9) and facilitate/accelerate disease in microscopic tumors. Legend: (EC) endothelial cell, (BM) basement membrane, (BMDC) bone marrow-derived cell, compensatory proangiogenic molecules (CAMs), (TAFs) tumor-associated fibroblasts.
*illustration note — we welcome suggestions for additional material and/or figure improvement to increase clarity. Ideally we would like to have the colors of the tumor and host-mediated color squares (or other suggested shape) fuse together.
Resistance to VEGF Pathway-Targeting Agents
Tumor verses host-mediated pathways
The initial non-response seen in a subset of patients receiving VEGF pathway-targeted therapy imply that various tumor cells possess certain intrinsic properties which could allow for immediate resistance upon treatment initiation. These could be dependent on a multitude of factors, such as patient treatment history, stage of disease, genetic factors, as well as inherent tolerability to hypovascular environments — something observed in certain cancer types (recently reviewed in 8). Some mechanisms of resistance thought to be mediated largely by the tumor include co-option of established vessels 9 and pre-existing expression by the tumor of multiple alternative proangiogenic pathways (i.e., PDGFs, PlGFs, FGFs) 10 — which could compensate immediately for the loss of VEGF signalling. Additional compensatory mechanisms may also be acquired by the tumor as a response to elevated tumor hypoxia induced by blockade of VEGF signalling and include the upregulation of alternate proangiogenic mediators, such as bFGF and SDF1α, that could allow for persistent neovascularisation despite continued anti-VEGF therapy 11,12. It is also evident that therapy-induced hypoxia plays a critical role in facilitating the selection of tumor cells which are able to tolerate, and perhaps even thrive, in low oxygen environments 13,14 (recently reviewed in 15).
While resistance to VEGF pathway targeted therapy may be mediated in large part by these intrinsic or acquired characteristics of the tumor cells, it is increasing clear that mechanisms involving the tumor microenvironment -- either directly (in response to drug action) or indirectly (in response to cues from the tumor) — can also be involved in mediating eventual tumor relapse and regrowth. For example, stromal cells such as tumor associated fibroblasts (TAFs) can upregulate PDGF-C in response to VEGF inhibition 16. Pericytes could also play a role by retaining vascular function following endothelial cell (EC) disruption 17, regulating EC proliferation 8,18, and/or providing a scaffold (along with remaining basement membrane associated cells) for rapid revascularization after cessation of therapy 19. Moreover, various types of proangiogenic bone marrow-derived cells (BMDCs) may home to the tumor microenvironment and mediate resistance to VEGF pathway blockade via the production of the aforementioned compensatory proangiogenic factors 3. Examples include circulating cells such as Gr1+CD11b+ myeloid suppressor-type cells 20 via Bv8 (prokineticin) and G-CSF-dependent mechanisms 21, TIE2 expressing monocytes via upregulation of angiopoeiten-2 22, and tumor-associated macrophages (also via upregulation of Bv8) 23, and there are likely others 24. Taken together, these tumor- and host-mediated mechanisms, either alone or together in concert, may diminish response to antiangiogenic agents despite continued therapy. But what are the phenotypic characteristics of tumors that progress after initial benefit with antiangiogenic therapy?
Implications for tumor invasion and metastasis
The progression of a locally growing primary tumor to the growth of distant metastases involves a number of steps, including a loss of cellular adhesion; augmented motility and invasion capabilities; intravasation into the bloodstream; honing and survival; extravasation and seeding of micrometastases; and finally colonization and growth in a new distant site 25. Because of the integral role of the vasculature in this process, one obvious theoretical advantage of antiangiogenic therapy would be that some of these steps may be compromised, particularly in primary tumors (e.g. via the destruction of the immature vasculature to prevent/suppress intravasation), as well as in distant sites (e.g., the prevention of avascular metastases which require angiogenesis for continued growth) To date, extensive preclinical and clinical studies using VEGF pathway-targeting drugs have indeed been shown to stop or slow the growth localized primary tumors or established metastatic disease but it remains largely unknown how effective antiangiogenic therapy is at blocking earlier stages of metastatic disease. Clues that antiangiogenic agents may not sufficiently suppress metastasis in many cases and, even more provocatively, possibly select for more invasive and metastatic tumor phenotypes, have recently emerged. For example, in preclinical tumor models of GBM where VEGF or HIF1α was genetically or therapeutically blocked 9,26,27, initially tumors shrank but elevated hypoxia in the tumor microenvironment eventually caused or facilitated recurrent tumor growth in existing and adjacent sites. Moreover, Paez-Ribes et al. recently showed that therapy with anti-VEGFR-antibodies or various VEGF RTKIs in genetically engineered _RIP1_-Tag2 pancreatic islet cell tumors and in orthotopically transplanted GBMs eventually resulted in tumors capable of increased invasion/intravasation and metastasis in distant organs (such as the liver) 28. Thus, in response to hypoxia induced by anti-VEGF pathway targeted therapy, tumors may acquire adaptive/evasive behaviour. Interestingly, tumor-independent (host-mediated) pathways of resistance to angiogenesis inhibition may also facilitate tumor growth and metastasis in certain instances. For example, following short-term (7 day) treatment with various VEGF RTKIs to mice prior to intravenous inoculation of human tumor cells, or immediately after removal of a primary tumor, accelerated metastasis could be observed concomitant with decreased survival 29.
But how can this be explained? Many possible mechanisms could be involved. For example, therapy-induced increases in tumor hypoxia and HIF1α expression following VEGF-pathway inhibition can lead to i) increased c-met expression 30,31 or IL6 expression 32, ii) activation/upregulation of various matrix metalloprotienases 33, iii) mobilization of BMDCs 34, iv) instigation of tumor epithelial-to-mesenchymal (EMT) transition 35 - all of which could increase invasive/metastatic potential in a ‘tumor-mediated’ manner. Tumor-independent (‘host-mediated’) mechanisms could contribute as well, for example, via aforementioned therapy-induced upregulation of various proangiogenic molecules — many of which may increase the invasive/metastatic potential of cancer cells. For instance, it is now well documented that increases in VEGF and PlGF, and decreases in sVEGFR-2, can be observed in the plasma of patients receiving VEGF RTKIs (including sorafenib, sunitinib, and many others) such that it can be considered a ‘class effect’ for these agents (see Supplementary Table 1 in 36). Indeed this is a major reason why these proteins are currently being evaluated for use as potential surrogate biomarkers for tumor response 37,38. However many of these changes could derive, in large part, from a systemic host-mediated response to treatment rather than (or in addition to) from the tumor itself. This possibility was raised by recent experiments from our laboratory which showed that dose-dependent, reversible, elevations in VEGF/PlGF (and decreases in sVEGFR-2) could be recapitulated in healthy tumor-free mice treated with VEGF RTKIs and could include many ‘off-target’ molecules such as osteopontin, G-CSF, and SDF1α 12,36. Given that many of these circulating proangiogenic cytokines, chemokines, and growth factors have been implicated in promoting angiogenesis and/or metastasis 39-43, it is theoretically possible that they may assist in the aforementioned rebound revascularization 19 and/or increased extravasive potential for circulating tumor cells. Such induced systemic host responses to antiangiogenic drugs could facilitate an enhanced ‘pre-metastatic niche’ precipitated by mechanisms largely independent of the tumor 29,44. These include, i) BMDC mobilization, such as the recruitment of circulating VEGFR-1+ bone marrow cells to distant organ sites 45,46, ii) pericyte dysfunction, which may in turn make vessels less mature and leaky, and allow for increased extravasive/metastatic tumor potential 47, iii) increased prothrombotic events, which may be caused directly or indirectly by vessel damage as a result of therapy and allow for increased tumor cell ‘seeding’ and growth in distant organs 48, iv) altered EC adhesion molecule function, a possibility that was raised in a recent study which showed that inhibitors of αvβ3/αvβ5 administered continuously at low doses can enhance VEGF-driven angiogenesis and tumor growth 49, and v) inflammatory pathway activation, which may lead to alterations (or injury) to the endothelial microenvironment which could collectively increase both intra- and extravasive potential for tumor cells 50. Thus both tumor and host-mediated responses to antiangiogenic therapy, at least in certain instances, can facilitate proinvasive and metastatic potential after treatment in early-stage/micrometastatic disease.
While eventual enhancement of metastasis in response to an anti-cancer therapy may, at first glance, seem a counterintuitive concept (irrespective of whether mediated by tumor or host related mechanisms) it is important to note that similar findings have been reported for over 30 years with cytotoxic treatments, including radiation and various chemotherapeutic drugs 51-53. Of course a presumed difference is that chemotherapy and radiation treatments act mainly by direct tumor cytotoxicity, e.g., by non-specifically targeting proliferating cells, whereas antiangiogenic agents primarily target host processes. Furthermore, cytotoxic (and toxic) chemotherapy and radiation are administered for defined periods, e.g. 3-6 months, whereas antiangiogenic agents are (at least theoretically) meant to be administered for longer periods of time, if not indefinitely.
Clinical-Translational Advances
Is there clinical evidence of increased invasion/metastasis after VEGF pathway targeted therapy?
While many host- and tumor-mediated pathways of resistance may explain, at least in part, some of the modest benefits attained in the majority of patients treated with anti-VEGF pathway targeted agents, it remains unclear whether antiangiogenic therapy will lead to increased invasion and/or metastasis after either prolonged or short-term treatments in the clinic. To date, the literature regarding this point remains largely anecdotal and limited to case reports or small studies, but there are some clues that suggest relapsed tumors may have an altered/increased progression after therapy stops working and/or when therapy is halted altogether. For example, in many instances human GBM patients treated with bevacizumab in combination with chemotherapy experience eventual tumor relapse/regrowth accompanied by a high rate of diffuse infiltrative lesions 54-58 — a finding suggestive of an adaptive/evasive response to antiangiogenic therapy leading to increased invasiveness. As well, there are instances where discontinuation of anti-VEGF pathway targeted therapy may support preclinical observations of ‘rebound revascularization’, something which in turn could influence tumor regrowth and/or metastasis. For example, cases of tumor ‘flares’ have been reported during drug-free break periods 59 or after treatment discontinuation in RCC patients receiving either sunitinib or sorafenib 60,61, or in CRC patients treated with bevacizumab in combination with chemotherapy 62. Furthermore, in addition to primary tumor regrowth after treatment cessation with various VEGF RTKIs, increases in local foci or metastatic spread in distant organs have been noted in certain retrospective analyses 63. Importantly, there are emerging clues that some patients having failed to respond to (or been taken off) VEGF RTKI treatment, may respond again with the same drug after a break period 63 or respond when the drug is switched for another (e.g. sunitinib to sorafenib or vice versa) 64,65.
Antiangiogenic therapy and micrometastatic disease: implications for adjuvant therapy?
To date, one of the difficulties in uncovering (and predicting) antiangiogenic drug resistance mechanisms is a general disconnect between how such drugs are evaluated in experimental and clinical settings. For example, in most cases, patients in early phase clinical trials receiving antiangiogenic agents (or any other type of anti-cancer drug/therapy for that matter) have late-stage metastatic disease, often in multiple sites, whereas the majority of preclinical work focuses on localized primary tumors 66. Thus it is essential that future testing of antiangiogenic (and other) therapies address this gap by investigating anti-cancer agents during various stages tumor progression, especially when advanced metastatic disease is already established or, conversely, when only microscopic metastasis are present. These considerations are of particular relevance because a) metastasis is generally the main reason for patient mortality rather than primary tumor growth 67, and b) antiangiogenic agents are now being evaluated in earlier stages of disease such as in the adjuvant setting, which may involve neither primary tumors or established metastasis, but rather early-stage occult micrometastatic disease. Indeed, surprisingly few preclinical studies have tested anti-VEGF targeted pathway drugs in early (micro) and late (established) stage metastasis, and even fewer still have directly compared anti-tumor efficacy in these indications to locally grown primary tumors. In such cases, mixed results have been observed, some of which may help explain the modest benefits seen in patients. For instance, VEGF RTKIs generally have been shown to slow or stop primary tumor growth in mice but the effects on established metastatic disease range from efficacious 68 to only a marginal or negligible benefit on the overall survival of mice 69. Moreover, in micrometastatic/early stage disease, the aforementioned studies by Ebos et al and Paez-Ribes et al show that VEGF pathway targeted therapy can result in increased rates or levels of tumor invasiveness and metastasis, in certain instances. Critically, such results contrasted in both studies with the potent tumor growth inhibitory effects the same drugs and treatment schedules had on locally grown primary tumors (Ebos et al. and Paez-Ribes et al.). It is likely that various experimental conditions - such as the animal model, tumors doses, drugs, treatment duration, combinations with chemotherapy — may explain some of these differences in experimental outcomes; however it is possible that differential efficacies with antiangiogenic therapy may be observed between micro- and macro-metastatic disease. Some studies with genetically engineered mouse models of intestinal adenomas (APCmin mice) show that tumor growth can be slowed and survival prolonged after treatment with various inhibitors of the VEGF pathway 70-72. Similarly, transgenic mouse models of NSCLC, generated by mutations in Kras and conditional Lkb1 deletions, had decreased metastasis and improved survival when sunitinib treatment started 4 weeks after metastatic tumor growth induction. Yet outcome in this latter instance was not improved if treatment started 2 weeks earlier 73,74 — raising the question whether observed benefits after treatment were because of effects against established metastasis rather than microscopic disease.
Such distinctions could be important for interpretation of both preclinical and clinical trials involving antiangiogenic agents in the adjuvant setting. In limited preclinical models where primary tumors are removed and antiangiogenic drug treatment is initiated, metastatic growth could be inhibited 68,75 or accelerated 29 — depending on the tumor models and drugs used, and when treatments are initiated. What is clear is that more studies should be conducted preclinically to test anti-VEGF pathway targeted therapy in an authentic adjuvant setting, i.e., very shortly or immediately after surgical resection of a primary tumor when only microscopic minimum residual disease is present — something that might be determined by various imaging techniques and/or other measures 76. Ironically, it might be that this question will be addressed and answered first in the clinical setting. Currently there are more than 40+ adjuvant clinical trials underway involving multiple VEGF pathway inhibitors, such as sorafenib and sunitinib 77, as well as bevacizumab (typically in combination with chemotherapy), in numerous cancer types, including breast cancer, renal cancer, prostate, head and neck cancers, NSCLC, ovarian, and there are others A. With respect to bevacizumab one such trial has been completed as of 2009, e.g. a Phase III study in postoperative colorectal patients with stage II-III disease, who were treated with the anti-VEGF antibody plus chemotherapy for 1 year and 6 months, respectively. The results of this trial (NSABP-C-08; clinicaltrials.gov: NCT00096278) showed no benefit in progression free survival when assessed three years after therapy initiation 78. Interestingly, a clear benefit in favour of the bevacizumab at one year (i.e., when therapy was completed) was observed — but this benefit gradually disappeared over time. The basis for this phenomenon is unknown and clearly highlights the urgent need for undertaking preclinical studies appropriate models to study the mechanisms by which antiangiogenic treatments such as VEGF pathway targeted drugs lose their activity and/or alter tumor progression and metastasis over time.
Taken together, it is now confirmed that anti-VEGF pathway targeted therapies represent an effective treatment of cancer in certain settings, however it may be necessary to recalibrate expectations and consider improved rational strategies to overcome these limitations at all stages of tumor progression.
Footnotes
Reference List
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat.Rev.Clin.Oncol. 2009 doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS. Tumor angiogenesis. N.Engl.J.Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N.Engl.J.Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Sun Y, Korfee S, Germonpré P, Saijo N, Zhou C, Wang J, Langmuir P, Kennedy J, Johnson BE. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small cell lung cancer (NSCLC): A randomized, double-blind phase III trial (ZODIAC) Journal of Clinical Oncology. 2009:31–5. doi: 10.1016/S1470-2045(10)70132-7.Ref Type: Abstract
- 7.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 8.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat.Rev.Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, Shuman MA. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 11.Casanovas O, Hicklin D, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev.Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Rak J, Coomber BL, Hicklin D, Kerbel RS. Effect of p53 status on tumor response to anti-angiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 15.Rapisarda A, Melillo G. Role of the hypoxic tumor microenvironment in the resistance to anti-angiogenic therapies. Drug Resist.Updat. 2009 doi: 10.1016/j.drup.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschi KK, D’Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. 419-28. [DOI] [PubMed] [Google Scholar]
- 19.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat.Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 21.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc.Natl.Acad.Sci.U.S.A. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 23.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat.Rev.Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: from promiscuity to surrogate marker and target identification. Nature Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat.Rev.Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fillbrandt R, Stavrou D, Westphal M, Lamszus K. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 27.Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP, Johnson RS, Bergers G. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 28.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 31.Steeg PS. Angiogenesis inhibitors: motivators of metastasis? Nat Med. 2003;9:822–823. doi: 10.1038/nm0703-822. [DOI] [PubMed] [Google Scholar]
- 32.Saidi A, Hagedorn M, Allain N, Verpelli C, Sala C, Bello L, Bikfalvi A, Javerzat S. Combined targeting of interleukin-6 and vascular endothelial growth factor potently inhibits glioma growth and invasiveness. Int.J.Cancer. 2009 doi: 10.1002/ijc.24380. [DOI] [PubMed] [Google Scholar]
- 33.Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr.Mol.Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- 34.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J.Clin.Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J.Transl.Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 39.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp.Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 41.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 42.Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Stoica G, Tasca SI, Kelly KA, Meininger CJ. Modulation of tumor angiogenesis by stem cell factor. Cancer Res. 2000;60:6757–6762. [PubMed] [Google Scholar]
- 44.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steeg PS. Cancer biology: emissaries set up new sites. Nature. 2005;438:750–751. doi: 10.1038/438750b. [DOI] [PubMed] [Google Scholar]
- 47.Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, Semb H. Pericytes limit tumor cell metastasis. J.Clin.Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elice F, Rodeghiero F, Falanga A, Rickles FR. Thrombosis associated with angiogenesis inhibitors. Best.Pract.Res.Clin.Haematol. 2009;22:115–128. doi: 10.1016/j.beha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat.Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 50.Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Putten LM, Kram LK, van Dierendonck HH, Smink T, Fuzy M. Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int.J.Cancer. 1975;15:588–595. doi: 10.1002/ijc.2910150408. [DOI] [PubMed] [Google Scholar]
- 52.Vollmer TL, Conley FK. Effect of cyclophosphamide on survival of mice and incidence of metastatic tumor following intravenous and intracardial inoculation of tumor cells. Cancer Res. 1984;44:3902–3906. [PubMed] [Google Scholar]
- 53.de Ruiter J, Cramer SJ, Lelieveld P, van Putten LM. Comparison of metastatic disease after local tumour treatment with radiotherapy or surgery in various tumour models. Eur.J.Cancer Clin Oncol. 1982;18:281–289. doi: 10.1016/0277-5379(82)90047-5. [DOI] [PubMed] [Google Scholar]
- 54.Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, Eagan P, Gruber ML. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J.Neurosurg. 2008 doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 55.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 56.Fischer I, Cunliffe CH, Bollo RJ, Raza S, Monoky D, Chiriboga L, Parker EC, Golfinos JG, Kelly PJ, Knopp EA, Gruber ML, Zagzag D, Narayana A. High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neuro.Oncol. 2008;10:700–708. doi: 10.1215/15228517-2008-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellis LM, Reardon DA. Cancer: The nuances of therapy. Nature. 2009;458:290–292. doi: 10.1038/458290a. %19. [DOI] [PubMed] [Google Scholar]
- 58.Mathews MS, Linskey ME, Hasso AN, Fruehauf JP. The effect of bevacizumab (Avastin) on neuroimaging of brain metastases. Surg.Neurol. 2008 doi: 10.1016/j.surneu.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 59.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, Deprimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J.Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 60.Wolter P, Beuselinck B, Pans S, Schoffski P. Flare-up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol. 2009;48:621–624. doi: 10.1080/02841860802609574. [DOI] [PubMed] [Google Scholar]
- 61.Desar IM, Mulder SF, Stillebroer AB, van Spronsen DJ, van der Graaf WT, Mulders PF, van Herpen CM. The reverse side of the victory: Flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol. 2009;1-4:1–4. doi: 10.1080/02841860902974167. %19. [DOI] [PubMed] [Google Scholar]
- 62.Cacheux W, Boisserie T, Staudacher L, Vignaux O, Dousset B, Soubrane O, Terris B, Mateus C, Chaussade S, Goldwasser F. Reversible tumor growth acceleration following bevacizumab interruption in metastatic colorectal cancer patients scheduled for surgery. Ann.Oncol. 2008;19:1659–1661. doi: 10.1093/annonc/mdn540. [DOI] [PubMed] [Google Scholar]
- 63.Johannsen M, Florcken A, Bex A, Roigas J, Cosentino M, Ficarra V, Kloeters C, Rief M, Rogalla P, Miller K, Grunwald V. Can Tyrosine Kinase Inhibitors be Discontinued in Patients with Metastatic Renal Cell Carcinoma and a Complete Response to Treatment? A Multicentre, Retrospective Analysis. Eur.Urol. 2008 doi: 10.1016/j.eururo.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Sablin MP, Negrier S, Ravaud A, Oudard S, Balleyguier C, Gautier J, Celier C, Medioni J, Escudier B. Sequential Sorafenib and Sunitinib for Renal Cell Carcinoma. J.Urol. 2009 doi: 10.1016/j.juro.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 65.Dudek AZ, Zolnierek J, Dham A, Lindgren BR, Szczylik C. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009;115:61–67. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 66.Kerbel RS. Human Tumor Xenografts as Predictive Preclinical Models for Anticancer Drug Activity in Humans: Better Than Commonly Perceived - But They Can Be Improved. Cancer Biol Ther. 2003;2:108–113. [PubMed] [Google Scholar]
- 67.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat.Clin.Pract.Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O’Connor P, Shalinsky DR, Bender SL. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin.Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 69.Schomber T, Zumsteg A, Strittmatter K, Crnic I, Antoniadis H, Littlewood-Evans A, Wood J, Christofori G. Differential effects of the vascular endothelial growth factor receptor inhibitor PTK787/ZK222584 on tumor angiogenesis and tumor lymphangiogenesis. Mol.Cancer Ther. 2009;8:55–63. doi: 10.1158/1535-7163.MCT-08-0679. [DOI] [PubMed] [Google Scholar]
- 70.Korsisaari N, Kasman IM, Forrest WF, Pal N, Bai W, Fuh G, Peale FV, Smits R, Ferrara N. Inhibition of VEGF-A prevents the angiogenic switch and results in increased survival of Apc+/min mice. Proc.Natl.Acad.Sci.U.S.A. 2007;104:10625–10630. doi: 10.1073/pnas.0704213104. %19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alferez D, Wilkinson RW, Watkins J, Poulsom R, Mandir N, Wedge SR, Pyrah IT, Smith NR, Jackson L, Ryan AJ, Goodlad RA. Dual inhibition of VEGFR and EGFR signaling reduces the incidence and size of intestinal adenomas in Apc(Min/+) mice. Mol.Cancer Ther. 2008;7:590–598. doi: 10.1158/1535-7163.MCT-07-0433. [DOI] [PubMed] [Google Scholar]
- 72.Goodlad RA, Ryan AJ, Wedge SR, Pyrah IT, Alferez D, Poulsom R, Smith NR, Mandir N, Watkins AJ, Wilkinson RW. Inhibiting vascular endothelial growth factor receptor-2 signaling reduces tumor burden in the ApcMin/+ mouse model of early intestinal cancer. Carcinogenesis. 2006;27:2133–2139. doi: 10.1093/carcin/bgl113. [DOI] [PubMed] [Google Scholar]
- 73.Gandhi L, McNamara KL, Li D, Borgman CL, McDermott U, Brandstetter KA, Padera RF, Chirieac LR, Settleman JE, Wong KK. Sunitinib prolongs survival in genetically engineered mouse models of multistep lung carcinogenesis. Cancer Prev.Res.(Phila Pa) 2009;2:330–337. doi: 10.1158/1940-6207.CAPR-08-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grandis JR, Argiris A. Targeting angiogenesis from premalignancy to metastases. Cancer Prev.Res.(Phila Pa) 2009;2:291–294. doi: 10.1158/1940-6207.CAPR-09-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mizobe T, Ogata Y, Murakami H, Akagi Y, Ishibashi N, Mori S, Sasatomi T, Shirouzu K. Efficacy of the combined use of bevacizumab and irinotecan as a postoperative adjuvant chemotherapy in colon carcinoma. Oncol.Rep. 2008;20:517–523. [PubMed] [Google Scholar]
- 76.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat.Rev.Clin.Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 77.Kapoor A, Gharajeh A, Sheikh A, Pinthus J. Adjuvant and neoadjuvant small-molecule targeted therapy in high-risk renal cell carcinoma. Curr.Oncol. 2009;16(Suppl 1):S60–S66. S60-6. [PMC free article] [PubMed] [Google Scholar]
- 78.Wolmark N, Yothers G, O’Connel MJ, Sharif S, Atkins JN, Seay TE, Feherenbacher L, O’Reilly S, Allegra CJ. A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: Results of NSABP Protocol C-08. Journal of Clinical Oncology. 2009;27(suppl abstr LBA4):18s, 31–5.Ref Type: Abstract