Renal Ontogeny in the Rhesus Monkey (Macaca mulatta) and Directed Differentiation of Human Embryonic Stem Cells Towards Kidney Precursors (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 1.
Published in final edited form as: Differentiation. 2009 Jun 4;78(1):45–56. doi: 10.1016/j.diff.2009.05.001
Abstract
The development of the metanephric kidney was studied immunohistochemically across gestation in monkeys to identify markers of cell specification, and to aid in developing experimental paradigms for renal precursor differentiation from human embryonic stem cells (hESC). PAX2, an important kidney developmental marker, was expressed at the tips of the ureteric bud, in the surrounding condensing mesenchyme, and in the renal vesicle. Vimentin, a mesenchymal and renal marker, was strongly expressed in the metanephric blastema then found to be limited to the glomerulus and interstitial cells of the medulla and cortex. A model of gene expression based on human and nonhuman primate renal ontogeny was developed and incorporated into studies of hESC differentiation. Spontaneous hESC differentiation revealed markers of metanephric mesenchyme (OSR1, PAX2, SIX2, WT1) that increased over time, followed by upregulation of kidney precursor markers (EYA1, LIM1, CD24). Directed hESC differentiation was also evaluated with the addition of Retinoic Acid, Activin-A, and BMP-4 or BMP-7, and using different culture substrate conditions. Of the culture substrates studied, gelatin most closely recapitulated the anticipated directed developmental pattern of renal gene expression. No differences were found when BMP-4 and BMP-7 were compared to baseline conditions. PAX2 and Vimentin immunoreactivity in differentiating hESC was also similar to the renal precursor patterns reported for human fetal kidneys and findings described in rhesus monkeys. The results of these studies: (1) provide additional data to support that rhesus monkey kidney development parallels that of humans, and (2) provide a useful model for hESC directed differentiation towards renal precursors.
Keywords: Human embryonic stem cells, renal precursors, kidney ontogeny, mesoderm
INTRODUCTION
Abnormalities that result from disruption of normal developmental processes account for approximately one-third of all childhood kidney-related illnesses, and represent the single most important need for dialysis and transplantation in the pediatric population (NIH and ASPN, 2000). Common childhood problems include urinary tract obstruction and other inherited kidney diseases, structural malformations, and focal segmental glomerulosclerosis. Fetal urinary tract obstruction is a relatively common problem for which little progress has been made towards developing effective methods for treatment. Research priorities for pediatric kidney disorders include characterizing renal morphogenesis and gene expression patterns associated with ontogeny and function, and elucidating the cell types and interactions in health and disease (DHHS, 2006). While prior studies have focused on identifying renal progenitor cell populations in the kidney (Challen et al., 2004; Lazzeri et al., 2007; Little, 2006; Nishinakamura, 2008), additional methods are needed to develop effective cell transplant techniques to regenerate kidneys damaged by disease. Human embryonic stem cells (hESC) provide a source of cells that can be differentiated towards mesoderm and, thus, could be useful for obtaining renal precursors for repair. Although complete reconstruction of the kidney may prove difficult in vivo, incremental improvements in renal function using cell transplantation strategies could avoid the need for dialysis or organ transplantation.
In order to obtain the necessary cell types for regenerative medicine purposes, differentiation of hESC must recapitulate the developmental paradigm of renal ontogeny. Several studies have focused on kidney development, from early precursors to the events associated with the formation of the definitive or metanephric kidney (Burrow, 2000; Dressler, 2006; Rodriguez et al., 1996). The essential process for metanephric kidney formation is the reciprocal induction of the mesoderm-derived metanephric mesenchyme (blastema) and the branching ureteric bud (derived from the mesonephric duct), which forms the excretory component and collecting system of the kidney, respectively, and is under the control of key genes and their protein products (Saxen, 1987). Normal kidney development includes fundamental processes of branching morphogenesis, vasculogenesis, mesenchymal-epithelial transformation, and segment-specific epithelial determination (Dressler, 2006; Nishinakamura and Osafune, 2006; Rothenpieler and Dressler, 1993). Cells relevant for kidney repair are those derived from intermediate mesoderm and express markers such as PAX2, LIM1, OSR1, and WT1; these cells have the capability to contribute to epithelial components of the nephron and regenerate damaged tubules. While the expression of these markers does not ensure renal lineages because these genes are not specific to the kidney, obtaining early precursors based on established renal markers in combination will provide cells that retain regenerative potential and are likely to be useful for this purpose. The addition of “nephrogenic factors” to mouse ESC culture has been shown to increase early renal marker expression, and these cells have been reported to contribute to tubule epithelia in mouse kidney explant culture (Kim and Dressler, 2005). Bone morphogenic protein (BMP) family proteins may also influence kidney development as the addition of BMP-4 to serum-free mouse ESC culture was reported to enhance intermediate mesoderm gene expression (Bruce et al., 2007).
Nonhuman primates have been shown to be important models for human health and disease; monkeys and humans share many characteristic features because of their close phylogenetic relationship. Developmental similarities include spatial and temporal pattern of organ development, placental structure, and growth characteristics, to name a few (Lee et al., 2001; Rodriguez et al., 1996; Tarantal, 2005; Tarantal and Gargosky, 1995; Tarantal et al., 2001). In these studies, rhesus monkey kidneys at sequential gestational ages were assessed by immunohistochemistry for the expression of renal developmental markers PAX2, an essential marker of kidney differentiation (Dressler, 1996), and Vimentin, a mesenchymal (Holthöfer et al., 1984; Naruse et al., 2000; Carev et al., 2008) and renal prognostic marker (Moll et al., 1991; Gonlusen et al., 2001), to provide additional insights relevant to hESC differentiation protocols. These and other ongoing ontogeny studies were incorporated into a model of gene expression of mesodermal differentiation through definitive kidney formation. Expression of such genes was explored in spontaneously differentiating human embryoid bodies (hEBs). In addition, directed differentiation of hESC towards renal precursors was pursued using suspension and monolayer culture on gelatin and laminin substrates with Retinoic Acid (RA), Activin-A, and BMP-4 or BMP-7 (RA4 and RA7, respectively). The results of these studies demonstrate that hESC can be directed towards early kidney precursors with the addition of RA4 or RA7 to the culture medium, and that the efficiency of differentiation is influenced by the culture conditions and substrates used. PAX2 and Vimentin immunoreactivity noted over time in differentiating hESC was similar to patterns reported in human fetal kidneys and to renal ontogeny in rhesus monkeys. The results of these studies: (1) support the use of these culture conditions to enhance renal differentiation of hESC; (2) provide additional data to support that rhesus monkey kidney development parallels that of humans (Lee et al., 2001); and (3) substantiates that expression of early developmental markers provides a useful paradigm for directed differentiation of hESC.
MATERIALS AND METHODS
Animals
All animal procedures conformed to the requirements of the Animal Welfare Act and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. Normally cycling, adult female rhesus monkeys (Macaca mulatta) (N=19) with a history of prior pregnancy were bred and identified as pregnant, using established methods (Tarantal, 2005). Pregnancy in the rhesus monkey is divided into trimesters by 55-day increments with 0 – 55 days gestation representing the first trimester, 56 – 110 days gestation representing the second trimester, and 111 – 165 days gestation the third trimester (term 165±10 days) (Tarantal and Gargosky, 1995). Activities related to animal care (diet, housing) were performed as per standard California National Primate Research Center (CNPRC) operating procedures. Normal fetal growth and development was confirmed by ultrasound (Tarantal, 2005) and hysterotomies performed using established methods for tissue harvests as previously described (Tarantal et al., 2001). The time points assessed in these studies included the first trimester (40 and 55 days gestation; N=4), second trimester (70 and 90 days gestation; N=6), and third trimester (110, 130, and 150 days gestation; N=9). Postnatal (3 and 6 months; N=4) and adult kidneys (N=2) were also collected according to standard protocols for comparison.
Ontogeny - PAX2 and Vimentin
Collected specimens were placed in 10% buffered formalin, embedded in paraffin, then sectioned at 5–6 µm. Sections were prepared for PAX2 and Vimentin immunohistochemical staining by deparaffinization followed by rehydration in graded ethanol according to established protocols (Lee et al., 2001; Tarantal et al., 2001). Heat induced epitope retrieval was accomplished in 0.01 M citrate buffer (pH 6.0, Invitrogen, Carlsbad, CA) using a decloaking chamber (Biocare Medical, Concord, CA) according to the manufacturer’s recommendations. Slides were washed in phosphate buffered saline (PBS) (two washes, 5 min each), incubated with Background SNIPER® (Biocare Medical) for 20 min to remove background staining, and washed in PBS with Triton X (PBST) (Invitrogen, twice for 2 min each). Non-specific binding was blocked by incubation for 1 hr in blocking solution consisting of PBS with 1% Fraction V bovine serum albumin (BSA, Invitrogen), 0.1% fish skin gelatin (Sigma, St. Louis, MO), 0.1% Triton X-100 (Sigma), 0.05% Tween 20 (Sigma), and 2% normal serum of the species of the secondary antibody host (Butt et al., 2007). Primary and secondary antibodies were incubated for 1 hr each in PBS with 1% BSA and 0.1% fish skin gelatin with intermediary washes in PBST (two washes for 5 min each). The following primary antibodies and dilutions were used: PAX2 (1:100; Invitrogen) and Vimentin (1:100, Invitrogen). Secondary antibodies included goat anti-rabbit Alexa Fluor® 594 (1:100) and goat anti-mouse Alexa Fluor 488® (1:100) (Invitrogen). A coverslip was placed on the slides with ProLong Gold® antifade reagent with DAPI (Invitrogen), and sections visualized with an Olympus BX61 fluorescent microscope, equipped with a Photometrics CoolSnap HAQ black and white camera. Black and white images were collected using the appropriate filter set for each fluorophore and color composite images were generated using MicroSuite™ software (Olympus America Inc., Melville, NY).
Cell Culture
The NIH Registry hESC line HSF-6 (passage 46) was cultured on a monolayer of irradiated mouse embryonic fibroblasts (MEFs) in DSR medium consisting of high-glucose Dulbecco’s Modified Eagle Medium (DMEM), 20% Knockout™ Serum Replacement, 2 mM L-Glutamine, 0.1 mM MEM, Non-Essential Amino Acids Solution, 4 ng/ml Recombinant Human FGF basic (Invitrogen), and 0.1 mM -mercaptoethanol (Sigma) using established protocols (Bodnar et al., 2004). This cell line has been maintained with a similar morphology for >70 passages and several cryopreservation-thaw cycles, and has a normal documented human XX karyotype. At the time of expansion, cells were tested for expression of select markers by quantitative RT-PCR including OCT-4, α-fetoprotein and transthyretin (endoderm), Brachyury and Flk-1 (mesoderm), and NCAM (neural ectoderm), immunofluorescence analysis (Tra-1-60, Tra-1-81, SSEA-3, SSEA-4) (data not shown), and to ensure a normal karyotype. Thus, a well-characterized cell bank was established and used for all experiments. Cells were passaged with 1 mg/ml collagenase Type IV (Invitrogen) every 3 to 4 days for expansion.
Feeder-free hESC cultures were established from the same cell bank according to protocols described by Beattie et al. (2005). Briefly, hESC were plated on laminin-coated plates (BD Biocoat™ Laminin Cellware, BD Biosciences, Bedford, MA) in DSR medium with 10 mM nicotinamide (Sigma), 50 ng/ml human recombinant Activin-A (PeproTech Inc, Rocky Hill, NJ), and 50 ng/ml human recombinant keratinocyte growth factor (PeproTech). Medium was changed every other day and cells were passaged weekly at 1:3 dilution by collagenase treatment for 5 min. Cultures were maintained in an undifferentiated state for > 25 passages.
Differentiation
For differentiation protocols, hESC colonies were removed from underlying MEF feeder layers by collagenase treatment for 30 min followed by gentle pipetting. Colonies were pooled, washed, and dissociated to small clusters by manual pipetting. The clusters were plated on ultra-low attachment plates (Corning, Acton, MA) in 2 ml of differentiation medium at a concentration ratio of 3 plates of hEBs per plate of hESC and cultured at 37°C in 5% CO2. For initial assessment of expression of kidney-specific genes, hEBs were differentiated in basal medium consisting of MEM medium (Invitrogen) with 10% FBS (Hyclone, Logan, UT), 1% Pen-Strep, 1% L-Glutamine, and 100 µM 1-thioglycerol (Sigma) in suspension culture. Directed differentiation studies were conducted with hEBs grown as above in the presence (RA4 or RA7) or absence (control) of growth factors including 0.1 µM trans-RA (EMD Biosciences, San Diego, CA), 10 ng/ml Activin-A (R&D Systems, Minneapolis, MN), and 50 ng/ml recombinant human BMP-4 or BMP-7 (R&D Systems). To evaluate the effect of monolayer culture with growth factors, hEBs were cultured for 2 days as described above, then plated on 0.1% gelatin-coated plates and cultured as a monolayer in high glucose DMEM with 10% FBS, 1% Pen-Strep, 1% L-glutamine, and in the presence or absence of RA4 or RA7. Directed differentiation was also assessed in feeder-free hESC cultures grown on laminin by the addition of RA4 or RA7 factors to DSR culture medium beginning at day 2 after passaging.
Quantitative RT-PCR
Total RNA was extracted using the RNeasy kit (RNeasy® Micro Kit, Qiagen, Valencia, CA) following the manufacturer's instructions. cDNA was synthesized using random primers (Promega, Madison, WI) and the PowerScript™ Reverse Transcriptase kit (Clontech, Mountain View, CA), as previously described (Jimenez et al., 2005). Primers were designed to span an intron and thus eliminate the possibility of amplifying genomic DNA templates remaining in the RNA preparations (Table 1). Real-Time PCR was carried out in 96-well optical plates using the 7900® ABI Sequence Detection System (Applied Biosystems, Foster City, CA) and the QuantiTect™ SYBR® Green PCR Kit (Qiagen) according to the manufacturer's protocols. PCR reactions were run in duplicate in separate wells and contained 1X SYBR Green master mix and 500 nM of forward and reverse primers in a 25 µl reaction volume. The PCR protocol consisted of one cycle of 2 min at 50°C, 15 min at 95°C, followed by 40 cycles at 15 sec at 95°C, and 60 sec at 60°C. RNA expression was quantified relative to the housekeeping gene, HPRT1, and to normalize the amount of sample RNA and to compensate for sample variation. Positive and negative controls (water) were included in each run. Data from replicate experiments were pooled by calculation of the fold change from day 0 (baseline). Differences in gene expression were determined as described in the User’s Bulletin #2 (P/N 4303859) (Applied Biosystems, updated 2001). The results were initially calculated as relative transcription or the n-fold difference relative to a calibrator cDNA.
Table 1.
Primer sequences for assessment of human embryonic stem cell (hESC) differentiation towards renal precursors
| Gene | Marker | Forward primer (5’ – 3’) | Reverse primer (5’ – 3’) |
|---|---|---|---|
| NANOG | Undifferentiated ESC | TTCCTTCCTCCATGGATCTG | TCTGCTGGAGGCTGAGGTAT |
| OCT-4 | Undifferentiated ESC | ATTTGCCAAGCTCCTGAAGCAG | TTGATCGCTTGCCCTTCTGG |
| BRY | Mesoderm | CCCGCGCACTACACACCCCTCACC | CCTTGGGCTGCGGCGTCGTACTG |
| OSR1 | IM (MM) | AGAGTCCTGGACTGGCAGAATC | AGACCACAGATATATTCACTCCAAAGAG |
| PAX-2 | IM (UB, Induced MM) | GCTTTGGATCGGGTCTTTGA | CTCGTTCCCCTGTTCTGATTTG |
| SIX2 | IM | AAGGAAAGGGAGAACAACGAGAA | TCCTCCGAGCTGCCTAACAC |
| WT1 | IM (Induced MM, renalvesicle, early podocytes) | CTTCAGAGGCATTCAGGATGTG | TCTCAGATGCCGACCGTACA |
| CD24 | Early renal precursors | GGTGCCCTGCAGTCAACAG | CGTTTCTTGGCCTGAGTCTCTTAA |
| CDH11 | Early renal precursors | TCTTTGCAGCAGAAATCCACAA | CTTGGGAGCATTATCGTTGACA |
| EYA1 | Early renal precursors | AATAATGTTGGAGGTCTGCTTGGT | GTCGGTCAGGGCTTCAATTTC |
| LIM1 | Early renal precursors (MM) | AGCAGTGTTGCCAAAGAGAACAG | TCTTGGGAATCCGGAGACAA |
| SIX1 | Early renal precursors (MM) | AAACTATTCTCTCCCGGGCTTAA | CCCCACTTAGGACCCCAAGT |
| GDNF | Developing kidney -branching morphogenesis | GGGTCTGGGCTATGAAACCA | TCTCAGCTGCATCGCAAGAG |
| RET | Developing kidney -branching morphogenesis | TGTGGAGACCCAAGACATCAAC | GCTCGTGTGTCCCCCAACAA |
| WNT4 | Developing kidney -renal vesicle induction | CAGGAAGGCCATCCTGACA | CTCGCCAGCACGTCTTTACC |
| AQP2 | Maturing kidney -water channel | GTGCGCCGAAAATTTCCA | CCTCGACTTCTCCTTGAAGCA |
| CDH16 | Maturing kidney - cadherin | GCACCCTGGTAGCAATAGGAATC | GGTTGATCCGGGTCCTTCTT |
| CLCN5 | Maturing kidney - Cl ionchannels and transporters | ACCCATGGAGATCGTAGTGGATA | CAATCGCCCGTTGTGTGTAA |
| CYP27* | Maturing kidney | TTTGGCCCAGATCCTAACACA | AGTCCGGGTCTTGGGTCTAAC |
| NPHS1 | Maturing kidney - nephrin | GAGGACCGAGTCAGGAACGA | TGACCGTGGAGCTCTGAGTGT |
| NPHS2* | Maturing kidney - podocin | CCTTTTCATGAGATCGTGACCAA | GCATTTTCCATTCGGTAGTAGCA |
| PODXL* | Maturing kidney - podocytes | AAGGACCAGCAGCGGCTAA | CATCACTTCCAGTGTTGGGTTGT |
| SLC12A1* | Maturing kidney - Na/K/Cltransporters (LOH) | CAGGGATGGTGTCCGAAGTC | TCTTATATCCAATCACCAGAGTGTTTG |
| HPRT1 | Housekeeping gene | TTTTATCAGACTGAAGAGCTATTGTAATGA | CATCGTTTTGCCAGTGTCAATTAT |
Immunocytochemistry and Immunofluorescence
Aliquots of cells were plated in 2-well chamber slides coated with either gelatin or laminin and grown until collected at the appropriate time point (2, 4, 6, or 8 days) in control, RA4, or RA7 medium. Cells were fixed in ice-cold methanol for 20 min at room temperature and frozen at •−20°C until analysis. For staining, slides were washed two times in PBS for 5 min each, then incubated for 1 hr with blocking solution as described above. Cells were then incubated with the primary antibody overnight at 4°C, followed by incubation with the secondary antibody for 1 hr at room temperature in the dark as previously described. Coverslips were secured with ProLong Gold® with DAPI and slides imaged with an Olympus BX61 fluorescent microscope, equipped with a Photometrics CoolSnap HAQ black and white camera.
Data analysis
Data are shown as mean ± standard error mean of three independent experiments. Significance was determined by Student’s t-test analysis at p • 0.05.
RESULTS
Renal Ontogeny
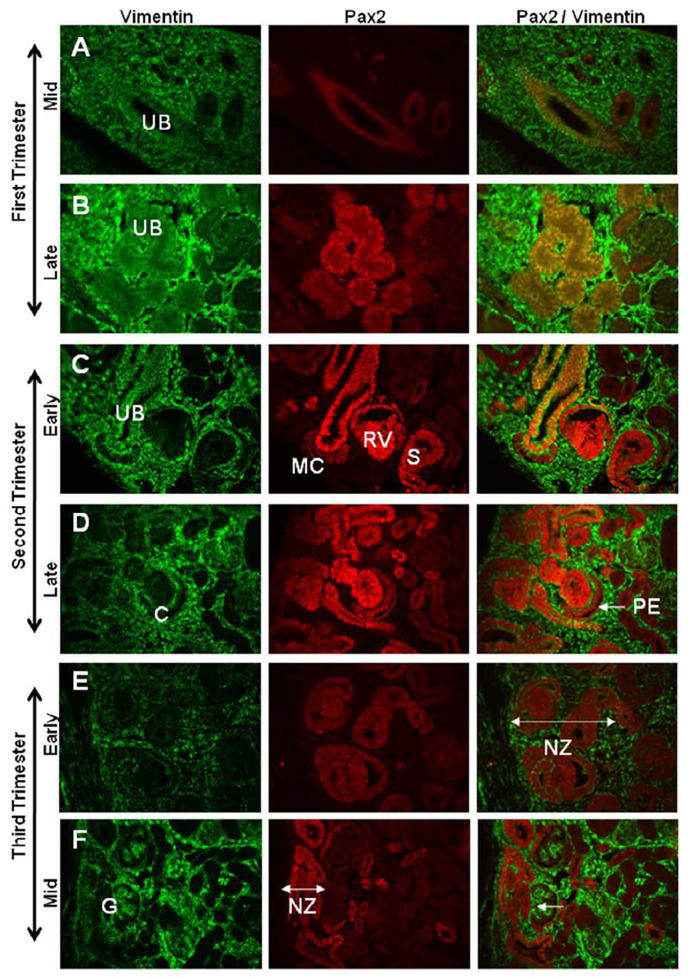
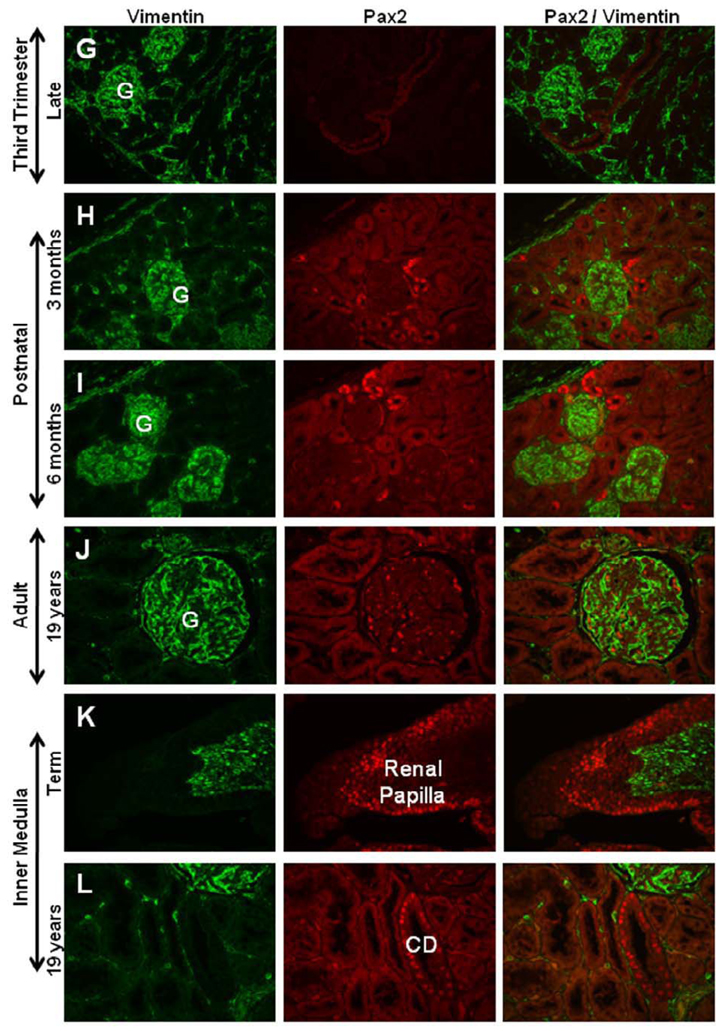
The development of the metanephric kidney was studied across sequential gestational ages in the rhesus monkey using immunohistochemical techniques for developmental markers PAX2 and Vimentin (Fig. 1). Mesenchymal cells of the first trimester kidney (40 days gestation) were strongly Vimentin positive, with mesonephric tubules PAX2 positive/Vimentin negative, and the developing ureteric bud positive for both PAX2 and Vimentin (Fig. 1A). Late first trimester kidneys (55 days gestation) showed strong Vimentin staining of cells of the metanephric blastema and faint PAX2 expression in ureteric bud tips (Fig. 1B). In the second trimester, PAX2 and Vimentin were co-expressed in the ureteric bud while condensing mesenchymal cells clustered at the bud tips were dimly positive for PAX2 and with no Vimentin expression. The developing renal vesicles were strongly PAX2 positive and Vimentin negative (Fig. 1C). Differentiating glomeruli in late second trimester kidneys (90 days gestation) were observed with PAX2 staining in visceral and parietal layers of S- and C-shaped bodies. Vimentin was co-expressed with PAX2 in the parietal layer of the C-shaped body and, with advancing gestational age, was also noted in the basal membrane of the PAX2 positive visceral layer (Fig. 1D). At the beginning of the third trimester (110 days gestation), PAX2 expression was restricted to a narrow nephrogenic zone at the edge of the cortex, cortical collecting ducts, and inner medullary regions lining the duct of Bellini or papillary (collecting) ducts (Fig. 1E, K). Vimentin staining was evident in the immature glomerulus of the mid-third trimester (130 days gestation) (Fig. 1F), and was then apparent in immature visceral glomerular cells during nephron maturation. Near term (150 days gestation), Vimentin expression was noted in the visceral glomerular cells or podocytes and glomerular tuft, the capsule, and in cortical and medullary interstitial cells (Fig. 1G). PAX2 expression was largely absent by the late third trimester except for a few small clusters of positive cells just below the cortical surface, medullary collecting ducts, and regions lining the renal papilla (Fig. 1H–K). Vimentin expression in the adult kidney was restricted to the glomerulus and occasional interstitial cells while PAX2 expression was limited to a few medullary collecting ducts (Fig. 1L).
Figure 1. PAX2 and Vimentin expression in developing rhesus monkeys.
The developing ureteric bud (UB) is positive for both PAX2 and Vimentin in mid-first trimester kidney (Fig. 1A). Late first trimester kidneys showed strong Vimentin staining of cells of the metanephric blastema and faint PAX2 expression in UB tips (Fig. 1B). In the second trimester, PAX2 and Vimentin were co-expressed in the UB while condensing mesenchymal cells (MC) clustered at the bud tips were dimly positive for PAX2 and with no Vimentin expression. The developing renal vesicles (RV) were strongly PAX2 positive and Vimentin negative (Fig. 1C). Differentiating glomeruli in late second trimester kidneys (90 days gestation) were observed with PAX2 staining in visceral and parietal layers of S- and C-shaped bodies (denoted S, C, respectively). Vimentin was co-expressed with PAX2 in the parietal layer (PE, arrow) of the C-shaped body (Fig. 1D). In the early third trimester, PAX2 expression was restricted to a narrow nephrogenic zone (NZ) at the edge of the cortex, cortical collecting ducts, and inner medullary regions lining the duct of Bellini (Fig. 1E, K). Vimentin staining was evident at the base of the immature glomerulus in the mid-third trimester (Fig. 1F, arrow), then was apparent in immature visceral glomerular cells during nephron maturation. Near term, Vimentin expression was noted only in the visceral glomerular cells or podocytes, the capsule, and in cortical and medullary interstitial cells (Fig. 1G). PAX2 expression was largely absent by the late third trimester except for a few small clusters of positive cells just below the cortical surface, medullary collecting ducts, and regions lining the renal papilla (Fig. 1H–K). Vimentin expression in the adult kidney was restricted to the glomerulus and occasional interstitial cells while PAX2 expression was reduced to a few medullary collecting ducts (Fig. 1L). Images taken at 40X magnification and arranged with cortex to the left (Fig. A–I).
Spontaneous Differentiation of hESC
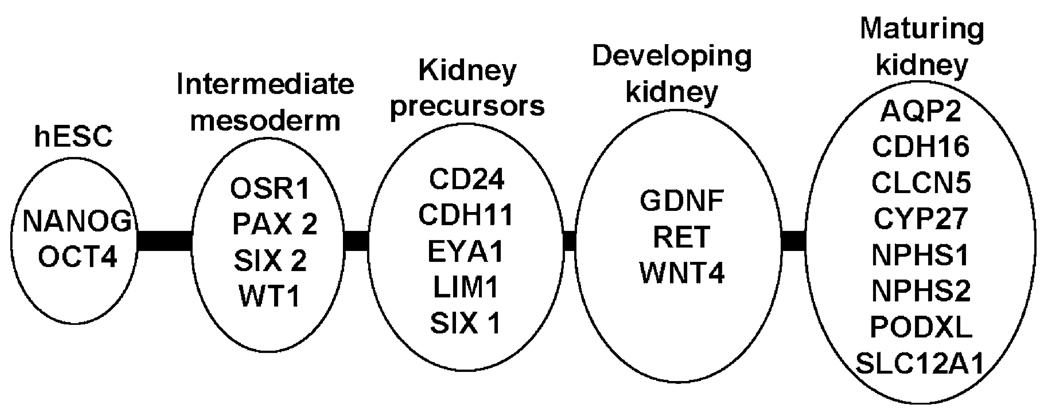
Genes associated with renal ontogeny were assessed in hESC grown in culture using the paradigm of intermediate mesoderm (OSR1, PAX2, SIX2, WT1), early kidney precursors (CD24, CDH11, EYA1, LIM1, SIX1), developing kidney (GDNF, RET, WNT4), and more mature renal phenotypes (AQP2, CDH16, CLCN5, CYP27, NPHS1, NPHS2, PODXL, SLC12A1) (see Fig. 2 and Table 1). hEBs were maintained in culture for 6 days and aliquots of cells removed for quantitative real-time RT-PCR daily using established techniques (Jimenez et al., 2005). Experiments were repeated 3 or more times and one experiment (Fig. 3) was continued until day 22 to assess upregulation of markers of mature kidney cell types. OCT4 and NANOG were used as markers for undifferentiated hESC prior to spontaneous and directed differentiation protocols with RA4 and RA7. Brachyury (BRY), a transcription factor important in mesodermal differentiation, was also included as a marker of early mesodermal commitment.
Figure 2. hESC renal differentiation.
Markers used to define renal ontogeny from early primitive structures (intermediate mesoderm) to more differentiated cell populations.
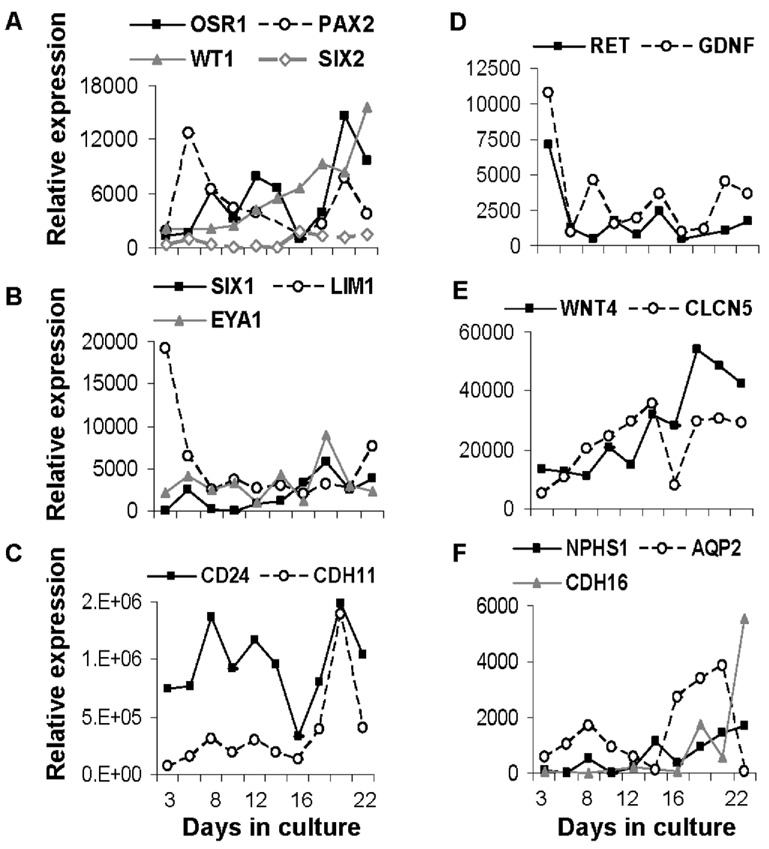
Figure 3. Gene expression in differentiating human embryoid bodies (hEBs).
Outcome over time in culture is expressed as the number of copies per 50,000 copies housekeeping gene (HPRT1). (A) Expression of markers of intermediate mesenchyme OSR1, PAX2, WT1, and SIX2, markers of kidney precursor populations (B) SIX1, LIM1, and EYA1, and (C) CD24 and CDH11. (D) Developing kidney markers RET and GDNF. Markers of maturing kidney cell types (E) WNT4 and CLCN5 and (F) NPHS1, AQP2, and CDH16. Markers of early kidney specification and precursor populations were upregulated early in culture while specific markers of mature kidney cell types were not upregulated until after two wks in culture.
The expression pattern of markers of the uninduced metanephric mesenchyme or blastema (PAX2, SIX2, WT1) and an early marker of kidney specification (OSR1) is shown in Figure 3A. Expression of CD24 and CDH11, genes upregulated in specification of the caudal intermediate mesoderm, increased with advancing days in culture (Fig. 3C). Transcription factors EYA1, LIM1, and SIX1 (Fig. 3B) were also expressed early and may have contributed to glial cell line-derived neurotrophic factor (GDNF) expression (Fig. 3D) together with WNT4, which are important in the differentiation of the ureteric bud (Fig. 3E). Markers of more mature kidney phenotypes including NPHS1, a glomerular-filter barrier protein, and several kidney-specific proteins including CDH16 (cadherin), CLCN5 (chloride channel), and AQP2 (aquaporin 2, water channel) were expressed at later stages of cell culture (Fig. 3E, F).
Directed Differentiation of hESC
RA7
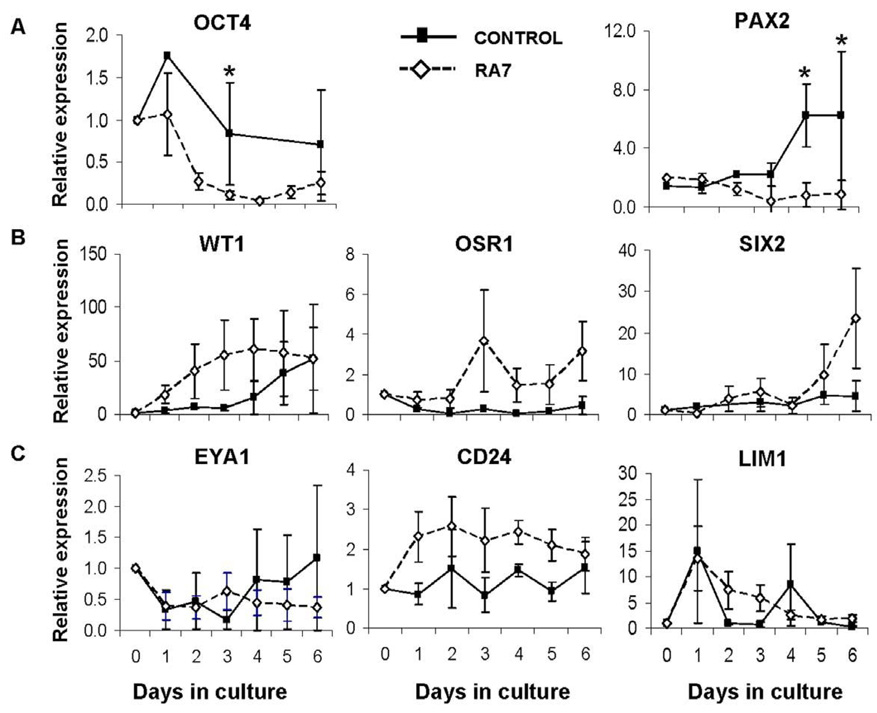
The expression of renal genes in hEBs was evaluated with the addition of the nephrogenic factors previously reported to enhance the expression of renal markers in mouse ESC (Kim and Dressler, 2005). Expression of OCT4 was similar in both control and RA7 cultures at baseline, and declined more rapidly in RA7 cultures (Fig. 4). WT1 expression gradually increased from day 0 to day 3 of culture while OSR1 was strongly upregulated beginning on day 3. In contrast, other markers of metanephric mesenchyme induction were not upregulated until day 6 (SIX2) or were downregulated (PAX2) in RA7 cultures. No significant differences were noted in the expression of kidney precursor markers EYA1, CD24, and LIM1 when these factors were added, however expression of LIM1 was upregulated nearly 15-fold within the first 4 hr of culture suggesting early differentiation towards mesodermal lineages in both RA7 and control cultures. Other markers of developing and mature kidney cell populations did not change significantly with RA7 when compared to untreated control cultures (data not shown).
Figure 4. Expression of markers of early renal ontogeny in differentiating hEBs.
hEBs were cultured in suspension in basal medium in the presence (RA7) or absence (control) of Retinoic Acid (RA), Activin-A, and BMP-7. Gene expression relative to HPRT1 was calculated and pooled from three separate experiments as the fold change (± standard error mean, SEM) from day 0 (baseline); *p < 0.05. Top left, marker of undifferentiated hESC (OCT4). Top right (PAX2) and middle (WT1, OSR1, SIX2) panels, markers of intermediate mesoderm. Bottom panel, markers of kidney precursor populations (EYA1, CD24, LIM1).
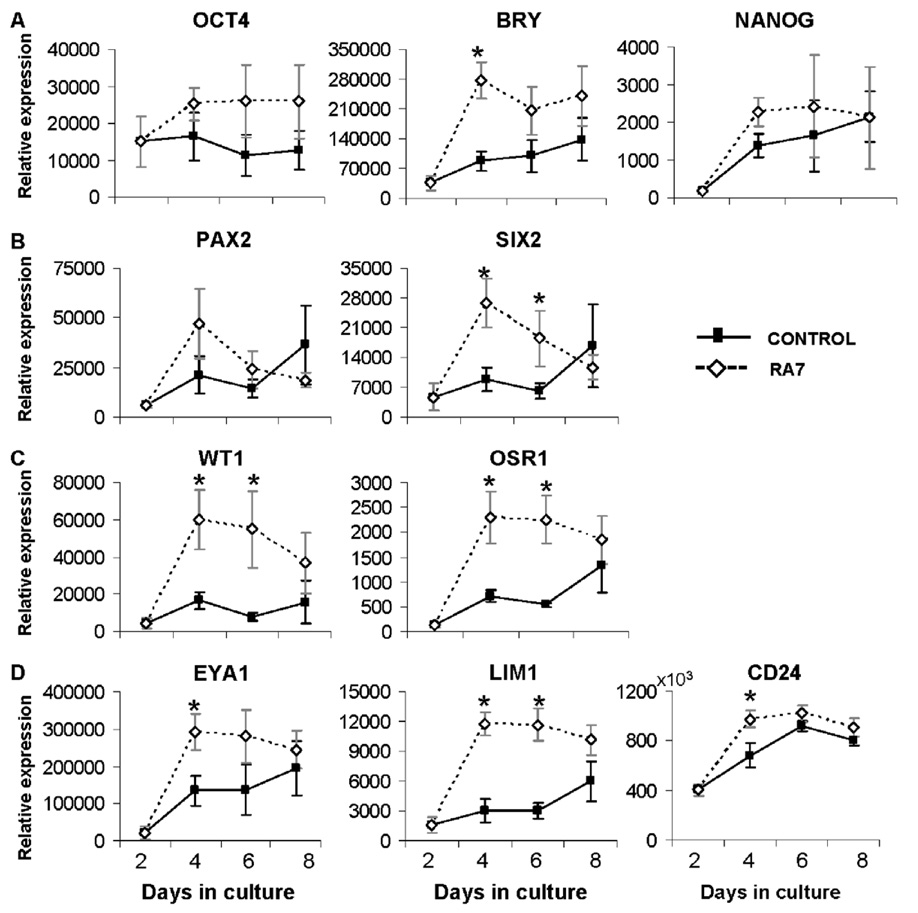
In an effort to increase the expression of intermediate mesenchymal markers OSR1, PAX2, SIX2, and WT1, hEBs were placed in suspension culture for 2 days, followed by differentiation on gelatin-coated plates in medium supplemented with RA7. Under these conditions, hEBs became adherent and formed monolayer cultures. While expression of OCT4 and NANOG were similar in RA7 and control cultures, expression of BRY was 4-fold greater on day 4 in RA7 cultures suggesting enhanced differentiation of mesodermal populations with this culture strategy (Fig. 5A). Intermediate mesenchymal markers (Fig. 5B, C) were increased 2- to 5-fold by day 4 of culture; these differences were significant for SIX2, WT1, and OSR1 (p < 0.05). Similarly, expression of kidney precursor markers EYA1, LIM1, and CD24 was also strongly upregulated (Fig. 5D) suggesting a positive influence of the monolayer culture system and gelatin substrate on intermediate mesodermal differentiation.
Figure 5. Renal differentiation of hESC on gelatin substrate.
Expression of markers of early renal ontogeny in hESC differentiated for 2 days as hEBs in suspension, then plated on gelatin-coated plates in high-glucose DMEM in the presence (RA7) or absence (control) of nephrogenic factors. Gene expression relative to HPRT1 was calculated and pooled from 3 separate experiments as mean ± SEM; *p < 0.05. (A) Markers of undifferentiated hESC (OCT4, NANOG), and an early mesodermal marker BRACHYURY (BRY). (B, C) Markers of intermediate mesoderm (PAX2, SIX2, WT1, OSR1). (D) Markers of kidney precursors (EYA1, LIM1, CD24). Gene expression patterns are suggestive of increased differentiation toward intermediate mesodermal lineages in the presence of RA7.
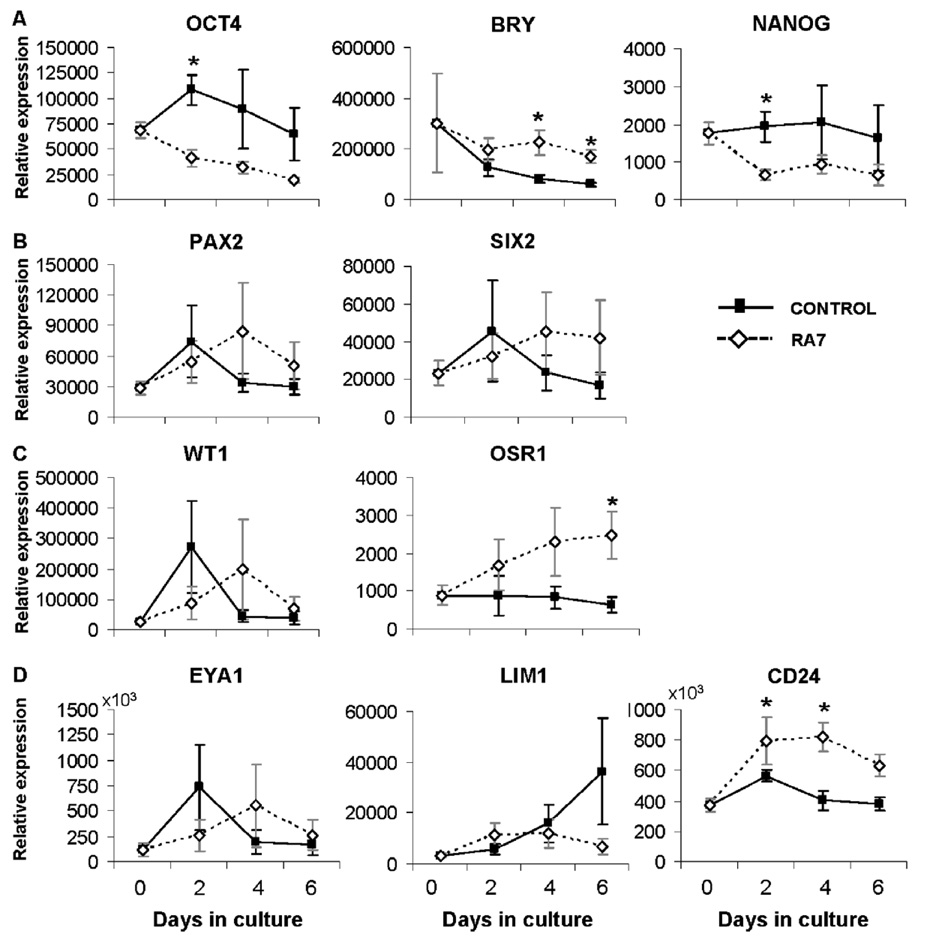
To further explore interactions of hESC in monolayer differentiation systems, feeder-free cultures of hESC were plated on laminin-coated plates and differentiated in the presence or absence of RA7 (Fig. 6). As feeder-free hESC cultures were previously adapted to the laminin substrate as part of the protocol for maintenance of pluripotency, this strategy was focused on increasing differentiation efficiency with cells already adapted to a monolayer culture system. Expression of hESC markers OCT4 and NANOG declined significantly by day 2 of culture when medium contained RA7 factors (Fig. 6A). Expression of BRY and OSR1 were upregulated and appreciably increased in RA7 conditions by days 4 and 6, respectively (Fig. 6A, C). Of the kidney precursor markers studied, only CD24 was upregulated with RA7 (Fig. 6D) suggesting that this culture condition was not effective in directing differentiation towards renal precursors.
Figure 6. Renal differentiation of hESC on laminin substrate.
Expression of markers of early renal ontogeny in hESC grown in feeder-free culture conditions on laminin-coated plates in the presence (RA7) or absence (control) of nephrogenic factors. Gene expression relative to HPRT1 was calculated and presented as mean ± SEM; *p < 0.05. (A) Markers of undifferentiated hESC (OCT4, NANOG), and BRY. (B, C) Markers of intermediate mesoderm (PAX2, SIX2, WT1, OSR1). (D) Markers of kidney precursors (EYA1, LIM1, CD24).
RA4
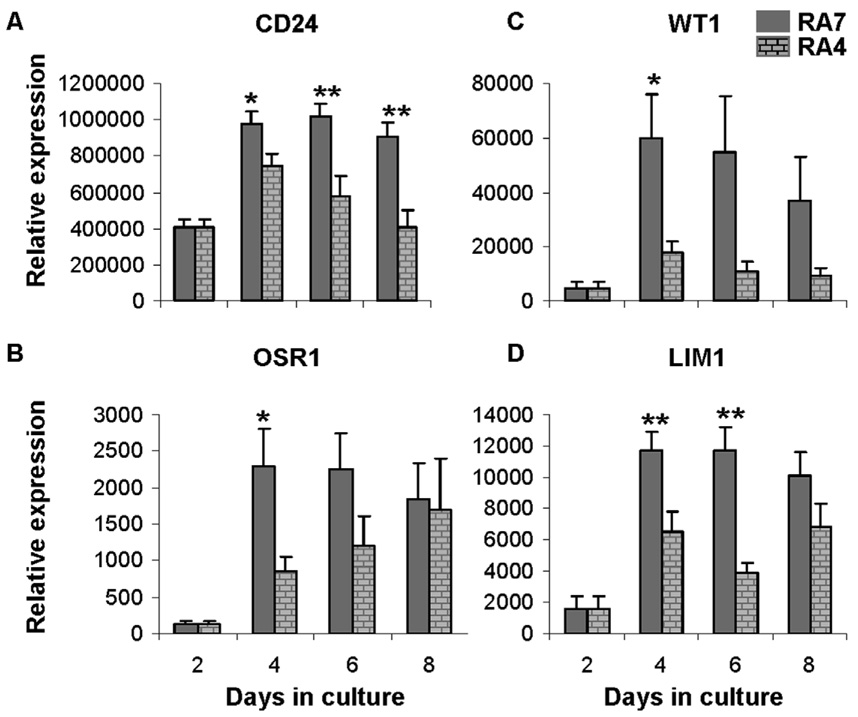
Subsequent efforts to maximize renal precursors focused on comparisons of gene expression when BMP-4 was included (RA4) as an alternative to BMP-7. The culture strategy for this study included formation of hEBs by 2 days of suspension culture followed by monolayer culture on 0.1% gelatin with the addition of RA4. Gene expression patterns for cultures that included RA4 were similar to cultures with RA7 for most genes studied (data not shown) with the only differences detected for CD24, OSR1, WT1, and LIM1. Expression of these four genes was increased in RA7 medium (Fig. 7). Differences were noted after 4 days in culture and were highly significant for kidney precursor markers CD24 and LIM1 (p < 0.01). This outcome supports the hypothesis that BMP-4 and BMP-7 do not exert differential effects early in mesodermal lineage commitment but may play a role later in development in directing specific kidney precursor populations.
Figure 7. Effect of BMP-4 or BMP-7 on renal differentiation of hESC.
Comparison of expression of renal developmental markers CD24 (A), OSR1 (B), WT1 (D), and LIM1 (D) in differentiating hESCs cultured in RA4 or RA7 over time. Note outcome with RA7 was significantly greater at specific time points for all genes. Gene expression relative to HPRT1 was calculated and pooled from 3 separate experiments as the mean ± SEM; *p < 0.05, **p < 0.01.
Immunocytochemistry
To further characterize the nature of renal progenitor differentiation from hESC, markers of renal development were examined by immunofluorescence. The mesodermal marker, Vimentin, was strongly expressed prior to addition of RA4 or RA7 to the culture medium and declined over 4 days in culture when compared to control cultures (Fig. 8) in a pattern similar to that observed in the immunohistochemical analysis of renal ontogeny in rhesus monkeys (Fig. 1). Conversely, intermediate mesodermal markers such as PAX2 were noted to increase over time in cultures with RA4 or RA7 (Fig. 8) and were distributed in small groups of positive cells in a similar manner when compared to PAX2 expression during kidney development in monkeys. All RA4 or RA7 cultures contained PAX2 positive aggregates that were qualitatively greater in fluorescence intensity when compared to control cultures.
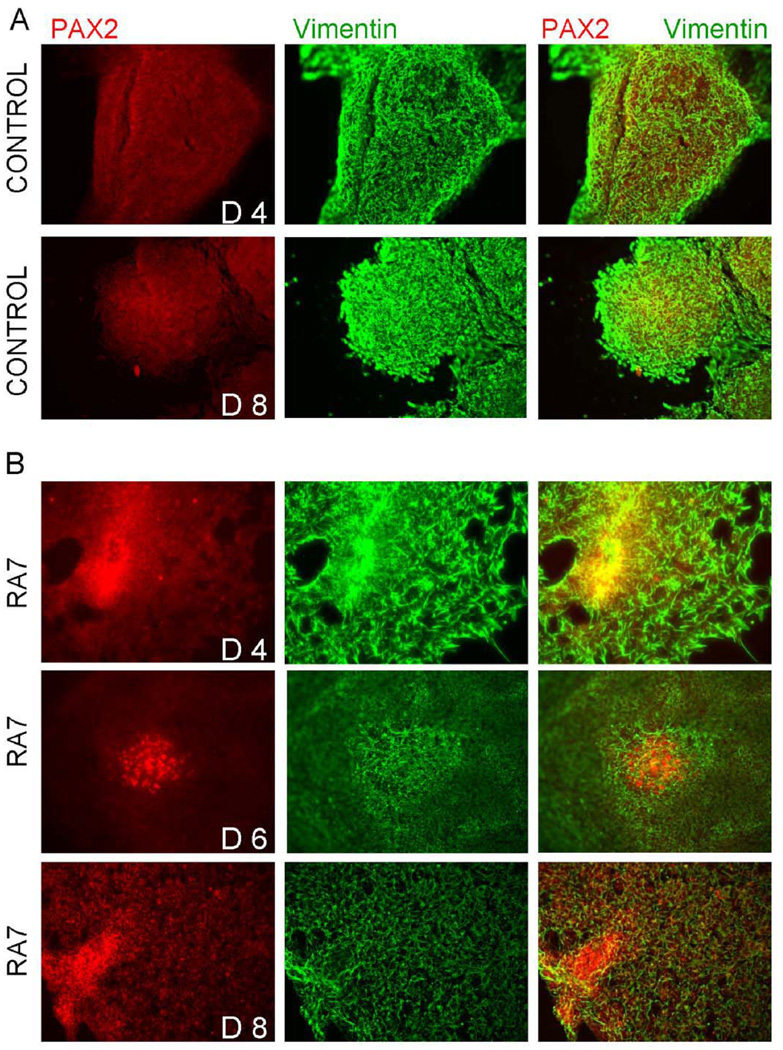
Figure 8. Immunocytochemistry of hESC cultured in the presence or absence of RA7.
Cells were cultured on chamber slides coated with gelatin or laminin and fixed in icecold methanol prior to staining. hESC were assessed on day 4, 6, and 8 in culture. hESC with RA7 were noted to increase PAX2 and decrease Vimentin expression over time (lower three panels; days (d) 4, 6, and 8 in culture shown) when compared to controls (upper two panels; days 4 and 8 shown. These findings parallel gene expression shown with quantitative real-time RT-PCR (see text).
DISCUSSION
The mammalian metanephric kidney develops from the intermediate mesoderm by a series of reciprocal inductive interactions between the metanephric blastema and the ureteric bud (Dressler, 2006). Signals from the metanephric blastema drive branching morphogenesis of the ureteric bud which, in turn, induces aggregation and differentiation of mesenchymal cells or the blastema surrounding the bud tips. The renal vesicle is the first recognizable structure to form in the differentiating mesenchyme, followed by development of S- and C-shaped bodies as the vesicle matures. Endothelial cell migration into the cleft of the S-shaped body results in development of glomerular tuft capillaries and the ensuing interactions between endothelial cells and surrounding mesenchymal-derived epithelium results in further maturation of the renal filtration unit, the glomerular basement membrane.
PAX2 and Vimentin immunoreactivity shown in developing rhesus monkeys during kidney ontogeny is similar to findings in human specimens at similar gestational ages (Winyard, et al., 1996, Naruse et al., 2000) and further supports the importance of the monkey as a model for human nephrogenesis (Lee et al., 2001; Rodriguez et al., 1996). Similar to findings in humans and rodents, PAX2 is an important marker of kidney development in the monkey with strong expression at the ureteric bud tips, the condensed mesenchyme and renal vesicle, and in the developing visceral and parietal layers of the capsule. Intense PAX2 staining was also notable in late gestation medullary regions and may be consistent with proposed progenitor cell populations in the renal papilla (Al-Awqati and Oliver, 2006; Oliver et al., 2004). In the developing glomerulus, Vimentin expression was initially noted in the early glomerular tufts and with maturation was shown additionally in the visceral epithelium in mature glomeruli, in accordance with previous reports in humans (Naruse et al., 2000; Holthöfer et al., 1984).
This complex and intricate process of kidney development provides a logical blueprint for in vitro production of precursors from hESC that may be useful for kidney repair. The panel of markers used in these studies includes genes known to be expressed in the intermediate mesoderm, early kidney precursors, differentiating medullary and cortical regions, and more mature kidney cell populations of the collecting system and excretory component. The expression pattern of spontaneously differentiating hEBs showed greater expression of genes important in early kidney ontogeny when compared to those genes indicative of more mature kidney cell types, which was not unexpected (Fig. 3). When RA7 was added to the culture medium, hEBs differentiated rapidly over time as evident by declining levels of OCT4 and NANOG expression (Fig. 4 and Fig. 6) in both suspension and laminin-substrate culture systems. These findings were not observed in gelatin-substrate monolayer culture where OCT4 expression remained fairly constant over time while NANOG expression increased 2-fold (Fig. 5) despite strong upregulation of intermediate mesodermal markers SIX2, WT1, and OSR1. Although additional characterization is needed to determine the exact nature of these cells, the results are suggestive of a population of renal precursors that express OCT4 together with renal differentiation markers, similar to the multipotent renal progenitor cells characterized by Gupta et al. (2006).
Expression of intermediate mesodermal markers (OSR1, WT1, PAX2, SIX2), while not increased with RA4 or RA7 in suspension culture, was rapidly and strongly upregulated in monolayer culture on gelatin-coated dishes (Fig. 5B, C). These findings are supported by immunocytochemical analyses (Fig. 8) that showed increased PAX2 and decreased Vimentin expression in RA7 cultures when compared with controls over time; and mirror trends in PAX2 and Vimentin expression observed in developing monkey kidneys (Fig. 1). A similar trend was noted in monolayer culture on a laminin substrate (Fig. 6B, C), although the response was delayed and less in magnitude. Similarly, expression of kidney progenitor markers (EYA1, LIM1, CD24) was increased 2- to 4-fold in monolayer culture on gelatin-coated dishes (Fig. 5D). Of this set of markers, only CD24 was increased when the culture substrate was laminin (Fig. 6D). Since LIM1 was shown to be important in formation of the cranium (Shawlot and Behringer, 1995), and EYA1 (Grifone et al., 2004) and CD24 (Pirruccello and LeBien, 1986) are expressed in muscle and lymphatic tissue, respectively, the data presented here must be interpreted with caution in terms of differentiation toward definitive renal phenotypes. In order to ensure renal cells for regenerative medicine purposes a combination of markers representative of specific stages of kidney differentiation are necessary to ensure lineage specificity. Methods for selecting these different populations will be important for exploring new regenerative techniques in animal models.
Extracellular matrix (ECM) molecules are important in kidney development and influence the physical organization of the cells, modulate signal transduction pathways, or regulate cell growth and proliferation through growth factor interactions (Kanwar et al., 2004). Laminin, an adhesive glycoprotein, has been shown to play a role in epithelial and endothelial migration, proliferation, and function while collagen family proteins act as structural and regulatory proteins in a wide variety of mammalian tissues. In knockout mouse experiments, laminin and collagen have been shown to regulate formation and function of the glomerulus, as well as other related structures, and play crucial roles in the formation of the glomerular basement membrane (Lelongt and Ronco, 2003). In the present studies, hESC differentiated in monolayer culture on substrates of gelatin (a heterogenous mixture composed primarily of collagen) or laminin were more responsive than hESC differentiated in suspension cultures as EBs. These findings support the idea that signaling through ECM molecules is important in directing early kidney differentiation.
RA, the active form of vitamin A, has a critical role in vertebrate embryonic development and has been shown to have wide-ranging effects on many anatomical areas such as the heart, brain, sensory systems, developing limbs, and respiratory system (Ross et al., 2001; Zile, 2001). The morphogenic properties of RA are likely mediated through tissue concentration gradients achieved in concert with distributions of various receptor isotypes and synthetic and catabolic enzymes in the developing embryo, and are perhaps best characterized in the emerging retina (Ross et al., 2001). In the embryonic kidney, RA has been shown to regulate c-ret expression and ureteric bud branching morphogenesis (Mendelsohn et al., 1994; Moreau et al., 1998) in the mouse and rat.
Other cytokines/growth factors considered important in the posterior patterning and survival of the intermediate mesoderm include the BMP family of growth factors (Bush et al., 2004; Xiao et al., 2007). BMP-7-deficient mice begin early nephrogenic inductive events but show a reduction in the extent of branching morphogenesis, mesenchymal condensation, epithelial formation, and glomerular development (Dudley et al., 1995; Luo et al., 1995). Reduction of BMP-4 expression by its antagonist, gremlin, has been shown to be necessary for normal ureteric bud outgrowth and branching morphogenesis and for the establishment of the GDNF-RET signaling loop between the metanephric mesenchyme and the ureteric bud epithelium (Michos et al., 2007). That BMP-4 is able to rescue BMP-7 mutant metanephric kidney development in mice suggests that, despite structural differences, BMP family members have redundant functions in kidney development (Oxburgh et al., 2005). Notably, gene expression was similar for most genes when BMP-4 was substituted for BMP-7 in the present experiments providing additional in vitro support for this hypothesis. Differential expression of CD24, OSR1, WT1, and LIM1 suggests that BMP-7 may promote more specific effects on this subset of genes.
In order to consider hESC for transplant purposes, graft purity will be necessary to ensure that contamination with undifferentiated cells does not occur and that only those cells directed towards a renal lineage are used for transplantation (Eiges et al., 2001; Halme and Kessler, 2006). Studies that focused on in vitro differentiation include those in mice where an enriched population of mouse ESC was obtained by sorting for brachyury. Selected cells were noted to integrate into the nephrogenic zone in metanephric kidney organ culture and in the proximal tubules in newborn in vivo transplant experiments (Vigneau et al., 2007). Mouse EBs have been shown to express markers of the mature kidney such as podocin, nephrin, and Wt1 in later stages of cell culture (Kramer et al., 2006). The addition of hepatocyte growth factor and Activin-A to mouse ESC transfected with Wnt4 cDNA was also shown to increase expression of Wt1, Wnt4, and Aqp_2_ in the later stages of mouse EB differentiation (Kobayashi et al., 2005). Further, these cells were found to contribute to Aqp_2_-positive tubules after injection into the renal cortex of host mice (Kobayashi et al., 2005). Likewise, as noted above, the addition of RA, Activin-A, and BMP-7 to mouse ESC culture medium resulted in increased expression of early renal markers including Pax2 and Wt1, and enhanced efficiency of incorporation into tubule epithelia following injection into embryonic kidney cultures (Kim and Dressler, 2005).
In summary, these studies have focused on spontaneous and directed differentiation of hESC toward renal lineages using a combination of factors. Taken together, these data support the interpretation that monolayer RA7 culture conditions may contribute to increased, but not definitive, differentiation of hESC along the renal pathway. Future investigations of renal ontogeny in monkeys with other developmental markers may illuminate critical branch points of renal developmental pathways and guide enhancement of renal differentiation protocols for hESC. Conversely, further investigations utilizing hESC as a model for mammalian kidney development will enhance our understanding of kidney precursor specification, and will be necessary for the development of new cell-based therapies for the treatment of human disease.
ACKNOWLEDGMENTS
These studies were supported by the National Institutes of Health (NIH) Center of Excellence in Translational Human Stem Cell Research (NIH grant #HL069748), the California Institute for Regenerative Medicine (CIRM) (#RC1-00144), the UC Davis CIRM Stem Cell Training Program (#T-00006), and the Primate Center base operating grant (#RR00169).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al-Awqati Q, Oliver JA. The kidney papilla is a stem cell niche. Stem Cell Rev. 2006;2:181–184. doi: 10.1007/s12015-006-0046-3. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- Bodnar MS, Meneses JJ, Rodriguez RT, Firpo MT. Propagation and maintenance of undifferentiated human embryonic stem cells. Stem Cells Dev. 2004;13:243–253. doi: 10.1089/154732804323099172. [DOI] [PubMed] [Google Scholar]
- Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75:337–349. doi: 10.1111/j.1432-0436.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- Burrow CR. Regulatory molecules in kidney development. Pediatr Nephrol. 2000;14:240–253. doi: 10.1007/s004670050049. [DOI] [PubMed] [Google Scholar]
- Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF- superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Butt MJ, Tarantal AF, Matsell DG. Collecting duct epithelial-mesenchymal transition in fetal urinary duct obstruction. Kidney Int. 2007;72:936–944. doi: 10.1038/sj.ki.5002457. [DOI] [PubMed] [Google Scholar]
- Carev D, Saraga M, Sraga-Babic M. Expression of intermediate filaments, EGF and TGF- in early human kidney development. J Mol Hist. 2008;39:227–235. doi: 10.1007/s10735-007-9157-7. [DOI] [PubMed] [Google Scholar]
- Challen GA, Martinez G, Davis MJ, Taylor DF, Crowe M, Teasdale RD, Grimmond SM, Little MH. Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol. 2004;15:2344–2357. doi: 10.1097/01.ASN.0000136779.17837.8F. [DOI] [PubMed] [Google Scholar]
- DHHS, NIH, and NIDDK. Strategic Plan for Pediatric Urology. 2006 NIH Publication No. 06-5879. Available online at http://www2.niddk.nih.gov/NR/rdonlyres/0FBD6429-3793-4F96-8CB1-628CEB9D3DE7/0/Pediatric_Urology_Feb_2006_Document.pdf.
- Dressler GR. Pax-2, kidney development, and oncogenesis. Med Pediatr Oncol. 1996;27:440–444. doi: 10.1002/(SICI)1096-911X(199611)27:5<440::AID-MPO9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Gonlusen G, Ergin M, Paydas S, Tunali N. The expression of cytoskeletal proteins (α-SMA, vimentin, desmin) in kidney tissue: A comparison of fetal, normal kidneys, and glomerulonephritis. Int Urol Nephrol. 2001;33:299–305. doi: 10.1023/a:1015226426000. [DOI] [PubMed] [Google Scholar]
- Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, Daegelen D, Concordet JP, Maire P. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol. 2004;24:6253–6267. doi: 10.1128/MCB.24.14.6253-6267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;35:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- Holthöfer H, Miettinen A, Lehto VP, Lehtonen E, Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult kidneys. Lab Invest. 1984;50:552–559. [PubMed] [Google Scholar]
- Jimenez DF, Lee CI, O’Shea CE, Kohn DB, Tarantal AF. HIV-1-derived lentiviral vectors and fetal route of administration on transgene biodistribution and expression in rhesus monkeys. Gene Ther. 2005;12:821–830. doi: 10.1038/sj.gt.3302464. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Wada J, Lin S, Danesh FR, Chugh SS, Yang Q, Banerjee T, Lomasney JW. Update of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Renal Physiol. 2004;286:F202–F215. doi: 10.1152/ajprenal.00157.2003. [DOI] [PubMed] [Google Scholar]
- Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3547–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- Kramer J, Steinhoff J, Klinger M, Fricke L, Rohwedel J. Cells differentiated from mouse embryonic stem cells via embryoid bodies express renal marker molecules. Differentiation. 2006;74:91–104. doi: 10.1111/j.1432-0436.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tanaka H, Kuwana H, Inoshita S, Teraoka H, Sasaki S, Terada Y. Wnt-4 transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Comm. 2005;336:585–595. doi: 10.1016/j.bbrc.2005.08.136. [DOI] [PubMed] [Google Scholar]
- Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- Lee CI, Goldstein O, Han VKM, Tarantal AF. Insulin-like growth factor (IGF-II) and IGF binding protein (IGFBP-1, IGFBP-3) gene expression in fetal monkey tissues during the second and third trimesters. Pediatr Res. 2001;49:379–387. doi: 10.1203/00006450-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Lelongt B, Ronco P. Role of extracellular matrix in kidney development and repair. Pediatr Nephrol. 2003;18:731–742. doi: 10.1007/s00467-003-1153-x. [DOI] [PubMed] [Google Scholar]
- Little MH. Regrow or repair: Potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390–2401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Décimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development. Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enable ureteric bud outgrowth and GDNF/WNT11 feedback signaling during kidney branching morphogenesis. Development. 2007;134:2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- Moll R, Hag C, Thoenes W. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult kidneys. Lab Invest. 1991;50:552–559. [PubMed] [Google Scholar]
- Moreau E, Vilar J, Lelièvre-Pégorier M, Merlet-Bénichou C, Gilbert T. Regulation of c-ret expression by retinoic acid in rat metanephros: Implication in nephron mass control. Am J Physiol. 1998;275:F938–F945. doi: 10.1152/ajprenal.1998.275.6.F938. [DOI] [PubMed] [Google Scholar]
- Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Toi M, Fukui T, Kuroda N, Hiroi M, Kurashige T, Enzan H. An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int. 2000;27:1836–1846. doi: 10.1046/j.1523-1755.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- NIH, NIDDK, and ASPN. Research Needs in Pediatric Kidney Disease: 2000 and Beyond. Pediatric Nephrology Task Force Publication. 2000 Available online at http://www2.niddk.nih.gov/NR/rdonlyres/DC7B952F-9CDD-44DDB0ABBFE79A774AE5/0/Pediatric_Kidney_Disease_2000_and_Beyond.pdf.
- Nishinakamura R. Stem cells in the embryonic kidney. Kidney Int. 2008;73:913–917. doi: 10.1038/sj.ki.5002784. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Osafune K. Essential roles of Sall family genes in kidney development. J Physiol Sci. 2006;56:131–136. doi: 10.2170/physiolsci.M95. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxburgh L, Dudley AT, Godin RE, Koonce CH, Islam A, Anderson DC, Bikoff EK, Robertson EJ. BMP4 substitutes for loss of BMP7 during kidney development. Dev Biol. 2005;286:637–646. doi: 10.1016/j.ydbio.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Pirruccello SJ, LeBien TW. The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. Immunol. 1986;136:3779–3784. [PubMed] [Google Scholar]
- Rodriguez CG, Makori N, Cukierski MA, Hendrickx AG. Development of the definitive kidney in the cynomolgus monkey (Macaca fascicularis) J Med Primatol. 1996;25:122–132. doi: 10.1111/j.1600-0684.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Rothenpieler U, Dressler GR. Pax2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2001;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the kidney. In: Barlow PW, Green PB, White CC, editors. Developmental and Cell Biology Series 19. Cambridge, UK: Cambridge Univ. Press; 1987. pp. 1–171. [Google Scholar]
- Tarantal AF. Ultrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fascicularis) macaques: Reproductive and research applications. The Laboratory Primate, Elsevier Academic Press; 2005. Chapter 20; pp. 317–351. [Google Scholar]
- Tarantal AF, Gargosky SE. Characterization of the insulin-like growth factor (IGF) axis in the serum of maternal and fetal macaques (Macaca mulatta and Macaca fascicularis) Growth Regul. 1995;5:190–198. [PubMed] [Google Scholar]
- Tarantal AF, Hendrickx AG. Prenatal growth in the cynomolgus and rhesus macaque (Macaca fascicularis and Macaca mulatta) - A comparison by ultrasonography. Am J Primatol. 1988;15:309–323. doi: 10.1002/ajp.1350150405. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Han VKM, Cochrum KC, Mok A, daSilva M, Matsell DG. Fetal rhesus monkey model of obstructive renal dysplasia. Kidney Int. 2001;59:446–456. doi: 10.1046/j.1523-1755.2001.059002446.x. [DOI] [PubMed] [Google Scholar]
- Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, Fehling HJ, Keller G, Burrow C, Wilson P. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18:1709–1720. doi: 10.1681/ASN.2006101078. [DOI] [PubMed] [Google Scholar]
- Winyard PJD, Risdon RA, Sams VR, Dressler GR, Woolf AS. The PAX2 transcription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J Clin Invest. 1996;98:451–459. doi: 10.1172/JCI118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362:550–553. doi: 10.1016/j.bbrc.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Zile MH. Function of vitamin A in vertebrate embryonic development. J Nutr. 2001;131:705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]