Cells with regulatory function of the innate and adaptive immune system in primary Sjögren's syndrome (original) (raw)
Abstract
The aim of the present study was to describe subsets of cells with regulatory properties in primary Sjögren's syndrome (pSS), and to correlate these cell populations with clinical symptoms. Among the 32 investigated patients, 23 had extraglandular manifestations (EGMs), while nine had only glandular symptoms. Twenty healthy individuals served as controls. The percentages of natural killer (NK), natural killer T cells (NK T), interleukin (IL)-10 producing T regulatory type 1 (Tr1) cells and CD4+CD25+ regulatory T cells (Treg) cells were determined by flow cytometry and serum cytokine levels of IL-4, IL-6, IL-10, tumour necrosis factor (TNF)-α and interferon (IFN)-γ were evaluated by enzyme-linked immunosorbent assay (ELISA). Functional tests were carried out to assess the suppressor properties of Treg cells in patients and controls. Peripheral NK, NK T and Tr1 cell percentages were elevated in pSS, while CD4+CD25+ Treg cells showed reduced frequencies in patients compared to controls. In pSS, elevated percentages of NK T, Tr1 and CD4+CD25+ Treg cells were observed in patients with EGMs, when compared to patients with sicca symptoms only. CD4+CD25+ Treg cell percentages showed a negative correlation with sialometry values. The in vitro functional assay demonstrated lower suppression activity of CD4+CD25+ Treg cells in patients compared to controls. Serum IL-6 and TNF-α levels were elevated, while IL-10 was decreased in patients compared to controls. Negative correlation was found between IL-10 levels and the percentages of Tr1 cells. Changes in the investigated subsets of regulatory cells in pSS may contribute to the development and progression of the disease.
Keywords: innate and adaptive immune system, regulatory cells, Sjögren's syndrome
Introduction
A complex system of immune-competent cells with regulatory function plays a key controlling role in avoiding the clonal expansion of autoreactive cells, and therefore maintains the inhibition of tissue and organ damage. The protection conferred by self-tolerance is crucial at all levels of immune response and, accordingly, cells with regulatory activity have been described both in the innate and adaptive immune systems [1–3].
In the innate immune system, natural killer (NK) NK1 and NK2 subsets have been shown to display regulatory roles in immune responses, similar to T helper type 1 (Th1) and Th2 cells [4].
NK T cells, which also have regulatory function, develop from thymocyte progenitor cells and express T cell receptors (TCR); however, the NK T TCR recognize glycolipids rather than protein antigens [5]. The potential role of NK T cells in autoimmunity was underlined by studies demonstrating decreased numbers of NK T cells in several autoimmune diseases, including systemic lupus erythematosus (SLE), insulin-dependent type I diabetes (IDDM) and rheumatoid arthritis (RA) [6–8]. The adoptive transfer of NK T cells improved disease severity in numerous immune-mediated animal models [9,10], thereby underscoring the regulatory potential of NK T cells.
As well as the innate immune system, the regulatory T cell (Treg) system/network is responsible for the maintenance of self-tolerance on the level of adaptive immunity. Among others, the two major representatives of this system are denoted as induced Treg cells (iTreg) and natural Treg cells [11].
iTreg CD4+ T cells gain their suppressor function following activation. In the regulation of peripheral T cell immune responses, several types of iTreg cells participate, where the most widely investigated subsets are the interleukin (IL)-10-producing T regulatory type 1 (Tr1) cells and transforming growth factor (TGF)-β-producing Th3 cells [12,13].
Natural Treg cells are produced in the thymus and exhibit an unresponsive phenotype in vitro, failing to proliferate and produce IL-2 after either polyclonal, or antigen-specific, stimulation. Natural Tregs express increased levels of the IL-2 receptor alpha chain (CD25) compared to activated T cells [14]. For the identification of CD4+CD25+ Treg cells, assessment of forkhead box P3 (FoxP3) expression is the most specific marker. Activation of the FoxP3 transcription factor is characterized by transcriptional events leading to inhibition of IL-2 production, which is crucial for the adequate activation of T cells [15].
Quantitative and functional modifications of regulatory cells have also been described in human autoimmune diseases. In patients with SLE, decreased percentages of natural CD4+CD25+ Treg cells and increased proportions of IL-10-producing Tr1 cells have been found in the peripheral blood, compared to healthy individuals [16–18]. Recent findings reveal a decrease in absolute numbers of the natural CD4+CD25+ Treg cells and an increase in IL-10-producing Tr1 cells in patients with mixed connective tissue disease (MCTD) compared to healthy controls [19]. Importantly, these observed changes in Treg populations were proportional with disease activity [19,20], indicating a functional role in disease progression. In patients with autoimmune myasthenia gravis, CD4+CD25+ T cells in the thymus were found to produce decreased levels of FoxP3, indicating damaged cell function [21,22]. Additionally, in the peripheral blood of these patients, fewer CD4+CD25+ Treg cells were found compared to controls, reflecting a complex disorder of the regulatory machinery in this disease [23].
Primary Sjögren's syndrome (pSS) is a common systemic autoimmune disease that affects primarily the exocrine glands, leading to decreased lachrymal and salivary secretion. Besides the characteristic glandular symptoms, other systemic symptoms, denoted as extraglandular manifestations (EGM), can also be found in a subset of patients [24]. In pSS, similar to the above-mentioned autoimmune diseases, changes in the suppressor function of the regulatory T cells may play an important role in pathogenesis. In pSS salivary gland biopsies, the number of CD4+CD25+ Treg cells and expression of FoxP3 protein were found to be reduced significantly compared to healthy individuals. In the peripheral blood of patients with pSS, the number of Treg cells and FoxP3 expression were decreased similarly [25]. Other studies have reported lower proportions of CD4+CD25+ Treg cells in the peripheral blood and also in salivary glands [26,27]. Contrary to these studies, Gottenberg et al. reported increased regulatory T cell numbers in the peripheral blood of patients with pSS [28]. A recent study found that FoxP3+ Treg cell frequency in minor salivary gland biopsy specimens varied proportionally with the extent of the intraglandular lesions; in the peripheral blood, a reverse distribution of these cells was also observed [29]. FoxP3+ Treg frequency in the salivary gland lesions of pSS patients showed a correlation with the grade of inflammation and certain risk factors for lymphoma development [29]. In addition to controversial findings regarding modifications of the CD4+CD25+ Treg cells, the role of Tr1 cells, as well as regulatory cells of the innate immune system in pSS, has not been assessed previously. Changes of peripheral regulatory immune parameters have not been evaluated until now in association with the presence of EGMs, which is the most relevant characteristic feature of systemic activation and disease flare in pSS. Although previous studies have pointed out the involvement of different regulatory cell compartments in the pathogenesis of pSS, further studies were warranted.
The aim of this study was to evaluate peripheral cell types with regulatory properties, reflecting overall immune-regulatory disturbances, characteristic to patients with pSS. We also assessed EGMs (generalized, systemic features) within these patients, and calculated the relation between the evaluated cell proportions and the clinical symptoms of the disease. We compared the functional suppressor-capability of Tregs in controls and in pSS patients with or without EGMs. Because we believe that pSS, in line with other systemic autoimmune diseases, is characterized by a general, systemic immune-regulatory defect, in many instances also accompanied by EGMs, in this study we describe the circulating components of the regulatory system in these patients.
Materials and methods
Patients
Thirty-two newly diagnosed patients with pSS (one male, 31 female; mean age 49·5 ± 7·2 years) were enrolled in the present study, recruited from the Autoimmune Outpatient Clinic of the Division of Clinical Immunology, 3rd Department of Medicine, Medical and Health Science Center, University of Debrecen. The diagnosis of pSS was established according to the European–American consensus criteria [30]. In all cases serum and blood samples were taken prior to the introduction of any medical treatment. Serum samples were stored at −70°C until further analysis, while cell samples were processed immediately. Among patients with pSS, 23 had EGMs, while nine patients had only sicca symptoms. The distribution of EGMs of pSS patients was as follows: thyroiditis n = 1; pulmonary involvement n = 2; myositis/myalgia n = 3; polyneuropathy n = 5; vasculitis n = 15; Raynaud's phenomenon n = 14; and polyarthritis n = 18. A cohort of age- and sex-matched healthy individuals taking no immunosuppressive or immune-modulating medications served as controls (n = 20). No patients or controls enrolled in this study had ongoing infections, either viral or bacterial. The study has been approved by the ethics committee of the University of Debrecen.
Analysis of NK and NK T cells
In order to determine NK and NK T cells from heparinized blood samples, the following monoclonal antibodies to cell surface markers were used: CD3 and CD56 (BD Biosciences, San Jose, CA, USA; and Immunotech, Marseille, France). Samples were processed according to the Coulter Q-PREP protocol and system (Beckman Coulter Inc., Miami, FL, USA). Briefly, cells from 100 µl of whole blood were stained with 10 µl of monoclonal antibody. After 30 min incubation, red blood cells were haemolysed and leucocytes were washed in phosphate-buffered saline (PBS) supplemented with bovine serum albumin (BSA) (10 mg/l) and sodium-azide (2 mg/l). The cells were fixed subsequently using 800 µl of 1% paraformaldehyde. Mouse immunoglobulin (Ig)G1 antibodies were used as isotype control throughout the experiments. Measurements were performed and events were collected on a Coulter EPICS XL-4 flow cytometer (Beckman Coulter Inc.). Lymphocytes, monocytes and granulocytes were separated based upon their size and granulation pattern on forward- and side-scatter scattergrams. Lymphocyte subpopulations were quantified as their percentage in the entire population.
Determination of CD4+ Tr1 cells
For the evaluation of intracellular cytokines of CD4+ T cells, 1 ml whole heparinized blood was diluted 1:2 in RPMI-1640 supplemented with 80 mg/l gentamycin and 2 nM glutamine. Cells were stimulated using 25 ng/ml phorbol–myristate–acetate (Sigma Aldrich, St Louis, MO, USA) and 1 ng/ml ionomycin (Sigma Aldrich) for 4 h at 37°C in an atmosphere containing 5% CO2. The transport of de novo synthesized cytokines from the Golgi apparatus was inhibited by 10 µg/ml brefeldin-A (Sigma Aldrich). Unstimulated cells served as controls. Following stimulation, cells were stained for CD4 for 30 min at room temperature. Cell staining was followed by red blood cell lysis using fluorescence activated cell sorter (FACS) lysing solution (Becton Dickinson, San Jose, CA, USA) for 10 min at room temperature in the dark. Leucocytes were then centrifuged (500 g for 10 min) and cell membranes were permeabilized using FACS permeabilizing solution (BD Biosciences, San Diego, CA, USA) for 10 min at room temperature. Samples were washed in PBS and were incubated further for 30 min in the dark with the specific monoclonal antibody: phycoerythrin (PE)-conjugated anti-human-IL-10 (Caltag Laboratories, Burlingame, CA, USA) for 30 min at room temperature in the dark. Subsequently, cells were fixed with 1% of paraformaldehyde. The samples were evaluated by a Coulter EPICS XL-4 flow cytometer (Beckman Coulter Inc.). Lymphocytes, granulocytes and monocytes were gated and separated based on their morphological properties.
Analysis of CD4+CD25+bright Treg cells
Cell surface (CD4, CD25) staining and intracellular (FoxP3) staining was carried out on freshly isolated peripheral blood mononuclear cells (PBMCs) from heparinized blood by an intracellular staining kit (eBioscience, San Diego, CA, USA), according to the manufacturer's instructions. Briefly, 100 µl cell suspension was stained with 10–10 µl cell surface anti-CD4, anti-CD25 molecules. Cells were incubated in the dark at room temperature for 30 min. Prior to the intracellular staining, cells were washed in cold PBS, permeabilized by fixation/permeabilization buffer. To prevent the non-specific binding of FoxP3, cells were blocked by rat serum at 4°C for 15 min. After this step, 20 µl anti-FoxP3 antibody was used for intracellular staining. Finally, cells were washed in PBS and suspended in flow cytometry staining buffer. Lymphocytes were gated on the basis of their forward- and side-scatter properties using FACSCalibur flow cytometry (Becton Dickinson). Data were analysed using CellQuest software (Becton Dickinson). The CD4+CD25high suppressor T cells were also positive for FoxP3. The mean fluorescence intensity (MFI) of FoxP3 was significantly higher in CD4+CD25high suppressor T cells compared to the CD4+CD25low or CD4+CD25– cells (P < 0·01). The following reagents were used: Ficoll and CD4-fluorescein isothiocyanate (FITC) monoclonal antibody (Sigma Aldrich), CD25-PC5 (Immunotech), FoxP3-PE, clone: PCH101 (eBioscience) and intracellular staining kit (eBioscience).
Suppression functional assay of CD4+CD25+ Treg cells
PBMCs were isolated from heparinized whole blood by density gradient centrifugation over Ficoll/Hystopaque (Sigma Aldrich). CD4+CD25+ T cells were isolated from PBMCs using a regulatory T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, non-CD4+ T cells were depleted by indirect magnetic labelling with biotin–antibody cocktail and anti-biotin microbeads. The magnetic separation was carried out by using LD columns. CD25+ T cells were purified from the pre-enriched CD4+ T cell fraction by positive selection process. In these steps, CD4+ T cells were labelled directly with anti-CD25 microbeads and CD4+CD25+ T cells were eluted from the MS column.
Magnetically isolated 1 × 105 CD4+CD25+ and CD4+CD25– T cells were cultured in 200 µl of cRPMI-1640 in 96-well U-bottomed plates for 72 h in co-culture, both separately and together. CD4+CD25+ and CD4+CD25– cells were cultured in a 1:1 ratio in the mixed lymphocyte reaction. For polyclonal stimulation, cells were stimulated with anti-CD3/CD28 T cell expander microbeads (Dynal, Oslo, Norway) in one bead/cell concentration. Proliferation was investigated by using a terazolium-based assay (EZ4U Proliferation Kit; BioMedica Inc, San Diego, CA, USA). The substrate was added in the last 2·5 h to the culture and finally the optical density (OD) values were detected at 450 nm by enzyme-linked immunosorbent assay (ELISA) reader. OD values of mixed lymphocyte reaction were corrected by OD values of CD4+CD25+ T cells cultured alone. Suppression activity was determined as the ratio of OD values of the CD4+CD25– T cell cultures and mixed lymphocyte reactions.
Assessment of anti-SS-A and anti-SS-B
As part of the routine diagnostic evaluation, anti-SS-A and anti-SS-B autoantibodies were determined by the indirect immunofluorescence technique at the Clinical Immunology Diagnostic Laboratory of the 3rd Department of Medicine, Medical and Health Science Center, University of Debrecen.
Determination of serum soluble cytokines
Serum IL-4, IL-6, IL-10, tumour necrosis factor (TNF) and interferon (IFN)-γ were measured by BD OptEIA ELISA kits (BD Biosciences), according to the manufacturer's instructions.
Statistical analyses
The Kolgomorov–Smirnov test was used to analyse the data distribution. In cases of normal distribution, we determined mean ± standard deviation (s.d.) values and used a two-sample _t_-test for statistical evaluation of the experimental data. In cases of distributions different from normal, median, minimum and maximum values were calculated, and the Mann–Whitney _U_-test was used. When the strength of the linear relationship between two variables was evaluated Pearson's correlation coefficient was used, while in cases of non-normal distribution, Spearman's correlation coefficient was applied. Differences were considered statistically significant at P < 0·05.
Results
Quantification of peripheral NK cells in pSS patients and in healthy individuals
Peripheral NK cell percentages were increased significantly in pSS patients when compared to controls (15·31 ± 9·81% versus 10·87 ± 4·74%, respectively, P = 0·034) (Fig. 1a). We found similar NK cell percentages in the subset of pSS patients with EGMs compared to pSS patients without EGMs. Although both subgroups of patients have elevated NK cell percentages compared to controls, the difference did not a reach a statistically significant level (Fig. 1b).
Fig. 1.
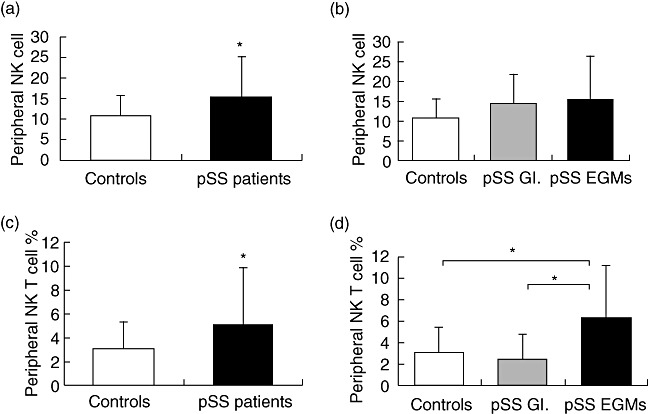
Assessment of cell percentages with regulatory function in the innate immune system. Bars show the mean and standard deviation (s.d.). Statistically significant differences are indicated by (*). (a) Percentages of natural killer (NK) cell in patients with primary Sjögren's syndrome (pSS) (n = 32) compared to healthy blood donors (n = 20) (P = 0·034). (b) Percentages of peripheral NK cells of patients with extraglandular manifestations (EGMs) (n = 23), patients without EGMs (n = 9) and controls (n = 20). (c) Percentages of NK T cells in patients with pSS (n = 32) compared to healthy individuals (n = 20) (P = 0·033). (d) Percentages of peripheral NK T cells of patients with EGMs (n = 23), patients without EGMs (n = 9) and controls (n = 20).
Assessment of peripheral NK T cells in pSS patients and in controls
In the overall pSS patient population, we found significantly elevated NK T cell percentages compared to controls (5·18 ± 4·60% versus 3·07 ± 2·28%, respectively, P = 0·033) (Fig. 1c). An increased number of NK T cells in the subset of pSS with EGMs, compared to pSS patients without EGMs (6·26 ± 4·85% versus 2·41 ± 2·30%, respectively, P = 0·005) was determined, as well as pSS with EGMs compared to healthy individuals (pSS with EGMs versus control: 6·26 ± 4·85% versus 3·07 ± 2·28%, respectively, P = 0·005) (Fig. 2b). NK T cell percentages in patients without EGMs and controls were similar (Fig. 1d).
Fig. 2.
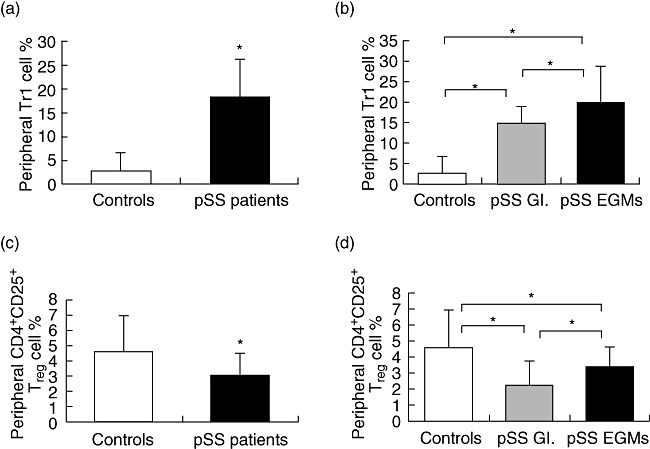
Assessment of cell percentages with regulatory function in the adaptive immune system. Bars show the mean and standard deviation (s.d.). Statistically significant differences are indicated by (*). (a) Percentages of T regulatory type 1 (Tr1) cells in patients with primary Sjögren's syndrome (pSS) (n = 32) compared to healthy controls (n = 20) (P < 0·001). (b) Percentages of peripheral Tr1 cell of patients with extraglandular manifestations (EGMs) (n = 23), patients without EGMs (n = 9) and controls (n = 20). (c) Percentages of CD4+CD25+ regulatory T cells (Tregs) in patients with pSS (n = 32) compared to healthy blood donors (n = 20). (d) Percentages of peripheral CD4+CD25+ Tregs of patients with EGMs (n = 23), patients without EGMs (n = 9) and controls (n = 20).
Quantification of peripheral Tr1 cells in pSS patients and in healthy individuals
Peripheral Tr1 cell percentages were increased strongly in pSS patients compared to those found in healthy individuals (18·51 ± 7·83% versus 2·73 ± 4·06%, respectively, P < 0·001) (Fig. 2a). We found Tr1 cell percentages in patients with EGMs significantly higher than in patients without EGMs (20·02 ± 8·57% versus 14·81 ± 3·96%, respectively, P = 0·028). Both subgroups of patients have significantly elevated Tr1 cell percentages compared to controls (pSS without EGMs versus control: 14·81 ± 3·96% versus 2·73 ± 4·06%, respectively, P < 0·001; pSS with EGMs versus control: 20·02 ± 8·57% versus 2·73 ± 4·06%, respectively, P < 0·001) (Fig. 2b).
Evaluation of peripheral CD4+CD25+ Treg cells in pSS patients and in controls
Peripheral CD4+CD25+ Treg cells were decreased in pSS patients when compared to healthy individuals (3·07 ± 1·41% versus 4·59 ± 2·34%, respectively, P = 0·005) (Fig. 2c). Percentages of these cells were increased significantly in the pSS with EGMs group compared to patients without EGMs (3·39 ± 1·26% versus 2·25 ± 1·51%, respectively, P = 0·038). In both subgroups of patients, the percentages of CD4+CD25+ Treg cells were significantly lower than in controls (pSS without EGMs versus control: 2·25 ± 1·51% versus 4·59 ± 2·34%, respectively, P < 0·003; pSS with EGMs versus control: 3·39 ± 1·26% versus 4·59 ± 2·34%, respectively, P < 0·038) (Fig. 2d).
Functional in vitro suppression assay of CD4+CD25+ Treg cells of pSS patients with or without EGMs compared to controls
Seven pSS patients with EGMs, seven patients without EGMs and seven controls were selected randomly for the functional in vitro suppression assay. We found that the suppression capability of CD4+CD25+ Treg cells was lower in the overall pSS patient population compared to healthy individuals (1·526 ± 0·66 versus 2·395 ± 0·87, P = 0·019). There was no significant difference in suppression capability when pSS patients with or without EGMs were compared.
Circulating cytokines in pSS patients and in healthy controls
Of serum soluble cytokines, TNF-α was elevated significantly in pSS patients compared to controls [median: 0·8 (0·1–16·5) pg/ml versus median: 0·4 (0·1–1·2) pg/ml, respectively, P = 0·001]. There was no significant difference in the serum levels of IFN-γ between pSS patients and healthy individuals [median: 14·6 (0–272·88) pg/ml versus median: 10·47 (0·1–57·6) pg/ml, respectively, P = 0·469]. IL-4 was found to be present in similar titres between the two groups [median: 1·44 (0–38·06) pg/ml versus median: 0·95 (0·1–47·38) pg/ml respectively, P = 0·685]. Serum IL-6 levels were shown to be elevated significantly in patients compared to healthy individuals [median: 0·8 (0·8–32·5) pg/ml versus median: 0·8 (0·8–2·2) pg/ml, respectively, P < 0·001]. We found a significant decrease in serum IL-10 levels in pSS patients when compared to healthy individuals [median: 0·79 (0–72·86) pg/ml versus median: 5·14 (0·1–31·91) pg/ml, respectively, P = 0·017]. No significant difference was found in concentrations of the investigated cytokines when compared in pSS patients with or without EGMs.
Correlation between regulatory cell populations and soluble cytokines
A negative correlation was found between serum IL-10 levels and the numbers of Tr1 cells (r = −0·369, P = 0·019); moreover, a positive correlation was detected between circulating IL-4 and IFN-γ (r = 0·444, P = 0·004).
Association of sicca syndrome and autoantibodies with peripheral immune parameters
A significant negative correlation was observed between the percentage of peripheral CD4+CD25+ Treg cells and sialometry values (r = –0·538, P = 0·003).
Among pSS patients, we found 28 SS-A-positive and 9 SS-B-positive individuals. No association between the presence of autoantibodies and percentages of any regulatory cell types was detected.
Discussion
Our findings indicated that several immune-competent cell types with regulatory capability were represented with disproportional levels in pSS compared to healthy individuals. According to our hypothesis, the elevation of NK and NK T cell levels in pSS could be part of an increased counter-regulatory reaction, presumably compensating the derailed, disproportional immune responses. The observation that NK T levels in patients with EGMs were increased significantly compared to pSS without EGMs also supports our hypothesis that in pSS, in parallel with the severity of the disease, these regulatory cells, especially NK T cells, counter-regulate further the proinflammatory processes.
Additionally, significant elevation of Tr1 cells in pSS patients compared to controls was determined, underlining further a possible compensatory mechanism in pSS.
In pSS exhibiting a systemic, more pronounced course of disease (pSS with EGMs), an additional increase of Tr1 cell levels was shown. Taken together, these observations are in line with the idea that the ongoing proinflammatory autoimmune machinery in pSS could lead to a counter-regulatory reaction, and IL-10-producing cells appear more frequently in these clinical entities.
In our study, decreased percentages of CD4+CD25+ Treg cells in patients with pSS were found, assuming that the reduced numbers of this key regulatory cell type is insufficient to dampen the autoimmune processes in pSS. Interestingly, significant elevated levels of CD4+CD25+ Treg cells in patients with EGMs were determined when compared to patients without EGMs. Nevertheless, these values were still significantly below control values and our results suggest that, in pSS, quantitative changes of CD4+CD25+ Treg cells have an important role in the failure of immunoregulation, which leads finally to the perpetuation of the disease. In addition to quantitative changes, qualitative disorders can also occur; moreover, the function of CD4+CD25+ Treg cells is highly dependent upon the local milieu. It has been shown that inflammatory cytokines such as TNF-α and IL-6 can temporarily impair the peripheral generation and function of Tregs[31–33], and render auto-aggressive T cells resistant to Treg-mediated regulation [34]. Interestingly, we found significantly elevated levels of both TNF-α and IL-6 in serum of pSS compared to healthy blood donors, raising the possibility that the increased levels of these cytokines impair the counter-regulatory function of Tregs. As the next step, we carried out an in vitro assay of CD4+CD25+ Treg cells in order to reveal a possible functional defect in the suppression capability of these cells in pSS. We established a decreased suppression effect of Tregs in pSS, which indicates that not only are the decreased peripheral CD4+CD25+ Treg levels responsible for their insufficient operation, but for their altered function as well.
A significant decrease in serum IL-10 levels was measured in pSS patients when compared to healthy individuals. The serum cytokine IL-10, which is secreted by several regulatory cells such as NK and Tr1 cells, plays a crucial role in controlling immune responses [4,12]. Interestingly, our results indicate that although as a counter-regulatory process the number of IL-10-producing Tr1 cells is increasing in patients with pSS, serum IL-10 levels are not elevated compared to healthy subjects. The significant negative correlation between the levels of Tr1 cells and of its product assume an inappropriate anti-inflammatory cytokine secreting function of Tr1 cells.
Our study revealed an association between changed peripheral immune parameters and the impairment of an important feature of exocrine secretory capability in pSS (sialometry). Decreased secretory capacity is associated with disease severity, and the deterioration of exocrine secretory function is a cornerstone of pSS pathogenesis. We found a significant negative correlation between the level of peripheral CD4+CD25+ Treg cells and sialometry values. Along with the loss of secretory function, the intensification of a counterbalance-mechanism appears; thus, levels of CD4+CD25+ Treg cells increase as a feedback process attempting to compensate the progression of disproportional immune responses. We assume that the aforementioned qualitative and quantitative changes could be caused at least partly by elevated IL-6 and TNF-α levels in patients with pSS.
In conclusion, we believe that the characterization of the complex interplay of immune-competent cells with regulatory properties may have an important role in future diagnostics, and the modulation of these cells could be a potentially powerful element of the novel therapeutic selection in pSS.
Disclosure
None of the authors has potential conflicts of interest, including relevant financial interests in any company or institution that might benefit from the publication.
References
- 1.Sprent J, Kishimoto H. The thymus and central tolerance. Phil Trans R Soc Lond B Biol Sci. 2001;356:609–16. doi: 10.1098/rstb.2001.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodnow CC, Cyster JG, Hartley SB, et al. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 3.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–71. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deniz G, Erten G, Kucuksezer UC, et al. Regulatory NK cells suppress antigen-specific T cell responses. J Immunol. 2008;180:850–7. doi: 10.4049/jimmunol.180.2.850. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 6.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IGG production. Arthritis Rheum. 2007;56:303–10. doi: 10.1002/art.22326. [DOI] [PubMed] [Google Scholar]
- 7.Novak J, Griseri T, Beaudoin L, Lehuen A. Regulation of type 1 diabetes by NKT cells. Int Rev Immunol. 2007;26:49–72. doi: 10.1080/08830180601070229. [DOI] [PubMed] [Google Scholar]
- 8.Yanagihara Y, Shiozawa K, Takai M, Kyogoku M, Shiozawa S. Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;118:131–6. doi: 10.1046/j.1365-2249.1999.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahng AW, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trop S, Samsonov D, Gotsman I, Alper R, Diment J, Ilan Y. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 1999;29:746–55. doi: 10.1002/hep.510290334. [DOI] [PubMed] [Google Scholar]
- 11.Fehérvári Z, Sakaguchi S. A paragon of self-tolerance. CD25+ CD4+ regulatory T cells and the control of immune responses. Arthritis Res Ther. 2004;6:19–25. doi: 10.1186/ar1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 14.Baecher-Allan C, Brown J, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 15.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FoxP3 in the development and function of human CD4+CD25+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 16.Barath S, Aleksza M, Tarr T, Sipka S, Szegedi G, Kiss E. Measurement of natural (CD4+CD25high) and inducible (CD4+IL-10+) regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2007;16:489–96. doi: 10.1177/0961203307080226. [DOI] [PubMed] [Google Scholar]
- 17.Crispin JC, Martinez A, Alococer-Varela J. Quantification of regulatory T cells in patient with systemic lupus erythematosus. J Autoimmun. 2003;21:273–6. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 18.Liu MF, Wang CR, Fung LL, WU CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 19.Baráth S, Sipka S, Aleksza M, et al. Regulatory T cells in peripheral blood of patients with mixed connective tissue disease. Scand J Rheumatol. 2006;35:300–4. doi: 10.1080/03009740600709790. [DOI] [PubMed] [Google Scholar]
- 20.Hassan BA, Gunnarsson I, Karlsson G, Klareskog L, Forslid J, Lundberg IE. Longitudinal study of interleukin-10, tumor necrosis factor-alpha, anti-U1-snRNP antibody levels and disease activity in patients with mixed connective tissue disease. Scand J Rheumatol. 2001;30:282–9. doi: 10.1080/030097401753180363. [DOI] [PubMed] [Google Scholar]
- 21.Balandina A, Lécart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–41. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177:5296–306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Xiao BG, Xi JY, Lu CZ, Lu JH. Decrease of CD4(+)CD25(high)Foxp3(+) regulatory T cells and elevation of CD19(+)BAFF-R(+) B cells and soluble ICAM-1 in myasthenia gravis. Clin Immunol. 2008;126:180–8. doi: 10.1016/j.clim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson R, Moen K, Vestrheim D, Szodoray P. Current issues in Sjögren's syndrome. Oral Dis. 2002;8:130–40. doi: 10.1034/j.1601-0825.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Li X, Qian L, et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjögren's syndrome. J Rheumatol. 2007;34:2438–45. [PubMed] [Google Scholar]
- 26.Li XM, Li XP, Qian L, et al. Expression of CD4+ CD25+ regulatory T cells in peripheral blood and salivary gland of patients with primary Sjögren's syndrome. Zhonghua Yi Xue Za Zhi. 2007;87:1034–6. [PubMed] [Google Scholar]
- 27.Liu MF, Lin LH, Weng CT, Weng MY. Decreased CD4+CD25+bright T cells in peripheral blood of patients with primary Sjogren's syndrome. Lupus. 2008;17:34–9. doi: 10.1177/0961203307085248. [DOI] [PubMed] [Google Scholar]
- 28.Gottenberg JE, Lavie F, Abbed K, et al. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjögren's syndrome. J Autoimmun. 2005;24:235–42. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM, Moutsopoulos HM. Foxp3+ T-regulatory cells in Sjogren's syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am J Pathol. 2008;173:1389–96. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitali C, Bombardieri S, Jonsson R, et al. European Study Group on Classification Criteria for Sjögren's syndrome, classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 32.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 33.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]