CA-074Me Protection against Anthrax Lethal Toxin (original) (raw)
Abstract
Anthrax lethal toxin (LT) activates the NLRP1b (NALP1b) inflammasome and caspase-1 in macrophages from certain inbred mouse strains, but the mechanism by which this occurs is poorly understood. We report here that similar to several NLRP3 (NALP3, cryopyrin)-activating stimuli, LT activation of the NLRP1b inflammasome involves lysosomal membrane permeabilization (LMP) and subsequent cytoplasmic cathepsin B activity. CA-074Me, a potent cathepsin B inhibitor, protects LT-sensitive macrophages from cell death and prevents the activation of caspase-1. RNA interference knockdown of cathepsin B expression, however, cannot prevent LT-mediated cell death, suggesting that CA-074Me may also act on other cellular proteases released during LMP. CA-074Me appears to function downstream of LT translocation to the cytosol (as assessed by mitogen-activated protein kinase kinase cleavage), K+ effluxes, and proteasome activity. The initial increase in cytoplasmic activity of cathepsin B occurs at the same time or shortly before caspase-1 activation but precedes a larger-scale lysosomal destabilization correlated closely with cytolysis. We present results suggesting that LMP may be involved in the activation of the NLRP1b inflammasome.
Bacillus anthracis, the organism that causes the disease anthrax, secretes three proteins that form two virulence factors: lethal toxin (LT) and edema toxin (ET). Anthrax LT is a combination of the protective antigen (PA) and lethal factor (LF) polypeptides. PA is responsible for the translocation of LF into the cytoplasm whereby LF, a metalloproteinase, cleaves mitogen-activated protein kinase kinases (MEKs) (11, 37, 52, 53). In macrophages from certain inbred strains of mice, LT treatment leads to a unique and rapid cell death (15, 45). Strain-specific macrophage death is attributed to the LT sensitivity locus Nlrp1b (Nalp1b) which encodes a member of the NOD-like receptor (NLR) family of proteins, known for their role in the assembly of the inflammasome (8). The inflammasome is a multiprotein complex involved in the activation of caspase-1, a cysteine protease responsible for cleaving the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 to their mature forms (30). The activation of caspase-1, likely through the formation of the NLRP1b inflammasome, is necessary for LT-mediated cell death in a manner independent of proinflammatory cytokine production and release (8, 12, 34, 59).
Although some of the cellular events downstream of MEK cleavage which lead to the activation of the NLRP1b inflammasome by LT are known, the mechanism by which LT leads to caspase-1 activation is not clear. MEK cleavage has not been linked to LT-mediated macrophage death (37). We do know that unlike other classic inflammasomes, degradation of some unidentified protein(s) by the proteasome is required for caspase-1 activation by the LT-induced NLRP1b inflammasome (12, 46, 59). Similar to other inflammasomes, however, a general K+ efflux signal has also been linked to LT-dependent caspase-1 activation (12, 59). Although macrophages undergo an osmotic lysis after caspase-1 activation, the mechanism by which caspase-1 induces cell death is unknown (12, 59).
The well-characterized NLRP3 inflammasome is also an activator of caspase-1 in response to an ever-growing number of stimuli, including bacterial pathogens, bacterial RNA, antiviral molecules, ATP, ionophoric bacterial toxins, low intracellular K+ concentrations, uric acid crystals, amyloid β, silica crystals, aluminum salts, and asbestos (for recent reviews, see references 14 and 62). In the past few years, evidence has arisen suggesting that one of the common mechanisms required for activation of the NLRP3 inflammasome by some of these diverse stimuli may be lysosome or phagosome destabilization and release of lysosomal proteases, including cathepsin B (16, 19, 21, 22, 43, 60). Recently, microglial phagocytosis of amyloid β (responsible for forming the amyloid plaques characteristic of Alzheimer's disease) was shown to cause cathepsin B-dependent activation of the NLRP3 inflammasome. Thus, pharmacological or genetic inhibition of cathepsin B prevented caspase-1 activity and IL-1β release from these cells (19). Similarly, silica crystals, aluminum salts, and microparticle vaccine adjuvants all lead to lysosomal damage and cathepsin B release, which activates the NLRP3 inflammasome and caspase-1 (22, 43). Nigericin, a K+ ionophore and potent activator of the NLRP3 inflammasome, induces cathepsin B release from lysosomes in THP-1 monocytes, and activity of the resulting cytoplasmic cathepsin B is required for caspase-1 activity and cell death (21). The antiviral molecule R837, which is known to activate the NLRP3 inflammasome and caspase-1, induces cell death through a cathepsin B-dependent pathway (16). In other systems, cathepsin B is required for caspase-1-independent death. For example, Shigella flexneri infection results in a NLRP3- and cathepsin B-dependent but caspase-1-independent cell death (60). Disease-associated NLRP3 mutations lead to downstream lysosomal leakage and lysosomal cathepsin B-dependent but caspase-1-independent cell death (16, 60). The wide range of stimuli now associated with increased cytoplasmic cathepsin B activity and subsequent activation of the inflammasome suggests that lysosomal damage might be the common mechanism through which many stimuli trigger the NLRP3 inflammasome.
In the present report we describe a protection of murine macrophages from LT lysis by a potent cathepsin B inhibitor, CA-074Me. Considering K+ efflux has been linked to LMP, cathepsin B release from lysosomes, and caspase-1 activation, we hypothesized that LT-induced ion fluxes may also result in the release of proteases from lysosomes and subsequent activation of caspase-1. We report that LT treatment does induce the release of cathepsin B into the cytoplasm. CA-074Me prevents LT-mediated caspase-1 activation in a manner independent of effects on LF translocation. Our results suggest that LMP and/or cytoplasmic proteases that are inhibited by CA-074Me may be a contributing factor in the activation of the NLRP1b inflammasome.
MATERIALS AND METHODS
Materials.
PA and LF were purified from B. anthracis by our laboratory as described previously (36, 38, 51). The concentrations of LT given for each experiment correspond to the concentration of each toxin component (i.e., 1 μg of LT/ml has 1 μg of PA/ml and 1 μg of LF/ml). l-3-_trans_-(Propylcarbamoyl)oxirane-2-carbonyl-l-isoleucyl-l-proline methyl ester (CA-074Me) and quinidine were from Sigma (St. Louis, MO). l-3-_trans_-(Propylcarbamoyl)oxirane-2-carbonyl-l-isoleucyl-l-proline (CA-074) was purchased from Biomol (Plymouth Meeting, PA). Boc-Asp (OBzl)-chloromethylketone (Boc-d-CMK) was obtained from AnaSpec (San Jose, CA). The anti-MEK1 NT antibody, z-Arg-Arg-aminomethylcoumarin (zRR-AMC), Ac-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK), ultrapure lipopolysaccharide (LPS), and lactacystin were purchased from Calbiochem (San Diego, CA). Anti-caspase-1 p10 (sc-514), anti-MEK2 NT (sc-524), and anti-MEK3 NT (sc-959) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Z-Val-Ala-Asp(OMe)-fluoromethylketone (Z-VAD-FMK), Z-Trp-Glu(OMe)-His-Asp(OMe)-fluoromethylketone (Z-WEHD-FMK), anti-IL-1β (AF-401-NA), and anti-cathepsin B antibodies (AF-965) were from R&D Systems (Minneapolis, MN). The anti-XIAP antibody was from BD Transduction (San Jose, CA). Anti-goat infrared dye (IRDye 800CW) secondary antibody (catalog no. 605-731-125) and anti-mouse infrared dye (IRDye 700DX) secondary antibody (catalog no. 610-130-121) were both from Rockland (Gilbertsville, PA). Anti-rabbit infrared dye (800CW) secondary antibody was from Licor Biosciences (Lincoln, NE). Active recombinant human caspase-1 and active recombinant murine caspase-1 were purchased from BioVision (Mountain View, CA). l-Leucine-l-leucine methyl ester (Leu-Leu-OMe) was purchased from Bachem (Torrance, CA).
Cell culture.
RAW 264.7 and L929 mouse fibroblast cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 5 mM HEPES, and gentamicin (50 μg/ml) (all obtained from Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Bone marrow-derived macrophages (BMDMs) were cultured from BALB/cJ mice (Jackson Laboratories, Bar Harbor, ME) in complete DMEM (as described above) with 30% L929 cell culture supernatant. BMDMs were grown for 7 to 9 days to allow time for differentiation before use in assays. Cells were heat shocked in a cell culture incubator between 41 and 45°C with 5% CO2. All experiments involving animals were performed under protocols approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Cytotoxicity assays.
Macrophages were grown in 96-well plates to 90% confluence. All cells were pretreated with CA-074Me or vehicle (dimethyl sulfoxide) at the indicated concentrations for various lengths of time and then treated with LT (1 μg/ml) for 2.5 h. Viability was assessed by addition of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide] (USB Corp., Cleveland, OH) to a final concentration of 0.6 mg/ml in DMEM. After a 30 to 45 min of incubation with MTT dye, cell culture medium was removed, and the cells were dissolved with 0.5% sodium dodecyl sulfate (SDS), 25 mM HCl in 90% isopropanol. A microplate reader was used to read the _A_570 of each well, and the percent viabilities were calculated relative to untreated controls.
MEK, caspase-1, and IL-1β cleavage.
BALB/cJ BMDM cells grown in supplemented DMEM at 37°C were primed with LPS (1 μg/ml) for 2 h and then pretreated with CA-074Me or vehicle prior to LT addition (1 μg/ml) for various lengths of time. Cell lysates were prepared with lysis buffer (1% Nonidet-P40, 0.5% sodium deoxycholate, and 0.1% SDS in phosphate-buffered saline [PBS]) containing EDTA-free Complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and protein concentrations quantified by using a BCA protein assay (Pierce, Rockford, IL) prior to electrophoresis and Western blotting using anti-MEK1 NT (1:7,500), anti-MEK2 NT (1:2,000), anti-MEK3 NT (1:500), anti-caspase-1 (1:250), anti-IL-1β (1:700), anti-cathepsin B (1:5,000), or anti-XIAP (1:2,000) antibodies. Primary antibodies were detected using IRDye-conjugated secondary antibodies (anti-rabbit 800CW, 1:30,000; anti-goat 800CW, 1:5,000; anti-mouse 700DX, 1:15,000) and the Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, NE).
In vitro caspase-1 assay.
RAW 264.7 cells were grown in supplemented DMEM and treated with ultrapure LPS (1 μg/ml) for 2 h and sucrose lysates prepared as previously described (29). Lysates containing pro-IL-1β substrate were then treated with recombinant caspase-1 (1 U per 50-μl reaction) in the presence of inhibitors and, incubated for 2 h at 37°C, and subjected to SDS-polyacrylamide gel electrophoresis. Western blotting was performed using the primary antibody anti-IL-1β (1:700).
Cytoplasmic cathepsin B activity assay.
BMDMs were plated in 96-well plates to 90% confluence. Cells were washed once with phenol red-free and HEPES-free DMEM nutrient mixture F-12 (DMEM/F12; Invitrogen, Carlsbad, CA). Inhibitors, LT, or Leu-Leu-OMe were then applied to the cells in DMEM/F12 and cells were incubated at 37°C in 5% CO2 for the indicated times. Saponin (S-4521) (Sigma, St. Louis, MO) dilutions prepared in 1× cold PBS in separate 96-well plates were applied to cells after removal of medium. After a 10-min incubation on ice, supernatants were removed and centrifuged at 2,000 rpm for 10 min at 4°C in round-bottom 96-well plates. A 10-μl portion of the supernatant was then mixed with 90 μl of the cathepsin B substrate buffer (80 μM zRR-AMC, 50 mM sodium acetate [pH 6.0], 4 mM EDTA, 10 mM dithiothreitol, 1 mM Pefablock) (13, 21). The rate of generation of free AMC was determined by recording fluorescence (excitation, 355 nm; emission, 460 nm) for 20 min at 30°C in a Wallac 1420 Multilabel Counter, Victor 3V (Perkin-Elmer Life and Analytical Sciences, Waltham, MA). Cathepsin B activity was determined by measuring the slope of the increase in AMC fluorescence (ΔAMC _t_−1) over 20 min. In parallel, 25 μl of the supernatant was used to measure lactate dehydrogenase (LDH) activity via the CytoTox-ONE homogeneous membrane integrity assay (Promega, Madison, WI) according to the manufacturer's protocol. Endpoint LDH activities are reported. To evaluate cathepsin B inhibition by various molecules, we used BALB/cJ BMDMs treated with concentrations of saponin that fully permeabilized lysosomal membranes (5 mg/ml, 20 min, room temperature) as a source of enzyme. Cell lysate supernatants were then treated with various inhibitor concentrations for 10 min prior to assessment of cathepsin B activity. The 50% inhibitory concentration (IC50) was calculated with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA).
Time-lapse fluorescence microscopy.
BALB/cJ BMDM cells grown in Lab-Tek chambered coverglass slides (Thermo Fisher Scientific, Rochester, NY) were stained for 30 min at 37°C with 100 nM LysoTracker Red DND-99 (Invitrogen, Carlsbad, CA) prepared in phenol red-free/HEPES-free DMEM/F12. After washes in phenol red-free and HEPES-free DMEM/F12, the cells were treated with LT (with or without drug pretreatment), and imaging was performed on a Leica TCS SP5 laser scanning confocal microscope with stacks (2-μm distances between images) collected every 5 min. Image stacks were analyzed as maximum intensity projections of each stack over time. Image processing was performed in Imaris (v6.1.5; Bitplane AG, Zurich, Switzerland).
siRNA cathepsin B knockdown.
Cells were nucleofected with Dharmacon ON-TARGET_plus_ SMARTpool small interfering RNA (siRNA; Thermo Scientific, Rochester, NY) targeted to the murine cathepsin B gene (Ctsb, NM_007798) using kits appropriate to each cell type and according to the manufacturer's protocols (Amaxa, Inc., Gaithersburg, MD). Negative controls were nucleofected in the absence of siRNA. Nucleofected cells were grown at 37°C for 40 h prior to making lysates for Western blotting with anti-cathepsin B (1:2,500) and anti-XIAP (loading control; 1:2,000). As before, primary antibodies were detected using IRDye-conjugated secondary antibodies and the Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, NE). Parallel LT toxicity assays were performed on the cells 40 h after nucleofection.
RESULTS
CA-074Me protects macrophages against LT.
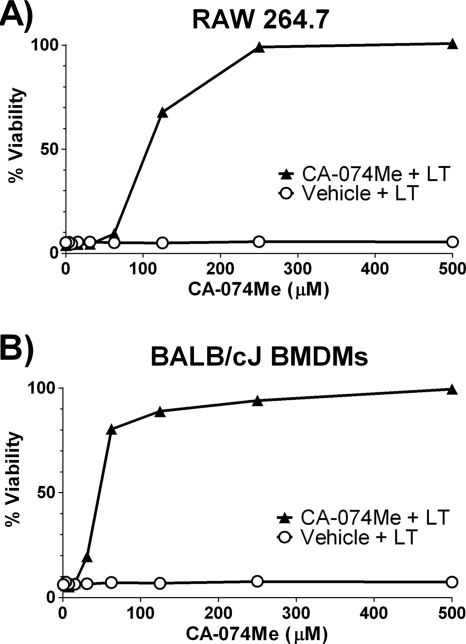
CA-074Me is a potent, cell-permeable, irreversible cathepsin B inhibitor (10). BALB/cJ BMDMs and RAW 264.7 cells pretreated with a range of CA-074Me concentrations for 2 h before exposure to LT were protected against toxin-dependent cell death (Fig. 1). The drug was used at doses previously shown to be effective for the inhibition of cytoplasmic cathepsin B activity required for NLRP3-mediated cell death (16, 21, 60). Late addition of some inhibitors of LT-dependent cell death (such as proteasome inhibitors) can still protect LT-sensitive macrophages (59). In similar add-back experiments with CA-074Me, 50% protection was observed when the drug was added up to 40 min after the start of LT treatment, although no protection was observed when CA-074Me was added at any later times (data not shown). Because CA-074Me is thought to require removal of its methyl ester group by intracellular esterases before forming active CA-074, this inhibitor may not be able to accurately measure the stage at which the activity it inhibits is required in the cell death process (10). This is also consistent with our observation that lower doses of CA-074Me can protect macrophages with increasing preincubation times (data not shown).
FIG. 1.
CA-074Me treatment protects against LT-induced macrophage death. (A) RAW 264.7 cells and (B) BALB/cJ BMDMs were incubated with the cathepsin B inhibitor CA-074Me or the vehicle for 2 h at 37°C. Cells were then treated with LT (1 μg/ml) (equal concentrations of PA and LF). Cell viability was accessed after 2.5 h, and the percent viability was calculated relative to control CA-074Me-treated cells.
CA-074Me prevents caspase-1 activation.
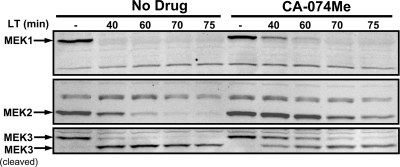
We next tested whether CA-074Me affected LF translocation or activity. BALB/cJ BMDMs were treated with LT in the presence of CA-074Me, and MEK cleavage was monitored over time by Western blotting. CA-074Me did not prevent MEK cleavage but did delay cleavage at the higher protective concentrations used in this experiment (Fig. 2). MEK1 cleavage was not delayed when lower but still protective concentrations of CA-074Me were used in conjunction with longer preincubation times, whereas MEK2 and MEK3 cleavages remained delayed by 20 min (data not shown). However, cells were protected even after completion of MEK cleavage. This suggests that, although CA-074Me treatment may affect initial delivery of LF to MEKs, it does not mediate protection through inhibition of LF activity or translocation.
FIG. 2.
CA-074Me delays but does not prevent MEK cleavage by LF. BALB/cJ BMDMs were primed with LPS (1 μg/ml) for 2 h, pretreated with either 500 μM CA-074Me or vehicle for 10 min, and then treated with LT (1 μg/ml) for various amounts of time. Western blotting was performed with antibodies against the N termini of MEK1, MEK2, and MEK3.
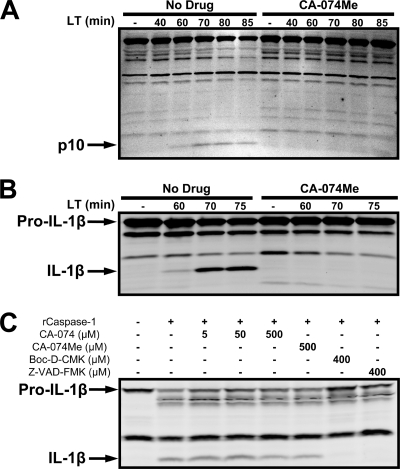
We then tested whether the drug had any effect on the caspase-1 activation that is required for LT-mediated macrophage death (8, 12, 34, 59). LPS priming was used to increase cellular IL-1β levels for ease of detection of the cleavage of this cytokine. Induction of this cytokine does not affect LT-mediated cytotoxic events. CA-074Me completely prevented caspase-1 cleavage (Fig. 3A) and processing of pro-IL-1β to mature IL-1β (Fig. 3B). The delay of MEK cleavage by 20 to 30 min at the highest tested concentrations of CA-074Me did not account for this blockage of caspase-1 activation, since we did not find any caspase cleavage at late LT treatment times (100 min) when MEK proteins were cleaved (data not shown). Furthermore, at lower protective concentrations of CA-074Me, at which MEK1 cleavage was unaffected, no evidence of caspase-1 activation was observed.
FIG. 3.
CA-074Me blocks activation of caspase-1 by LT. LPS-primed BALB/cJ BMDMs (LPS, 1 μg/ml, 2 h) were pretreated with either 500 μM CA-074Me or vehicle for 10 min, followed by LT (1 μg/ml) for various amounts of time. Western blots with antibodies for the p10 subunit of activated caspase-1 (A) and IL-1β (B) are shown. (C) LPS-primed RAW 264.7 cell sucrose lysates were incubated with active recombinant caspase-1 in the presence of the indicated inhibitors for 2 h and then subjected to Western blotting with an IL-1β antibody to monitor IL-1β cleavage.
Although nonspecific inhibitory activity of both CA-074 and CA-074Me on caspase-1 have been previously ruled out (21, 39), we also wanted to exclude the possibility that CA-074Me was acting as a nonspecific caspase-1 inhibitor in our system. We tested both CA-074Me and CA-074 in an in vitro recombinant caspase-1 activity assay measuring IL-1β cleavage. Neither CA-074 nor CA-074Me inhibited human or mouse caspase-1 activity, in contrast to two known caspase-1 inhibitors, Boc-d-CMK and Z-VAD-FMK (46, 59) (Fig. 3C). These data corroborate previous reports demonstrating that CA-074Me and CA-074 are not caspase-1 inhibitors. Thus, CA-074Me appears to act downstream of LF translocation to the cytosol (as assessed by MEK cleavage) and upstream of caspase-1 activation.
LT treatment increases cytoplasmic cathepsin B activity and induces LMP.
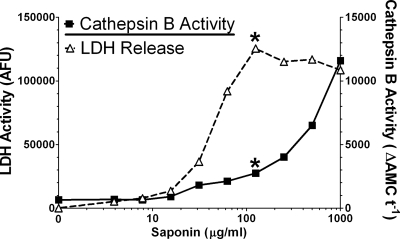
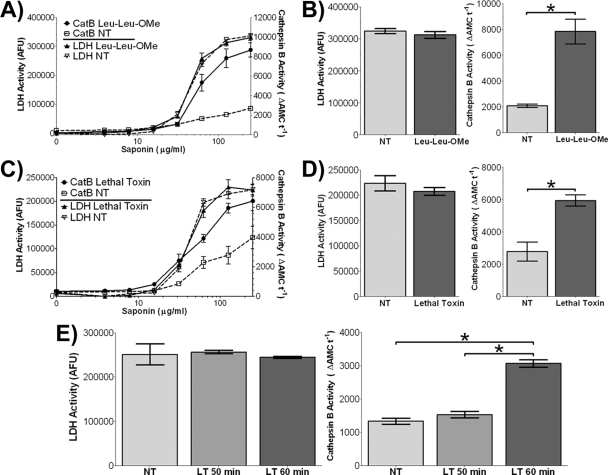
We next wanted to investigate whether LT treatment could induce lysosomal destabilization. Selective plasma membrane permeabilization techniques are available that allow release of a cell's cytoplasmic contents. When used with appropriate controls, these methods can detect whether LT treatment causes lysosomal protease translocation from lysosomes to the cytoplasm. In the present case, a saponin-based method was used to isolate the cytosol for measurement of cathepsin B activity, since this enzyme is the primary enzyme often associated with lysosomal leakage (13, 21, 50, 56). To optimize this assay, we exposed untreated BALB/cJ BMDMs to a range of saponin concentrations and assayed the supernatants for LDH and cathepsin B activity. A 10-min treatment with saponin (125 μg/ml) was found to give selective release of the cytosolic LDH marker and limited lysosomal release (Fig. 4). These conditions were used in all subsequent permeabilization experiments.
FIG. 4.
Saponin-mediated differential permeabilization of plasma and lysosomal membranes. BALB/cJ BMDMs were treated with a range of saponin concentrations in PBS for 10 min on ice. LDH activity and cathepsin B activity of the supernatant were measured in parallel assays. An asterisk indicates the optimum concentration of saponin (125 μg/ml) that results in minimal baseline cathepsin B activity (minimal lysosomal permeabilization) and maximum released LDH activity (maximum cytoplasmic release).
We then exposed BALB/cJ BMDMs to a nontoxic dose of the lysosomal destabilizing dipeptide Leu-Leu-OMe (48) as a positive control and verified LMP-mediated cathepsin B release to the cytosol (Fig. 5A and B). Having confirmed that the assay was measuring LMP, we proceeded to treat the BALB/cJ BMDMs with LF, which we found caused a twofold increase in cytoplasmic cathepsin B activity relative to untreated cells after 60 min of LT treatment. The similar amounts of LDH activity in LT-treated and untreated cells verified equal degrees of plasma membrane permeabilization and cytoplasmic release (Fig. 5C and D). Importantly, there was also no difference in cathepsin B or LDH activity in the unpermeabilized LT-treated cells, indicating that prior to and during the saponin treatment, the cells retained intact cell membranes. Saponin-released cytoplasmic cathepsin B levels were assessed every 10 min after LT treatment. We determined that the LMP corresponding to measurable changes in cytosolic cathepsin B release begins between 50 and 60 min after the application of LT (Fig. 5E). Although cathepsin B levels measured by this assay did continue to increase beyond 60 min (normally doubling by 80 min), because we observed a 10% baseline cathepsin B release without any saponin permeabilization at 80 min we assumed cells had already begin to undergo cytolysis by these later time points, contributing at minimum 10% of the cathepsin B activity (data not shown). Thus, we selected the 50 to 60 min window for assessment of cytosolic cathepsin B activity, prior to any cell lysis. This time frame corresponds closely to the activation of caspase-1 (Fig. 3). Despite the fact that changes in cytoplasmic cathepsin B activity which represented a small fraction of the total cathepsin activity in the cells were unlikely to be caused by an increase in cathepsin B proteins levels, we did verify that cathepsin B levels remained unchanged in LT-treated cells in the presence or absence of CA-074Me (data not shown).
FIG. 5.
LT induces an increase in cytoplasmic cathepsin B activity. BALB/cJ BMDMs were first treated with Leu-Leu-OMe (1 mM) for 30 min (A and B), LT (2 μg/ml) for 60 min (C and D), or left untreated (identified by “NT”). LDH activity and cathepsin B activity after saponin permeabilization of plasma membranes was measured as described in Materials and Methods. Panels B and D show the cathepsin B activity (right panel) and LDH activity (left panel) at the ideal saponin permeabilization concentration (125 μg/ml) in cells treated with Leu-Leu-OMe and LT, respectively. (E) In a similar experiment, BALB/cJ BMDMs were treated with LT (2 μg/ml) for either 50 or 60 min and subjected to a similar analysis of cytoplasmic cathepsin B (right panel) and LDH activities (left panel). An asterisk indicates statistically significant differences (P < 0.005; two-tailed Student t test). No statistically significant differences were noted between all other treatments.
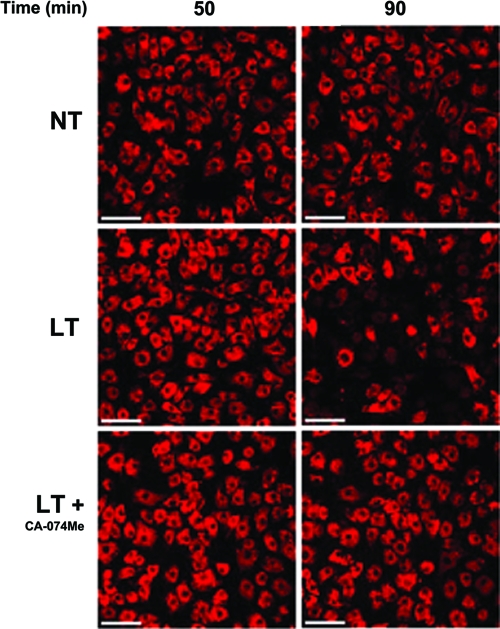
In order to confirm the LT-mediated LMP, we investigated toxin-dependent changes in lysosomal stability by monitoring the fluorescence of LysoTracker Red by confocal microscopy. LysoTracker Red is a lysomotrophic dye that only fluoresces in the acidic lysosomal compartment and thus provides an indicator of lysosomal integrity. We observed the beginning of a rapid loss of LysoTracker Red fluorescence in BALB/cJ BMDMs in some cells by 70 min after administration of LT (Fig. 6). This loss of fluorescence was not apparent in a majority of the cells until 90 min, followed closely by cytolysis, and was prevented in cells pretreated with CA-074Me (Fig. 6). These data strongly suggest that LT initiates LMP that is measurable at early times as an increase in cytoplasmic cathepsin B activity, but the accompanying pH changes or more extensive lysosomal destabilization in lysosomes can only be monitored visually at later times. The temporal distinction between cytoplasmic cathepsin B activity and large-scale lysosomal destabilization is consistent with previous reports suggesting that cathepsin B, L, and D can be translocated into the cytosol, while the lysosomes still retain their pH gradient and thus retain their LysoTracker fluorescence (5, 13). In fact, it can take between 30 and 90 min from the initial LMP events before it is possible to visualize lysosomal destabilization with lysomotropic dyes (47).
FIG. 6.
LT induces late large-scale lysosomal membrane permeabilization. BALB/cJ BMDMs were stained with LysoTracker Red (100 nM, 30 min) and the same field was imaged every 5 min after LT treatment (2 μg/ml), with or without CA-074Me pretreatment (500 μM, 10 min). NT represents untreated cells stained with the dye. The scale bars represent 50 μm.
LT-induced increases in cytoplasmic cathepsin B activity in the context of other toxin-mediated events.
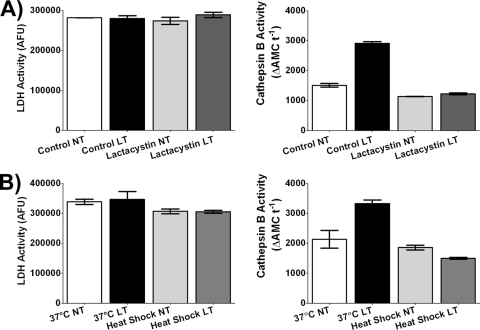
We then examined the role of cytoplasmic cathepsin B activity in the context of the proteasome activity and K+ efflux required for LT-mediated caspase-1 activation. The potent proteasome inhibitor lactacystin fully prevented the LT-induced increase in cytoplasmic cathepsin B activity (Fig. 7A). Similarly, we tested the K+ channel inhibitor quinidine and found that although this drug substantially increased the baseline cytoplasmic cathepsin B activity in untreated control cells, it also inhibited (or simply masked) any further LT-dependent increases in cytoplasmic cathepsin B activity (data not shown). This suggests that K+ efflux induced by LT precedes LMP. Finally, we wanted to relate LMP to the event(s) altered by heat shock, which prevents LT-induced cell death at an unknown step upstream of caspase-1 activation (29). In this regard, we found that the LT-dependent increase in cytoplasmic cathepsin B activity was also inhibited by this treatment (Fig. 7B). We conclude that increases in cytoplasmic cathepsin B activity (and the associated initiation of LMP) are likely downstream of the proteasome activity and K+ efflux required for caspase-1 activation.
FIG. 7.
LT-induced increases in cytoplasmic cathepsin B activity and other known LT-dependent events. BALB/cJ BMDMs were pretreated with lactacystin (10 μM) for 10 min (A) or heat shocked at 45°C for 2 h (B). The cells were then treated with LT (2 μg/ml) for 60 min or left untreated (NT). LDH activity and cathepsin B activity after saponin permeabilization of plasma membranes was measured as described in Materials and Methods. Cathepsin B activities (right panels) and LDH activities (left panels) are indicated at the ideal saponin permeabilization concentration (125 μg/ml). Lactacystin- and heat shock-treated samples treated with toxin were not statistically different for cathepsin B activity from those not treated with toxin (P values are 0.0455 [A] and 0.0473 [B]).
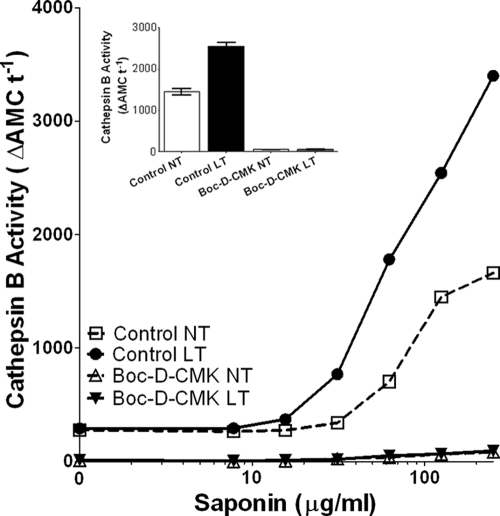
Because the appearance of cathepsin B in the cytosol and caspase-1 activation occur at similar times in response to LT and there is evidence of LMP augmentation by both NLRP3 activation (16) and other caspase activities (13, 18, 57), we sought to determine whether lysosomal leakage events were upstream or downstream of caspase-1 activation by LT. We pretreated cells with the potent caspase-1 inhibitor Boc-d-CMK (46) prior to performing the cytoplasmic cathepsin B assay. Interestingly, we found that Boc-d-CMK strongly inhibited cathepsin B activity in both LT-treated and untreated cells at all saponin concentrations, including high doses that permeabilized the lysosomal membranes (Fig. 8). Suspecting that Boc-d-CMK, like many other caspase inhibitors (17, 21, 39, 41, 49), could act as a potent cathepsin B inhibitor, we compared it and other known caspase-1 and cathepsin B inhibitors in the in vitro cathepsin B activity assay. As expected, both CA-074 and CA-074Me inhibited cathepsin B activity, with CA-074 being much more potent (approximate IC50s of 60 nM and 6 μM, respectively [data not shown]). Caspase inhibitors, including Boc-d-CMK, Z-VAD-FMK, Ac-YVAD-CMK, and Z-WEHD-FMK, also strongly inhibited cathepsin B activity (approximate IC50s of 9, 1, 1, and 6 μM, respectively), whereas lactacystin did not inhibit cathepsin B activity (data not shown). Thus, almost all caspase-1 inhibitors are also cathepsin B inhibitors and may contribute to protection against LT-mediated lysis through acting on both enzymes, as well as other potential targets. Therefore, because cathepsin B activity appears in the cytosol at the same time as caspase-1 activation, it is still possible that LT-induced LMP actually requires caspase-1 activation and is a consequence of inflammasome activation rather than the initiating event. If this is true, CA-074Me protection may act at a different step of LT cytotoxicity.
FIG. 8.
The caspase inhibitor Boc-d-CMK acts as a cathepsin B inhibitor. (A) Boc-d-CMK (80 μM, 60 min)-pretreated BALB/cJ BMDMs were left untreated or were treated with LT for 60 min (2 μg/ml). Cytoplasmic cathepsin B activity was assessed after saponin permeabilization as described in Materials and Methods for a range of saponin concentrations. Cathepsin B activity inhibition by Boc-d-CMK after permeabilization at 125 μg of saponin/ml is shown in the inset.
Effects of cathepsin B expression inhibition on LT-induced cell death.
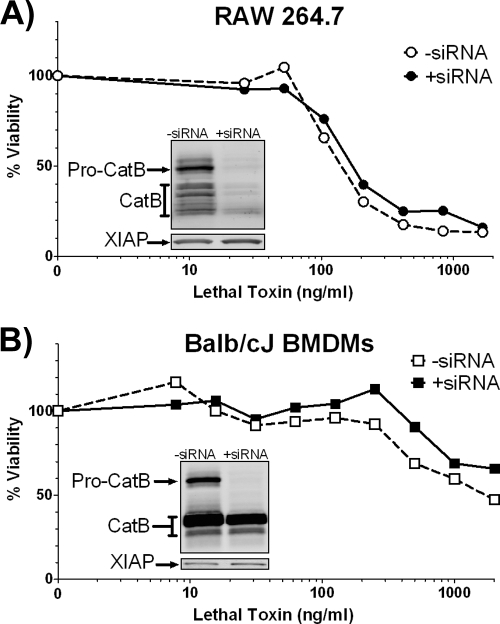
Because LMP is associated with the release of numerous other enzymes to the cytoplasm and there have been examples of CA-074Me inhibiting some related proteases, we wanted to test whether cathepsin B was specifically essential for LT cytotoxicity in macrophages. We inhibited cathepsin B expression by RNA interference in both RAW 264.7 and BALB/cJ BMDMs. In RAW 264.7 cells we obtained a strong knockdown of both pro-cathepsin B and mature cathepsin B protein levels, as indicated by a Western blot (Fig. 9A, inset). However, we did not observe protection against or delay of LT-mediated cell death associated with reduced cathepsin B levels (Fig. 9A). In BALB/cJ BMDMs we noticed a nearly complete knockdown of pro-cathepsin B without a corresponding reduction of the mature forms of cathepsin B (Fig. 9B, inset). Thus, it was not surprising that there was no difference in LT toxicity in BMDMs (Fig. 9B). We also found that LT-induced caspase-1 activation occurred similarly between the knockdown and control cells (data not shown). Curiously, even in the absence of LT, nucleofection with cathepsin B siRNA resulted in some baseline caspase-1 activation, likely due to the pore formation associated with this process (data not shown). This small amount of activated caspase-1 may have been sufficient as the first step in NLRP1b-dependent LT-mediated cell death. However, it is possible that cathepsin B activity is not required for LT-dependent cell death or that very small levels of this enzyme are sufficient for LT-mediated effects. Alternatively, other cathepsins and lysosomal enzymes may also be involved in LT cell death and compensate for cathepsin B.
FIG. 9.
Cathepsin B alone may not be necessary for LT-dependent cell death. RAW 264.7 (A) and BALB/cJ BMDMs (B) were nucleofected with cathepsin B siRNA. At 40 h postnucleofection cells were treated with a range of LT concentrations for 105 min (RAW 264.7 cells) (A) or 75 min (BALB/cJ BMDMs) (B) prior to accessing cell viability by the MTT assay. Insets indicate cathepsin B Western blots run in parallel to the cytotoxicity assays. Blots were reprobed with a XIAP antibody to confirm equal loading.
DISCUSSION
In the present study, we demonstrate that CA-074Me, a drug described as a potent and specific cathepsin B inhibitor, protects macrophages against LT-dependent cell death without inhibiting LF-mediated translocation or activity (as assessed by MEK cleavage), but instead by blocking some step upstream of caspase-1 activation. LT induced an increase in cytoplasmic cathepsin B activity, indicating a LMP event which occurred earlier than the large-scale lysosomal destabilization coupled to cell lysis (as indicated by a complete loss of LysoTracker Red fluorescence). LMP and cathepsin translocation into the cytosol can begin prior to the loss of lysosomal acidic pH, measurable by LysoTracker fluorescence, sometimes taking 30 to 90 min before dye-based visualization is possible (5, 13). We hypothesize that there is an initial event triggering LMP that correlates with increased cytoplasmic cathepsin B activity (as measured by our activity assay) and a later, large-scale LMP event immediately preceding cell death (as measured by fluorescence microscopy). It is unclear, however, whether CA-074Me protects against LT-mediated lysis through inhibition of cathepsin B itself or other cellular proteases because a reduction of the enzyme by siRNA in RAW 264.7 cells does not protect against toxin. Furthermore, we cannot state whether CA-074Me protection actually targets protease release associated with LT-induced LMP.
There is an increasing recognition that cathepsin B is critically involved in a number of different functions separate from its established role in lysosomal protein degradation and protein processing (33). The release of cathepsin B from lysosomes is involved in both apoptosis (typically after partial LMP) and necrosis (after large-scale LMP) (9). Furthermore, cathepsin B release has been shown to act both upstream and downstream of NLRP3 inflammasome activation (16, 19, 21, 22, 43, 60). Indeed, lysosomal destabilization and the cytoplasmic activity of lysosomal proteases such as cathepsin B may be a universal mechanism through which a variety of stimuli induce the proinflammatory responses associated with inflammasome activation (reviewed in references 14 and 62).
Release of cathepsin B into the cytoplasm by LT corresponds closely in time with the activation of caspase-1 by LT. This suggests that a feedback amplification mechanism might be operative, one in which traces of cytosolic proteases associated with the lysosome leakage process might act on the NLRP1b inflammasome or its procaspase-1 component to begin an activation cascade. In this scenario, the CA-074Me inhibitor may actually act on the putative cytoplasmic enzyme required for NLRP1b-mediated caspase-1 activation, preventing further LMP-based enzyme release.
Interestingly, the relationship between NLRP3-activated caspase-1 and cathepsin B is also unclear. Lysosomal destabilization and the release of cathepsin B have been implicated in the activation of the NLRP3 inflammasome/caspase-1 after exposure to nigericin, amyloid β, aluminum salts, silica crystals, microparticle vaccine adjuvants, osmotic lysosomal rupture, and Leu-Leu-OMe (19, 21, 22, 43). However, in a contrary view, cathepsin B release from lysosomes has been described as resulting from the upstream activation of NLRP3 that occurs upon transfection of disease-associated mutant forms of NLRP3, treatment with certain antiviral drugs, and S. flexneri infection, although cell death in these scenarios is caspase-1 independent (16, 60). Thus, LMP has been shown to both cause the release of cathepsin B and be dependent on the activity of cathepsin B, contrasting observations that taken together could support the cathepsin-dependent positive-feedback loop mentioned above (16, 58).
Cathepsin B has also been shown to activate caspase-1 and caspase-11 in vitro and in vivo, further suggesting that there could be important interactions between cathepsin B and the inflammasome (4, 21, 42, 49). In a recent study, both caspase-1 and caspase-11 were found to associate with NLRP1b in response to LT treatment (35). Caspase-11 has also been reported to be required for caspase-1 activation, and cathepsin B may act on caspase-11 (25, 30, 55).
We tried to relate caspase-1 activation to cytoplasmic cathepsin B activity by observing cytoplasmic cathepsin B activity in the presence of a pan-caspase inhibitor. We hypothesized that the inhibition of caspase-1 activity would lead to inhibition of cathepsin B release if caspase-1 was necessary for amplification of the cathepsin B release. These experiments were complicated by the discovery that the potent caspase inhibitor Boc-d-CMK, previously used to implicate caspase-1 activity in LT-mediated macrophage death (46), is also a potent cathepsin B inhibitor, much in the manner previously shown for other caspase inhibitors (17, 21, 39, 41, 49). This inhibitor, a potent protector against LT-mediated lysis (12, 34, 59), may act through targeting both caspase-1 and cathepsin B. These results underscore the need to interpret data from pharmacological approaches with caution and to use inhibitors in conjunction with other methods to clarify the role of cathepsins and caspases in various cellular pathways. For this very reason, we performed siRNA experiments which suggest that CA-074Me may protect LT by acting on other cellular enzymes in addition to cathepsin B. However, the half-life of previously transcribed and processed mature cathepsin B is around 24 h (6, 26, 27), and it is likely that some active enzyme remained during toxicity testing. Only a few molecules of cathepsin B could be sufficient for inducing the events that lead to caspase-1 activation, making it difficult to observe a significant change in LT-protection with these knockdown experiments. Furthermore, it has been demonstrated that CA-074Me and CA-074 can inhibit cathepsins L and X (enzymes also released during LMP), and concerns remain that they may also act on other unknown targets (28, 31, 32). Therefore, it is possible that although cytoplasmic cathepsin B is a clear measure for LT-induced LMP, the enzyme itself is not involved in LT-mediated cell death.
The mechanism(s) by which LT induces LMP remain unknown. One membrane-active enzyme that could play a role is phospholipase A2 (PLA2). PLA2 is known to be a strong inducer of LMP (61, 63, 64), and it has previously been implicated in LT-mediated cell death (44). Various forms of PLA2 are activated by both lysosomal proteases and K+ effluxes, and in turn, PLA2 can regulate caspase-1 activity (3, 24, 54, 63). Our preliminary data suggest that the iPLA2β-specific inhibitor (S)-bromoenol lactone (23) does, in fact, protect macrophages against LT-dependent cell death (data not shown).
Another organelle affected by LT that may be involved in the regulation of LT-mediated LMP is the mitochondrion. Strong interdependent relationships exist between the state of lysosomes and mitochondria (7, 47). Mitochondrial membrane potential disruption is an essential, proteasome-dependent step in LT-induced cell death (2). A key mediator of mitochondrial or lysosomal damage could be LT-induced reactive oxygen species (20), since reactive oxygen species molecules can easily damage lysosomal membranes (7, 47). Finally, the channel formed by PA in endosomes could possibly act as a potential small-scale LMP trigger. In cells engineered to overexpress anthrax toxin receptor 1 (TEM8), exposure to PA induces cell death through what was proposed to be an LMP-like event (40). One can imagine that a membrane-inserted PA channel might traffic to lysosomes and cause small-scale content release, which could act in concert with other LF-dependent events to produce cell death and inflammasome activation.
To summarize, we report that anthrax LT-induced cell death results in LMP events and CA-074Me, an inhibitor of lysosomal proteases, prevents cell death. Although CA-074Me inhibited the NLRP1b inflammasome and caspase-1 activation by LT, it is possible that caspase-1 activation also contributes to LT-induced LMP.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
We thank Devorah Crown and Sharmina Miller-Randolph for bone marrow isolation and Rasem Fattah for toxin preparation. We also thank Owen Schwartz, Lily Koo, Juraj Kabat, and Steven Becker of the National Institute of Allergy and Infectious Diseases Research Technologies Branch, Biological Imaging Section, for help with the microscopy and image analysis.
Footnotes
▿
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Abrami, L., M. Lindsay, R. G. Parton, S. H. Leppla, and F. G. van der Goot. 2004. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 166**:**645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alileche, A., R. C. Squires, S. M. Muehlbauer, M. P. Lisanti, and J. Brojatsch. 2006. Mitochondrial impairment is a critical event in anthrax lethal toxin-induced cytolysis of murine macrophages. Cell Cycle 5**:**100-106. [DOI] [PubMed] [Google Scholar]
- 3.Andrei, C., P. Margiocco, A. Poggi, L. V. Lotti, M. R. Torrisi, and A. Rubartelli. 2004. Phospholipases C and A2 control lysosome-mediated IL-1β secretion: implications for inflammatory processes. Proc. Natl. Acad. Sci. USA 101**:**9745-9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benchoua, A., J. Braudeau, A. Reis, C. Couriaud, and B. Onteniente. 2004. Activation of proinflammatory caspases by cathepsin B in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 24**:**1272-1279. [DOI] [PubMed] [Google Scholar]
- 5.Bidere, N., H. K. Lorenzo, S. Carmona, M. Laforge, F. Harper, C. Dumont, and A. Senik. 2003. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J. Biol. Chem. 278**:**31401-31411. [DOI] [PubMed] [Google Scholar]
- 6.Bohley, P. 1987. Intracellular Proteolysis, p. 307-332. In A. Neuberger and K. Brocklehurst (ed.), New comprehensive biochemistry, vol. 16. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 7.Boya, P., and G. Kroemer. 2008. Lysosomal membrane permeabilization in cell death. Oncogene 27**:**6434-6451. [DOI] [PubMed] [Google Scholar]
- 8.Boyden, E. D., and W. F. Dietrich. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38**:**240-244. [DOI] [PubMed] [Google Scholar]
- 9.Brunk, U. T., H. Dalen, K. Roberg, and H. B. Hellquist. 1997. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 23**:**616-626. [DOI] [PubMed] [Google Scholar]
- 10.Buttle, D. J., M. Murata, C. G. Knight, and A. J. Barrett. 1992. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch. Biochem. Biophys. 299**:**377-380. [DOI] [PubMed] [Google Scholar]
- 11.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280**:**734-737. [DOI] [PubMed] [Google Scholar]
- 12.Fink, S. L., T. Bergsbaken, and B. T. Cookson. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA 105**:**4312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foghsgaard, L., D. Wissing, D. Mauch, U. Lademann, L. Bastholm, M. Boes, F. Elling, M. Leist, and M. Jaattela. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153**:**999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi, L., T. Eigenbrod, R. Munoz-Planillo, and G. Nunez. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10**:**241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedlander, A. M., R. Bhatnagar, S. H. Leppla, L. Johnson, and Y. Singh. 1993. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect. Immun. 61**:**245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisawa, A., N. Kambe, M. Saito, R. Nishikomori, H. Tanizaki, N. Kanazawa, S. Adachi, T. Heike, J. Sagara, T. Suda, T. Nakahata, and Y. Miyachi. 2007. Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood 109**:**2903-2911. [DOI] [PubMed] [Google Scholar]
- 17.Gray, J., M. M. Haran, K. Schneider, S. Vesce, A. M. Ray, D. Owen, I. R. White, P. Cutler, and J. B. Davis. 2001. Evidence that inhibition of cathepsin-B contributes to the neuroprotective properties of caspase inhibitor Tyr-Val-Ala-Asp-chloromethyl ketone. J. Biol. Chem. 276**:**32750-32755. [DOI] [PubMed] [Google Scholar]
- 18.Gyrd-Hansen, M., T. Farkas, N. Fehrenbacher, L. Bastholm, M. Hoyer-Hansen, F. Elling, D. Wallach, R. Flavell, G. Kroemer, J. Nylandsted, and M. Jaattela. 2006. Apoptosome-independent activation of the lysosomal cell death pathway by caspase-9. Mol. Cell. Biol. 26**:**7880-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halle, A., V. Hornung, G. C. Petzold, C. R. Stewart, B. G. Monks, T. Reinheckel, K. A. Fitzgerald, E. Latz, K. J. Moore, and D. T. Golenbock. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9**:**857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna, P. C., B. A. Kruskal, R. A. Ezekowitz, B. R. Bloom, and R. J. Collier. 1994. Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol. Med. 1**:**7-18. [PMC free article] [PubMed] [Google Scholar]
- 21.Hentze, H., X. Y. Lin, M. S. Choi, and A. G. Porter. 2003. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 10**:**956-968. [DOI] [PubMed] [Google Scholar]
- 22.Hornung, V., F. Bauernfeind, A. Halle, E. O. Samstad, H. Kono, K. L. Rock, K. A. Fitzgerald, and E. Latz. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9**:**847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins, C. M., X. Han, D. J. Mancuso, and R. W. Gross. 2002. Identification of calcium-independent phospholipase A2 (iPLA2) β, and not iPLA2γ, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells: enantioselective mechanism-based discrimination of mammalian iPLA2s. J. Biol. Chem. 277**:**32807-32814. [DOI] [PubMed] [Google Scholar]
- 24.Kahlenberg, J. M., and G. R. Dubyak. 2004. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 286**:**C1100-C1108. [DOI] [PubMed] [Google Scholar]
- 25.Kang, S. J., S. Wang, H. Hara, E. P. Peterson, S. Namura, S. Amin-Hanjani, Z. Huang, A. Srinivasan, K. J. Tomaselli, N. A. Thornberry, M. A. Moskowitz, and J. Yuan. 2000. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149**:**613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katunuma, N., N. Wakamatsu, K. Takio, K. Titani, and E. Kominami. 1983. Structure, function, and regulation of endogenous thiol proteinase inhibitor, p. 135-145. In N. Katunuma, H. Umezawa, and H. Holzer (ed.), Proteinase inhibitors: medical and biological aspects. Japan Scientific Societies Press, Tokyo, Japan.
- 27.Kirschke, H., and A. J. Barrett. 1987. Chemistry of lysosomal proteases, p. 193-238. In H. Glanmann and J. Ballard (ed.), Lysosomes: their role in protein breakdown. Academic Press, London, England.
- 28.Klemencic, I., A. K. Carmona, M. H. Cezari, M. A. Juliano, L. Juliano, G. Guncar, D. Turk, I. Krizaj, V. Turk, and B. Turk. 2000. Biochemical characterization of human cathepsin X revealed that the enzyme is an exopeptidase, acting as carboxymonopeptidase or carboxydipeptidase. Eur. J. Biochem. 267**:**5404-5412. [DOI] [PubMed] [Google Scholar]
- 29.Levin, T. C., K. E. Wickliffe, S. H. Leppla, and M. Moayeri. 2008. Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell. Microbiol. 10**:**2434-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10**:**417-426. [DOI] [PubMed] [Google Scholar]
- 31.Mihalik, R., G. Imre, I. Petak, B. Szende, and L. Kopper. 2004. Cathepsin B-independent abrogation of cell death by CA-074-OMe upstream of lysosomal breakdown. Cell Death Differ. 11**:**1357-1360. [DOI] [PubMed] [Google Scholar]
- 32.Montaser, M., G. Lalmanach, and L. Mach. 2002. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol. Chem. 383**:**1305-1308. [DOI] [PubMed] [Google Scholar]
- 33.Mort, J. S., and D. J. Buttle. 1997. Cathepsin B. Int. J. Biochem. Cell Biol. 29**:**715-720. [DOI] [PubMed] [Google Scholar]
- 34.Muehlbauer, S. M., T. H. Evering, G. Bonuccelli, R. C. Squires, A. W. Ashton, S. A. Porcelli, M. P. Lisanti, and J. Brojatsch. 2007. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6**:**758-766. [DOI] [PubMed] [Google Scholar]
- 35.Nour, A. M., Y. G. Yeung, L. Santambrogio, E. D. Boyden, E. R. Stanley, and J. Brojatsch. 2009. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect. Immun. 77**:**1262-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, S., and S. H. Leppla. 2000. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr. Purif. 18**:**293-302. [DOI] [PubMed] [Google Scholar]
- 37.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 1999. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNγ-induced release of NO and TNFα. FEBS Lett. 462**:**199-204. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez, D. M., S. H. Leppla, R. Schneerson, and J. Shiloach. 2002. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J. Ind. Microbiol. Biotechnol. 28**:**232-238. [DOI] [PubMed] [Google Scholar]
- 39.Rozman-Pungercar, J., N. Kopitar-Jerala, M. Bogyo, D. Turk, O. Vasiljeva, I. Stefe, P. Vandenabeele, D. Bromme, V. Puizdar, M. Fonovic, M. Trstenjak-Prebanda, I. Dolenc, V. Turk, and B. Turk. 2003. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 10**:**881-888. [DOI] [PubMed] [Google Scholar]
- 40.Salles, I. I., D. E. Voth, S. C. Ward, K. M. Averette, R. K. Tweten, K. A. Bradley, and J. D. Ballard. 2006. Cytotoxic activity of Bacillus anthracis protective antigen observed in a macrophage cell line overexpressing ANTXR1. Cell. Microbiol. 8**:**1272-1281. [DOI] [PubMed] [Google Scholar]
- 41.Schotte, P., W. Declercq, H. S. Van, P. Vandenabeele, and R. Beyaert. 1999. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 442**:**117-121. [DOI] [PubMed] [Google Scholar]
- 42.Schotte, P., C. W. Van, M. Van de Craen, L. G. Van, M. Desmedt, J. Grooten, M. Cornelissen, R. L. De, J. Vandekerckhove, W. Fiers, P. Vandenabeele, and R. Beyaert. 1998. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 251**:**379-387. [DOI] [PubMed] [Google Scholar]
- 43.Sharp, F. A., D. Ruane, B. Claass, E. Creagh, J. Harris, P. Malyala, M. Singh, D. T. O'Hagan, V. Petrilli, J. Tschopp, L. A. O'Neill, and E. C. Lavelle. 2009. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 106**:**870-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin, S., Y. B. Kim, and G. H. Hur. 1999. Involvement of phospholipase A2 activation in anthrax lethal toxin-induced cytotoxicity. Cell Biol. Toxicol. 15**:**19-29. [DOI] [PubMed] [Google Scholar]
- 45.Singh, Y., S. H. Leppla, R. Bhatnagar, and A. M. Friedlander. 1989. Internalization and processing of Bacillus anthracis lethal toxin by toxin-sensitive and -resistant cells. J. Biol. Chem. 264**:**11099-11102. [PubMed] [Google Scholar]
- 46.Squires, R. C., S. M. Muehlbauer, and J. Brojatsch. 2007. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J. Biol. Chem. 282**:**34260-34267. [DOI] [PubMed] [Google Scholar]
- 47.Terman, A., T. Kurz, B. Gustafsson, and U. T. Brunk. 2006. Lysosomal labilization. IUBMB Life 58**:**531-539. [DOI] [PubMed] [Google Scholar]
- 48.Thiele, D. L., and P. E. Lipsky. 1990. Mechanism of l-leucyl-l-leucine methyl ester-mediated killing of cytotoxic lymphocytes: dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc. Natl. Acad. Sci. USA 87**:**83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vancompernolle, K., H. F. Van, G. Pynaert, M. Van de Craen, V. K. De, N. Totty, A. Sterling, W. Fiers, P. Vandenabeele, and J. Grooten. 1998. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 438**:**150-158. [DOI] [PubMed] [Google Scholar]
- 50.Vanden Berghe, T., G. Denecker, G. Brouckaert, D. V. Krysko, K. D'Herde, and P. Vandenabeele. 2004. More than one way to die: methods to determine TNF-induced apoptosis and necrosis. Methods Mol. Med. 98**:**101-126. [DOI] [PubMed] [Google Scholar]
- 51.Varughese, M., A. Chi, A. V. Teixeira, P. J. Nicholls, J. M. Keith, and S. H. Leppla. 1998. Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol. Med. 4**:**87-95. [PMC free article] [PubMed] [Google Scholar]
- 52.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352(Pt. 3)**:**739-745. [PMC free article] [PubMed] [Google Scholar]
- 53.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, and C. Montecucco. 1998. Anthrax lethal factor cleaves the N terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248**:**706-711. [DOI] [PubMed] [Google Scholar]
- 54.Walev, I., J. Klein, M. Husmann, A. Valeva, S. Strauch, H. Wirtz, O. Weichel, and S. Bhakdi. 2000. Potassium regulates IL-1β processing via calcium-independent phospholipase A2. J. Immunol. 164**:**5120-5124. [DOI] [PubMed] [Google Scholar]
- 55.Wang, S., M. Miura, Y. K. Jung, H. Zhu, E. Li, and J. Yuan. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92**:**501-509. [DOI] [PubMed] [Google Scholar]
- 56.Wassler, M., I. Jonasson, R. Persson, and E. Fries. 1987. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem. J. 247**:**407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Werneburg, N., M. E. Guicciardi, X. M. Yin, and G. J. Gores. 2004. TNF-α-mediated lysosomal permeabilization is FAN and caspase 8/Bid dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 287**:**G436-G443. [DOI] [PubMed] [Google Scholar]
- 58.Werneburg, N. W., M. E. Guicciardi, S. F. Bronk, and G. J. Gores. 2002. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 283**:**G947-G956. [DOI] [PubMed] [Google Scholar]
- 59.Wickliffe, K. E., S. H. Leppla, and M. Moayeri. 2008. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 10**:**332-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willingham, S. B., D. T. Bergstralh, W. O'Connor, A. C. Morrison, D. J. Taxman, J. A. Duncan, S. Barnoy, M. M. Venkatesan, R. A. Flavell, M. Deshmukh, H. M. Hoffman, and J. P. Ting. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2**:**147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Windelborn, J. A., and P. Lipton. 2008. Lysosomal release of cathepsins causes ischemic damage in the rat hippocampal slice and depends on NMDA-mediated calcium influx, arachidonic acid metabolism, and free radical production. J. Neurochem. 106**:**56-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, H. B., and B. B. Finlay. 2008. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe 4**:**198-208. [DOI] [PubMed] [Google Scholar]
- 63.Zhao, M., F. Antunes, J. W. Eaton, and U. T. Brunk. 2003. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur. J. Biochem. 270**:**3778-3786. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, M., U. T. Brunk, and J. W. Eaton. 2001. Delayed oxidant-induced cell death involves activation of phospholipase A2. FEBS Lett. 509**:**399-404. [DOI] [PubMed] [Google Scholar]