The role of CD40 and CD40L in Dendritic Cells (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 1.
Published in final edited form as: Semin Immunol. 2009 Jun 12;21(5):265–272. doi: 10.1016/j.smim.2009.05.010
Introduction
In this review, we focus on the function of CD40-CD40L (CD154) interactions in the regulation of dendritic cell (DC) - T cell and DC-B cell cross-talk. In addition, we examine differences and similarities between the CD40 signaling pathway in DCs and other innate immune cell receptors, and how these pathways integrate DC functions. As research into DC vaccines and immunotherapies progresses, further understanding of CD40 and DC function will advance the applicability of DCs in immunotherapy for human diseases.
Background on CD40 and CD154/CD40L
CD40 is a 48 kilodalton transmembrane glycoprotein surface receptor that is a member of the Tumor Necrosis Factor Receptor superfamily (TNFRSF). The importance of CD40 in regulating functions was not appreciated for many years after CD40 was discovered. Our initial studies emphasized that CD40 was expressed on B cells and that ligating CD40 induced B cells to proliferate [1, 2]. The cloning of human and mouse CD40 [3, 4] facilitated the identification of CD40 ligands and the development of anti-mouse CD40 monclonal antibodies (mAbs); analyses of mRNA and protein expression showed that CD40 is expressed on non-B cells like DCs, monocytes, epithelial cells [5–7] and even endothelial cells [8]. Nevertheless, most early studies of CD40 including our own focused on defining the role CD40 plays in B cell survival, ‘T cell help’ and isotype class switching [9–11]. The Ig deficiencies in CD40L (CD154)-impaired patients with hyper-IgM syndrome and in CD40 or CD40L knockout (KO) mice served to underscore the central role of CD40 in mature B cell functions [12–17]. However, the fact that these patients are more susceptible to certain opportunistic infections suggested CD40L-CD40 may regulate non-B functions.
More attention turned toward DCs after several groups reported that CD40 signaling induces changes in DCs, which make them more effective antigen presenting cells (APCs), such as upregulation of MHC class II and co-stimulatory molecules CD80/CD86 [18–20]. Although DCs resembled B cells in these responses to CD40 crosslinking [9], it soon became apparent that different cell types respond in distinct ways to CD40 stimulation; for instance, while CD40 ligation promotes B cells to survive, it promotes some epithelial to die [21] and others to produce GM-CSF [22]. Stimulation of CD40 was found to program monocytes and DCs to do things B cells do not do well, such as producing inflammatory cytokines and chemokines [23, 24].
The ligand for CD40, CD154 (also known as TRAP, T-BAM, CD40 Ligand or CD40L) is a 34–39 kilodalton type II integral membrane protein. Initially CD40L was reported to be expressed principally on activated CD4+ T cells, which led to models that the main function of CD40L was to provide T cell help to B cells [25]. Once again, further scrutiny revealed that CD154/CD40L is also expressed on activated B cells, platelets and smooth muscle cells [25]. Furthermore, functional CD40L is induced on human blood-derived DCs after CD40 stimulation [26], and CD40L has been detected on a variety of DCs including murine Langerhans cells [27], lung DCs [28]), plasmacytoid dendritic cells (pDCs) [29] and splenic DCs [30].
B cells can also express CD40L [31–33], and CD40 has been shown to be expressed on activated CD4+ and CD8+ T cells [34]. Thus, emphasizing the CD4+ T cell mediated functions of CD40L may be over simplistic; any discussion of CD40 function on DCs must consider that a CD40L signal to DCs or B cells may come from not only CD4+ T cells but also activated DCs or B cells (Fig. 1). Studies with a related TNF-TNFR family pair, RANKL-RANK, showed that RANKL can promote DC survival [35] and that RANKL is expressed on activated DCs [36]. Since DCs interact with each other in vivo, forming e.g., a network in the lamina propria [37], it is quite possible that DCs interact with each other to regulate their functions through CD40L-CD40 or RANKL-RANK pathways. The C4 binding protein (C4bp) was reported to be a second ligand for CD40 [38], suggesting that CD40 can receive and bridge innate and adaptive immune signals [39]. Little is known about the role of C4bp via CD40 in DC functions.
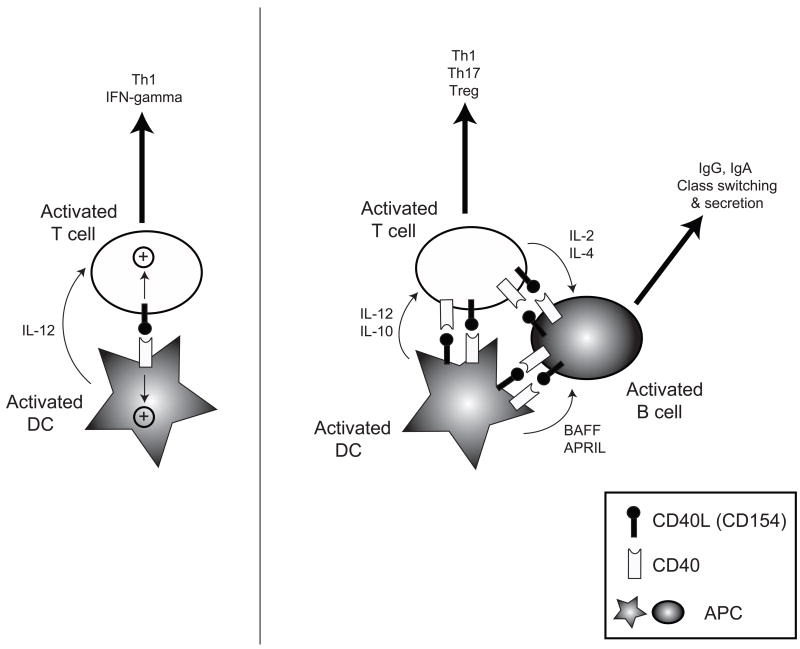
Figure 1. Schematic showing CD40 and CD40L expression on DCs and Lymphocytes. (Left) Initial model of CD40-CD40L interactions between T cells and DCs.
CD40 is upregulated on activated DCs and CD40L is expressed on activated T cells. Engagement of CD40 on DCs induces positive signaling that leads to expression of CD80/86 and the production of IL-12 that skews towards Th1 differentiation in CD4+ T cells. In addition to IL-12, CD40L signaling in T cells induces IFN-γ production. (Right) Model of CD40-CD40L crosstalk interactions between T cells, B cells and DCs. CD40 and CD40L are both reciprocally expressed on activated DCs and lymphocytes, and may engage in multi-directional crosstalk between cell types. CD40 signaling on DCs induces secretion of secrete IL-12 which promotes Th1 differentiation, IL-10 that induces Tregs or other cytokines that induces Th17 differentiation. CD40 signaling also induces BAFF and APRIL, which along with T cell-derived IL-2 and IL-4 [125], induces B cell class switching and secretion of IgG and IgA antibodies.
Another important consideration regarding the function of CD40 in DCs is the change in CD40 expression from immature to mature DCs. CD40 is expressed constitutively at relatively low levels on unactivated DCs [25]. In mice levels of CD40 are used as a marker to distinguish between inactivated and activated DCs, as its expression is up-regulated on DCs after encounter with microbial products (e.g. Toll-like receptor ligands) [40] and pathogens (e.g. viruses like HIV), as well as after uptake of apoptotic cells [41].
CD40 Signaling pathways in DCs
The role the CD40-CD40L pair plays in regulating B cell functions is well documented [9, 11, 42]. The signaling motifs within CD40’s cytoplasmic tail required for extrafollicular B cell differentiation versus germinal center formation in vivo are different [43]. CD40 ligation clearly inhibits the apoptosis of immature B cells including transitional B cells [44, 45]. A number of CD40-responsive genes have been identified including Bcl-2 family members cIAPs, c-myc, signaling elements like TRAF1 and Pim-1 kinase and cell surface molecules like CD40 itself, CD23 and CD21. A number of these genes are dependent on the NF-κB signaling pathway [42, 46–49]. These data suggest CD40 can program both survival and differentiation in B cells.
The signaling pathways induced downstream of CD40 engagement on DCs also have been studied and activate multiple genes important for DC function. The cytoplasmic tail of CD40 contains sites for the recruitment of TNF Receptor Associated Factor family of proteins (TRAFs). CD40 ligation results in the trimeric clustering and the signaling pathways activated downstream CD40 depends on the specific TRAF being recruited. The recruitment of TRAFs after CD40 ligation initiates signaling cascades that activate genes involved in cytokine production, as well as upregulation of co-stimulatory molecules such as CD80 and CD86, and other maturation markers [50]. CD40 signaling can recruit TRAFs 2, 3, 5 and 6, although TRAF6 appears to be the predominant player utilized in DCs [34]. The details of CD40 signaling have been reviewed elsewhere, and thus will not be discussed in great detail here [25, 34].The recruitment of TRAF2 and TRAF3 occurs downstream of CD40 signaling in human tonsillar B cells but not in monocyte-derived immature DCs (iDCs) [46]. Vidalain et al. reported TRAF3 (and to a lesser extent TRAF2) could be co-immunoprecipitated from CD40-containing lipid rafts stimulated human iDCs after CD40 ligation, and suggested that TRAF2 and TRAF3 may also be recruited downstream of CD40 signaling in DCs [51]. However, the authors did not show direct binding of TRAF2 or TRAF3 to the cytoplasmic tail of CD40, and in addition acknowledged that the use of detergent insolubility as the only criterion for whether a protein associated with the lipid raft was flawed. Since then no additional studies have been published to support this finding. At this time, TRAF6 appears to be the only member of the TRAF family that clearly is recruited downstream of CD40 ligation in DCs.
These signaling pathways downstream of CD40 ligation in DCs are similar to those activated by other receptors such as Toll-like receptors (TLRs) and RANK-RANKL (TRANCE R-TRANCE). However, CD40 ligation also induces functions distinct from signaling through TLRs or RANK. Thus CD40 induces important DC functions via signaling pathways that are both common with and unique from other receptor interactions.
CD40 and TLR signaling pathways
One of the major DC functions regulated by CD40 ligation is the production of cytokines. In DCs, TRAF6 is recruited downstream of CD40 ligation [52], resulting in activation of the p38 MAP Kinase (MAPK) and JNK (Jun Kinase) [53] leading to production of cytokines such as IL-12p40 [23] and IL-6 [54]. TRAF6 is also involved in the signaling pathways downstream of TLR ligation via MyD88 and TRIF signaling [40]. DCs from TRAF6 KO mice are unable to upregulate maturation markers MHC II and CD86, and cannot produce IL-6 or IL-12p40 in response to LPS or CD40L [55].
Both TLR and CD40 signaling can induce the activation of NFκB, which turns on a number of genes important to DC function. A recent study showed that CD40 ligation-induced DC antigen presentation required the activation of NF-κB –inducing kinase (NIK) to activate non-canonical NF-κB2 (p52/p100) [56]. DCs from alymphoplasia (aly) mice expressing a point mutation of NIK impairing its interaction with the IKK complex were unable to cross-present OVA antigen to CD8+ T cells. These aly DCs were also unable to cross-present OVA antigen to CD8+ T cells after LPS stimulation, which signals through both the MyD88 and TRIF pathways [40]. While it remains controversial whether TLR signaling truly induces NF-κB activation through the non-canonical pathway physiologically [40]. It appears that TLR and CD40 signaling overlap in the activation of NIK to turn on NF-κB2 through the non-canonical signaling pathway.
Although the cytoplasmic tail of CD40 can potentially recruit other TRAF family members, only TRAF6 has been clearly shown to be active in CD40 signaling pathways in DCs. While further research is required to elucidate if other TRAF members are involved in CD40 signaling in DCs, the finding that CD40 signaling in DCs depends on TRAF6 suggest that CD40 signaling of DCs normally only induces non-canonical activation of NF-κB. In contrast, signaling through MyD88 and TRIF can activate NF-κB through the canonical pathway (formation of Rel-A/p50 heterodimers) [40]. In addition, MyD88 and TRIF, activates unique pathways not triggered via CD40 signaling, such as the activation of interferon (IFN) response factors (IRFs).
CD40 signaling, however, can synergize with TLR9 signaling induce production of Type I IFN by human plasmacytoid DCs (pDCs): Kerkmann et al. detected enhanced production of IFNα by human pDCs stimulated with CpG-B and CD40L [57]. Since the addition of IFN-receptor (IFNR) blocking antibodies did not abrogate production of IFNα in response to CpG-B and CD40L, the authors concluded that CD40L enhancement of IFNα production was independent of an IFNR positive feedback loop. Neither IRF7 norIRF3 transcription was induced by CD40L stimulation, and thus CD40L appears to promote IFN production through a pathway distinct from that of TLR9 signaling.
CD40 and RANK-RANKL Signaling
Another member of the TNFRSF important in regulating DC function is RANK (Receptor Activator of NF-κB, also known as TRANCE-Receptor or TRANCE-R). Although a number of studies have examined the function of RANK in DCs, it is important to keep in mind that RANK expressed on cells other than DCs including osteoclasts, B cells and fibroblasts [58, 59]. Signaling through RANK is regulated through competitive binding of RANK to its ligand (RANKL, or TRANCE) by a soluble receptor called osteoprotegerin (OPG) [60]. CD40L stimulation of DCs upregulates the expression in DCs of both RANK [58] and OPG [61], underscoring the interconnectiveness of these two receptor-ligand pairs.
Similar to CD40, signaling through RANK-RANKL induces the production of pro-inflammatory cytokines. The production of cytokines by murine bone marrow-derived DCs (BMDCs) after either RANKL or CD40L stimulation induces the production of IL-1β, IL-1Rα, IL-6 as well as the T cell/natural killer (NK) cell-activating cytokine IL-15 [62]. However, CD40L stimulation also induces production of IL-12, which is important for inducing Th1 responses [23]. The RANK signaling pathway in DCs, like CD40 mainly utilizes TRAF6, but other TRAFs have also been implicated in RANK signaling of DCs, [59, 63]. Evidence that RANKL and CD40L trigger distinct signaling pathways in DCs comes from studies showing that RANKL signaling can enhance the survival of CD40L-stimulated DCs [64] and that DCs transduced with a CD40L adenoviral vector produce significantly more IL-12 and express more co-stimulatory molecules than DCs overexpressing RANKL [65]. Conversely, RANKL can enhance the expansion of CD8+ T cells in vivo under conditions where CD40L is not effective [66].
RANKL (TRANCE) -RANK signaling promotes the survival of DCs, most likely by the upregulation of Bcl-xL [59], although it upregulates Bcl-xL to a lesser extent than CD40L signaling [67]. The expression of Bcl-2 is also induced in RANKL-treated BMDCs [59]. Similar to RANK, ligation of CD40 induces signals that promote cell survival, thus making CD40 and an important molecular player involved in the regulation of DC apoptosis and survival. In murine DCs, CD40 ligation on DCs can induce survival by protection against Fas-induced apoptosis [67] as well as through upregulation of anti-apoptotic protein Bcl-xL [68]. Both CD40L and RANKL-induced DC survival requires NF-κB p50 and cRel [69]. In human iDCs, CD40 ligation induces upregulation of anti-apoptotic protein Bcl-2, which has been correlated to protection against Fas-induced apoptosis [70].
CD40 signaling of DCs induces expression of OPG [61]. Constitutive RANKL-RANK interactions between DCs sustain DC viability and Bcl-2 expression [36, 71]. However, OPG blocks both RANK-dependent DC survival and cytokine production [36]. Thus, CD40 signaling of DCs and OPG production may induce feedback regulation that limits the duration of DC-mediated activation.
CD40 and DC Subsets
There are multiple DC subsets in mice and humans [72, 73]; however, the growing expanse of DC subset definitions has limited the analysis of CD40 expression and function being conducted for each subset. Human peripheral blood pDCs (CD4+,CD123+, BDCA1+, BDCA4+) and myeloid DCs (mDCs: CD4+CD11+CD33+) upregulate CD40 in response to TLR9 (CpG) or TLR4 (LPS) agonists [74]. The pDC subset stimulated with CpG upregulated CD40 to a greater extent compared to mDCs stimulated with LPS in vitro, while conversely, CD40 upregulation was not induced in LPS-stimulated pDCs [74, 75]. pDCs and mDCs both upregulated CD40 to similar levels in response to the TLR7 ligand Imiquimod [75, 76]. Thus, the level of CD40 expression in peripheral blood DCs is not subset specific but rather dictated by the signaling pathway and receptor utilized when the DC becomes activated.
Indeed, a recent study examining the expression of maturation markers including CD40 on human pDCs versus mDCs in response to Hepatitis C virus (HCV) showed that HCV inhibits TLR7-mediated upregulation of CD40 in pDCs, but not TLR3-mediated upregulation of CD40 in mDCs [77]. Also, Veckman and Julkenen showed that human pDCs increased more CD40 after exposure to Streptococcus pyogenes and Influenza A compared to mDCs [78]. Influenza A induced greater fold CD40 induction in pDCs compared to S. pyogenes, whereas in mDCs, Influenza A failed to induce CD40 upregulation. Again differences in the upregulation of CD40 expression appear to be more dependent on the type of stimulation rather than the DC subset.
However, this may not always be the case. In human lungs, basal expression of CD40 and other co-stimulatory markers CD80 and CD86 appear to be higher in mDCs compared to pDCs [28]. In addition, mature DC subsets in the human thymus differentiated by the expression of CD11b show slightly different expression patterns of CD40 [79]. Additional work is required to compare expression of CD40 in subsets localized to other tissues, particularly in mucosal tissues, spleen and lymph nodes.
The broad classification of human DCs has been used in defining mouse DC subsets [73]. In the mouse spleen, there appears to be no difference in levels of basal CD40 expression between splenic CD8−CD4+ and CD8−CD4− myeloid DCs [80]. In the liver, CD11chiB220− DC subsets express higher basal levels of CD40 expression compared to that of CD11cintB220− subsets, and there appears to be little or no expression of CD40 on the B220+CD11cint population [81]. Activation of DC subsets using murine cytomegalovirus (MCMV) induces high upregulation of CD40 on the CD11chiB220− population, but not CD11cintB220+ DCs [81]. In addition, CD8 expression on hepatic DC subsets is correlated with higher basal expression of CD40 compared to CD8− DC populations [82].
Expression Levels of CD40 and DC Function
The term ‘regulatory DCs’ has been associated with subsets of DCs that are distinct from ‘immature’ or ‘tolerogenic’ DCs that present signal 1 (e.g. antigen peptide-MHC complex) but not a co-stimulator signal 2, and thus can induce anergy in cognate lymphocytes [83, 84]. It describes subsets of DCs found in both mice and humans that induce T cells with poor proliferative capacity, and produce IL-10 and TGF-β rather than Th1 or Th2 cytokines. Notably, these DCs also induce the generation of regulatory T cells (Tregs) [85–88]. DCs possessing these regulatory effector functions encompass a heterogenous and broad range of DCs that are induced under various types of stimuli and infection [89], so we will narrow this discussion to a specific subset identified in mice [86–88].
Wakkach et al. identified one particular population of regulatory CD11cintCD45RBhi DCs [88]. This population is found in the spleen and the lymph nodes and has the capacity to induce tolerance by promoting differentiation of Treg cells through secreting IL-10. Stromal cells may be required for the differentiation and support of this DC subset. One characteristic of the CD45RB+ regulatory DCs is that they do not upregulate CD40 even after infection or TLR stimulation; indeed, they do not upregulate surface CD40 even in response to LPS [86, 88] or Plasmodium falciparum infection [90].
Another recent study reported the induction of DCs possessing similar effector functions to the CD11cintCD45RBhi DCs, and which fail to upregulate CD40 in response to Trypanosome cruzi exposure in vitro [91]. However this study employed the use of bone-marrow derived DCs (BMDCs) and moreover, did not use CD45RB as a marker. Thus it is difficult to compare whether these cells are the same subset as identified by Wakkach et al.
Whether CD40 signaling is associated with the function of these DC subsets has yet to be determined. Given the low expression of CD40 on the regulatory DCs, it would be of interest to know if TNFR family members other than CD40 such as RANK regulate this DC subset. Furthermore, one could speculate that the decreased expression of co-stimulatory molecules on these DCs may contribute to their decreased capacity to induce effector T helper cells and preferential induction of Tregs. Indeed, it was shown that steady-state DCs without anti-CD40 activation were the most efficient at the induction of CD4+CD25+Foxp3+ Tregs from naive CD4+CD25− T cells in the periphery. [92]. The authors targeted splenic CD8α+ DC subsets with a fusion protein of an anti-DEC205 antibody conjugated to the hemagglutinin antigen from the Influenza virus (anti-DEC-HA), which by itself does not alter maturation status of steady-state DCs [93]. DCs targeted with low doses of anti-DEC205-HA were most efficient at inducing de novo CD25+Foxp3+ Tregs from naive CD4+CD25−Foxp3− progenitors when compared to DCs targeted with high dose antigen or in the presence of anti-CD40 stimulus.
A more recent study examined murine DCs that displayed a ‘semi-mature’ phenotype and its role in regulating T cell responses in collagen-induced rheumatoid arthritis (CIA) [94]. The authors stimulated murine BMDCs with naked DNA plasmid versus LPS and showed that treatment with plasmid induced levels of cytokines and expression of co-stimulatory molecules (including CD40) that were intermediate between LPS and unstimulated BMDCs, and hence termed these DCs as ‘semi-mature’. Functionally, semi-mature DCs were similar to LPS-stimulated mature DCs in ability to induce Tregs and provided protection against CIA. Although there was no enhanced efficacy of ‘semi-mature’ DCs to induce Tregs compared to LPS-stimulated DCs expressing higher levels of CD40, the levels of IL-10 and TGF-β transcription were the highest in animals that had received transfer of ‘semi-mature’ DCs in the peripheral lymph nodes early after transfer. Thus ‘semi-mature’ DCs may induce alternate T cell programming that result in protection from CIA.
Cytokine and pharmacological treatments also affect the expression of CD40 and other co-stimulatory molecules associated with maturation status of DCs. The use of pharmacological treatments such as Vitamin D can modulate the expression of surface CD40 and overall maturation of DCs. These DCs have been shown to induce Tregs and increase IL-10 production resulting in increased tolerance to transplants and decreased diabetes in mouse models [85].
Sato et al. initially showed that human or murine DCs cultured with immunomodulatory cytokines could result in suppression of effector T cell responses [87]. They showed that human DCs cultured in the presence of IL-10 and TGF-β could induce expansion of CD4+CD25+ and IL-10 secreting CD8+CD28− Treg cells that could suppress CD4+ T cell functions. In a model of xenogeneic graft-versus-host disease (XGVHD), murine BMDCs cultured with IL-10 and TGF-β (which also expressed low levels of co-stimulatory molecules and had decreased capacity to induce expansion of primed human T cells) were transferred into a SCID mouse grafted with human peripheral blood leukocytes (hu-PBL-SCID). The hu-PBL-SCID mice receiving murine BMDCs cultured with IL-10 and TGF-β had significantly higher rates of survival from XGVHD compared to hu-PBL-SCID mice that received murine BMDCs cultured in GM-CSF alone.
In another study, Lan et al. showed that Balb/c murine BMDCs generated in GM-CSF with IL-10 and TGF-β are more resistant to LPS-induced upregulation of CD40 and preferentially induced higher percentages of CD4+CD25+Foxp3+ Tregs in vitro compared to BMDCs generated with GM-CSF alone [95]. In an MHC-mismatch rejection model, BMDCs grafts from Balb/c mice were tolerated by C57BL/10J recipient mice if the BMDCs were generated in the presence of IL-10/TGF- β. In addition, tolerized grafts were shown to have significant numbers of CD4+Foxp3+ infiltrates compared to rejected grafts, thus supporting the notion that IL-10/TGF-β alters BMDC function in the regulation of T cell responses.
While the role of CD40 signaling and expression on DCs has not been directly connected to the induction or maintainence of Tregs, the levels of CD40 expression on DCs may influence CD40-CD40L interactions between DCs and T cells and thus lymphocyte programming by DCs. In this regard, it is relevant to consider how CD40 and/or CD40L signaling of DCs and how that affects regulation of B and T lymphocytes. Thus we next discuss the differential expression of CD40 and CD40L on DCs and lymphocytes and how crosstalk between these molecules may affect DC-lymphocyte interactions.
Regulation of Lymphocytes by CD40+ and CD40L+ DCs
CD40 not only is expressed on APCs such as B cells and DCs, but also has been detected on activated T cells (Fig. 1) [34]. Similarly, CD40L not only is expressed on activated T cells, but also on activated human and murine DCs (Fig. 1) [26, 30]. Thus, it is important to consider the role of possible CD40L-CD40-dependent bi-directional crosstalk that occurs between DCs and lymphocytes. Indeed it was shown early on that CD40-CD40L interactions between T cell and DCs provides reciprocal effects that both regulate T cells and DCs [96, 97]. While it is clear that CD40L crosslinking can signal T cells e.g., to produce IFN-γ [98, 99], it is not clear how ligation of CD40L on B cells or DCs affects their functions.
CD40 expression on DCs is clearly important for the effector functions regulating lymphocytes. As noted above ligation of CD40 via anti-CD40 mAbs or CD40L induces upregulation of co-stimulatory molecules, adhesion molecules, and the Th1-polarizing cytokine IL-12 in both mouse and human DCs [23, 100, 101]. The similarities between human and mouse CD40 enabled mouse genetics to be used for further understanding the role of CD40 in DC functions. CD40 KO (CD40−/−) mice were generated by two different groups, one with the CD40 defect targeted to hematopoietic cells [102], the other, with the defect restricted to lymphocytes [103]. Initially, both groups showed that CD40 deficient mice were impaired in the generation of T-dependent antibody responses. Later the CD40−/− mouse model was used to study DC-lymphocyte interactions initially in the context of cardiac allograft rejection [104] and subsequently by Mackey et al. [96].
Mackey et al. showed that transfer of CD40+/+ DCs into CD40−/− mice could rescue recipients from death by tumor challenge [96]. Exogenous IL-12 could partially rescue CD40L−/− mice from succumbing to tumor challenge. The authors concluded that the absence of CD40-induced IL-12 production following cognate T cell-DC interactions results in a defect in mounting effective Th1 responses and susceptibility to tumor load. However, a later study showed that CD40L−/− splenic APCs produced IL-12 to similar levels as that of CD40+/+ mice following a tumor allograft challenge, but this did not protect CD40L−/− mice from succumbing to tumor load [105]. The authors reported a defect in priming tumor-specific CTLs in CD40L−/− mice which could not be rescued with the addition of exogenous CD40L in vivo. Thus the requirement for CD40-CD40L interactions may not solely be due to the lack of IL-12 production in defective CD40 signaling on DCs, and suggests that CD40-CD40L interactions between APCs and T cells involves other mechanisms (e.g. production of other cytokines, direct signaling via CD40) in the priming of effector T cells.
CD40 may also be involved in differentiation of T cell subsets other than Th1 effectors. As mentioned, “regulatory DCs” expressing low levels of CD40 preferentially induces Treg generation. A recent study by Iezzi et al. illustrated a requirement for the expression of CD40 and interaction with CD40L on CD4+ T cells to drive to Th17, but not Th1 differentiation [106]. CD40−/− mice had lower numbers of IL-17 and IFN-γ-producing CD4+ T cells compared to wildtype mice after immunization with a lymphocytic choriomeningitis virus peptide compared to CD40+/+ mice, but the numbers of IFN-γproducing cells could be rescued in CD40−/− using a higher dose of antigen. However, both CD40−/− and CD40+/+ mice were able to induce comparable numbers of IFN-γproducing cells after immunization with Listeria monocytogenes. In addition, CD40−/− mice also are protected from MOG peptide-induced experimental autoimmune encephamyelitis (EAE), a disease whose immunopathology is associated with IL-17-producing CD4+ T cells [107, 108]. The protection in CD40−/− mice from EAE was correlated with decreased Foxp3+, IL-17+ or IFN-γ+ T cell infiltrates into the central nervous system.
Although Iezzi et al. did not test if transfer of CD40+/+ DCs or Tregs could induce Th17 effectors or IL-17 production resulting in exacerbation of EAE in CD40−/− mice, their data emphasize the importance of CD40 signaling in enabling DCs to induce effector populations such as Tregs and Th17 in certain antigen environments in vivo. Thus CD40 plays a role in DC-mediated priming of multiple effector T cell responses in addition to the Th1 subset. As more novel effector T cell subsets continue to be defined, further work will be required to understand the direct role of CD40 signaling in DCs in regulation of these subsets.
CD40 Expression on DCs and the Regulation of B cells
Both DCs and B cells express CD40 constitutively, and both cell types interact with and regulate T cells by ligating CD40L. As such, CD40-CD40L interactions have not been heavily studied in the context of DC-B cell interactions. However, CD40L is expressed on activated B cells and DCs (Fig. 1). Thus it is possible that B cells and DCs directly interact via CD40-CD40L. This seems all the more likely given the growing literature showing that DCs regulate B cell responses through cell contact-dependent mechanisms independent of CD4+ T cell help [109–111].
Wykes and MacPherson showed that the expression of CD40 on DCs and B cells is required for optimal proliferation of B cells in absence of any exogenous stimuli [111]. The loss of CD40 on B cells also diminishes long term survival of B cells in the presence of DCs. While the mechanism of this interaction has not been established, it suggested that CD40 expressed on DCs played a direct role in regulating B cell proliferation, and perhaps more in regulation of B cell homeostasis.
Bergtold et al. showed that BMDCs directly enhanced B cell proliferation in response to anti-CD40 compared to B cells cultured with anti-CD40 alone [109]. DCs lacking activating receptors FcγRI and FcγRIII utilize the inhibitory FcγRIIB to internalize immune complexes, and recycles antigens to the surface for presentation to B cells in B cell-DC clusters. The loss of FcγRI and FcγRIII on DCs also increased B cell proliferation and CD86 expression in response to anti-CD40 stimulation, especially if the DCs were pulsed with anti-IgM containing immune complexes. Thus the recycling of immune complexes taken up by DCs via FcγRIIB endocytosis induced optimal B cell activation. It remains unclear if CD40 stimulation of B cells, DCs or both cell types was necessary for optimal B cell proliferation. Since B cells and DCs can express both CD40 and CD40L (Fig. 1), it is possible that CD40-driven proliferation could occur in the absence of T cells.
Cytokines produced by DCs activated by CD40 signaling can regulate T cell-independent B cell antibody responses. T-cell dependent regulation of B cell antibody responses is thought to require CD40L on T cells interacting with CD40 on B cells [112]. Litinskiy et al. showed that DCs activated by CD40L induces the expression of TNF family member cytokines B lymphocyte stimulator protein (BLyS, also known as BAFF, TALL-1, THANK and zTNF4) and a proliferation-inducing ligand (APRIL) [113]. BAFF binds to BAFF Receptor (BAFF-R) and B cell maturation antigen (BCMA) on B cells, while APRIL binds to transmembrane activator and calcium modulator and cyclophylin ligand interactor (TACI) and BCMA on B cells [114]. BAFF and APRIL promoted B cell survival, class switch recombination and secretion of IgG and IgA antibodies. The addition of anti-BAFF, BCMA-Ig or TACI-Ig to neutralize DC-derived BAFF or APRIL abrogated antibody production and class switching, but was not observed with the addition of CD40-Ig. The authors concluded that B cell antibody responses are regulated by cytokines produced during CD40 signaling on DCs, and not through DC-induced CD40 signaling on B cells.
This model is supported by subsequent studies illustrating the importance of DC-derived BAFF and APRIL in regulating B cell antibody responses. Konrad et al. showed that BMDCs pulsed with intestinal bacteria induced IgA production from B cells in the Peyer’s patches and that IgA production could be abrogated with blockade of BAFF or APRIL by TACI-Ig fusion protein [115]. Later Tezuka et al. showed that mice deficient in nitric oxide production (iNOS KO mice) have DCs that secrete decreased amounts of APRIL [116]; as a result T-independent IgA class switching and secretion is impaired unless the mice are adoptively transferred with wildtype DCs which can produce APRIL. Finally, we found that BAFF produced by human monocyte-derived DCs enhances anti-IgM but not CD40L-activated B cells [117]. Collectively these studies illustrate that cytokines produced by DCs via CD40 signaling can regulate B cell antibody responses.
CD40L Expression on DCs and the Regulation of Adaptive Immunity
As mentioned, CD40L is expressed on activated T cells, and is important both in providing co-stimulation for effector T cell functions [118–121] as well as inducing proper ‘licensing’ of DCs and APCs to prime CD8+ T cells to become CTLs [83, 122–124]. Most of these studies were conducted using mice with CD40L deficiency in all cells of the hematopoietic compartment. Again it is important to remember that CD40L is expressed not only on T cells but also on human and murine DCs [26, 28–30].
We found that human peripheral blood DCs as well as tonsillar B cells bound to an anti-CD40L monoclonal antibody [26]. Expression of CD40L was upregulated via CD40 ligation or PMA/Ionomycin treatment of isolated DCs. DCs pre-activated through CD40 induced B cells to produce IgG and IgA antibodies by a CD40L-CD40 pathway since exogenous anti-CD40L blocked antibody production. These data support the idea that DCs also regulate B cells via CD40-CD40L interactions (Fig. 1).
Johnson et al. focused on the role of CD40L in CD4-independent CD8+ T cell responses [30]. As with human DCs [26], murine splenic DCs expressed CD40L after CD40 ligation and also after TLR stimulation. In an in vitro mixed lymphocyte reaction, CD40L−/− DCs induced less proliferation of CD40+/+ CD8+ T cells. Conversely, CD40−/−CD8+ T cells had decreased proliferative response to CD40L+/+ DCs. These finding strongly suggest that CD40L on DCs and CD40 on CD8+ T cells are required for efficient CD8+ T cell priming.
Other in vivo studies by Johnson et al. implicated CD40L and CD40 in the priming of CTLs by DCs [30]. Mixed bone marrow chimeric mice reconstituted with CD40L−/− and CD40L+/+CD11c+-DTR bone marrow were generated; depletion of CD40L+/+ CD11c-DTR DCs decreased specific lysis of OVA-coated splenocytes that had been transferred into the chimeric mice. CD4+ T cells were depleted with anti-CD4 antibody prior to transfer of OVA-coated splenocytes, showing that CD40L expression on DCs could induce CTL priming in the absence of CD4+ T cell help. Thus the authors concluded that CD40L expression on DCs primes CD4-independent CTL responses.
The mechanism by which CD40L expression on DCs induces CTL priming has not been elucidated. It is possible that CD40L signaling on DCs induces the production of cytokines that regulate lymphocytes. Another possibility is that DCs expressing CD40L induces CD40 signaling on activated lymphocytes. While the mechanism remains unknown, these studies reveal that DCs and lymphocytes can express CD40 and CD40L, and can participate in reciprocal crosstalk interactions (Fig. 1).
Concluding Remarks
CD40 was initially characterized as a co-stimulatory molecule expressed on APCs that played a central role in B and T cell activation. However, this molecular pair functions in the regulation of both APCs and effector lymphocytes (Fig. 1). As we understand more about the number of different DC and T cell subsets, we are likely to find that CD40-CD40L interactions play important and distinct roles in regulating these novel subsets. In addition, as we continue to understand how innate immunity cells directly regulate B cells and antibody responses, the influence of CD40 and CD40L in these interactions should be further clarified. Further insights into the functions of CD40-CD40L interactions will advance our understanding of immune cell crosstalk and interdependent regulation of the immune system.
Acknowledgments
Grant Information: Supported by NIH grants AI52203 and DE16381.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986;83:4494–8. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ledbetter JA, Shu G, Gallagher M, Clark EA. Augmentation of normal and malignant B cell proliferation by monoclonal antibody to the B cell-specific antigen BP50 (CDW40) J Immunol. 1987;138:788–94. [PubMed] [Google Scholar]
- 3.Stamenkovic I, Clark EA, Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. Embo J. 1989;8:1403–10. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres RM, Clark EA. Differential increase of an alternatively polyadenylated mRNA species of murine CD40 upon B lymphocyte activation. J Immunol. 1992;148:620–6. [PubMed] [Google Scholar]
- 5.Clark EA. CD40: a cytokine receptor in search of a ligand. Tissue Antigens. 1990;36:33–6. doi: 10.1111/j.1399-0039.1990.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 6.Hart DN, McKenzie JL. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988;168:157–70. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdic O, et al. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–9. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 8.Harnett MM. CD40: a growing cytoplasmic tale. Sci STKE. 2004:pe25. doi: 10.1126/stke.2372004pe25. [DOI] [PubMed] [Google Scholar]
- 9.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–8. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–31. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 11.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 13.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 14.Brown MP, Topham DJ, Sangster MY, Zhao J, Flynn KJ, Surman SL, et al. Thymic lymphoproliferative disease after successful correction of CD40 ligand deficiency by gene transfer in mice. Nat Med. 1998;4:1253–60. doi: 10.1038/3233. [DOI] [PubMed] [Google Scholar]
- 15.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 16.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–3. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 17.Korthauer U, Graf D, Mages HW, Briere F, Padayachee M, Malcolm S, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–41. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 18.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinchuk LM, Polacino PS, Agy MB, Klaus SJ, Clark EA. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity. 1994;1:317–25. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med. 1996;183:159–67. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galy AH, Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992;149:775–82. [PubMed] [Google Scholar]
- 23.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–25. [PubMed] [Google Scholar]
- 25.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 26.Pinchuk LM, Klaus SJ, Magaletti DM, Pinchuk GV, Norsen JP, Clark EA. Functional CD40 ligand expressed by human blood dendritic cells is up-regulated by CD40 ligation. J Immunol. 1996;157:4363–70. [PubMed] [Google Scholar]
- 27.Salgado CG, Nakamura K, Sugaya M, Tada Y, Asahina A, Koyama Y, et al. Functional CD40 ligand is expressed on epidermal Langerhans cells. J Leukoc Biol. 1999;66:281–5. doi: 10.1002/jlb.66.2.281. [DOI] [PubMed] [Google Scholar]
- 28.Masten BJ, Yates JL, Pollard Koga AM, Lipscomb MF. Characterization of accessory molecules in murine lung dendritic cell function: roles for CD80, CD86, CD54, and CD40L. Am J Respir Cell Mol Biol. 1997;16:335–42. doi: 10.1165/ajrcmb.16.3.9070619. [DOI] [PubMed] [Google Scholar]
- 29.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol. 2006;7:740–6. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30:218–27. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grammer AC, McFarland RD, Heaney J, Darnell BF, Lipsky PE. Expression, regulation, and function of B cell-expressed CD154 in germinal centers. J Immunol. 1999;163:4150–9. [PubMed] [Google Scholar]
- 32.Lipsky PE, Attrep JF, Grammer AC, McIlraith MJ, Nishioka Y. Analysis of CD40-CD40 ligand interactions in the regulation of human B cell function. Ann N Y Acad Sci. 1997;815:372–83. doi: 10.1111/j.1749-6632.1997.tb52088.x. [DOI] [PubMed] [Google Scholar]
- 33.Wykes M, Poudrier J, Lindstedt R, Gray D. Regulation of cytoplasmic, surface and soluble forms of CD40 ligand in mouse B cells. Eur J Immunol. 1998;28:548–59. doi: 10.1002/(SICI)1521-4141(199802)28:02<548::AID-IMMU548>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan B, Thomas R. Recent advances on the role of CD40 and dendritic cells in immunity and tolerance. Curr Opin Hematol. 2003;10:272–8. doi: 10.1097/00062752-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Williamson E, Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol. 2002;169:3606–12. doi: 10.4049/jimmunol.169.7.3606. [DOI] [PubMed] [Google Scholar]
- 36.Chino T, Santer DM, Giordano D, Chen C, Li C, Chen CH, et al. Effects of oral commensal and pathogenic bacteria on human dendritic cells. Oral Microbiol Immunol. 2009;24:96–103. doi: 10.1111/j.1399-302X.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 38.Brodeur SR, Angelini F, Bacharier LB, Blom AM, Mizoguchi E, Fujiwara H, et al. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity. 2003;18:837–48. doi: 10.1016/s1074-7613(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 39.Clark EA, Craxton A. A CD40 bridge between innate and adaptive immunity. Immunity. 2003;18:724–5. doi: 10.1016/s1074-7613(03)00146-8. [DOI] [PubMed] [Google Scholar]
- 40.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–9. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–96. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 42.Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunol Res. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- 43.Yasui T, Muraoka M, Takaoka-Shichijo Y, Ishida I, Takegahara N, Uchida J, et al. Dissection of B cell differentiation during primary immune responses in mice with altered CD40 signals. Int Immunol. 2002;14:319–29. doi: 10.1093/intimm/14.3.319. [DOI] [PubMed] [Google Scholar]
- 44.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–48. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Sater RA, Sandel PC, Monroe JG. B cell receptor-induced apoptosis in primary transitional murine B cells: signaling requirements and modulation by T cell help. Int Immunol. 1998;10:1673–82. doi: 10.1093/intimm/10.11.1673. [DOI] [PubMed] [Google Scholar]
- 46.Aicher A, Shu GL, Magaletti D, Mulvania T, Pezzutto A, Craxton A, et al. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J Immunol. 1999;163:5786–95. [PubMed] [Google Scholar]
- 47.Craxton A, Shu G, Graves JD, Saklatvala J, Krebs EG, Clark EA. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol. 1998;161:3225–36. [PubMed] [Google Scholar]
- 48.Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, et al. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proc Natl Acad Sci U S A. 2002;99:1497–502. doi: 10.1073/pnas.032665099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu N, Ramirez LM, Lee RL, Magnuson NS, Bishop GA, Gold MR. CD40 signaling in B cells regulates the expression of the Pim-1 kinase via the NF-kappa B pathway. J Immunol. 2002;168:744–54. doi: 10.4049/jimmunol.168.2.744. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6:1333–8. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Vidalain PO, Azocar O, Servet-Delprat C, Rabourdin-Combe C, Gerlier D, Manie S. CD40 signaling in human dendritic cells is initiated within membrane rafts. Embo J. 2000;19:3304–13. doi: 10.1093/emboj/19.13.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, et al. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–8. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 53.Pullen SS, Dang TT, Crute JJ, Kehry MR. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J Biol Chem. 1999;274:14246–54. doi: 10.1074/jbc.274.20.14246. [DOI] [PubMed] [Google Scholar]
- 54.Yanagawa Y, Onoe K. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells. Immunology. 2006;117:526–35. doi: 10.1111/j.1365-2567.2006.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, et al. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–63. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 56.Lind EF, Ahonen CL, Wasiuk A, Kosaka Y, Becher B, Bennett KA, et al. Dendritic cells require the NF-kappaB2 pathway for cross-presentation of soluble antigens. J Immunol. 2008;181:354–63. doi: 10.4049/jimmunol.181.1.354. [DOI] [PubMed] [Google Scholar]
- 57.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170:4465–74. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 58.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 59.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bharti AC, Aggarwal BB. Ranking the role of RANK ligand in apoptosis. Apoptosis. 2004;9:677–90. doi: 10.1023/B:APPT.0000045780.10463.c6. [DOI] [PubMed] [Google Scholar]
- 61.Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161:6113–21. [PubMed] [Google Scholar]
- 62.Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162:2562–8. [PubMed] [Google Scholar]
- 63.Galibert L, Tometsko ME, Anderson DM, Cosman D, Dougall WC. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. J Biol Chem. 1998;273:34120–7. doi: 10.1074/jbc.273.51.34120. [DOI] [PubMed] [Google Scholar]
- 64.Yu Q, Gu JX, Kovacs C, Freedman J, Thomas EK, Ostrowski MA. Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8+ T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals. J Immunol. 2003;170:1797–805. doi: 10.4049/jimmunol.170.4.1797. [DOI] [PubMed] [Google Scholar]
- 65.Yurkovetsky ZR, Shurin GV, Barry DA, Schuh AC, Shurin MR, Robbins PD. Comparative analysis of antitumor activity of CD40L, RANKL, and 4–1BBL in vivo following intratumoral administration of viral vectors or transduced dendritic cells. J Gene Med. 2006;8:129–37. doi: 10.1002/jgm.834. [DOI] [PubMed] [Google Scholar]
- 66.Miyahira Y, Akiba H, Katae M, Kubota K, Kobayashi S, Takeuchi T, et al. Cutting edge: a potent adjuvant effect of ligand to receptor activator of NF-kappa B gene for inducing antigen-specific CD8+ T cell response by DNA and viral vector vaccination. J Immunol. 2003;171:6344–8. doi: 10.4049/jimmunol.171.12.6344. [DOI] [PubMed] [Google Scholar]
- 67.Chen A, Xu H, Choi Y, Wang B, Zheng G. TRANCE counteracts FasL-mediated apoptosis of murine bone marrow-derived dendritic cells. Cell Immunol. 2004;231:40–8. doi: 10.1016/j.cellimm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol. 2004;5:583–9. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- 69.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–70. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 70.Bjorck P, Banchereau J, Flores-Romo L. CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int Immunol. 1997;9:365–72. doi: 10.1093/intimm/9.3.365. [DOI] [PubMed] [Google Scholar]
- 71.Cremer I, Dieu-Nosjean MC, Marechal S, Dezutter-Dambuyant C, Goddard S, Adams D, et al. Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood. 2002;100:3646–55. doi: 10.1182/blood-2002-01-0312. [DOI] [PubMed] [Google Scholar]
- 72.Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol. 2008;86:439–52. doi: 10.1038/icb.2008.28. [DOI] [PubMed] [Google Scholar]
- 73.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 74.Hellman P, Eriksson H. Early activation markers of human peripheral dendritic cells. Hum Immunol. 2007;68:324–33. doi: 10.1016/j.humimm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 75.Della Bella S, Giannelli S, Taddeo A, Presicce P, Villa ML. Application of six-color flow cytometry for the assessment of dendritic cell responses in whole blood assays. J Immunol Methods. 2008;339:153–64. doi: 10.1016/j.jim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–12. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang H, Russell RS, Yonkers NL, McDonald D, Rodriguez B, Harding CV, et al. Differential effects of hepatitis C virus JFH1 on human myeloid and plasmacytoid dendritic cells. J Virol. 2009 doi: 10.1128/JVI.02671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veckman V, Julkunen I. Streptococcus pyogenes activates human plasmacytoid and myeloid dendritic cells. J Leukoc Biol. 2008;83:296–304. doi: 10.1189/jlb.0707457. [DOI] [PubMed] [Google Scholar]
- 79.Vandenabeele S, Hochrein H, Mavaddat N, Winkel K, Shortman K. Human thymus contains 2 distinct dendritic cell populations. Blood. 2001;97:1733–41. doi: 10.1182/blood.v97.6.1733. [DOI] [PubMed] [Google Scholar]
- 80.Martin P, del Hoyo GM, Anjuere F, Ruiz SR, Arias CF, Marin AR, et al. Concept of lymphoid versus myeloid dendritic cell lineages revisited: both CD8alpha(-) and CD8alpha(+) dendritic cells are generated from CD4(low) lymphoid-committed precursors. Blood. 2000;96:2511–9. [PubMed] [Google Scholar]
- 81.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, et al. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–65. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 82.O’Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8 alpha+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 83.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 84.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 85.Adorini L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann N Y Acad Sci. 2003;987:258–61. doi: 10.1111/j.1749-6632.2003.tb06057.x. [DOI] [PubMed] [Google Scholar]
- 86.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–24. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 87.Sato K, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–79. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 88.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 89.Coquerelle C, Moser M. Are dendritic cells central to regulatory T cell function? Immunol Lett. 2008;119:12–6. doi: 10.1016/j.imlet.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Wong KA, Rodriguez A. Plasmodium infection and endotoxic shock induce the expansion of regulatory dendritic cells. J Immunol. 2008;180:716–26. doi: 10.4049/jimmunol.180.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poncini CV, Alba Soto CD, Batalla E, Solana ME, Gonzalez Cappa SM. Trypanosoma cruzi induces regulatory dendritic cells in vitro. Infect Immun. 2008;76:2633–41. doi: 10.1128/IAI.01298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 93.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Jaen O, Rulle S, Bessis N, Zago A, Boissier MC, Falgarone G. Dendritic cells modulated by innate immunity improve collagen-induced arthritis and induce regulatory T cells in vivo. Immunology. 2009;126:35–44. doi: 10.1111/j.1365-2567.2008.02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, et al. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–77. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 96.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094–8. [PubMed] [Google Scholar]
- 97.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 98.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, et al. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–7. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 99.Mackey MF, Barth RJ, Jr, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–28. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 100.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 102.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 103.Castigli E, Alt FW, Davidson L, Bottaro A, Mizoguchi E, Bhan AK, et al. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994;91:12135–9. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu L, Li W, Fu F, Chambers FG, Qian S, Fung JJ, et al. Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. 1997;64:1808–15. doi: 10.1097/00007890-199712270-00031. [DOI] [PubMed] [Google Scholar]
- 105.Shepherd DM, Kerkvliet NI. Disruption of CD154:CD40 blocks generation of allograft immunity without affecting APC activation. J Immunol. 1999;163:2470–7. [PubMed] [Google Scholar]
- 106.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L crosstalk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106:876–81. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 108.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–6. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 109.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–14. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 110.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–6. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 111.Wykes M, MacPherson G. Dendritic cell-B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100:1–3. doi: 10.1046/j.1365-2567.2000.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–60. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 113.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 115.Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050–9. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 116.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–33. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 117.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–71. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 118.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–20. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 119.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–3. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 121.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–31. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 122.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, et al. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–42. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol. 2000;67:607–14. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- 124.Kelleher M, Beverley PC. Lipopolysaccharide modulation of dendritic cells is insufficient to mature dendritic cells to generate CTLs from naive polyclonal CD8+ T cells in vitro, whereas CD40 ligation is essential. J Immunol. 2001;167:6247–55. doi: 10.4049/jimmunol.167.11.6247. [DOI] [PubMed] [Google Scholar]
- 125.DeKruyff RH, Rizzo LV, Umetsu DT. Induction of immunoglobulin synthesis by CD4+ T cell clones. Semin Immunol. 1993;5:421–30. doi: 10.1006/smim.1993.1048. [DOI] [PubMed] [Google Scholar]