Origins of correlated activity in an olfactory circuit (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 1.
Published in final edited form as: Nat Neurosci. 2009 Aug 16;12(9):1136–1144. doi: 10.1038/nn.2376
Abstract
Multineuronal recordings often reveal synchronized spikes in different neurons. How correlated spike timing affects neural codes depends on the statistics of correlations, which in turn reflects the connectivity that gives rise to correlations. However, determining the connectivity of neurons recorded in vivo can be difficult. Here, we investigate the origins of correlated activity in genetically-labeled neurons of the Drosophila antennal lobe. Dual recordings show synchronized spontaneous spikes in projection neurons (PNs) postsynaptic to the same type of olfactory receptor neuron (ORN). Odors increase these correlations. The primary origin of correlations lies in the divergence of each ORN onto every PN in its glomerulus. Reciprocal PN-PN connections make a smaller contribution to correlations, and PN spike trains in different glomeruli are only weakly correlated. PN axons from the same glomerulus reconverge in the lateral horn, where pooling redundant signals may allow lateral horn neurons to average out noise that arises independently in these PNs.
Introduction
Nearby neurons in the brain often show correlated spontaneous activity and correlated trial-to-trial variability in their responses to sensory stimuli. These correlations have important consequences for the way information is processed by neural circuits1–3. Correlations can be useful if they create additional coding capacity4, 5, confer robustness6, 7, or cancel shared noise in a population response8. Conversely, they can be detrimental if they limit the ability of downstream neurons to increase their signal-to-noise ratio by averaging multiple inputs9.
The way correlated activity arises within a neural circuit has important consequences for how correlations might be interpreted by subsequent layers of the circuit. For example, if correlated activity arises from common feedforward input, then correlated spikes could carry information that is not present when correlations are ignored; alternatively, if correlations arise purely from reciprocal connections, then ignoring correlations may not result in a loss of information10. Also, correlations will have no impact unless correlated spike trains converge onto some of the same postsynaptic neurons. Therefore, it is useful to know whether neurons having correlated activity share presynaptic inputs, and whether they also share postsynaptic targets. However, it can be difficult to precisely determine the connectivity of multiple specific neurons recorded simultaneously in vivo.
In this study we use the Drosophila antennal lobe as a model circuit for addressing these issues. In this preparation it is possible to genetically label small subclasses of neurons or individual neurons. The glomerular architecture of the antennal lobe also helps us define neural connectivity. By recording from pairs of labeled neurons in vivo, we can investigate how correlations depend on connectivity.
Previous studies have reported correlated activity in the insect antennal lobe, the vertebrate olfactory bulb, and analogous circuits in other invertebrates11–15. These correlations are thought to arise primarily from the action of GABAergic local interneurons, which coordinate synchronous oscillations in activity that are coherent across glomeruli16–18. Additional correlations are also thought to arise within each glomerulus through reciprocal dendrodendritic connections19–22. However, previous studies have focused mainly on the role of central circuits in creating correlations, and the contribution of peripheral ORNs to correlated activity has been largely neglected. Here we show that ORNs are the primary origin of correlations in the Drosophila antennal lobe.
Results
Homotypic PNs produce highly correlated spikes
In Drosophila, each odorant receptor is expressed by an average of ~40 ORNs23, 24. All the ORNs that express the same receptor project bilaterally onto a pair of glomeruli, one in each antennal lobe25 (Fig. 1a). There they synapse onto PNs, which in turn send olfactory information to two higher brain regions, the mushroom body and the lateral horn. Each PN has a single dendritic tuft which is confined to one glomerulus. There are on average ~3 PNs per glomerulus25. Local neurons (LNs) interconnect glomeruli25.
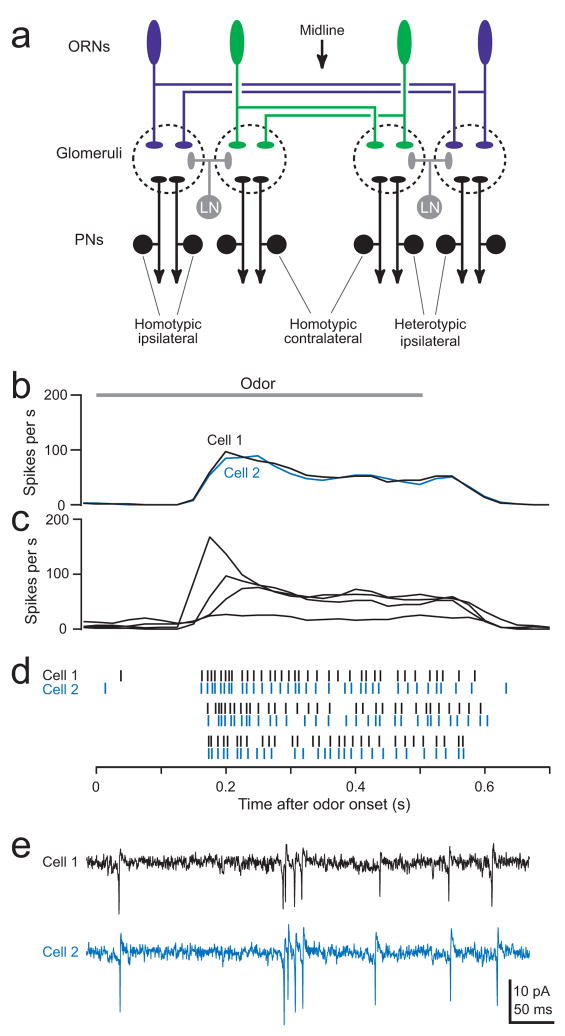
Figure 1. Homotypic PNs produce correlated spikes.
a. Schematic of the Drosophila antennal lobe circuit.
b. Results of simultaneous cell-attached recordings from homotypic ipsilateral PNs in glomerulus DM6. Average response of each PN to the odor 1-butanol is shown in black and blue (average of 13 trials). Bar indicates the 500-ms period of odor stimulation. Pearson’s r = 0.96 ± 0.02 for ipsilateral pairs (n = 15) and 0.96 ± 0.01 for contralateral pairs (n = 7) (p < 10−7 for all pairs).
c. Average 1-butanol responses of DM6 PNs in four different brains. Variability between trial-averaged responses is significantly larger between brains than within a brain (Pearson’s r = 0.49 ± 0.06, n = 52; p < 10−8 compared to both ipsi- and contralateral within-brain pairs, _t_-test).
d. Raster plots showing highly correlated responses of a DM6 PN pair to 1-butanol (same cells as in b).
e. Simultaneous cell-attached recordings of spontaneous spikes from the same cells as in b and d.
We began by performing simultaneous cell-attached recordings from pairs of PNs in the same glomerulus (“homotypic ipsilateral PNs”). We used flies where PNs in glomerulus DM6 are labeled with GFP to allow us to target our electrodes selectively to these cells. The trial-averaged odor responses of these PNs are similar across experiments, and are more similar within a brain (Fig. 1b) than between brains (Fig. 1c). Notably, we found that the precise timing of odor-evoked spikes is correlated in simultaneously-recorded PNs (Fig. 1d). The timing of spontaneous spikes is also correlated (Fig. 1e). Thus, spikes are not generated independently in these PNs.
These results illustrate two types of correlations: signal correlations and noise correlations3. We define the first of these as a correlation in the trial-averaged responses of neurons to a sensory stimulus (Fig. 1b). We would expect to find high signal correlation in homotypic PNs because these PNs receive direct input from the same ORN type. We define noise as the variance in neural activity that is not explained by the stimulus. Noise correlations appear as correlations in spontaneous activity (Fig. 1e) and correlations in the trial-to-trial fluctuations of stimulus-evoked responses (Fig. 1d). Our finding that homotypic PNs show substantial noise correlations is not a predictable consequence of the fact that these PNs receive direct input from the same ORN type. We focus on noise correlations in the remainder of this study (termed here simply “correlations”).
Correlated spontaneous activity
To investigate the mechanistic origin of these correlations, we made recordings from different types of PN pairs (schematized in Fig. 1a). In addition to homotypic PNs in the same brain hemisphere (“ipsilateral PNs”), we recorded from homotypic PNs in different hemispheres (“contralateral PNs”). Homotypic contralateral PNs share input from the same population of ORNs, but unlike ipsilateral PNs they cannot connect directly to each other. Also, because many LNs are unilateral25–29, ipsilateral PNs may share some LN input that contralateral PNs do not share. We also recorded from PNs innervating different glomeruli (“heterotypic PNs”) in the same hemisphere (Fig. 1a). Heterotypic ipsilateral PNs may share LN inputs but cannot share ORN inputs and cannot connect directly to each other. We reasoned that comparing the level of correlation among these pairs would indicate whether correlations arise from peripheral or central sources.
We found that the timing of spontaneous spikes is correlated in both ipsilateral and contralateral homotypic pairs and is uncorrelated in heterotypic pairs (Fig. 2a). We quantified the degree of spike timing correlation by computing the cross-correlation function30, which represents the probability of encountering a spike in one cell as a function of time before or after a spike in the other cell. The peak of the cross-correlation function is higher for ipsilateral pairs than for contralateral pairs, indicating that spikes are more tightly correlated (Fig. 2b). The cross-correlation function is almost flat for heterotypic pairs (Fig. 2b).
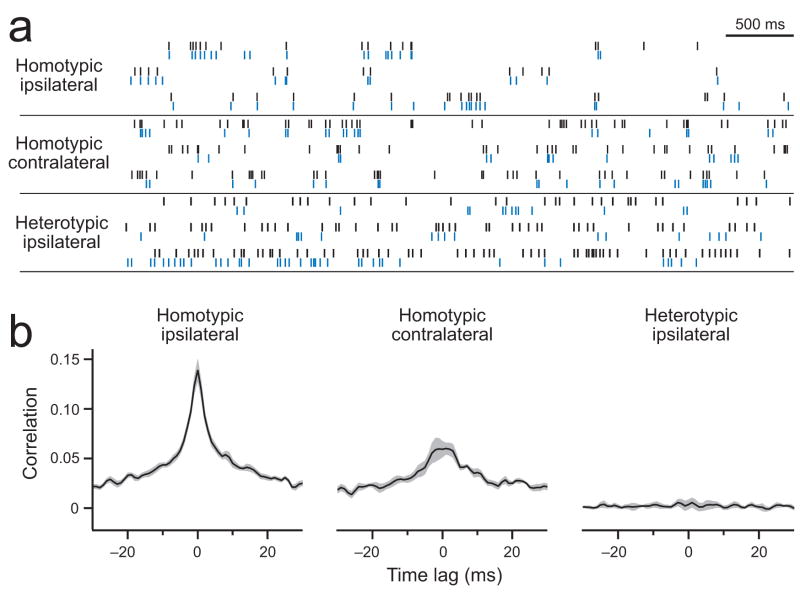
Figure 2. Correlations in spontaneous activity.
a. Simultaneous cell-attached recordings of spontaneous spikes from homotypic ipsilateral PNs (DM6), homotypic contralateral PNs (DM6), and heterotypic ipsilateral PNs (DM4 and DL5). Black and blue are cell 1 and 2, respectively.
b. Average cross-correlation functions for each type of pair (n = 15, 7, and 8 pairs for homotypic-ipsi, homotypic-contra, and heterotypic-ipsi, respectively). Gray band is ± s.e.m. across pairs.
These results demonstrate that correlated spontaneous activity occurs only in PNs that share input from the same type of ORN. Among these PNs, correlations are stronger in ipsilateral PNs than in contralateral PNs, and this suggests that central circuits make an additional contribution to correlated spontaneous activity.
Correlated activity during olfactory stimulation
We next asked whether different types of PN pairs show correlated fluctuations in their responses to repeated presentations of the same odor. Because the odor stimulus has high reproducibility in our experimental setup (Supplementary Fig. 1), most trial-to-trial variation in odor responses is not explained by stimulus fluctuations. In other words, this variation represents noise.
We found that odor-evoked spike trains are highly correlated in homotypic ipsilateral PNs (Fig. 3a–c). Indeed, olfactory stimulation significantly increases the percentage of spikes that occur synchronously in these PNs (16.7 ± 1.3% versus 13 ± 1.0% of spikes occur within a 1.6-ms window of a spike in the other cell, n = 15, p = 0.03, _t_-test; see Methods). This is true for virtually all odor stimuli, regardless of the firing rate they evoke in these PNs (Fig. 3d).
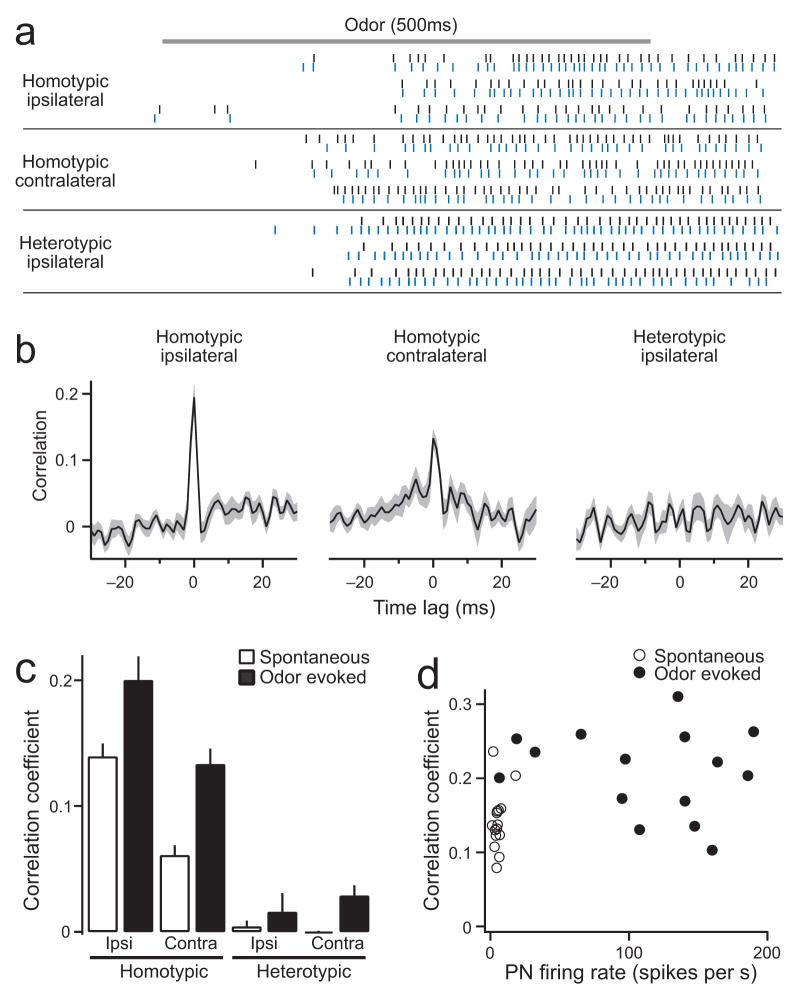
Figure 3. Correlations in odor-evoked activity.
a. Simultaneous cell-attached recordings of odor-evoked spikes from three different PN pairs. Cells are the same as in Fig. 2. Rasters show responses to methyl salicylate, which is a relatively weak stimulus for DM6 PNs. Note the expanded time scale compared to rasters in Fig. 2a.
b. Average cross-correlation functions for each type of pair (n = 15, 7, and 8 pairs for homotypic-ipsi, homotypic-contra, and heterotypic-ipsi, respectively). See Methods for olfactory stimuli. Gray band is ± s.e.m. across pairs.
c. Average correlation coefficient for four different types of PN pairs (n = 15, 7, 8, and 5 pairs for homotypic-ipsi, homotypic-contra, heterotypic-ipsi, and heterotypic-contra, respectively). Correlation differs among pairs (p < 10−4, two-way ANOVA) and becomes higher during olfactory stimulation (p < 10−4, two-way ANOVA). Bars are s.e.m.
d. Correlation coefficient increases near the transition between spontaneous firing rates and odor-evoked firing rates (homotypic ipsilateral pairs, n = 15).
In contralateral homotypic pairs, correlations during odor-evoked activity are also substantial, albeit weaker than in ipsilateral pairs (Fig. 3a–c). In these PNs, 7.0 ± 1.8% of spikes occur synchronously during odor-evoked activity (n = 7). Heterotypic PNs are almost uncorrelated (Fig. 3a–c). In these PNs, only 0.9 ± 0.7% of spikes are synchronous during odor-evoked activity (n = 15).
Divergent receptor neuron connectivity
The fraction of ORN inputs shared by PNs in the same glomerulus could in principle range from zero (if each ORN synapses onto just one PN) to 100% (if every ORN axon diverges onto all PNs). Our results so far imply that most correlated activity in the antennal lobe circuit arises from shared ORN input, and this in turn suggests that homotypic PNs share a large fraction of their ORN inputs.
To investigate this issue, we performed whole-cell recordings from PNs. These recordings reveal an ongoing barrage of large, fast, spontaneous excitatory postsynaptic currents (EPSCs; Fig. 4a–d). ORNs are the only source of large, fast EPSCs in PNs31, and every ORN spike reliably produces an EPSC32. Thus, we can assess the fraction of ORN inputs that are shared by a pair of PNs by measuring the fraction of synchronous EPSCs.
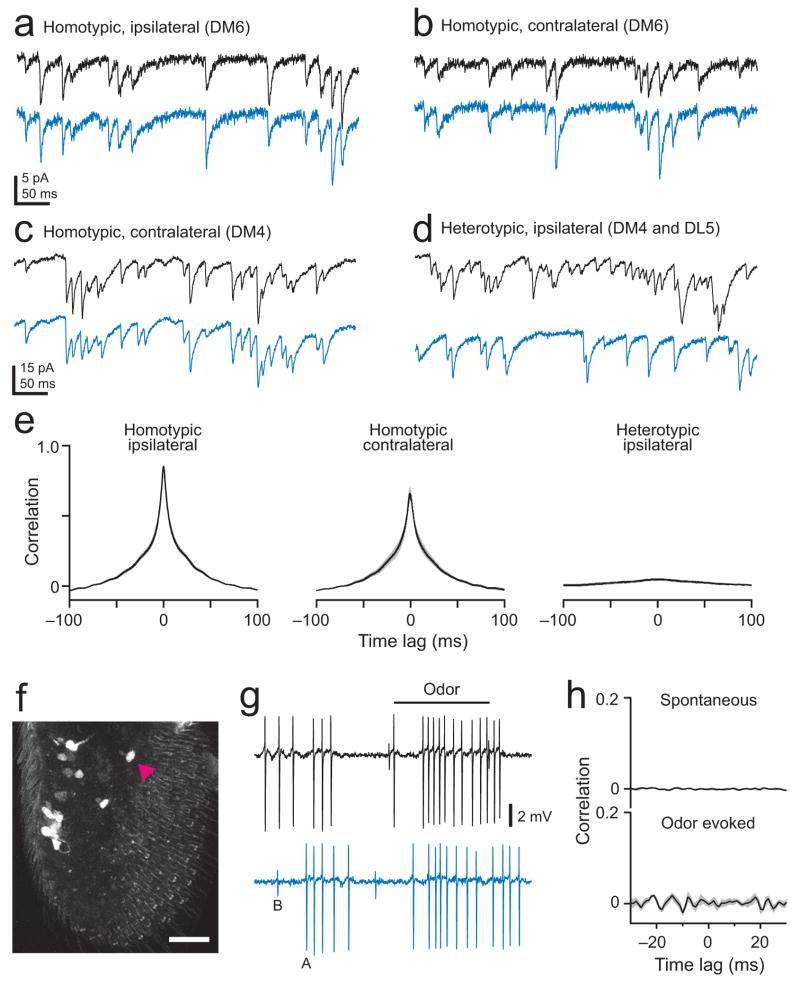
Figure 4. Each PN receives input from all the ORNs in its glomerulus.
a. Simultaneous whole-cell recordings from homotypic ipsilateral PNs (DM6). Spontaneous EPSCs are synchronous and correlated in amplitude. A pair with a relatively low rate of EPSCs is displayed so that individual EPSCs can be distinguished clearly.
b. Homotypic contralateral PNs (DM6, same brain as in a).
c. Homotypic contralateral PNs in a different glomerulus (DM4). One antenna was removed to decrease the rate of spontaneous EPSCs.
d. EPSCs are asynchronous in heterotypic ipsilateral PNs (DM4 and DL5). One antenna was removed to decrease the rate of spontaneous EPSCs.
e. Cross-correlation is higher in homotypic pairs compared to heterotypic pairs, and even higher in ipsi- compared to contralateral pairs (n = 5, 4, and 4 for homotypic-ipsi, homotypic-contra, and heterotypic-ipsi, respectively; p = 10−10, ANOVA; p < 0.01 in post hoc Tukey HSD for all combinations). Note that the absolute value of the cross-correlation calculated from continuous current traces (Figs. 5 and 7) cannot be directly compared with that calculated from spike trains (Figs. 2–4).
f. A projection of a confocal stack through an antenna. Each GFP-positive ORN soma (arrowhead) expresses the odorant receptor Or59b (Or59b-Gal4/+;UAS-nls:GFP/+). The number of these ORNs multiplied by their mean firing rate predicts the mean spontaneous EPSC rate in DM4 PNs.
g. A simultaneous recording from ORN pairs shows that spikes are independent in homotypic ORNs. Large spikes in this sensillum arise from DM4 ORNs (cell A) and small spikes arise from a different ORN type (cell B)23.
h. No correlation between DM4 ORN spike trains (computed over a 500-ms period beginning 100 ms after nominal stimulus onset, averaged across 6 pairs of DM4 ORNs, ± s.e.m. in gray, odor is 1-butanol or ethyl acetate).
We observed that spontaneous EPSCs virtually always occur synchronously in ipsilateral homotypic PNs (Fig. 4a). Spontaneous events are almost always coincident in contralateral homotypic pairs as well (Fig. 4b,c). Recordings where we removed one antenna allowed us to measure the time lag between the arrival of coincident EPSCs to ipsi- and contralateral PNs; this time lag is small (0.3 ± 0.10 ms, n = 3) compared to the average interval between spontaneous synaptic events (tens of ms). As a negative control, we confirmed that spontaneous EPSCs occur independently in heterotypic PNs (Fig. 4d).
To quantify this observation, we first identified all the EPSCs in each PN, here necessarily restricting our analysis to PN pairs where EPSCs were clearly distinguishable from the recording noise. Given an EPSC in one PN, we found that the probability of observing a time-locked EPSC in the other PN is 99.6 ± 0.1% for glomerulus DM4 (n = 3 pairs, all contralateral). This is good evidence that each of the DM4 ORNs makes precise synaptic contacts with each and every DM4 PN. The probability of observing a time-locked EPSC was 96.1% for glomerulus DM6 (n = 2 pairs, one ipsi- and one contralateral). The signal-to-noise ratio of DM6 recordings is lower than in DM4 recordings, and so it is most likely that some small EPSCs fall below the limit of our recording noise in the DM6 recordings. Alternatively, ORN-PN connectivity may be less precise in DM6 than in DM4.
For all paired whole-cell recordings, we also measured the overall similarity between their whole-cell currents by computing the cross-correlation function between the continuous current traces. This correlation is high for homotypic pairs, with a small but significant difference between ipsi- and contralateral pairs (Fig. 4e). By contrast, this correlation is low in heterotypic PN pairs (Fig. 4e).
The finding that virtually all EPSCs occur synchronously in homotypic pairs implies that each ORN axon branches to synapse onto every PN in its cognate glomerulus. This predicts that the rate of spontaneous EPSCs in a PN should equal the mean firing rate of its cognate ORNs multiplied by the number of those ORNs. In agreement with this prediction, we found that the rate of spontaneous EPSCs in DM4 PNs (74.9 ± 8.6 Hz, n = 8, one antenna removed) is not substantially different from the mean firing rate of DM4 ORNs (3.44 ± 0.16 Hz, n = 11) multiplied by the mean number of DM4 ORNs (17.4 ± 0.9 per antenna, n = 5 antennae). The latter figure was obtained by counting the number of GFP-positive cells in the antennae of flies where GFP expression is linked to the expression of the odorant receptor gene Or59b (Fig. 4f).
Finally, we considered an alternative interpretation of our results–namely, that synchronous EPSCs reflect coordinated spiking in ORNs rather than divergent ORN connections. This cannot be true because paired extracellular recordings from homotypic ORNs demonstrate that they spike independently (Fig. 4g,h). Thus, synchronous EPSCs in homotypic PNs must reflect shared input from the same ORN, not coordinated input from different ORNs.
Taken together, these results argue that each ORN axon is likely to synapse onto every PN in its target glomerulus.
Synaptic dynamics correlate synchronous EPSC amplitudes
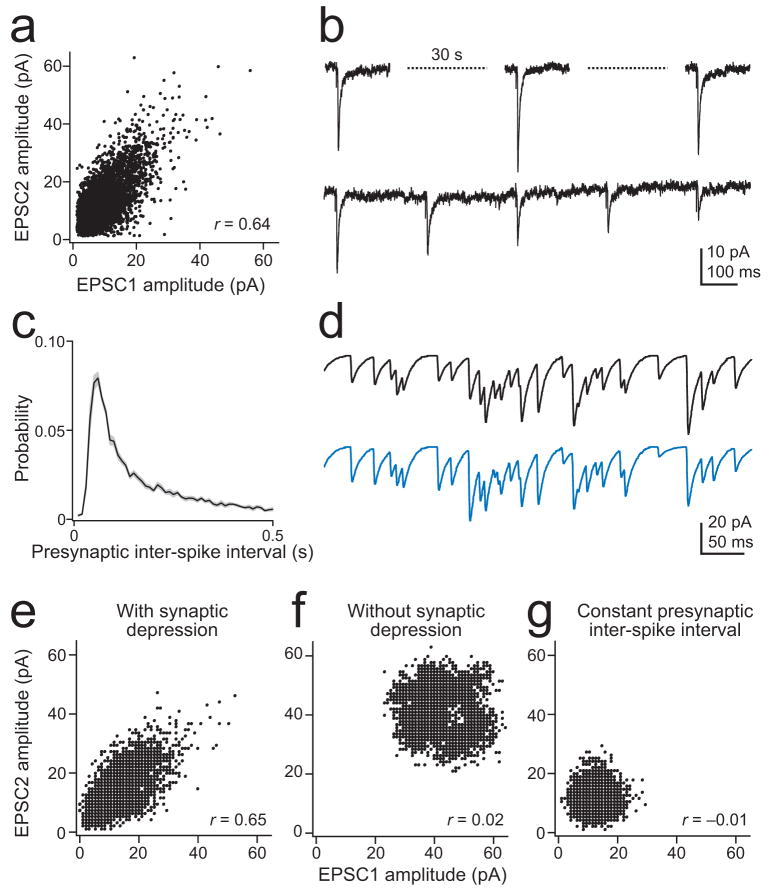
Another notable finding from these recordings is that synchronous EPSCs in homotypic PNs have correlated amplitudes (Fig. 4a–c and Fig. 5a). ORN-PN synapses depress when ORNs spike rapidly (Fig. 5b), and this should tend to produce a wide range of spontaneous EPSC amplitudes in PNs because ORNs fire spontaneously in an irregular fashion that includes many short inter-spike intervals (Fig. 5c). Because each ORN-PN synapse contains many vesicular release sites with a high probability of release, there is little stochastic variation in quantal content across trials or across synapses32. Thus EPSCs occurring after a short inter-spike interval are reliably small, and EPSCs occurring after a long interval are reliably large. Therefore, we hypothesized that short-term depression could suffice to produce strongly correlated EPSC amplitudes in homotypic PNs.
Figure 5. Short-term depression correlates the amplitudes of synchronous EPSCs.
a. Amplitude of synchronous EPSCs is correlated in a typical homotypic PN pair (DM4, contralateral, one antenna removed, Pearson’s r = 0.64, p < 10−10).
b. EPSCs recorded in response to electrical stimulation of the antennal nerve. When ORNs are stimulated with a long inter-pulse interval (30 s), EPSCs are consistently large (top). A short inter-pulse interval (0.25 s) produces short-term depression (bottom). See also Supplementary Fig. 2.
c. Inter-spike intervals are irregular in DM4 ORNs. Histogram shows the distribution of inter-spike intervals averaged across ORNs (n = 11 ORNs). Gray band is ± s.e.m.
d. Simulated synaptic currents in two PNs.
e. Simulation recapitulates the correlation between the amplitudes of synchronous EPSCs.
f. Correlation between EPSC amplitudes is absent in a model without short-term synaptic depression.
g. Correlation is also absent when presynaptic spikes occur with a constant inter-spike interval.
To test this idea, we modeled synaptic transmission at ORN-to-PN synapses. Recorded spontaneous ORN spike trains were fed into the model, and the quantal content for each spike-evoked synaptic event at an ORN-PN synapse was generated by drawing randomly from a binomial distribution defined by the number of release sites (N ) and the release probability (p) at this synapse32. Because EPSCs in different PNs are generated independently, this stochastic factor tends to decorrelate synchronous EPSCs in different PNs. Also, the mean number of release sites varies across ORN-PN synapses in our simulation, again in agreement with data32 (Supplementary Fig. 2). This is a second factor that tends to decorrelate synchronous EPSC amplitudes. On the other hand, short-term synaptic depression should tend to correlate EPSC amplitudes. In the model, the magnitude of depression and its rate of recovery were obtained from experimental data (see Methods and Supplementary Fig. 2).
This model produced a degree of correlation similar to that observed experimentally (Fig. 5d,e and Supplementary Fig. 2). As expected, correlation disappeared if synaptic depression was turned off (Fig. 5f) or if presynaptic spike trains were modeled with a constant inter-spike interval rather than real, irregular inter-spike intervals (Fig. 5g). These results demonstrate that short-term synaptic dynamics are sufficient to produce synaptic events with strongly correlated amplitudes.
Central circuits contribute to correlated noise
Next, we returned to the question of why ipsilateral homotypic PNs show more highly correlated activity than contralateral homotypic PNs. Some of this may reflect the fact that ipsilateral PNs receive more synchronous ORN input than do contralateral PNs. However, because the delay imposed by axonal conduction is only 0.3 ms, this is unlikely to completely explain the difference in correlation. Also, the strength and reliability of ORN-PN synapses is similar for both ipsi- and contralateral projections32. Thus, the higher correlation between ipsilateral PNs suggests a role for correlated central input to these cells which is not shared by contralateral pairs.
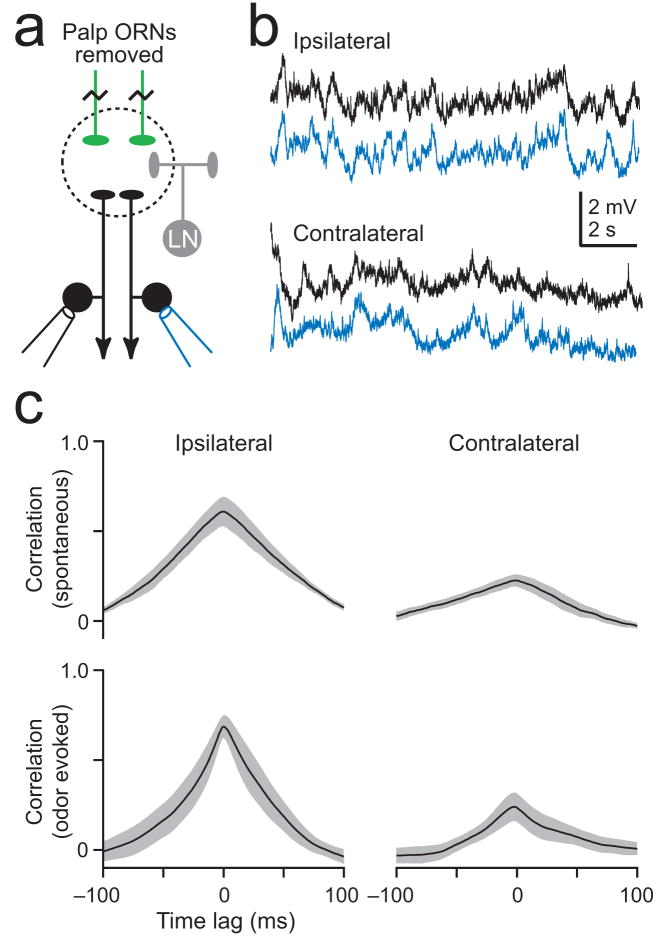
We therefore asked whether homotypic PNs receive correlated central input. Here we took advantage of the fact that a few glomeruli (~10%) receive ORN input from the maxillary palps rather than the antennae. Thus, by removing the palps we can selectively eliminate ORN input to palp glomeruli while leaving ORN input to most glomeruli intact. Recordings from pairs of denervated homotypic palp PNs (Fig. 6a) reveal correlated fluctuations in their membrane potential in both the absence and presence of odors (Fig. 6b,c). This demonstrates that homotypic PNs receive correlated central input.
Figure 6. Central circuits contribute to correlated noise.
a. In order to isolate central input to a glomerulus, direct ORN input to that glomerulus was acutely removed while preserving ORN input to most other glomeruli. This was achieved by removing the maxillary palps and recording from a pair of palp PNs (glomerulus VM7). The antennae (which provide input to most glomeruli) are intact.
b. Simultaneous whole-cell recordings from ipsi- and contralateral homotypic PN pairs (VM7).
c. Correlation is higher in ipsilateral pairs than in contralateral pairs (computed in a 500-ms window beginning 100 ms after nominal stimulus onset, n = 5 for each; p < 10−4, two-way ANOVA). Olfactory stimulation of the antennae did not affect correlation (p = 0.57, two-way ANOVA).
Interestingly, central input is correlated even in contralateral pairs (Fig. 6b,c). These correlations may arise through common bilateral ORN input to contralateral sets of LNs. Bilateral LNs and extrinsic input from other brain regions may also contribute25–27.
Although membrane potential fluctuations are highly correlated in PN pairs lacking ORN input, these fluctuations are much smaller than the correlated membrane potential fluctuations driven by ORNs (Supplementary Fig. 3). This suggests that correlated central input plays a smaller role than correlated input from ORNs.
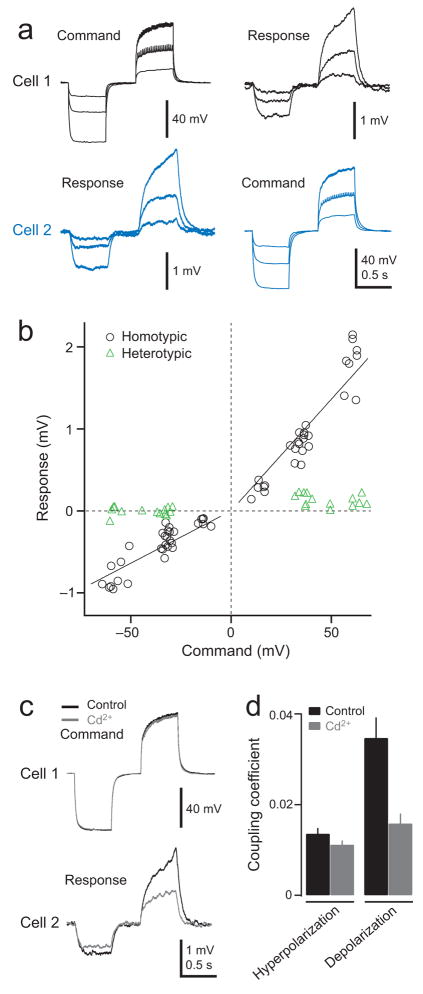
Reciprocal electrical and chemical connections between PNs
Finally, we asked whether some correlated central input could arise from PN-PN connections. In paired whole-cell recordings from the somata of ipsilateral homotypic PNs, we found that hyperpolarizing current injections in one cell induce a small hyperpolarization in the other cell, and coupling was always reciprocal (Fig. 7a). This implies that these PNs are electrically coupled. Depolarizing current injections in one cell always depolarized the other cell, but the strength of coupling was even larger than with hyperpolarizing current injections (Fig. 7a,b,d). This result would be consistent with a mixed electrical/chemical synapse because depolarization would produce both electrical and chemical coupling, whereas hyperpolarization would reveal only electrical coupling. Blocking chemical transmission with an antagonist of voltage-dependent calcium channels (100 μM Cd2+) significantly diminished but did not abolish reciprocal transmission of depolarizing steps, consistent with the idea that these are mixed electrical/chemical synapses. As expected, Cd2+ did not affect the transmission of hyperpolarizing steps (Fig. 7c,d). We did not observe coupling of this strength in recordings between heterotypic PNs (Fig. 7b).
Figure 7. PNs in the same glomerulus are reciprocally connected.
a. Simultaneous whole-cell recordings from two PNs in the same glomerulus (DM6). Current injection into cell 1 produces voltage changes which are transmitted to cell 2 (left) and vice versa (right). Antennae are removed to reduce spontaneous fluctuations. Note small action potentials when the Command neuron is depolarized above its threshold. Response traces are averages of 50 trials.
b. Membrane potential change in Response cell plotted as a function of membrane potential change in Command cell. Solid lines are linear fits to data from homotypic pairs in that quadrant. Note that the strength of coupling is stronger during depolarization as compared to hyperpolarization. Coupling is negligible between heterotypic PN pairs.
c. Blocking chemical transmission (100 μM Cd2+) selectively reduces but does not abolish the transmission of depolarizing steps to the responding cell.
d. Coupling coefficient (Response/Command) with and without chemical neurotransmission. Coupling coefficient is larger for depolarizing steps (p = 0.007, two-way ANOVA, p < 0.01 _post hoc_ Tukey HSD), but this effect is blocked by Cd2+ (_p_ > 0.05, post hoc Tukey HSD). Coupling coefficient for hyperpolarizing steps is not affected by Cd2+ (p > 0.05, post hoc Tukey HSD).
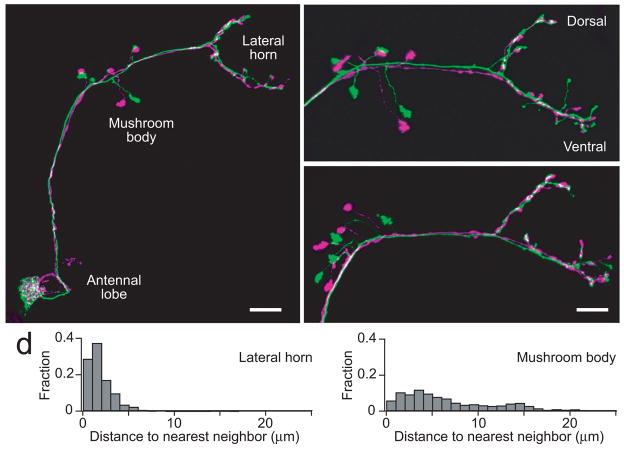
Postsynaptic targets of homotypic PNs
The implications of these findings depend on how correlated spike trains are integrated in higher brain regions. This motivated us to ask whether homotypic PNs target the same or different postsynaptic neurons. We filled pairs of ipsilateral homotypic PNs in glomerulus DM6 with different fluorescent dyes and visualized their axonal projections in two higher olfactory regions, the mushroom body and the lateral horn. In the mushroom body, we found that these axons project to different microdomains (Fig. 8a–c). Thus, these PNs may synapse onto different sets of postsynaptic mushroom body neurons. By contrast, in the lateral horn, their projections are overlapping (Fig. 8a–c), suggesting that they converge onto the same postsynaptic neurons in this region. The average distance between the nearest axonal processes of homotypic ipsilateral PNs is ~1 μm in the lateral horn and ~6 μm in the mushroom body (Fig. 8d). Previous studies have reached similar conclusions33, 34 (but see 35). These results provide a motivation to consider the implications of correlated PN activity from two perspectives: the perspective of a neuron that receives input from at most one PN per glomerulus, and the perspective of a neuron that integrates input from multiple homotypic PNs. Both these cases will be considered below.
Figure 8. Axonal projections of homotypic PNs.
a. A pair of ipsilateral DM6 PNs in the same brain filled with different fluorescent dyes. The image is a _z_-projection of a confocal stack. In the mushroom body, homotypic PN axons target different microdomains, but they are highly congruent in the lateral horn. Scale bar = 20 μm. The apparently overlapping boutons in the mushroom body are actually in different _z_-planes.
b,c. Pairs of DM6 PNs from two other brains. In the lateral horn, note that PN axons are especially congruent in the dorsal branch, with less congruence in the ventral branch. Scale bar = 15 μm.
d. Histograms of distances between the nearest axonal processes belonging to different homotypic ipsilateral PNs. The average distance is significantly shorter in the lateral horn than in the mushroom body (1.2 ± 0.0 vs 6.1 ± 0.1μm, p < 10−10, Mann-Whitney _U_-test).
Discussion
We can estimate the signal that a neuron carries about a stimulus by averaging its responses to many presentations of the same stimulus. Neural responses fluctuate across stimulus presentations, and this unexplained variability is termed noise. Here we have shown that both signals and noise are correlated in PNs postsynaptic to the same glomerulus.
Signal correlation
Some degree of signal correlation between homotypic PNs is expected because homotypic PNs receive direct input from ORNs expressing the same odorant receptor gene. However, several recent studies have speculated that homotypic cells might form different lateral connections and hence perform different computations36. Our results show this is not true of Drosophila antennal lobe PNs: signals in homotypic PNs are very strongly correlated.
Interestingly, we found that signal correlations are higher in homotypic PNs recorded simultaneously in the same brain, compared to homotypic PNs recorded in different brains. This suggests that variations in the olfactory responses of an identified PN type37, 38 do not reflect strictly cell-autonomous events. Rather, these variations are coordinated across all the PNs in a glomerulus, and might reflect slight differences in the genetic makeup or experience of different flies.
Signal correlation does not necessarily imply noise correlation3. Thus, the observation of strong noise correlations between homotypic PNs provides additional information about the connectivity of this circuit. It also has functional implications for how PN spike trains are integrated. The mechanisms and functions of noise correlations occupy the rest of this Discussion.
Origins of correlated activity: feedforward circuit
We found that PNs postsynaptic to the same glomerulus exhibit correlated spontaneous spikes, and correlated fluctuations in their spiking responses to repeated presentations of the same stimulus. This is true for both ipsi- and contralateral homotypic PNs. Because ORNs project bilaterally, this finding suggests that shared ORN input plays an important role in producing these correlations. By contrast, correlations are low in ipsilateral heterotypic PNs. This is in spite of the fact that heterotypic ipsilateral glomeruli are linked by a large network of LNs. This suggests that LNs play a relatively minor role in producing correlated noise in this circuit, as compared to the role of ORN input.
The underlying explanation for how ORNs generate correlated activity was revealed by dual whole-cell recordings, which showed that spontaneous EPSCs virtually always occur synchronously in homotypic PNs. This implies that ORN-PN connections are completely convergent, with each PN receiving input from all ORNs. This is equivalent to the statement that this circuit is completely divergent, meaning that each ORN synapses onto all PNs.
This connectivity has interesting consequences for the developing circuit. Each ORN-PN synapse consists of many release sites, and the number of release sites is consistent across synapses within a glomerulus32. These release sites must be distributed across many dendritic branches within each PN to ensure effective quantal summation39. Therefore, it may be a challenge to engineer these divergent, distributed connections between every ORN and every PN in a glomerulus.
Another striking finding from our paired whole-cell recordings is that the amplitudes of synchronous EPSCs are highly correlated. This is perhaps surprising because stochastic release should produce independent fluctuations in the responses of different PNs to the same ORN spike. However, at this synapse stochastic fluctuations are small compared to fluctuations resulting from short-term depression32. As a consequence, an irregular presynaptic spike train produces a correlated envelope of fluctuating synaptic currents in all postsynaptic neurons.
Origins of correlated activity: central circuits
In principle, there are potentially three types of central inputs to a PN: other PNs, LNs, and neurons in other brain regions. We found that PNs are indeed reciprocally connected to other PNs in the same glomerulus. These connections are mixed electrical/chemical synapses, much like the connections between homotypic mitral/tufted cells in the olfactory bulb19, 20. These reciprocal connections are likely to increase the level of correlation between the activity of homotypic mitral/tufted cells20, 21 and homotypic PNs. Thus, they may help explain why whole-cell currents and especially spike times are more highly correlated in ipsilateral homotypic PNs than in contralateral homotypic PNs.
LNs are a second source of central input to PNs. Some antennal lobe LNs are strictly unilateral25–29, and these could create a source of shared input to ipsilateral PNs that is not shared by contralateral PNs. This may also help explain why activity is more highly correlated in ipsilateral PNs. However, if shared LN input played a major role in generating correlations, we would expect to see this in heterotypic PNs as well. The absence of correlations in heterotypic ipsilateral PNs argues that LNs contribute relatively little correlated noise to PNs.
A third source of central input to PNs is extrinsic input from other brain regions. Individual extrinsic neurons generally arborize bilaterally throughout most glomeruli25, and so any correlated noise arising from these inputs could affect all types of PN pairs (ipsi- and contralateral, hetero- and homotypic). The absence of correlations in heterotypic PNs suggests that extrinsic neurons make little contribution to noise correlations.
Modulation of correlated activity
In the absence of odors, PNs fire spontaneously (typically 1–5 spikes/sec), and spiking in homotypic PNs is moderately correlated. We found that olfactory stimuli increase correlated activity among homotypic PNs. Odors that elicit only a modest increase in firing rate (10–30 spikes/sec above spontaneous firing rates) increase correlations just as much as odors that elicit a powerful increase in firing rate (>150 spikes/sec). This is suggestive of a mechanism that is engaged in the regime near spike threshold.
A potential explanation for this phenomenon was provided by a recent study which showed that spike correlations generically depend on firing rate as a consequence of the spike threshold40. If a pair of neurons receives both correlated and uncorrelated input, spike correlations between these neurons will be relatively low in the firing rate regime where threshold crossings are unreliable. As total input to both neurons increases, threshold crossings become more reliable and thus correlations increase. As total input increases further and both neurons are reliably in the super-threshold regime, correlations saturate. This mechanism would explain why even weak odor stimulation increases correlations among homotypic PNs.
Implications of correlated activity for neural codes
We have demonstrated that spikes in homotypic PNs (especially ipsilateral PNs) are highly correlated. The implications of this finding depend on how these spike trains are integrated by downstream neurons. Our dual dye fills show that in the mushroom body, homotypic PNs target largely non-overlapping microdomains. Each mushroom body neuron receives input from only ~10 PNs41. Therefore, some mushroom body neurons might receive input from at most one PN per glomerulus. By contrast, the axons of homotypic PNs are almost completely congruent in the lateral horn. This means that some neurons in this region could receive input from multiple homotypic PNs. If so, the consequences of correlated activity will be different for these two target regions.
In the mushroom body, if each neuron receives input from at most one PN per glomerulus, then the rate of information it receives from each glomerulus will be limited by the signal-to-noise ratio (SNR) of single PNs. We have seen that each PN pools input from all the ORNs that express a given odorant receptor gene, and this should, in theory, maximize the SNR of individual PNs. This would, in turn, maximize the SNR of individual mushroom body neurons.
In the lateral horn, if each neuron receives input from several homotypic PNs, then the SNR can be improved by pooling several redundant signals. Pooling in the lateral horn cannot remove noise originating in the ORN layer because this pooling has already occurred at the level of individual PNs. On the other hand, pooling in the lateral horn could diminish the impact of noise that arises independently in different PNs (e.g., noise arising at PN synapses onto lateral horn neurons). Thus, divergence (in the antennal lobe) and reconvergence (in the lateral horn) may be an important mechanism for ensuring the fidelity of information transmission. A similar phenomenon has been proposed for the transmission of visual information in the vertebrate brain6, 42. The success of this strategy depends critically on the reliability of transmission at the site of divergence, and accordingly ORN-PN synapses are extremely reliable32.
Importantly, spike-timing correlations among PNs increase in the presence of an olfactory stimulus. By increasing synchrony among PN spikes, an olfactory stimulus should increase the impact of PN spikes on any lateral horn neuron that pools input from homotypic PNs. This is because synchronous presynaptic spikes typically have a greater impact on a postsynaptic neuron as compared to asynchronous spikes1, 2. This may be important in discriminating a weak stimulus from spontaneous activity, and in discriminating among different weak stimuli, both of which represent difficult tasks for this system43, 44. In many sensory systems, “top-down” attentional shifts have been proposed to control the gain of sensory signals by modulating spike synchrony. Our results illustrate how “bottom-up” phenomena—arising in early layers of a sensory pathway—can also produce modulated synchrony in neural ensembles.
Comparisons with other circuits
Previous reports of correlated activity in the olfactory system have focused on the phenomenon of oscillatory synchrony. Local field potential recordings reveal fast odor-evoked oscillations (~20 Hz) in the vertebrate olfactory bulb, the locust and bee antennal lobe, and analogous invertebrate circuits12–15. Spikes in mitral/tufted cells or PNs tend to phase-lock with these oscillations11, 45. Oscillations are coherent across glomeruli, and arise through the action of GABAergic LNs16–18. In addition, spontaneous non-oscillatory synchronous bursting in different glomeruli has been observed on slower time scales (~seconds)46.
By contrast, the correlations we describe here arise primarily from shared ORN input. These correlations are non-oscillatory, are largely restricted to an individual glomerulus, and are insensitive to a GABA receptor antagonist (Supplementary Fig. 4). Thus, these two types of correlations are distinct phenomena. We have not observed odor-evoked oscillations in the Drosophila antennal lobe, but they have been observed by other investigators (Tanaka NK, Ito I, Stopfer M, J. Neurosci. 2009 29:8595-603). Thus, the Drosophila antennal lobe may be able to produce oscillatory synchrony, but oscillations are evidently less prominent than in some other species41.
Our results raise the question whether olfactory circuits in other species show the type of glomerulus-specific correlations that we have described here in the Drosophila antennal lobe. In the macroglomerular complex of the moth, correlations have been reported among pairs of homotypic PNs innervating specialized pheromone glomeruli47. In olfactory bulb slices, recordings from pairs of homotypic mitral/tufted cells showed that correlated activity can be induced19–21, 48, but feedforward correlations would not be revealed in slices because the ORN layer is not intact. Recently, correlated spontaneous calcium signals were reported in homotypic mitral/tufted cells of the Xenopus olfactory bulb in vivo22.
Finally, it is useful to compare the Drosophila antennal lobe with another well-characterized early sensory circuit, the retina. Nearby retinal ganglion cells exhibit strong signal and noise correlations4, 49, 50. These correlations are mainly due to the influence of shared photoreceptor and bipolar cell inputs, with secondary contribution made by reciprocal connections between ganglion cells49, 50. In these respects, the origins of correlated noise in the retina are similar to those in the Drosophila antennal lobe. Discovering common mechanisms of correlated activity that unite different neural circuits should help clarify the fundamental functional implications of correlated activity for neural computation.
Methods
Fly stocks
PN recordings
Whole-cell patch-clamp recordings from PN somata were performed essentially as previously described29; see Supplementary Methods for details. Voltage-clamp experiments were performed in a cesium aspartate-based internal pipette solution while current-clamp experiments were performed in a potassium-based internal solution. In voltage-clamp recordings, the command potential was either −60 or −65 mV. In current-clamp recordings, the membrane potential was held at around −50 or −60 mV by injecting current ranging from −10 to −40 pA. Unitary EPSCs evoked by electrical stimulation of the antennal nerve were recorded as described previously32. Cell-attached recordings were done in voltage-clamp mode using large saline-filled patch pipettes. The command potential was adjusted so that the amplifier did not pass any current. Signals were low-pass filtered at 1 kHz and digitized at 5 kHz. Voltages were uncorrected for liquid junction potential of 13 mV. The following strains were used to record from specific types of PNs: NP3062-Gal4,UAS-CD8:GFP (labels at least 3 PNs in DM6, 1 PN in DL5, and 1 PN in DM4), and NP7217-Gal4,UAS-CD8:GFP (labels at least 3 PNs in VM7). In the case of whole-cell recording, the morphology of each recorded PN was always visualized post hoc with biocytin-streptavidin labeling. In the case of cell-attached recording, the identity of each recorded PN was determined based on the PN’s characteristic responses to a panel of odor stimuli. In some experiments (Fig. 8), PNs were filled with internal solutions containing 1 mM Alexa Fluor 568 hydrazide or Alexa Fluor 633 hydrazide (Invitrogen). Biocytin and Alexa dyes did not reveal coupling between a PN and any other neurons in the antennal lobe. After recording, these brains were fixed with 4% formaldehyde in PBS for 15 min, washed in PBS, mounted in Vectashield (Vector Labs), and imaged with a Zeiss LSM 510 confocal microscope using a 40× oil-immersion objective.
ORN recordings
Flies were immobilized in the trimmed end of a plastic pipette tip. Sensilla were visualized using an Olympus BX51W1 microscope with a 50× air objective. A saline-filled reference electrode was inserted into the eye, and a sharp saline-filled electrode was inserted into a sensillum. Signals were low-pass filtered at 2 kHz and digitized at 5 kHz. ORN types were identified based on the morphology of the sensillum, spontaneous firing rate, spike size, and their characteristic responses to a panel of odor stimuli.
Olfactory stimulation
Odors used in this study were benzaldehyde, butyric acid, ethyl acetate, linalool, methyl salicylate, 1-octanol, and pentyl acetate. See Supplementary Methods for odor delivery details. The trial-to-trial stability of olfactory stimuli was confirmed with a photoionization detector (Aurora Scientific; coefficient of variation = 0.015 ± 0.003 for consecutive trials; Supplementary Fig. 1). This photoionization detector (miniPID, Model 200A) has a true frequency response of 330 Hz with 10–90% rise time of 0.6 ms. Another indication of the stability of our stimuli is the lack of correlation between trial-to-trial fluctuations in the responses of homotypic ORNs (Fig. 4g,h). In each experiment, a few stimuli were selected from our set (based on the identity of the recorded PNs) such that most of the stimuli that were used in that experiment produced an excitatory response in both the recorded PNs. The intensity of the response depended on the PN and the stimulus (see Fig. 3d for mean and range).
Data analysis
To quantify noise correlation, we computed the cross-correlation function30, which is the shift-corrected cross-covariance normalized by the variance in the firing rate of each cell. The shift-corrected cross-covariance is given by:
| CovAB(τ)=1nT∑i=1n∑t=0T(xAi(t)xBi(t+τ)−xAi(t)xBi+1(t+τ)). | (1) |
|---|
where t is a discretized (binned) time, xiA(t) and xiB(t) are responses of cell A and B in the _i_th trial, _τ_is the time lag of xiB(t) relative to xiA(t), n is the number of trials, and T is the duration of each trial. Spike trains were binned using 2-ms windows which overlapped by 1 ms. This window size was selected because it corresponds approximately to the width of the peak we observed in the shift-corrected cross-correlograms (see below). The shift correction removes correlations expected by chance given each cell’s average firing rate and (in trials where odor stimuli were presented) correlations arising from the stimulus itself. We chose to use a shift correction rather than an all-way shuffle correction because our olfactory stimulus drifts slowly for some odors (Supplementary Fig. 1) and thus stimuli on adjacent trials are most similar. The cross-covariance function was then normalized by the geometric mean of the response variance of each cell to produce the cross-correlation function which ranges from −1 to 1:
| CAB(τ)=CovAB(τ)σA2σB2 | (2) |
|---|
where σ_2_A is the variance in the spike count of cell A in each time bin averaged over all bins. The cross-correlation functions for odor-evoked PN spike trains were computed using a 100-ms window beginning at odor response onset (defined as the time when the trial-averaged peri-stimulus time histogram for that cell-stimulus combination reached 10% of its peak, generally about 100 ms after nominal stimulus onset); this time window generally corresponded to the peak of the odor response and the epoch of highest correlations. The cross-correlation functions for spontaneous activity was calculated during an 8-s period preceding stimulus onset; this time window was chosen to ensure that roughly similar number of spikes went into the two calculations. The cross-correlation functions for continuous current and voltage fluctuations (Figs. 4 and 6) were calculated as follows:
| CAB(τ)=1n(T−∣τ∣)1σA2σB2∑i=1n∑t=0T(xAi(t)−xA¯)(xBi(t+τ)−xB¯) | (3) |
|---|
where xA¯ denotes the time average. When stimulus is present, xiA(t) represents the difference between the individual response and the trial-averaged responses (i.e., the residual).
The correlation coefficient is the value of the cross-correlation function at τ = 0, which was generally also the peak of the cross-correlation function. In Fig. 4b, both the correlation coefficient and PN firing rates were computed over a 100-ms period beginning at odor response onset (the time when the trial-averaged peri-stimulus time histogram reached 10% of its peak).
Average correlation functions and correlation coefficients (Figs. 2–4, 6) are reported as mean ± s.e.m., averaged across pairs. For each pair, the correlation function and correlation coefficient were computed across 10–20 trials.
See Supplementary Methods for further details.
Model of synaptic transmission
Data for the model were obtained from PNs in glomerulus DM4, but the model is likely to generalize to synaptic transmission in other glomeruli as well, since the properties of ORN-PN synapses are similar across glomeruli32. The amplitude of each simulated EPSC was obtained as follows:
where N is the number of vesicular release sites per ORN axon, p is the probability of vesicular release at each release site, q is the quantal size, and A(t) is a factor representing the degree of synaptic depression at time t after the arrival of a previous ORN action potential (inter-spike interval). The number of sites that undergo vesicular release upon the arrival of each ORN action potential was drawn randomly from the binomial distribution B(N, p). Synaptic parameters obtained from a previous study32: N = 51, p = 0.79, and q = 1.05 pA. The variability in synaptic strength across ORN fibers (Fig. 5b) was modeled as variability in N: for each ORN fiber, a different value for N was chosen randomly from a Gaussian distribution with mean 51 and standard deviation 11. The number of ORN fibers per PN was set at 22 but the result of the simulation was insensitive to this number.
Short-term plasticity was incorporated by allowing A(t) to vary between 1 (not depressed) and 0 (fully depressed) according to the history of presynaptic spikes. After the arrival of each action potential, A(t) decreased to a fraction α of the previous value (αA(t)) and recovered exponentially with a time constant τ:
To characterize the dynamics of ORN-PN synapses, the antennal nerve was stimulated with a suction electrode at a constant frequency mimicking the mean spontaneous firing rate of DM4 ORNs (Supplementary Fig. 2). The parameters α = 0.72 and τ = 2.4 s were obtained from a least-squares regression using these data.
Spontaneous EPSCs were simulated by using recorded DM4 ORN spike trains (Fig. 4g) as the input to the model. In Fig. 5e, f, and g, α was set to 0.72, 1, and 0.72, respectively. In Fig. 5g, we simulated a presynaptic spike train with a constant inter-spike interval of 0.29 s.
Morphological analysis
Supplementary Material
1
Supplementary Figure 1 Trial-to-trial variability of olfactory stimuli is low.
Supplementary Figure 2 Short-term depression and its impact on the correlation between simulated EPSC amplitudes in a divergent feedforward circuit.
Supplementary Figure 3 Spontaneous fluctuations in membrane potential are mainly driven by direct ORN input, not central input.
Supplementary Figure 4 Noise correlation is insensitive to a GABAA receptor antagonist.
Acknowledgments
We thank K. Ito, L. Luo, and B. J. Dickson for gifts of fly stocks, and B.P. Bean for the loan of equipment. We are grateful to V. Jayaraman, A.W. Liu, O. Mazor, M. Meister, M. Stopfer, and members of the Wilson lab for comments on the manuscript. This work was funded by a postdoctoral fellowship from the NIH (F32DC009538 to H.K.), a research project grant from the NIH (R01DC008174), a Pew Scholar Award, a McKnight Scholar Award, a Sloan Foundation Research Fellowship, and Beckman Young Investigator Award (to R.I.W.).
Footnotes
Author contributions. H.K. performed the experiments and analysed the data. H.K. and R.I.W. designed the experiments and wrote the paper.
References
- 1.Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–56. doi: 10.1146/annurev.physiol.61.1.435. [DOI] [PubMed] [Google Scholar]
- 2.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–50. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–66. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- 4.Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–10. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- 5.Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci. 1998;1:501–7. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- 6.Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–9. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- 7.Puchalla JL, Schneidman E, Harris RA, Berry MJ. Redundancy in the population code of the retina. Neuron. 2005;46:493–504. doi: 10.1016/j.neuron.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Romo R, Hernandez A, Zainos A, Salinas E. Correlated neuronal discharges that increase coding efficiency during perceptual discrimination. Neuron. 2003;38:649–57. doi: 10.1016/s0896-6273(03)00287-3. [DOI] [PubMed] [Google Scholar]
- 9.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–3. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
- 10.Shlens J, Rieke F, Chichilnisky E. Synchronized firing in the retina. Curr Opin Neurobiol. 2008;18:396–402. doi: 10.1016/j.conb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–71. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- 12.Gelperin A, Tank DW. Odour-modulated collective network oscillations of olfactory interneurons in a terrestrial mollusc. Nature. 1990;345:437–40. doi: 10.1038/345437a0. [DOI] [PubMed] [Google Scholar]
- 13.Laurent G, Naraghi M. Odorant-induced oscillations in the mushroom bodies of the locust. J Neurosci. 1994;14:2993–3004. doi: 10.1523/JNEUROSCI.14-05-02993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–4. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 15.Adrian ED. Olfactory reactions in the brain of the hedgehog. J Physiol. 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagier S, Carleton A, Lledo PM. Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb. J Neurosci. 2004;24:4382–92. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galan RF, Fourcaud-Trocme N, Ermentrout GB, Urban NN. Correlation-induced synchronization of oscillations in olfactory bulb neurons. J Neurosci. 2006;26:3646–55. doi: 10.1523/JNEUROSCI.4605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malun D. Synaptic relationships between GABA-immunoreactive neurons and an identified uniglomerular projection neuron in the antennal lobe of Periplaneta americana: a double-labeling electron microscopic study. Histochemistry. 1991;96:197–207. doi: 10.1007/BF00271538. [DOI] [PubMed] [Google Scholar]
- 19.Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol. 2002;542:355–67. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoppa NE, Westbrook GL. AMPA autoreceptors drive correlated spiking in olfactory bulb glomeruli. Nature Neuroscience. 2002;5:1194–202. doi: 10.1038/nn953. [DOI] [PubMed] [Google Scholar]
- 21.Christie JM, et al. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46:761–72. doi: 10.1016/j.neuron.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Chen TW, Lin BJ, Schild D. Odor coding by modules of coherent mitral/tufted cells in the vertebrate olfactory bulb. Proc Natl Acad Sci U S A. 2009;106:2401–6. doi: 10.1073/pnas.0810151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–52. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 24.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–36. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 25.Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 26.Das A, et al. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 2008;3:33. doi: 10.1186/1749-8104-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai SL, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–93. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
- 28.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–56. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–79. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. II Simultaneous spike trains. Biophys J. 1967;7:419–40. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58:401–13. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–55. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 34.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–41. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 35.Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–17. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Fantana AL, Soucy ER, Meister M. Rat olfactory bulb mitral cells receive sparse glomerular inputs. Neuron. 2008;59:802–14. doi: 10.1016/j.neuron.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–70. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 38.Bhandawat V, Olsen SR, Schlief ML, Gouwens NW, Wilson RI. Sensory processing in the Drosophila antennal lobe increases the reliability and separability of ensemble odor representations. Nature Neuroscience. 2007;10:1474–82. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gouwens NW, Wilson RI. Signal propagation in Drosophila central neurons. J Neurosci. 2009;29:6239–49. doi: 10.1523/JNEUROSCI.0764-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature. 2007;448:802–6. doi: 10.1038/nature06028. [DOI] [PubMed] [Google Scholar]
- 41.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. Journal of Neurophysiology. 2007 doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 42.Reid RC. Divergence and reconvergence: multielectrode analysis of feedforward connections in the visual system. Progress in Brain Research. 2001;130:141–54. doi: 10.1016/s0079-6123(01)30010-9. [DOI] [PubMed] [Google Scholar]
- 43.Pelz C, Gerber B, Menzel R. Odorant intensity as a determinant for olfactory conditioning in honeybees: roles in discrimination, overshadowing and memory consolidation. J Exp Biol. 1997;200:837–47. doi: 10.1242/jeb.200.4.837. [DOI] [PubMed] [Google Scholar]
- 44.Silbering AF, Okada R, Ito K, Galizia CG. Olfactory information processing in the Drosophila antennal lobe: anything goes? J Neurosci. 2008;28:13075–87. doi: 10.1523/JNEUROSCI.2973-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–65. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 46.Galan RF, Weidert M, Menzel R, Herz AV, Galizia CG. Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Comput. 2006;18:10–25. doi: 10.1162/089976606774841558. [DOI] [PubMed] [Google Scholar]
- 47.Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nature Neuroscience. 2002;5:557–65. doi: 10.1038/nn0602-859. [DOI] [PubMed] [Google Scholar]
- 48.Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49:271–83. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 49.Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20:527–39. doi: 10.1016/s0896-6273(00)80992-7. [DOI] [PubMed] [Google Scholar]
- 50.Trong PK, Rieke F. Origin of correlated activity between parasol retinal ganglion cells. Nat Neurosci. 2008;11:1343–51. doi: 10.1038/nn.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
Supplementary Figure 1 Trial-to-trial variability of olfactory stimuli is low.
Supplementary Figure 2 Short-term depression and its impact on the correlation between simulated EPSC amplitudes in a divergent feedforward circuit.
Supplementary Figure 3 Spontaneous fluctuations in membrane potential are mainly driven by direct ORN input, not central input.
Supplementary Figure 4 Noise correlation is insensitive to a GABAA receptor antagonist.