Hypoxia and radiation therapy: Past history, ongoing research, and future promise (original) (raw)
. Author manuscript; available in PMC: 2010 May 1.
Abstract
Tumor hypoxia influences the outcome of treatment with radiotherapy, chemotherapy and even surgery, not only for the treatment of large bulky tumors with extensive necrosis, but also in the treatment of very small primary tumors and recurrences, micrometastases, and surgical margins with microscopic tumor involvement. Because hypoxic tumor cells are resistant to radiation and to many anticancer drugs, many approaches to circumventing the therapeutic resistance induced by hypoxia have been examined in laboratory studies and clinical trials. In this review, these approaches and the results of past laboratory and clinical studies are described and the limitations of the past agents and their testing are discussed. We describe the importance of new technologies for measuring hypoxia in human tumors, which allow assessment of pretreatment tumor oxygen levels and changes in hypoxia over the course of prolonged treatment regimens. These offer the possibility of improving the design of clinical trials and the selection of patients who will benefit from hypoxia-directed therapies, as well as the possibility of facilitating the development of better agents and regimens for use in hypoxia-directed therapy. We also discuss how the improved understanding of the abnormal vascular beds in solid tumors and of the effects of hypoxia and related microenvironmental insults, resulting from recent and ongoing research, offers the potential for finding new therapeutic targets, that may lead to the development of new agents and novel therapeutic approaches for selectively targeting cells in the adverse microenvironments within solid tumors.
Introduction
Research on the effects of hypoxia has blossomed in recent years, with the realization that hypoxia is not only an abnormal stress associated with injury and disease, but also a physiologic factor modulating a variety of normal developmental and metabolic processes. This has led to extensive studies of the many and varied molecular signaling pathways and cellular responses triggered by or modulated by moderate and severe hypoxia. This interest in and appreciation of the many effects of hypoxia has as its roots 100 years of research by radiation biologists and radiation oncologists, which was stimulated by the radiobiological effects of oxygen and by the implications of hypoxia for the treatment of cancer [1-6].
The history of research on hypoxia is intimately interwoven with the history of radiation therapy. From its earliest roots in the late nineteenth century, radiotherapy was a unique area of medicine that required the integrated use of physics, engineering, and medicine every day, in the treatment of every patient. The history of clinical and experimental radiotherapy reflects this intersection of science and medicine. Collaborations between physicians, physicists, engineers, chemists, and biologists have always been the norm in academic radiation oncology departments and professional organizations and an intrinsic part of the training and culture of the specialty [1,3-13]. Perhaps equally important in the development of radiotherapy has been the implicit assumption that the improvement of clinical care is a science that requires quantitative planning and documentation of treatments, quantitative, objective measurements of the beneficial and toxic responses to treatments, and careful evaluation of new therapeutic approaches and technologies through rigorous comparisons with the standard of care [3,5-7,9]. As noted by Béclère in 1901, “La radiotherapie ne saurent être une science sans mesures exactes” (Radiotherapy will never be a science without precise measurements) [3]. The modern concept of “evidence-based medicine” is presaged in this history.
It is probably not happenstance that the first rigorous, quantitative measurements of the survival of cells treated with cancer therapeutic agents in cell cultures [14], in tumors [15], or in normal tissues (beginning with the discovery of pluripotent bone marrow stem cells [16]) were all performed in radiobiology laboratories. Similarly, quantitative, functional endpoints for assessing injury to normal tissues and quantitative model-based analyses of tumor growth curves and of dose-response curves for tumor cure were standard models in experimental radiotherapy 50 years ago [1,5,6], when other disciplines in experimental oncology routinely used less informative endpoints such as extension of lifespan (reflecting a mixture of antitumor effects and toxicities) or ratios of the weights of treated and control tumors at a single predetermined time. Experimental and clinical studies of the therapeutic implications of tumor oxygenation therefore reflect the unique culture in the radiation oncology community, with its emphasis on close interactions between scientists and physicians, on the formulation of model-driven hypotheses, on rigorous testing of these hypotheses in laboratory studies using quantitative biological endpoints, and on clinical testing of new approaches and agents in rigorous and objective clinical trials.
As early as 1904, Hahn [17] and Schwarz [18] observed that compression that compromised blood flow changed the effects of low energy X-rays and superficial radium plaques. These observations rapidly influenced the use of these sources, although the importance of oxygen to the effects was not immediately recognized [2]. In the 1930's, Crabtree and Cramer [19] showed that molecular oxygen was a critical determinant of the response of cells to irradiation, while Mottram's histological observations of tumors in hamsters [20] led to the hypothesis that growing tumors should have areas where vascular insufficiencies caused the development of regional hypoxia.
Since these early observations, advances in science, medicine, and technology have altered radiobiology laboratories and radiation therapy clinics. This has not been a gradual, continual process; instead specific developments have suddenly and dramatically altered research and patient care. Advances in technology, including the development of high energy x-ray radiation sources and linear accelerators and improved diagnostic imaging approaches, changed radiation therapy from a palliative modality focusing on short term improvements in patient comfort into a curative modality focusing on long-term tumor control and concerned with late toxicities. This change increased the importance of developing therapies to attack radioresistant cell populations, because killing (or even inhibiting the growth of) most of the radiosensitive cells will produce good short-term palliation, but curing the tumor requires eradicating all of the malignant cells, including those which are radioresistant because of hypoxia on for other reasons. The development of inbred rodent strains, transplanted syngeneic tumor lines, and rigorous, quantitative assays to measure and compare the effects of therapies on tumors and normal tissues [1] are not ancient history: they revolutionized experimental cancer therapy during the careers of many scientists still active in the field. Similarly, the development of cell culture methodologies allowing study of well-characterized cell populations in defined media and rigorously controlled environmental conditions using quantitative clonogenic assays of cell survival [1,10,13] revolutionized studies of the effects of radiation in the 1950's and 1960's. All of these techniques were applied immediately to study the effects of oxygen and to test approaches to circumventing the radioprotective effects of hypoxia.
Attempts to use differentials in blood flow and oxygenation in tumors and normal tissues for therapeutic advantage began a century ago. They continue today. The approaches have changed as knowledge of the pathophysiology underlying tumor hypoxia has improved and as new technologies have been developed. Periods of great expectation, when major therapeutic advances were predicted to be imminent, have alternated with periods of disillusionment when these overly optimistic predictions proved false [2,4-7]. Neither extreme was probably warranted either at the time it occurred or in retrospect. In this review we will present a brief overview of past laboratory and clinical studies aimed at improving the outcome of radiotherapy by considering the implications of tumor oxygen, discuss the lessons that this history offers, and consider some ways in which recent insights into the molecular effects of hypoxia now offer new tools and technologies for use in research, new targets for therapeutic interventions and new opportunities for improving the care of cancer patients.
Effects of oxygen on radiation response
Molecular oxygen (O2) is a potent chemical radiosensitizer. This radiosensitization does not result from any of the metabolic or physiological effects of oxygen, but instead reflects the fact that O2 is an extremely electron-affinic molecule that participates in the chemical reactions that lead to the production of DNA damage after the absorption of energy from ionizing radiation [21,22]. Cells that are anoxic during irradiation are about three times more resistant to radiation than cells that are well oxygenated at the time of irradiation Fig. (1). Very low levels of O2 are required for radiosensitization. Radiosensitivity increases as the oxygen tension increases from anoxia to ∼10 Torr Fig. (2), but then plateaus, with no further significant increase as the oxygen tension increases through the range found in healthy tissues or even in cells exposed to 100% oxygen [24]. Because the underlying chemical reactions are essentially complete within a few milliseconds after irradiation, O2 must only be present during irradiation to produce full radiosensitization; the presence of oxygen before or after irradiation is irrelevant to this mode of radiosensitization.
Figure 1. Survival curves for hypoxic and aerobic cells in cell culture.
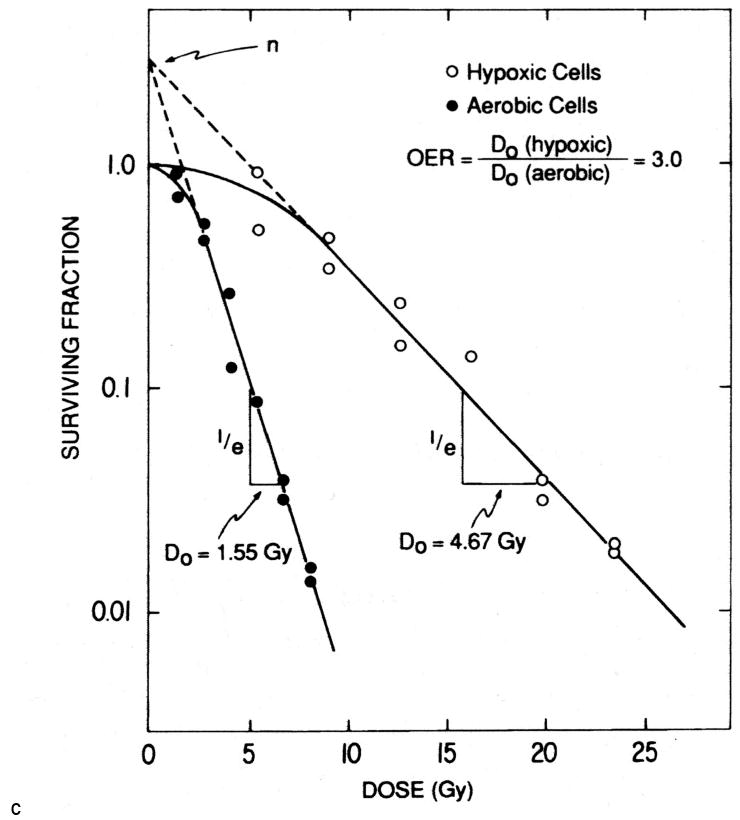
Exponentially growing EMT6 mouse mammary tumor cells were irradiated under aerobic conditions or were made severely hypoxic just before and during irradiation with 250 kV x-rays. Cell survival was measured immediately after irradiation using a colony formation assay. The hypoxic cells are three times as resistant to irradiation as the aerobic cells. Reprinted from [23].
Figure 2. Schematic representation of the relationship between radiosensitivity and oxygen tension.
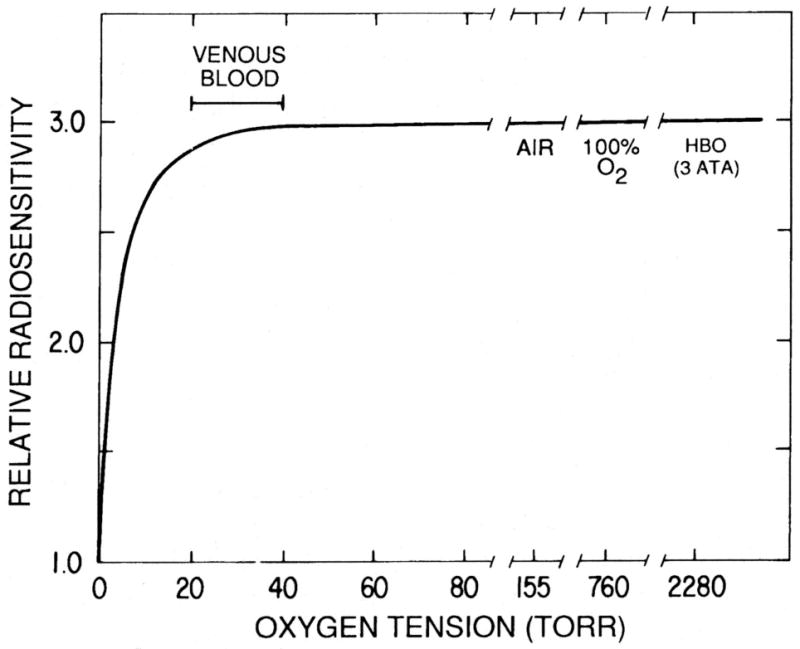
The relative radiosensitivity of aerobic and acutely hypoxic cells was determined from the slopes of the survival curves measured at different oxygen tensions using protocols similar to those described in the experiments shown on Figure 1. The radiosensitivity of the cells increases rapidly as the oxygen tension rises from anoxia to ∼10 Torr; at higher concentrations of O2, similar to those found in venous blood, the radiosensitivity plateaus and does not increase greatly as the oxygen tension rises to that of cells equilibrated with air or with 100% O2 at normal atmospheric pressure or even hyperbaric pressures (HBO). Reprinted from [23].
The classic work of Gray and his collaborators during the 1950's [24-27] defined the effects of oxygen on the radiation response of a wide variety of microbial, plant, cellular and animal model systems and explored the potential implications of the “oxygen effect”. Thomlinson and Gray's classical histological studies of human lung cancers and their mathematical modeling of oxygen delivery, oxygen consumption and regional O2 distributions within these tumors suggested that these tumors contained areas in which viable cells were subjected to chronic, severe hypoxia [25]. Later studies by others, beginning with Reinhold and his collaborators, showed that tumors not only contained areas of severe, chronic hypoxia and anoxia, but also areas in which fluctuations in blood flow through individual tumor vessels led to transient exposures to moderate or severe hypoxia [28,29]. Chronic and fluctuating hypoxia would, respectively, produce persistent or transient radioresistance, both of which make tumor cells resistant to radiotherapy.
One of the major problems limiting research in this area before the 1990s was the lack of physical or chemical methods for detecting or measuring oxygen levels in tissues over the pO2 range of interest in radiotherapy. The very low oxygen levels needed for radiosensitization (see Fig. (2)) and the need to measure oxygen levels in small regions within tumors produced formidable barriers, which were not breached until relatively recently. As a result, hypoxia was detected indirectly, from the resultant radioresistance, and “radiobiological hypoxic fractions” were calculated for experimental rodent tumors by comparing the radiation responses of the tumors of interest with the responses of tumors made completely hypoxic. These techniques for measuring radiobiological hypoxic fractions were time consuming and expensive, because they required the use of very large numbers of mice or rats to measure and compare dose-response curves for tumor cure, tumor regrowth, or tumor cell survival (using endpoint dilution or colony formation assays) under different experimental conditions [30-32]. An example of the experiments performed to measure the radiobiological hypoxic fraction of a tumor line in mice is shown on Fig. (3). These radiobiological techniques for measuring hypoxic fractions could not be used in the clinic. The relatively recent development of techniques that allow the measurement of regional and temporal variations in oxygenation in experimental tumors, and especially in the tumors of patients, is revolutionizing both laboratory and clinical work in this area as described below.
Figure 3. Illustration of the measurement of the “Radiobiological Hypoxic Fraction” of an experimental tumor.
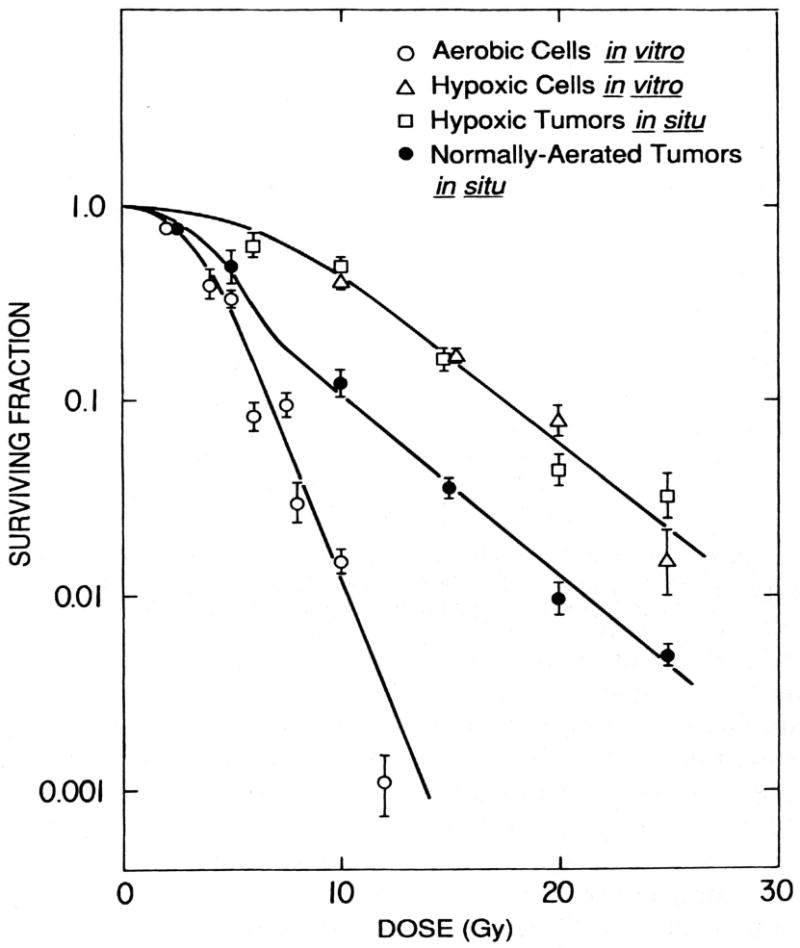
EMT6 mouse mammary tumors were irradiated in vivo, either in unanesthetized, air-breathing mice (normally aerated tumors in situ) or in tumors made completely fully anoxic (hypoxic tumors in situ). The in vivo survival curves were compared at doses over 10 Gy and found to be parallel. The difference of the elevation of these survival curves was compared using the mathematical techniques described in [30]; the surviving fraction of cells in the normally aerated tumors is ∼ 20% of that in fully hypoxic tumors, indicating that about 20% of the viable (clonogenic) cells in the “normally aerated tumors” are sufficiently hypoxic to be fully radioresistant.
The importance of the naturally hypoxic cells in determining the response of tumors to radiation can be seen from the difference between the survival curve for normally aerated tumors and that for cells in culture under fully aerobic and hypoxic conditions. At low radiation doses, the survival curve in normally-aerated tumors is similar to that of cells in culture (reflecting the radiosensitivity of the tumor cells that are radiobiologically well oxygenated); at higher radiation doses, where most of these aerobic cells have been killed, the tumor cell survival curve breaks and becomes parallel to that of hypoxic cells (reflecting the radioresistance of the naturally hypoxic tumor cells). Reprinted from [23.]
During the 1960-80's, studies in a variety of tumor models showed that virtually all primary and transplanted rodent tumors and human tumor xenografts contained viable, radioresistant hypoxic cells [reviews 30-33]. In most experimental solid tumors, ∼10-20% of the viable tumor cells were found to be sufficiently hypoxic to be fully radioresistant as measured by analyses of tumor cell survival, tumor growth, or tumor cure. Even microscopic primary tumors and micrometastases were found to have significant “radiobiological hypoxic fractions”, indicating that hypoxia was a factor to be considered in the treatment of small primary tumors, involved surgical margins, and micrometastases as well as in treatment of large bulky tumors where gross necrosis was observed. Hypoxic cells were shown to dominate the response of tumors to large doses of radiation Fig. (3). Moreover, tumors could be made more radioresistant by making them more hypoxic and made more radiosensitive by manipulations expected to improve their oxygenation. Such observations stimulated widespread interest in seeking and testing approaches to improving cancer therapy by circumventing the radioprotective effects of hypoxia. Three basic approaches were considered: improving tumor oxygenation, increasing the radiosensitivity of the hypoxic tumor cells, and killing the hypoxic tumor cells.
Approaches to Circumventing Hypoxia
Modulating tumor oxygenation
Beginning in the middle decades of the twentieth century, there was great optimism that the outcome of radiotherapy could be improved by increasing tumor oxygenation during irradiation. Many approaches to improving tumor oxygenation were considered and tested in animal model systems and several were tested in clinical trials [reviews: 1,2,5,6,34]. The simplest was breathing of 100% oxygen during irradiation, to ensure saturation of hemoglobin and also deliver extra oxygen dissolved in plasma. Carbogen (a mixture of oxygen and a small amount [2-7%] of carbon dioxide) was also tested, on the basis of studies suggesting that CO2 would inhibit the vasoconstriction sometimes seen with pure oxygen. Hyperbaric oxygen (HBO), 100% oxygen delivered at pressures of 2-4 Atmospheres, was also used to increase the plasma pO2 further. Because the diffusion distance of oxygen through respiring tissue increases with the initial oxygen tension, HBO should theoretically allow O2 to diffuse further through tissue than normobaric oxygen breathing, oxygenate more hypoxic cells and produce greater radiosensitization [35].
Encouraging results with HBO were reported in small series of patients as early as the 1950s [2,34,36,37]. However, early trials of HBO proved challenging because of the difficulties of setting up and treating patients with the relatively primitive hyperbaric chambers (steel chambers with small glass portholes through which radiation to be delivered to very restricted fields) and the irradiators then available. As a result, many trials were small, many used nonstandard, suboptimal hypofractionated radiation regimens, and many used historical controls or control patients treated with radiotherapy regimens different from those used with HBO; the results were therefore equivocal and difficult to interpret. Physicians expecting dramatic improvements were disappointed with the outcomes of these trials and the approach fell into disuse [2,7,34,38]. However, retrospective metaanalyses of the well-controlled HBO trials [2,34] show that HBO provided a statistically significant improvement in local control, along with a statistically significant increase in disease free and overall survival. Ironically, an analogous improvement in local control in common solid malignancies would be considered clinically significant in trials of a new anticancer drug, and would probably result in changes in the standard of care. Studies with HBO are continuing in Europe and Japan [39], but the approach is not currently in routine clinical use with radiotherapy, despite the relatively wide availability of greatly improved HBO chambers and the routine use of hyperbaric oxygen in several other areas of medicine.
Administration of O2 and Carbogen at normal atmospheric pressure has also been examined in clinical trials [2,34,40-42]. The animal data on these regimens is mixed [33,43-45]. Large improvements in tumor oxygenation and radiation response are produced by oxygen or Carbogen breathing in some rodent tumor models; no effect is seen in others. In many animal models, HBO produces larger effects than O2 or Carbogen administered at atmospheric pressure. Carbogen produces greater effects than oxygen in some animal model systems, but similar effects in others. Many experimental variables (duration of breathing, anesthesia, temperature) influence the effects of O2 or Carbogen. These data raise the possibility that the effects of oxygen and Carbogen breathing may vary in different patient populations and even in individual patients. A recent review of the clinical trials by Overgaard [2] supports the predictions from animal studies: there is greater variability in the trial outcomes with Carbogen or oxygen than with HBO, and the overall data set shows a trend toward improved outcome, but the change is not statistically significant.
In many clinical studies, Carbogen breathing has been combined with other modalities that might also improve tumor response. For example, the ARCON trials [41,42] combine Carbogen (which would be expected to reduce diffusion-limited hypoxia) with nicotinamide (a vasoactive agent improving tumor blood flow and reducing perfusion-limited hypoxia) and accelerated radiotherapy (delivering multiple treatments per day over a short overall treatment time to decrease repopulation during treatment). Preliminary data from the phase I/II ARCON trials were very promising; results of ongoing phase III trials have not yet been published. The fact that hemoglobin levels were not predictive of outcome in ARCON patients [41,42] suggests that modulation of tumor oxygenation is involved in producing the promising results seen with the ARCON regimens. Measurements of tumor oxygenation in patients in the ongoing ARCON trials [46] should provide interesting insights on the importance of hypoxia in the outcome.
Tumor oxygenation, and therefore tumor radiosensitivity, should also be improved by increasing the ability of the blood to transport oxygen. This hypothesis is supported by the fact that 40-60% of all cancer patients are anemic at the start of radiotherapy and the fact that anemic patients have a worse prognosis than patients with normal hematocrits [47-52]. Many laboratory and clinical studies therefore have examined the effects of transfusions on the radiation response of solid tumors. The laboratory data warn that the effects of anemia and of its resolution by transfusions are complex. In inbred mice, transfusions that slightly increase hematocrits do indeed improve the response of tumors to radiation given shortly thereafter [53-56]. However, larger increases in hematocrit are less effective and can even induce radioresistance, probably because high red cell concentrations increase blood viscosity, change hemodynamics and decrease blood flow through tumor microvessels. With time after transfusion, the tumor returns to the baseline radiation response, reflecting the induction of homeostatic processes that lead to normalization of perfusion. Counter-intuitively, acute reductions in red cell numbers can actually sensitize tumors to irradiation in some model systems, probably because the acute decrease in blood viscosity leads to improved tumor perfusion. It should be remembered that the model systems used in most animal studies offer an ideal system for transfusion: the experiments generally use fresh blood that was harvested from a syngeneic mouse only a few moments before the transfusion. Most transfusions in the clinic use stored blood or packed red cells, which transport oxygen less effectively than fresh blood. In addition, clinical blood donors generally will be of an appropriate blood type, but not fully HLA matched to the recipient; immunologic effects that are absent in syngeneic mouse studies therefore complicate clinical use of transfusions [47].
Although the poor prognosis for anemic cancer patients may in part reflect the resistance of hypoxic cells to radiation (and to many anticancer drugs), the complex relationship between anemia and tumor hypoxia and the fact that anemia can also be an indicator of poor prognosis after surgery suggest that the situation is not this simple, and that anemia may also be associated with patient or tumor characteristics that adversely affect prognosis [47-52]. Moreover, as described briefly above, data from studies with animal models show that blood transfusions do not always lead to increased tumor radiosensitivity. It is therefore not surprising that clinical trials examining the effects of transfusions on the outcome of therapy have produced mixed results, and that many studies showed no improvement, or even poorer prognosis for patients who were transfused before or during radiotherapy [47-52].
Clinical trials using erythropoietin to improve red blood cell production and increase hematocrits during radiotherapy have likewise produced mixed results, and some recent trials have even shown significantly higher mortality rates in patients treated with erythropoietin [47,57,58]. This negative finding may in part reflect the same confounding factors seen with transfusions. However, it may also reflect the direct effects of erythropoietin, which has many actions in addition to stimulating red blood cell production. Erythropoietin receptors are found on many normal and malignant cells. Erythropoietin can be mitogenic and can reduce apoptotic responses in many cell lines. This multifunctional molecule therefore may have a wide range of effects on tumor cells and on critical cell populations in normal tissues, some of which would be expected to be deleterious when erythropoietin is used as an adjunct to cancer therapy [46,57,58]. More laboratory work and critical analyses of clinical data in this area are needed, given the wide use of erythropoietin by cancer patients and the aggressive campaign of direct marketing of the drug to cancer patients.
During the 1980's investigators from several laboratories investigated the use of the artificial intravenous oxygen transport agents (often, but inaccurately, called artificial blood substitutes) being developed for use as alternatives to blood transfusions [59-62]. Interest in these agents was spurred by concern over HIV, hepatitis viruses, and other blood-borne pathogens, by the desire to develop shelf-stable products that could be used without blood typing in emergencies and mass casualty situations, and by the desire to find agents that could be used by patients who refused blood transfusions on religious grounds. Development focused primarily on use during surgery and after trauma, i.e. situations where short term use was needed because of extensive blood loss. Several radiobiology groups examined the potential use of these agents in cancer therapy, because of the observations in mice, described above, which showed improvements in tumor response after transfusions and because of theoretical reasons to expect that the differences between oxygen uptake, transport, and delivery by red blood cells and by some synthetic O2 transport agents might result in better delivery of oxygen to hypoxic areas within tumors by these agents than by blood [35].
Two basic types of oxygen transport agents have been developed: those using hemoglobin as the transport molecule and those using perfluorochemicals [59-62]. The first generation hemoglobin-based agents, which used stroma-free hemoglobins, proved to have very short half lives and to produce both cardiovascular complications and renal injuries. As a result, subsequent studies focused on the development of more complex preparations. At present, several commercial and academic institutions are working on a wide variety of preparations [59-62]. These use several different crosslinked human and animal hemoglobins, polymerized hemoglobins, PEG-conjugated hemoglobins, fish and analid hemoglobins, and genetically-engineered hemoglobins. The hemoglobins are often encapsulated in artificial red blood cells, vesicles and nanoparticles, sometimes in combination with other agents. Recent nanotechnology advances offer improved approaches to packaging hemoglobins for greater stability and efficacy [62]. In addition, new insights into the molecular interactions and signaling pathways that mediate the physiological effects of hemoglobins on the vasculature that can lead to vasoconstriction and impaired perfusion offer possible approaches for developing hemoglobin-based agents that circumvent the problems seen with first and second generation agents [62]. Laboratory studies by Teicher and others during the 1980's showed that appropriate regimens of treatment using a crosslinked bovine hemoglobin-based preparation could improve the response of solid rodent tumors to radiation [63]. Studies examining the effects of the newer agents as adjuncts to radiotherapy have not been reported.
Perfluorochemical emulsions (PFC-E) consist of small droplets of liquid perfluorochemicals, stabilized with a lipid coat and suspended in a physiological saline-based diluent [59-62]. When exposed to high O2 concentrations, perfluorochemicals dissolve very large amounts of oxygen; in hypoxia, O2 diffuses out of the perfluorochemical and into the surrounding milieu. The entry and efflux of O2 from the liquid perfluorochemical differs dramatically from the tightly regulated uptake and release of O2 from the O2-binding sites of hemoglobin, resulting in significant differences in oxygen transport and delivery, which could be advantageous in the delivery of oxygen to hypoxic regions of tumors [35,64]. Several different PFC-E have been tested as adjuncts to radiotherapy in animal systems, with extremely encouraging results [35,45,65]. An example of these studies is shown on Fig. (4). Clinical trials in cancer patients were limited, were complicated by the non-optimal formulations of the early emulsions available for clinical use at the time of the trials, and were often stopped for reasons having little relationship to the potential of the agents to improve the outcome of cancer therapy [66,67].
Figure 4. Effects of HBO and an artificial oxygen transport agent.
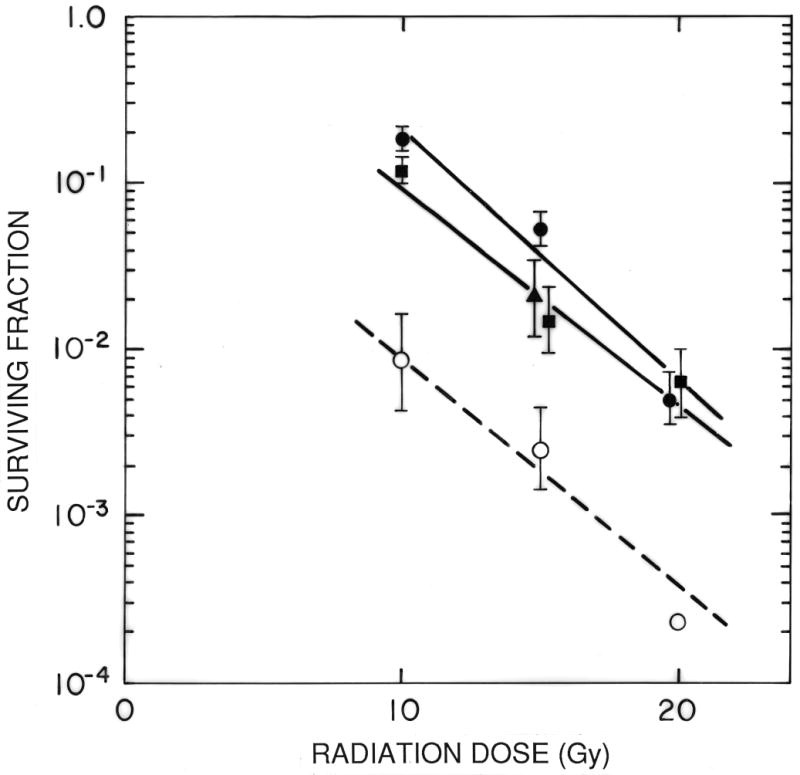
The graph shows the survival of cells from BA1112 tumors irradiated in vivo after treatment with radiation in air breathing rats (■), rats under HBO (●), rats treated with 4 ml/kg of the perfluorochemical emulsion Oxygent ® and irradiated under HBO (○), or rats treated with the diluent for the perfluorochemical emulsion and irradiated under HBO (□). Points are means ± SEMs from multiple experiments. Treatment with HBO produced a small change in the oxygenation and radiation response of the tumors. Treatment with Oxygent plus HBO produced a dramatic improvement over that seen in air-breathing and HBO-treated rats. Reprinted from [45].
It became clear during the 1990s that the development and clinical use of synthetic oxygen transport agents would be more difficult than originally envisioned [59-62,64]. In addition, the commercial manufacturers became increasingly aware that the use of these agents with radiotherapy would require many administrations of high doses of the agents – regimens that would be far more intensive than those needed in many other potential applications and therefore would be more likely to result in toxicities. Moreover, the fragile status of the patients generally entered into Phase I/II oncology trials, the effects of their advanced malignancies, and the intensive irradiation regimens used in their treatment all increased the potential for toxicities that could impact FDA approval. Companies developing these agents therefore backed away from potential cancer therapeutic applications and focused their development efforts on uses in diagnostic imaging, during surgery, and after trauma, all of which required smaller doses and acute administration. Extensive research and developmental efforts for these applications are ongoing [59-62,64]. The potential value of these agents in clinical cancer therapy still remains to be rigorously explored. Ironically, the increasing use of stereotactic radiosurgery, IMRT, intraoperative radiotherapy, and high dose rate brachytherapy regimens that use short radiation courses and high radiation doses per fraction offers increased potential for administration of oxygen-modulating agents for shorter times and in fewer treatments. If clinical trials of oxygen transport agents for other indications lead to marketed products with well documented efficacy and manageable toxicities in other patient populations, it will be interesting to revisit the question of whether these more advanced oxygen transport agents have value as adjuncts to radiotherapy.
Another approach to modulating tumor oxygen is to modulate hemoglobin-oxygen binding affinity and thereby increase delivery of oxygen to hypoxic regions. Efaproxiral (RSR13) was the first of this class of agents to reach clinical trials with radiotherapy. Efaproxiral binds noncovalently in the central water cavity of the hemoglobin tetramer, altering the conformation of the hemoglobin and reducing its oxygen-binding affinity. Studies in a number of rodent tumor model systems [68,69] showed that pretreatment with Efaproxiral plus oxygen breathing increased the oxygenation of solid rodent tumors and significantly improved the response of the tumors to irradiation. Initial clinical trials with this agent [49,70,71] showed improved response to radiotherapy in patients with brain metastases but confirmatory trials were disappointing; the manufacturer is not currently pursuing development of the product.
Modulation of tumor blood flow to increase tumor oxygen levels during irradiation also provides a potential approach to increasing tumor radiosensitivity. A number of pharmacological agents have been proposed as potential agents to improve tumor blood flow, including pentoxifylline and nicotinamide. These drugs have been shown to be effective in increasing the radiation response of animal tumors [41,72-74]. However, clinical trials using these agents to modulate blood flow during radiation therapy proved to be complex and difficult. This is in part because of the complex effects of the agents and the toxicities encountered when these agents are used repeatedly in high doses [41,72-75]. In addition, radiation itself induces very significant changes in tumor blood flow, and oxygenation [76] complicating extrapolation from the effects seen in single-dose animal experiments to plan and interpret studies with fractionated radiotherapy regimens.
Changes in tumor blood flow after irradiation, and the concomitant changes in oxygen delivery to tumor tissue, are partially responsible for the observed differences in the efficacy of different radiotherapy regimens. Fraction size, interfraction interval, overall treatment duration, dose rate (for brachytherapy) and radiation quality (LET; x and γ-rays vs. neutrons or heavy charged particles) all influence radiation-induced changes in tumor oxygenation [5-7,76]. Moreover, the importance of oxygen as a chemical radiosensitizer also varies with these treatment parameters. It is widely forgotten that one of the major theoretical advantages of continuous low dose rate irradiation, which was discussed extensively in the middle of the last century and which provided one of the primary rationales for low-dose-rate brachytherapy at that time, is the reduced OER for radiation cytotoxicity observed at low radiation doses and with low dose rates [77-79]. It is probable that some of the effects seen with the oxygen-modulating regimens described above reflect the changes in tumor blood flow induced by the agents as well as changes in blood oxygen levels. For example, both Carbogen breathing and perfluorochemical emulsions alter blood flow in animal tumor models. Changes in blood flow induced by prior treatments with hyperthermia [80], chemotherapy [81], and antiangiogenic therapies and antivascular therapies [82,83] have been shown to be responsible for some beneficial effects of combined modality therapy regimens using these agents in rodent tumors.
Inhibition of the consumption of O2 by tumor cells also has great potential to increase tumor oxygenation. Theoretical considerations suggest that it should be much easier to increase tumor oxygenation by decreasing oxygen consumption than by increasing oxygen delivery [84,85]. The effects of agents that directly inhibit oxygen metabolism and of physiologic interventions that reduce O2 consumption have been examined in rodent systems. Changes in oxygen consumption can result from treatments with radiation, hypothermia, hyperthermia, anticancer drugs, hormones, antivascular drugs, anesthetics, and anti-inflammatory drugs such as Cox-2 inhibitors [32,80,81,84-86]. These changes in O2 consumption, and the resulting changes in tumor oxygenation, have been suggested or shown in animal models to be important in determining the outcome of treatment regimens in which these agents are used. Thus, changes in blood flow and oxygen consumption during the course of multifraction and multiagent therapy alter tumor oxygenation and are probably critical in determining the efficacy of many widely used therapeutic regimens. Such changes are seen even with agents such as NSAID [86], which are routinely used by cancer patients but not thought of as influencing the efficacy of cancer therapy.
Until recently, the problem in using these changes to therapeutic advantage has been the lack of techniques to monitor tumor oxygen levels quantitatively and repeatedly during the course of protracted therapeutic regimens. As a result, it has been impossible to assess the nature, magnitude, timing and therapeutic importance of these intercurrent changes in oxygenation. This limitation has impacted interpretation of studies using experimental animals, but the impact on study design, patient selection and interpretation of data is even greater for clinical studies. With the development of minimally invasive and non-invasive techniques for measuring oxygenation within tumors [46,86-93], it is becoming possible to monitor tumor oxygen distributions not only before treatment, but also repeatedly throughout and after complex therapeutic regimens. Moreover, patient-to-patient differences in pretreatment tumor oxygenation and in the changes occurring during therapy can be examined. These techniques allow rigorous evaluation of the implications of the pretreatment tumor oxygenation and of the changes in oxygenation during therapy for “conventional” therapeutic regimens, as well as for regimens that are still investigational or that are specifically intended to alter oxygenation. This holds great promise for facilitating the development of improved treatment regimens that use changes in tumor oxygenation to therapeutic advantage and may also lead to individualized treatment regimens that are optimized for specific patients.
Oxygen-mimetic radiosensitizers
Oxygen-mimetic radiosensitizers, which selectively sensitize hypoxic cells to ionizing radiation by replacing oxygen in the chemical reactions that lead to the production of DNA damage, have been widely studied as potential adjuncts to radiotherapy. Metronidazole, misonidazole, nimorazole, piminidazole and several other hypoxic cell radiosensitizers were found to be extremely effective in sensitizing hypoxic cells in vitro and in rodent tumors [2,34,95,96], as illustrated on Fig. (5) and Fig. (6). However, the rapid biodistribution and clearance of these agents in mice was critical in producing the high therapeutic ratio in these mouse model systems. In humans the long half-lives of the drugs result in greater toxicities, which preclude administration of these drugs at doses that produce the high tumor drug concentrations needed for effective radiosensitization and therefore prevent maximally effective use of the radiosensitizers with fractionated radiotherapy. Clinical evaluation of these agents suffered from the resulting compromises in the structure of the clinical trials: sensitizers could be given only in low doses and only with a few radiation treatments [2,34]. Rodent studies with analogous regimens showed no or only very small improvements in tumor response, with at best marginal statistical significance [96]. Considering the inherently greater variability in the patient populations than in the experimental models, most clinical trials with oxygen mimetic radiosensitizers were doomed to failure, because they lacked the statistical power needed to detect the effects that could have been anticipated from the rodent studies even with the most optimistic assumptions. Only the largest, most tightly controlled clinical trials (most notably those performed in Denmark) and metaanalyses including many randomized clinical trials [2,34] revealed positive effects of radiosensitizers in human patients. The metaanalyses showed the approach to be successful in some tumors, with small but statistically significant improvements in local control and disease free survival.
Figure 5. Effects of hypoxic cell radiosensitizers on aerobic and hypoxic cells in vitro.
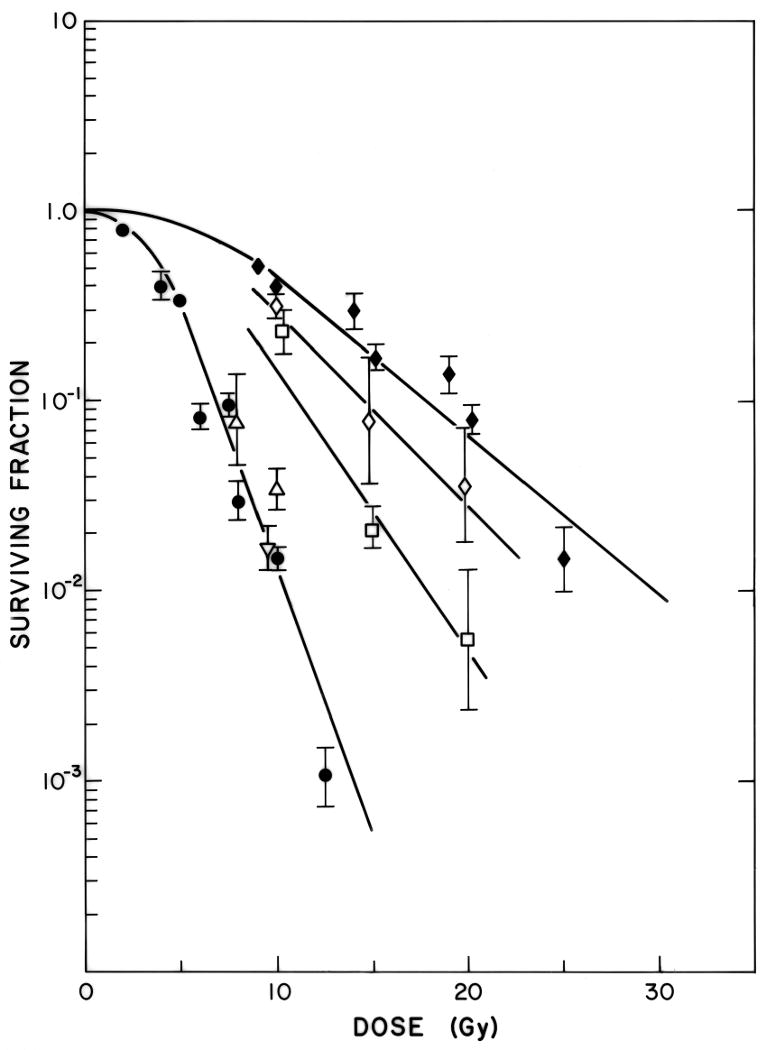
EMT6 cells were irradiated under aerobic (●) and severely hypoxic conditions (◆), under hypoxic conditions in the presence of 0.1 mM (◇) or 1 mM (□) misonidazole, or under aerobic conditions in the presence of 0.1 mM (▽) or 1 mM (△) misonidazole. Misonidazole produced a dose-dependent increase in the radiosensitivity of the hypoxic cells, but had no effect on the radiosensitivity of the aerobic cells. Reprinted from [23].
Figure 6. Effect of misonidazole on the response of mouse tumors in vivo to large single doses of radiation.
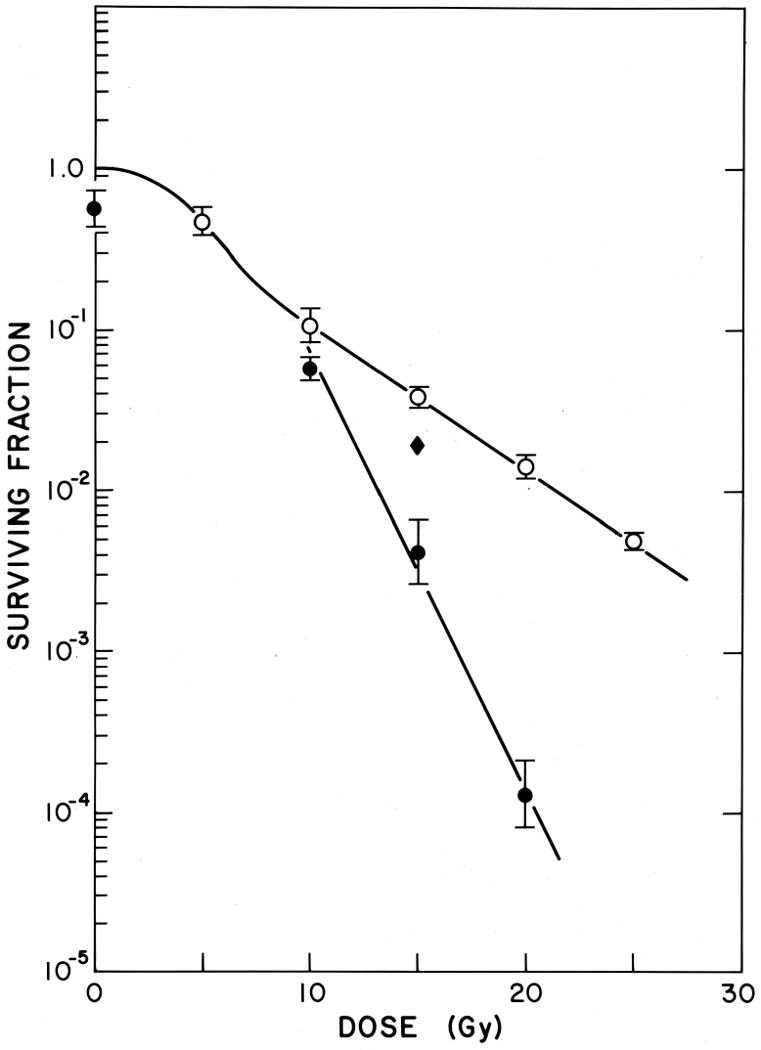
EMT6 tumors were treated with radiation alone (○) or one hour after treatment with 1 mg/kg misonidazole (●); this dose produced peak levels of misonidazole in tumors of 1-2 mM at that time. Misonidazole produces marked radiosensitization of the tumors. When misonidazole was given just after irradiation (◆) a small change in tumor cell survival was seen, which reflects the cytotoxicity of misonidazole to the hypoxic tumor cells. Reprinted from [23].
As with HBO, it would be interesting to re-explore the potential value of the best oxygen-mimetic radiation sensitizers as adjuvants to investigational radiotherapy regimens using large radiation doses and small numbers of fractions, as it is likely that appropriate use of hypoxic cell radiosensitizers as adjuncts to certain stereotactic radiosurgery, intraoperative radiotherapy and high-dose-rate brachytherapy regimens could produce therapeutic gain, especially if potential subjects were prescreened for tumor oxygenation and only patients with hypoxic tumors were entered into the trials.
Oxygen-mimetic radiosensitization is not the only approach to selectively increasing the radiosensitivity of hypoxic cells. Information now developing on the biology of hypoxic cells offers the possibility of using the physiological changes induced by hypoxia to identify other pathways and targets that can be exploited to sensitize these cells to the cytotoxic effects of radiation [95, 97-99]. We will discuss this below.
Bioreductive Drugs and Other Hypoxic Cell Cytotoxins
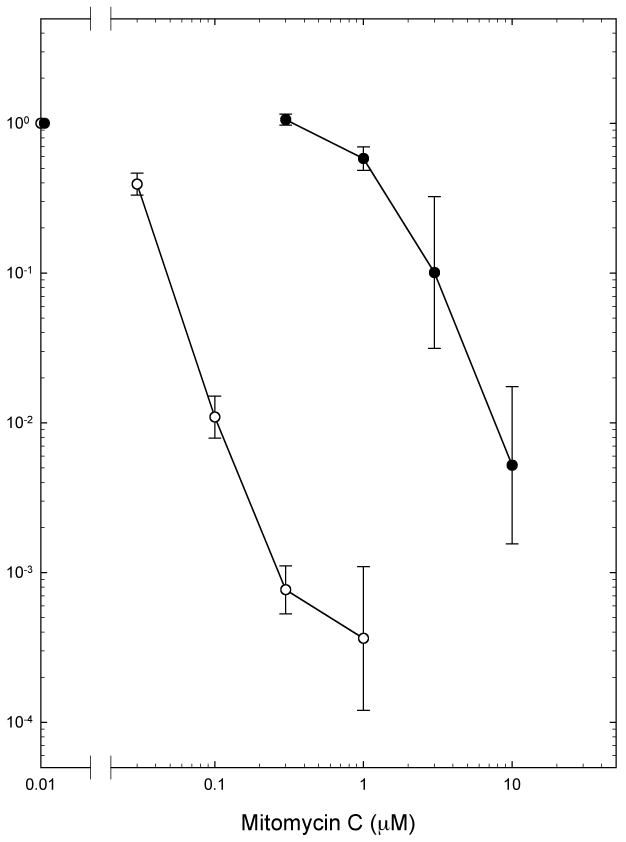
Agents with selective toxicity to hypoxic cells could likewise play a valuable role in combination with radiation therapy, and also in combination with conventional anticancer drugs, which are generally more toxic to rapidly proliferating, well-perfused, tumor cells than to the hypoxic cells in unperfused regions of tumors [100]. The prototype hypoxic cell cytotoxins were the bioreductive alkylating agents, originally described by Sartorelli and his collaborators [101]. The lead compound of this class, the quinone mitomycin C, was shown to have preferential toxicity to cells that were severely hypoxic at the time of drug treatment, Fig. (7). The duration of pre-treatment hypoxia is unimportant, Fig. (8), as long as cells are severely hypoxic at the time of treatment. The preferential toxicity of the drug to hypoxic cells reflects differences in the pathway of reductive activation of MC in air and hypoxia, which lead to increased production under hypoxia of reactive intermediates of capable of bi-functional alkylation, and therefore to the production of greater numbers of highly toxic interstrand and intrastrand DNA crosslinks under hypoxic conditions [100-103]. Severe hypoxia, at oxygen levels below those required for full radioprotection, is required for maximal sensitivity to MC. Under aerobic conditions, cells are more sensitive to MC at low pH than at physiologic pH, Fig. (9).
Figure 7.
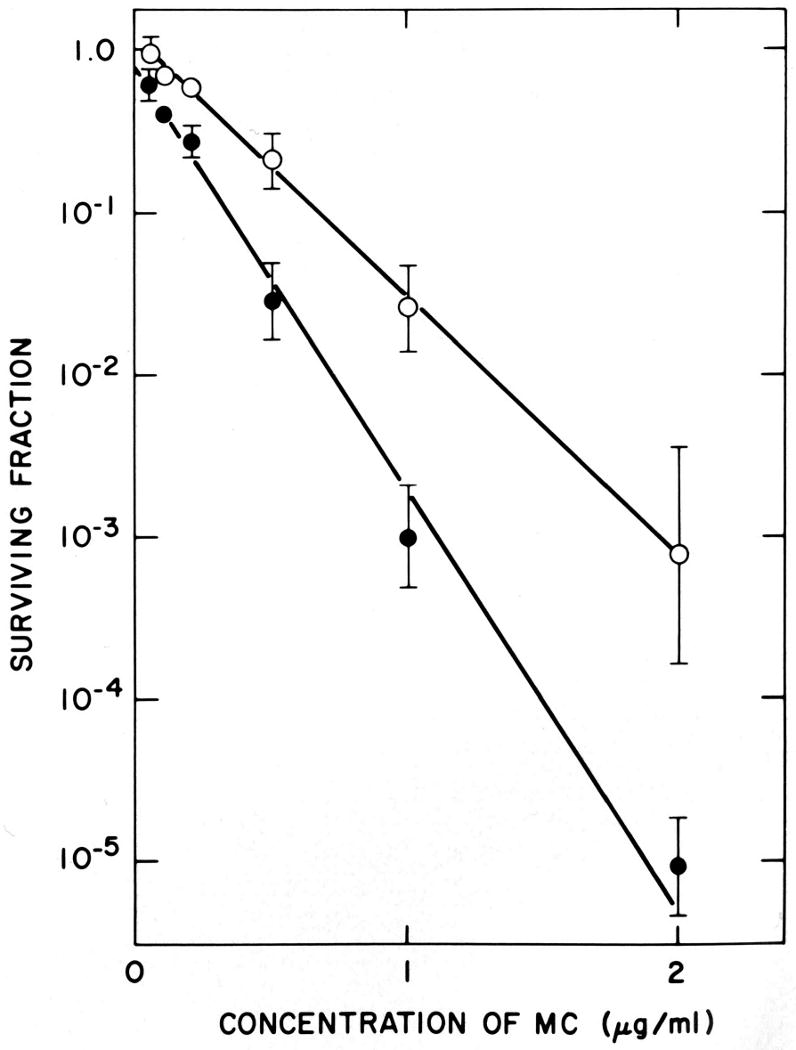
Survival of EMT6 cells in exponential growth in cell culture after treatment for 2 hours with graded doses of Mitomycin C under aerobic (○) or severely hypoxic (●) conditions. Survival was determined using a clonogenic assay. Points are means ± SEMS for 4 or more experiments. Reprinted from [102].
Figure 8. The duration of hypoxia does not affect the survival of EMT6 cells treated with mitomycin C.
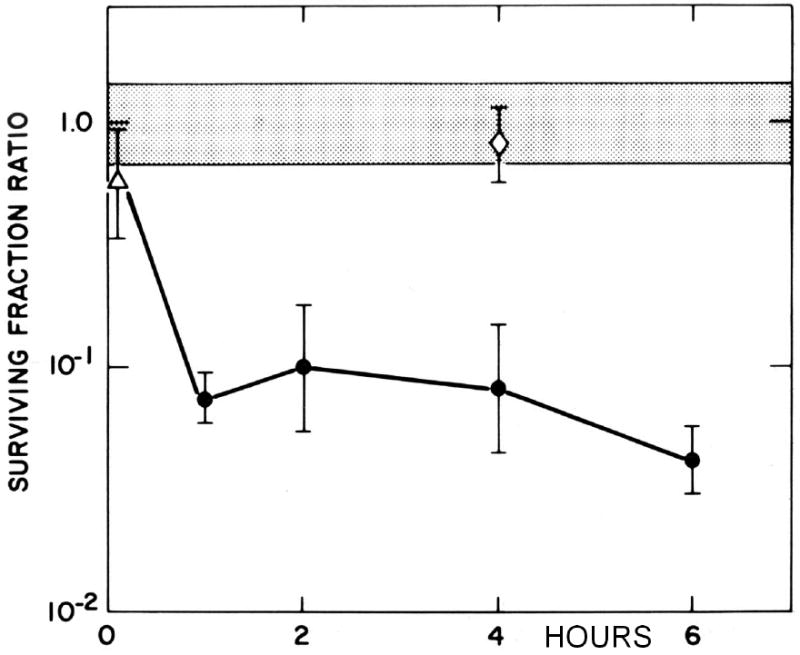
The surviving fraction ratio was calculated relative to the survival of aerobic cells treated with MC in the same experiment. Cells that became hypoxic during a 1 hour treatment with 1.5 μM MC (△) were only slightly more sensitive than aerobic cells (stippled area). Cells hypoxic for 30 min to 6 hour before addition of MC showed similar survivals (●), while cells held in hypoxia for 4 hours and then aerated before addition of MC (◇) had sensitivities indistinguishable from those of cells that had never been hypoxic. Only the oxygenation of the cells at the time of MC treatment affected the sensitivity of the cells to the drug. Reprinted from [102].
Figure 9. Effect of pH on the cytotoxicity of Mitomycin C in air.
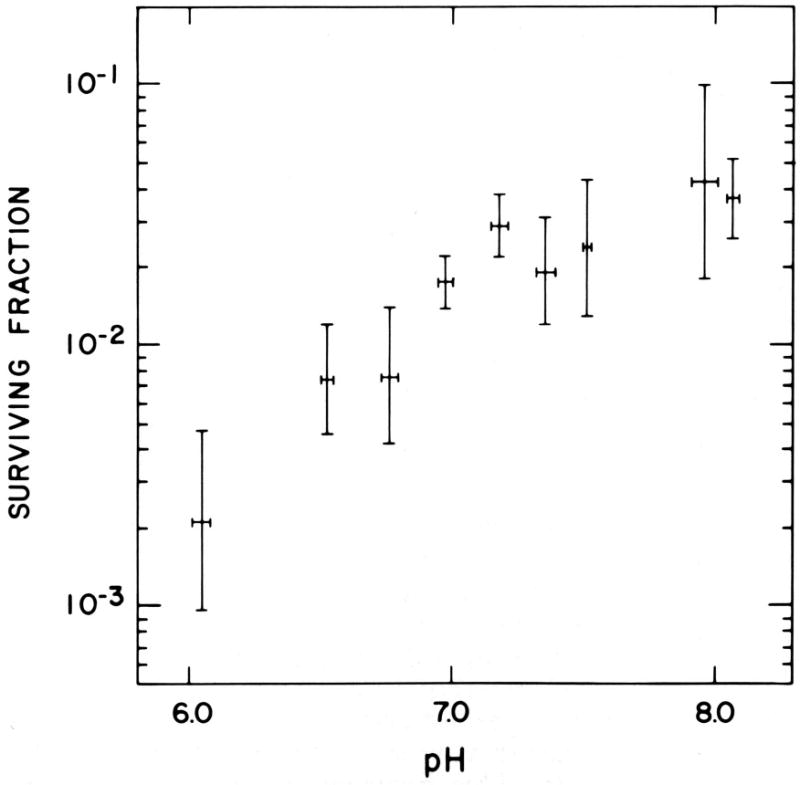
Survival of EMT6 cells in exponential growth in cell culture after treatment for 30 minutes with 2 μg/ml MC under aerobic conditions at different pH. Points are means from 4-6 experiments, with SEMs shown for both the surviving fraction and the pH. Reprinted from [102].
Prospective randomized clinical trials of mitomycin C (MC), showed statistically significant improvements in local control and disease free survival when MC was used as an adjunct to radiotherapy in carcinomas of the cervix and of the head and neck [104-105]. The magnitude of the effect in cervix cancer was similar to that obtained with adjuvant treatments with cis-platinum, but the toxicities with MC were less severe. Trials of MC, alone and in combination with other drugs, as an adjunct to radiation therapy are continuing at several institutions [106]. A major problem in the interpretation of these trials is the small and variable differential in the cytotoxicities of MC to hypoxic and aerobic cells. Toxicities of this drug to aerobic cells, especially cells in acidic environments Fig. (9) or cells with the unusual high levels of critical reductases found in certain malignancies (especially DT diaphorase, NQO1), could be partially responsible for the increases in local control seen with this drug; high levels of NQO1 may also decrease the hypoxia selectivity of this drug, as they do with the indolequinone EO9 [106]. Therapy with MC has often been limited not by the toxicities routinely seen with this drug, but rather by rare, unpredictable, serious and sometimes fatal idiosyncratic toxicities that occur at doses well below those expected to be toxic. It seems reasonable to ask whether polymorphisms in a reductase that activates this drug lead to greater aerobic activation of this drug in some patients and, if so, whether a pretreatment screen could be developed to identify such patients, so the MC could be avoided or the dose reduced in hypersensitive patients, thereby allowing safer and wider use of this drug. Recent studies have also shown that cells lacking functional BRCA2 are hypersensitive to MC [107], Fig. (8), suggesting that BRCA2-/- patients would be hypersensitive to MC, but also suggesting that breast cancers exhibiting a BRAC2 repair-deficient phenotype, either because of mutations in the tumor or because of downregulation of this DNA repair pathway in hypoxia [108], might respond unusually well to MC.
Many other quinones have been studied in our laboratory and elsewhere; the potency, the nature of the DNA lesions, and the magnitude of the aerobic/hypoxic cytotoxicity differential vary dramatically for different quinones [103,106,109]. Porfiromycin, which showed greater selectivity to hypoxic cells than MC in vitro and in animal models, did not improve the outcome of radiotherapy in clinical trials [109]. The promising hypoxia-selective indolequinone EO9, studied by McKeown and her collaborators, is currently in clinical trials in Europe [106].
In addition, other classes of drugs with preferential toxicity to hypoxic cells are being developed and tested in preclinical and clinical studies. The benzotriazine di-N-oxide Tirapazamine has a much larger differential toxicity to hypoxic cells than the quinones and requires only moderate hypoxia for enhanced activity. Combined treatments with Tirapazamine and radiation are extremely effective in mouse models [110]. Clinical trials using Tirapazamine in multi-agent regimens have shown great promise [49,106,110,111]. It is somewhat difficult to assess the importance of the selective hypoxic cytotoxicity of Tirapazamine to this success, as the clinical trials reported to date have used this drug in combination with cis-platinum, other drugs, or chemoradiotherapy, and pharmacologic interactions of Tirapazamine with other drugs may be important to the outcomes. Reports of the value of Tirapazamine as an adjunct to radiotherapy, the use in which its activity against hypoxic cells would be most critical, have yet to be published.
Several newer bioreductive alkylating agents are in various stages of laboratory and clinical testing. The bioreductive agent KS119, developed by Sartorelli and his collaborators, releases the alkylating agent 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine (also called 90CE) after enzymatic reduction in hypoxia. KS119 has a much larger differential in aerobic and hypoxic cytotoxicity than the quinones in vitro Fig. (11) and is effective in killing hypoxic cells in mouse tumors [112]. KS119 has very little toxicity to aerobic cells and is remarkably non-toxic in mice. The insolubility of this lead compound limited its development, but a water-soluble analog, KS119W, looks extremely promising in preclinical studies in vitro and in vivo [Belcourt et al., proceedings, AACR, 2005; Kim, Liu and Rockwell, proceeding, Radiation Research Society, 2008].
Figure 11.
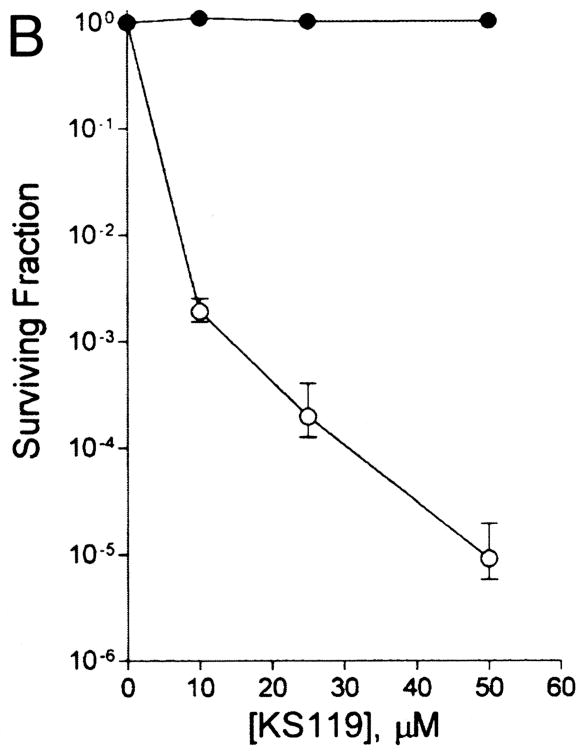
Toxicity of the bioreductive alkylating agent KS119 to aerobic (●) and severely hypoxic (○) cells. Points are means ± SEMs from 3 experiments. Reprinted from [112].
Several nitroaromatic compounds have been explored as potential hypoxia-selective cytotoxins [27,106], based on the observation that the nitroimidazole radiosensitizer Misonidazole has selective cytotoxicity to hypoxic cells in vitro at high drug concentrations and has been shown to kill hypoxic cells in animal tumors by its small effects on tumor growth and tumor cell survival when given after irradiation (i.e. when it would not produce radiosensitization), as shown on Fig. (6). The 2-nitroimidazoles RSU1069 and RB6145 were both effective as hypoxic cytotoxins in model tumor systems, but proved to have GI and retinal toxicities, respectively, which precluded clinical development. Nitroaromatics with weak DNA intercalating activity have been studied by Cowen and Wilson. The lead compound in this series, NLCQ-1, has both sensitizing and cytotoxic activities and is being considered for clinical development [106].
Another class of bioreductive drugs, the alaphilic N-oxides, has also entered clinical trials [106,113]. The bis-N-oxide banoxantrone, AQ4N, is reduced to yield AQ4, a stable analog of mitoxantrone, which has high DNA binding affinity and topoisomerase II inhibitory activity. Bioreductive activation occurs in the microsomal fraction and appears to be hypoxia selective. Banoxatrone looks promising in combination with radiation in rodent tumor studies and is now in early clinical trials.
Ongoing work [106,114, Sartorelli, personal communication] is exploring the development of drugs which are activated in hypoxia to produce reactive intermediates with lifetimes considerably longer than those of the active metabolites of previous bioreductive drugs. These long lifetimes allow the active species to diffuse from the regions of hypoxia where they are produced into adjacent tumor regions and to kill aerobic cells in these areas. Conceptually, this approach uses hypoxic tumor cells as factories to convert non-toxic prodrugs into cytotoxic drugs locally, within the tumor, to kill both hypoxic and aerobic cells in a somewhat larger volume of the tumor. The dinitrobenzyl mustards are currently the most well developed compounds of this class. The lead compound, PR104 (Proacta) is in Phase 1 clinical trials.
Another variation on the approach of targeting drugs using hypoxia has been the use of bacteria that preferentially home to and proliferate in hypoxic or acidic areas, secrete cytotoxic moieties into their environment and thereby deliver therapy selectively within the tumor [111,115].
Interest in the development of hypoxia-selective cytotoxic agents is intense. The possibility of developing improved bioreductive drugs with selective toxicities to hypoxic cells will undoubtedly increase with increasing understanding of the identities and activities of the enzymes that perform the selective reductive activation and produce the preferential cytotoxicity of the existing drugs. It should be noted that the existing hypoxia-directed drugs were all developed to target cells that are hypoxic at the moment of drug treatment: these are activated by pathways that are inhibited or reversed in the presence of oxygen. It seems very likely that the extensive ongoing studies of the changes in gene expression, enzyme activities, and cell physiology induced by cells that are held under hypoxia for longer periods of time will result in the identification of new signal transduction pathways and new molecular targets that will provide new drugs and novel therapeutic approaches. Similarly, studies of the oxidative stresses associated with reoxygenation and with repeated cycles of hypoxia and reoxygenation are revealing potential targets for therapy. These studies will undoubtedly lead to new classes of drugs, which have not been explored in the past and ongoing studies with the hypoxia-directed drugs described above.
The Biotechnology Revolution
Recent developments in molecular biotechnology are revolutionizing studies of the effect of hypoxia, by allowing researchers to move from observations of changes in the phenotype and viability of cell populations and of overall changes at the tissue level to studies of the molecular pathways and processes that underlie these changes. The ability to monitor gene expression, protein levels, or enzyme activities simultaneously in thousands of microscopic cell or tissue samples changes the parameters and limitations of experimental design in ways most biologists have not yet appreciated and utilized. The availability of cell lines and animals genetically engineered to have predetermined changes in specific genetic loci allows the effects of specific genes, mutations, proteins, and signal transduction pathways to be studied with a power that improves daily. The ability to define the gene sequences and thereby detect critical genomic heterogeneity in biological systems, alters the choice of experimental models. The ability to make such measurements in the tumors and normal tissues of individual patients offers the tantalizing possibility of individualizing treatment to maximize therapeutic efficacy or minimize toxicity. The speed offered by automated chemical and molecular assays and the data management and data analysis capacity offered by current and developing computer technologies have also dramatically impacted research in experimental radiotherapy. Vast chemical libraries, produced by collection or by automated combinatorial chemistry, combined with automated array assays defining the interactions of these chemicals with specific therapeutic targets or determining their effects on specific cellular pathways or processes now allow screening of thousands of compounds for a desired activity within days, rather than years. The impact of these developments in experimental cancer therapy is enormous and will undoubtedly affect the development of new hypoxia-directed therapies.
Close collaboration between basic scientists and translational researchers will be critical to ensure that insights obtained from studies in molecular biology, cell biology, and chemistry laboratories are rapidly converted into therapeutic application. This will mean that radiation oncologists must be in close communication with basic science researchers whose work may at first appear to be completely unrelated to their clinical interests, as well as with the translational researchers who can move new ideas, new findings and new agents toward clinical application, thus adding new elements to the already complex web of interactions of the experimental radiotherapy community.
Identification and Quantification of Hypoxic Cells in Tumors
Clinical trials of interventions based on tumor oxygenation during the twentieth century were limited by an inability to measure oxygen levels in human tumors. By the 1980's the importance of oxygen in determining the outcome of radiotherapy in animal tumors had been well established in studies using tumor growth, tumor cure, and tumor cell survival endpoints (as reviewed in 30-33) but these radiobiological endpoints were not applicable for use in humans. Proof of the importance, and indeed the existence, of hypoxia in human tumors remained limited and largely indirect. It was even argued by some that hypoxia was an artifact of rapidly growing transplanted animal tumors, and that human tumors did not, in fact, contain hypoxic cells.
The landscape in this area has now changed dramatically. Studies using oxygen microelectrodes, several different MRI and EPR approaches, and detection of exogenous [e.g. misonidazole, EF5, or piminidazole] or endogenous [e.g. HIF1, carbonic anhydrase IX, GLUT-1] molecular markers of cellular hypoxia with immunohistochemical or noninvasive imaging techniques all show that hypoxia is a common feature of tumors in patients presenting for therapy, as well as in animal tumors [27,46,87-94,97-99,116-119]. Perhaps more importantly, the data show that the amount and severity of hypoxia varies among individual patients with histologically similar diseases. Several studies now show that hypoxia is a prognostic factor predictive of poor outcome after therapy. Extensive hypoxia can be correlated with failure after surgery and with increased risk of metastases as well as with increased risk of local failure. This indicates that hypoxia can be a marker of aggressive disease, rather than reflecting only environmentally-induced resistance to radiation and/or chemotherapy. This should not be surprising, given the extensive laboratory investigations showing that treatment of cells with hypoxia increases in the ability of tumor cells to form metastases [120,121], results in the development of genomic instability, and fosters the evolution of model cell populations in vitro to increasingly aggressive phenotypes [108,122,123]. Measurements of hypoxia in human tumors therefore are being coordinated with studies that characterize the tumors to assess other, potentially related, prognostic factors and phenotypes.
Data from past clinical trials of hypoxia-directed therapies, which used unselected patients, suggested that unidentified tumor or patient factors influenced the therapeutic value of these approaches in these trials [2,35]. The availability of simple, repeatable, relatively non-invasive assays that allow repeated measurements of tumor oxygenation before and during therapy offers new tools for the development of improved clinical protocols testing hypoxia-directed therapies [94]. Studies of the prognostic significance of pretreatment oxygen distributions and of changes in oxygenation during therapy are rapidly shedding light on the relative importance of these two metrics in specific diseases and with specific therapies. Selection or stratification of patients by pretreatment oxygen levels could potentially decrease sample heterogeneity and reduce the number of patients needed to achieve adequate statistical power in trials testing new hypoxia-directed therapies [2,94]. Patients identified as having unusually well oxygenated tumors might be poor candidates for any therapeutic approach targeting or modulating hypoxia, but might well benefit from another therapy aimed at a different target. Pretreatment studies examining the efficacy of a potential intervention in modulating oxygen in the tumors of individual patients [46,118] could conceivably be used to identify those patients who respond well to that specific approach to modulating pO2 and who might benefit from its use. Similarly, as more information on the molecular pathways modulated by oxygen becomes available, it may be possible to identify patients who would benefit from chemotherapeutic approaches targeting specific oxygen-modulated pathways or, equally important, to identify and exclude patients who should not be treated with specific hypoxia-directed therapies. Application of new tools for measuring tumor hypoxia and for studying the biology of individual tumors therefore has the potential to offer improvement in the use of existing hypoxia-directed therapies as well as the potential to facilitate the development of new agents and new approaches that will improve the treatment of solid tumors.
Studies of the Tumor Vasculature
It has long been known that tumor vasculature is abnormal in its structure, function, and response to normal homeostatic signals. Understanding of the biological basis for the observed defects is increasing rapidly. Studies of angiogenesis and neovascularization are providing new insights into these complex processes, which is critical to tumor growth and metastasis, at the molecular and cellular levels [82,83,124,125]. A large number of agents that target the tumor vasculature, either by preventing the formation of new blood vessels or by selectively damaging the abnormal blood vessels within tumors, are being tested in laboratory studies. Many have entered clinical trials and several have FDA approval. These approaches and agents generally are likely to be more effective for the treatment of established tumors when used in combination with effective cytotoxic therapies like radiation than when used as single agents. There is also the exciting possibility of using these agents to prevent individual metastatic cells or microscopic metastases from developing into invasive, symptomatic cancers. The possibility of preventing hyperplastic premalignant lesions from initiating angiogenesis and evolving into invasive malignancies can also be envisioned. Of interest in this regard is the accumulating evidence that the initiation of angiogenesis in nascent tumors (the “angiogenic switch”) involves not only tumor cells, but also host cells including macrophages [126] and bone marrow derived endothelial progenitor cells [127], suggesting the possibility of therapies directed at the participating host cells, which may offer a more uniform and stable target than the cancer cells. As with all anticancer therapies, it will be critical that these new agents exhibit selective effects on tumors without excessive toxicities to critical normal tissues, including normal tissues that are mildly hypoxic, so that the therapeutic ratio is enhanced by their use.
It was originally assumed by many that use of these vascular-targeted agents would damage the vasculature and therefore increase tumor hypoxia. However, the nature and magnitude of the effects of antivascular agents vary with time. Studies by Jain and his collaborators and by others have shown that treatments with these agents can actually result in normalization of the tumor vasculature, producing improvements in tumor perfusion, increased tumor blood flow and decreased interstitial pressure [82,83,127]. The use of vascular-targeted agents to improve tumor oxygenation during radiotherapy and to increase the delivery and efficacy of anticancer drugs has only begun to be explored, and offers great promise [82,129-131].
Studies of the Physiological and Molecular Effects of Hypoxia
One of the common features of past therapeutic approaches aimed at circumventing the effects of hypoxic cells is that their effects depend only on the oxygenation of the cells at the moment of treatment. Approaches to modulating tumor oxygenation have largely focused on oxygenating the hypoxic tumor cells acutely, during the radiation treatment. The radiosensitizing activity of the oxygen-mimetic hypoxic cell sensitizers results from their presence at the moment of irradiation. Preferential activation of the bioreductive alkylating agents and other exiting hypoxia-activated prodrugs results from their reduction via pathways involving enzymes or reactive radical intermediates that are inactivated by the presence of molecular oxygen. The activity of these agents requires that the cells be hypoxic at the time of drug treatment, but the activity does not depend on how long the cells have been hypoxic before treatment Fig. (8). All of the current treatments would be effective against either acutely or chronically hypoxic cells. Moreover, most of these drugs were developed to attack cells that were radiobiologically hypoxic. They are therefore targeted to cells in very severe hypoxia. Tirapazamine is the first hypoxia-directed drug that was effective against moderately hypoxic cells [110,111], and its efficacy argues for the success of this approach.
With the ability to measure and image hypoxia repeatedly, on a regional level within tissues, it is becoming clear that malignancies contain not only cells that are chronically exposed to severe hypoxia and are doomed to die if not rescued by irradiation, as originally proposed by Thomlinson and Gray [25], but also cells that are exposed to mild, moderate, or severe hypoxia for times of seconds, minutes or hours before they are reoxygenated [28,29]. The characteristics of cells that have been moderately or severely hypoxic for hours or days differ from those of cells made acutely hypoxic; the differences will depend on the severity of the hypoxia, the duration of the hypoxia, and the nature and severity of any other concomitant environmental deficits [108,122-123,131-139]. Moreover, cells that have been hypoxic and are then reoxygenated will not return to their pre-hypoxia condition immediately, because some of the changes in proliferation, gene expression, and protein levels may require hours or days to normalize [108,137]. In addition, the acute oxidative stress induced by sudden reoxygenation will also induce injury and stress responses that alter the physiology and therapeutic responses of the cells [108,131,137]. It is also becoming clear that not only the malignant cells, but also the stromal cells within the tumor are altered by these exposures to hypoxia [83,84,124,126,127]. The responses of the stromal cells may, in fact, offer especially interesting targets for the development of therapeutic agents, because these cells may show less genomic instability than the tumor cells and because they offer targets that may be applicable across a wide spectrum of tumor types. All of these hypoxic cell populations within tumors have characteristics that offer potential new targets for cancer therapy.
Moreover, it is becoming clear that hypoxia is not just important in cancer. Hypoxia may be important during embryonic development [138], in the physiology of certain normal tissues [124], and in the maintenance of the phenotypes of certain stem cells [139]. Moreover, both the acute induction of severe chronic hypoxia and the oxidative stress occurring when that hypoxia is alleviated are critical factors in determining the extent of injury after acute medical emergencies such as traumatic injury, stroke and myocardial infarction. Chronic moderate hypoxia is also being implicated in the pathogenesis of certain benign diseases, including certain retinopathies and complications of diabetes. Mild hypoxia and hypoxia-associated angiogenesis may also be critical in the fetal development, as shown by the devastating side effects that thalidomide (now known to be a potent antiangiogenic agent) produced when it was used for nausea by pregnant women [138]. Research in the area of hypoxia is therefore moving from a narrow focus on hypoxic radioresistance and methods to circumvent the effects of this radioresistance to merge with studies of hypoxia in other contexts and with studies of mild hypoxia that produces only minimal radioresistance.
It has long been known from studies of cells in vitro and in tumors in vivo that hypoxia and the environmental insults that accompany hypoxia (e.g. low pH; nutrient levels) inhibit cell proliferation [140,141], alter glucose metabolism and energy balance, cellular redox status and drug metabolism [142,143], and inhibit certain DNA repair pathways [144,145]. Studies as early as the 1960's and 70's clearly documented these effects of hypoxia and pointed out their possible implications for fractionated radiotherapy, brachytherapy, chemotherapy, and combined modality therapies [140-145]. However, current studies at the cellular and molecular level differ fundamentally from this previous work, in that they are probing the gene expression changes, signal transduction pathways, and enzyme activities and molecular mechanisms underlying these phenomena. Research has therefore moved to a new level, which allows identification of molecular targets and offers opportunities for the development of highly targeted therapies. This takes studies of hypoxia into a new era, which, as described elsewhere in this volume, offers new horizons for improving the treatment of cancer, as well as many other diseases.
Conclusions
Solid tumors contain hypoxic areas from the earliest phases of their development. Tumors of the smallest sizes that are currently detectable by physical examination, diagnostic imaging, or analysis of markers such as PSA are already large enough to have initiated angiogenesis, developed regional and temporal variations in perfusion, and developed microenvironmental heterogeneity. Tumor hypoxia therefore must be considered as a factor influencing the outcome of treatment with radiotherapy, chemotherapy, and even surgery, not only for the treatment of large bulky tumors with extensive necrosis, but also for the treatment of small primary and recurrent tumors, micrometastases and surgical margins with microscopic tumor involvement. Because hypoxic tumor cells are resistant to radiation and to many anticancer drugs, many approaches to circumventing the effects of hypoxia have been examined extensively in laboratory and clinical studies. Only limited success was achieved in past studies of these approaches, but it should be remembered that improvements in local control and disease free survival seen with HBO, hypoxic cell sensitizers, and bioreductive drugs were of the same magnitude as the incremental improvements seen for adjuvant treatments with anticancer drugs that are widely regarded as therapeutic successes by the oncology community. New technologies for measuring hypoxia in human tumors and for assessing changes in hypoxia over the course of prolonged treatment regimens offer the possibility of identifying those patients who are most likely to benefit from hypoxia-directed therapies and of facilitating the development of better regimens for the use of existing hypoxia-directed agents. Agents now being tested in preclinical studies and clinical trials offer the possibility that better hypoxia-directed therapies will become available in the near future. Moreover, improved understanding of the abnormal vascular beds in solid tumors and of the effects of hypoxia and its related microenvironmental insults offer the potential for finding new targets, thereby leading to the development new agents and novel therapeutic approaches. There is therefore ample reason to believe that this century-old area of research will soon yield new therapeutic benefits.
Figure 10. Effect of BRCA2 deficiency on the sensitivity of cells to Mitomycin C.
Exponentially-growing cultures of VC-8 cells defective in BRCA2 (XRCC11) (○) and the parental V79 cells (BRCA2 wild type) (●)m were treated with mitomycin C under aerobic conditions for 2 hrs, then suspended, counted, and assayed for viability using a colony formation assay. Points are means ± SEM of survivals determined in 4 independent experiments.
Acknowledgments
Research in the authors' laboratory is supported in part by research grants from the NIH and the Susan G. Komen Foundation, research fellowships from the STARS program at Yale and the Yale-HHMI Future Scientists Program.
References
- 1.Rockwell S. Radiat Res. 1998;150(Suppl):S157–S169. [PubMed] [Google Scholar]
- 2.Overgaard J. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 3.Tubiana M, Dutreix J, Pierquin B. Int J Radiat Oncol Biol Phys. 1996;35:227–242. doi: 10.1016/0360-3016(96)00091-0. [DOI] [PubMed] [Google Scholar]
- 4.Rockwell S. Rev Cancer Biol Ther. 2007:211–232. [Google Scholar]
- 5.Suit HD. Textbook of Radiotherapy. Lea & Febiger; Philadelphia, PA: 1966. Radiation Biology: A Basis for Radiotherapy. [Google Scholar]
- 6.Fowler JF. Radiother Oncol. 1983;1:1–22. doi: 10.1016/s0167-8140(83)80003-6. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan HS. Radiat Res. 1970;43:460–476. [PubMed] [Google Scholar]
- 8.Laughlin JS. Radiat Res. 1995;144:129–140. [PubMed] [Google Scholar]
- 9.Phillips TL. Radiat Res. 2002;158:389–417. doi: 10.1667/0033-7587(2002)158[0389:rrs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Whitmore GF. Radiat Res. 1995;144:148–159. [PubMed] [Google Scholar]
- 11.Inokuti M, Seltzer SM. Radiat Res. 2002;158:3–12. doi: 10.1667/0033-7587(2002)158[0003:paaeor]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Zimbrick JD. Radiat Res. 2002;158:127–140. doi: 10.1667/0033-7587(2002)158[0127:rrsrca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Bedford JS, Dewey WC. Radiat Res. 2002;158:251–291. doi: 10.1667/0033-7587(2002)158[0251:hachir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Puck TT, Marcus PI. Proc Natl Acad Sci USA. 1955;41:432–437. doi: 10.1073/pnas.41.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt HB, Wilson CW. Nature. 1959;183:1060–1061. doi: 10.1038/1831060a0. [DOI] [PubMed] [Google Scholar]
- 16.Till JE, McCulloch EA. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 17.Hahn R. Fortscnr Geb Rontgenstr Nuklearmed. 1904;8:120–121. [Google Scholar]
- 18.Schwarz G. Munchener Medizinische Wochenschrift. 1909;24:1–2. [Google Scholar]
- 19.Crabtree HG, Cramer W. Proc R Soc Lond. 1933;113:238–250. [Google Scholar]
- 20.Mottram JC. Br J Radiol. 1936;9:606–614. [Google Scholar]
- 21.Quintiliani M. Int J Radiat Biol. 1986;50:573–594. doi: 10.1080/09553008614550981. [DOI] [PubMed] [Google Scholar]
- 22.Ward JF. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 23.Rockwell S. Manual de Radiotherapia Oncologia. Yale University; New Haven CT: 1989. Principios de radiobiologia. [Google Scholar]
- 24.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OCA. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 25.Thomlinson RH, Gray LH. Br J Cancer. 1955;11:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray LH. Am J Roentgenol Radium Ther Nucl Med. 1961;85:803–815. [PubMed] [Google Scholar]
- 27.Dendy PP, Wardman P. Brit J Radiol. 2006;79:545–549. doi: 10.1259/bjr/13634453. [DOI] [PubMed] [Google Scholar]
- 28.Reinhold HS, Blachiwiecz B, Blok A. Biblio Anat. 1977;15:270–272. [PubMed] [Google Scholar]
- 29.Brown JM. Brit J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 30.Moulder JE, Rockwell S. Int J Radiat Oncol Biol Phys. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 31.Moulder JE, Rockwell S. Cancer Metastasis Rev. 1987;5:313–341. doi: 10.1007/BF00055376. [DOI] [PubMed] [Google Scholar]
- 32.Rockwell S, Moulder JE. Int J Radiat Oncol Biol Phys. 1990;19:197–202. doi: 10.1016/0360-3016(90)90154-c. [DOI] [PubMed] [Google Scholar]
- 33.Rockwell S. Oncol Res. 1997;9:383–390. [PubMed] [Google Scholar]
- 34.Overgaard J, Horsman MR. Sem Radiat Oncol. 1996;6:10–21. doi: 10.1053/SRAO0060010. [DOI] [PubMed] [Google Scholar]
- 35.Fischer JJ, Rockwell S, Martin DF. Int J Radiat Oncol Biol Phys. 1986;12:95–102. doi: 10.1016/0360-3016(86)90421-9. [DOI] [PubMed] [Google Scholar]
- 36.Churchill-Davidson I, Sanger C, Thomlinson RH. Lancet. 1955;268:1091–1095. doi: 10.1016/s0140-6736(55)90589-4. [DOI] [PubMed] [Google Scholar]
- 37.Dische S, Anderson PJ, Sealy R, Watson ER. Br J Radiol. 1983;56:251–255. doi: 10.1259/0007-1285-56-664-251. [DOI] [PubMed] [Google Scholar]
- 38.Rockwell S, Knisely JPS. In: Regulation of Angiogenesis. Goldberg ID, Rosen EM, editors. Birkhauser Verlag Basel; Switzerland: 1997. pp. 335–360. [Google Scholar]
- 39.Mayer R, Hamilton-Farrell MR, van der Kleij AJ, Schmutz J, Granström G, Sicko Z, Melamed Y, Carl UM, Hartmann KA, Jansen EC, Ditri L, Sminia P. Strahlenther, Onkol. 2005;181:113–123. doi: 10.1007/s00066-005-1277-y. [DOI] [PubMed] [Google Scholar]
- 40.Mendenhall WM, Morris CG, Amdur RJ, Mendenhall NP, Siemann DW. Cancer. 2005;104:332–227. doi: 10.1002/cncr.21146. [DOI] [PubMed] [Google Scholar]
- 41.Kaanders JH, Bussink J, van der Kogel AJ. Lancet Oncol. 2002;3:728–737. doi: 10.1016/s1470-2045(02)00929-4. [DOI] [PubMed] [Google Scholar]
- 42.Hoogsteen IJ, Pop LA, Marres HA, Merkx MA, van den Hoogen FJ, van der Kogel AJ, Kaanders AH. Int J Radiat Oncol Biol Phys. 2006;64:83–89. doi: 10.1016/j.ijrobp.2005.07.003. 2006. [DOI] [PubMed] [Google Scholar]
- 43.Inch WR, McCredie JA, Sutherland RM. Cancer. 1970;25:926–931. doi: 10.1002/1097-0142(197004)25:4<926::aid-cncr2820250428>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Grau C, Horsman MR, Overgaard J. Int J Radiat Oncol Biol Phys. 1992;22:415–419. doi: 10.1016/0360-3016(92)90844-8. [DOI] [PubMed] [Google Scholar]
- 45.Rockwell S. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:1097–1108. doi: 10.3109/10731199409138805. [DOI] [PubMed] [Google Scholar]
- 46.Ljungkvist ASE, Bussink J, Kaanders HAM, van der Kogel AJ. Radiat Res. 2007;167:127–145. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]
- 47.Varlotto J, Steveson MA. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 48.Girinski T, Pejovic-Lenfant MH, Bourhis J, Campana F, Cosset JM, Petit C, Malaise EP, Haie C, Gerbaulet A, Chassagne D. Int J Radiat Oncol Biol Phys. 1989;16:37–42. doi: 10.1016/0360-3016(89)90007-2. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg A, Knox S. Int J Radiation Oncol Biol Phys. 2006;64:343–354. doi: 10.1016/j.ijrobp.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Bush RS. Int J Radiat Oncol Biol Phys. 1986;12:2047–2050. doi: 10.1016/0360-3016(86)90146-x. [DOI] [PubMed] [Google Scholar]
- 51.Dische S. Radiother Oncol. 1991;1:35–40. doi: 10.1016/0167-8140(91)90184-i. [DOI] [PubMed] [Google Scholar]
- 52.De Los Santos JF, Thomas GM. Gynecol Oncol. 2007;105:517–529. doi: 10.1016/j.ygyno.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 53.Siemann DW, Alliet KL, Macler LM. Int J Radiat Oncol Biol Phys. 1989;16:1169–1172. doi: 10.1016/0360-3016(89)90276-9. [DOI] [PubMed] [Google Scholar]
- 54.Hill RP, Bush RS, Yeung P. Brit J Radiol. 1971;44:299–304. doi: 10.1259/0007-1285-44-520-299. [DOI] [PubMed] [Google Scholar]
- 55.Hirst DG, Wood PJ. Int J Radiat Biol. 1987;51:597–609. doi: 10.1080/09553008414552131. [DOI] [PubMed] [Google Scholar]
- 56.Hirst DG, Hazelhurst JL, Brown JM. Int J Radiat Biol Relat Stud Phys Chem Med. 1984;46:345–354. doi: 10.1080/09553008414551521. [DOI] [PubMed] [Google Scholar]
- 57.Boogaerts M, Mittelman M, Vaupel P. Oncology. 2005;21:22–30. doi: 10.1159/000088285. [DOI] [PubMed] [Google Scholar]
- 58.Henke M, Mattern D, Pepe M, Bézay C, Weissenberger C, Werner M, Pajonk F. J Clin Oncol. 2006;24:4708–4713. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- 59.Spahn DR, Kocian R. Curr Pharm Design. 2005;11:4099–4114. doi: 10.2174/138161205774913354. [DOI] [PubMed] [Google Scholar]
- 60.Ness PM, Cushing MM. Pathol Lab Med. 2007;131:734–741. doi: 10.5858/2007-131-734-OTPOAA. [DOI] [PubMed] [Google Scholar]
- 61.Kim HW, Greenburg AG. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:537–550. doi: 10.1080/10731190600973725. [DOI] [PubMed] [Google Scholar]
- 62.Chang TM. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:551–566. doi: 10.1080/10731190600973808. [DOI] [PubMed] [Google Scholar]
- 63.Robinson MF, Dupuis NP, Kusumoto T, Liu F, Menon K, Teicher BA. Artif Cells Blood Substit Immobil Biotechnol. 1995;23:431–438. doi: 10.3109/10731199509117959. [DOI] [PubMed] [Google Scholar]
- 64.Riess JG. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- 65.Teicher BA, Herman TS, Frei E. Important Adv Oncol. 1992:39–59. [PubMed] [Google Scholar]
- 66.Dowling S, Fischer JJ, Rockwell S. Biomater Artif Cells Immobilization Biotech. 1992;20:903–905. doi: 10.3109/10731199209119738. [DOI] [PubMed] [Google Scholar]
- 67.Evans RG, Kimler BF, Morantz RA, Batnitzky S. Int J Radiat Oncol Biol Phys. 1993;26:649–652. doi: 10.1016/0360-3016(93)90283-2. [DOI] [PubMed] [Google Scholar]
- 68.Rockwell S, Kelley M. Radiat Oncol Invest. 1998;6:199–208. doi: 10.1002/(SICI)1520-6823(1998)6:5<199::AID-ROI1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 69.Teicher BA, Ara G, Emi Y, Kakeji YI, Ikebe, Maehara Y, Buxton D. Drug Devel Res. 1996;38:1–11. [Google Scholar]
- 70.Shaw E, Scott C, Suh J, Kadish S, Stea B, Hackman J, Pearlman A, Murray K, Gaspar L, Mehta M, Curran W, Gerber M. J Clin Oncol. 2003;21:2364–2371. doi: 10.1200/JCO.2003.08.116. [DOI] [PubMed] [Google Scholar]
- 71.Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, Mercier JP, Senzer N, Chang EL, Boyd AP, Cagnoni PJ, Shaw E. J Clin Oncol. 2006;24:106–114. doi: 10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 72.Collingridge DR, Rockwell S. Int J Cancer (Radiat Oncol Invest) 2000;90:256–264. [PubMed] [Google Scholar]
- 73.Horsman MR, Chaplin DJ, Brown JM. Radiat Res. 1989;118:139–150. [PubMed] [Google Scholar]
- 74.Stone HB, Minchinton AI, Lemmon M, Menke D, Brown JM. Int J Radiat Oncol Biol Phys. 1992;22:79–86. doi: 10.1016/0360-3016(92)90985-q. [DOI] [PubMed] [Google Scholar]
- 75.Ward A, Clissold SP. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 76.Kallman RF. Radiology. 1972;105:135–142. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 77.Alpern T, editor. Cell survival after low doses of radiation: Theoretical and clinical implications. Wiley; London: 1975. [Google Scholar]
- 78.Palcic B, Skarsgard LD. Radiat Res. 1984;100:328–339. [PubMed] [Google Scholar]
- 79.Ling CC, Spiro IJ, Mitchell J, Stickler R. Int J Radiat Biol Phys. 1985;11:1367–1373. doi: 10.1016/0360-3016(85)90253-6. [DOI] [PubMed] [Google Scholar]
- 80.Dewhirst MW, Vujaskovic ZE, Jones D. Int J Hyperthermia. 2005;21:779–790. doi: 10.1080/02656730500271668. [DOI] [PubMed] [Google Scholar]
- 81.Griffon-Etienne G, Boucher CY. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 82.Jain RK. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 83.Kerbel RS. Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 84.Biaglow J, Dewhirst M, Leeper D, Burd R, Tuttle S. Adv Exp Med Biol. 2005;566:317–323. doi: 10.1007/0-387-26206-7_42. [DOI] [PubMed] [Google Scholar]
- 85.Kirkpatrick JP, Brizel DM, Dewhirst MW. Radiat Res. 2003;159:336–344. doi: 10.1667/0033-7587(2003)159[0336:ammoto]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 86.Crokart N, Radermacher K, Jordan BF, Baudelet C, Cron GO, Grégoire V, Beghein N, Bouzin C, Feron O, Gallez B. Cancer Res. 2005;65:7911–7916. doi: 10.1158/0008-5472.CAN-05-1288. [DOI] [PubMed] [Google Scholar]
- 87.Evans SM, Judy KD, Dunphy I, Jenkins WT, Nelson PT, Collins R, Wileyto EP, Jenkins K, Hahn SM, Stevens CW, Judkins AR, Phillips P, Geoerger B, Koch CJ. Cancer Res. 2004;64:1886–1892. doi: 10.1158/0008-5472.can-03-2424. [DOI] [PubMed] [Google Scholar]
- 88.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Juergen D, Terris DJ, Overgaard J. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 89.Isa AY, Ward TH, West CML, Slevin NJ, Homer JJ. Brit J Radiol. 2006;79:791–798. doi: 10.1259/bjr/17904358. [DOI] [PubMed] [Google Scholar]
- 90.Wilson GD. Frontiers Biosci. 2007;12:3502–3518. doi: 10.2741/2330. [DOI] [PubMed] [Google Scholar]
- 91.Vaupel P, Mayer A. Cancer Metast Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 92.Okunieff P, Fenton B, Chen Y. Adv Exp Med Biol. 2005;566:213–222. doi: 10.1007/0-387-26206-7_29. [DOI] [PubMed] [Google Scholar]
- 93.Foo SS, Abbott DF, Lawrentschuk N, Scott AM. Mol Imaging Biol. 2004;6:291–305. doi: 10.1016/j.mibio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Tatum JL, Kelloff J, Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 95.Wardman P. Clin Oncol. 2007;19:397–417. doi: 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 96.Moulder JE, Dutreix J, Rockwell S, Siemann DW. Int J Radiat Oncol Biol Phys. 1988;14:913–927. doi: 10.1016/0360-3016(88)90014-4. [DOI] [PubMed] [Google Scholar]
- 97.Brown JM, Wilson WR. Nature Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 98.Moeller BJ, Richardson RA, Dewhirst MW. Cancer Metast Rev. 2007;26:241–248. doi: 10.1007/s10555-007-9056-0. [DOI] [PubMed] [Google Scholar]
- 99.Kaufman B, Scharf O, Arbeit J, Ashcroft M, Brown JM, Bruick RK, Chapman JD, Evans SM, Giaccia AJ, Harris AL, Huang E, Johnson R, Kaelin W, Koch CJ, Maxwell P, Mitchell J, Neckers L, Powis G, Rajendran J, Semenza GL, Simons J, Storkebaum E, Welch MJ, Whitelaw M, Melillo G, Ivy SP. Cancer Res. 2004;64:3350–3356. doi: 10.1158/0008-5472.can-03-2611. [DOI] [PubMed] [Google Scholar]
- 100.Rockwell S. Semin Oncol. 1992;19:29–40. [PubMed] [Google Scholar]
- 101.Lin AJ, Pardini RS, Cosby LA, Lillis BJ, Shanksy CW, Sartorelli AC. J Med Chem. 1973;16:1268–1271. doi: 10.1021/jm00269a010. [DOI] [PubMed] [Google Scholar]
- 102.Rockwell S. Int J Cancer. 1986;38:229–235. doi: 10.1002/ijc.2910380213. [DOI] [PubMed] [Google Scholar]
- 103.Palom Y, Kumar GS, Tang LQ, Paz MM, Musser SM, Rockwell S, Tomasz M. Chem Res Toxicol. 2002;15:1398–1405. doi: 10.1021/tx020044g. [DOI] [PubMed] [Google Scholar]
- 104.Roberts KB, Urdaneta N, Vera R, Vera A, Gutierrez E, Aguilar Y, Ott S, Medina I, Sempere P, Rockwell S, Sartorelli AC, Fischer DB, Fischer JJ. Int J Cancer (Radiat Oncol Invest) 2000;90:206–223. doi: 10.1002/1097-0215(20000820)90:4<206::aid-ijc4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 105.Rewari AN, Haffty BG, Wilson LD, Son YH, Joe JK, Ross DA, Papac RJ, Sasaki CT, Fischer JJ. The Cancer Journal. 2006;12:123–129. [PubMed] [Google Scholar]
- 106.McKeown SR, Cowen RL, Williams KJ. Clinical Oncol. 2007;19:427–442. doi: 10.1016/j.clon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Kraakman-van de Zwet M, Overkamp WJI, van Lange REE, Essers J, van Duijn-Goedhart A, Wiggers I, Swaminathan S, van Buul PPW, Errami A, Tan RTL, Jaspers NGJ, Sharan SK, Kanaar R, Zdzienicka MZ. Mol Cell Biol. 2002;22:669–679. doi: 10.1128/MCB.22.2.669-679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bindra RS, Crosby ME, Glazer PM. Cancer Metast Rev. 2007;26:249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 109.Haffty BG, Son YH, Wilson LD, Papac R, Fischer D, Rockwell S, Sartorelli AC, Ross D, Sasaki CT, Fischer JJ. Radiat Oncol Investig. 1997;5:235–245. doi: 10.1002/(SICI)1520-6823(1997)5:5<235::AID-ROI4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 110.Brown JM. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 111.Brown JM, Wilson WR. Nature. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 112.Seow HA, Penketh PG, Shyam K, Rockwell S, Sartorelli AC. Proc Natl Acad Sci USA. 2005;102:9282–9287. doi: 10.1073/pnas.0409013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patterson LH, McKeown SR. Brit J Cancer. 2000;83:1589–1593. doi: 10.1054/bjoc.2000.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson WR, Hicks KO, Pullen SM, Ferry DM, Helsby NA, Patterson AV. Radiat Res. 2007;167:625–636. doi: 10.1667/RR0807.1. [DOI] [PubMed] [Google Scholar]
- 115.Platt J, Sodi S, Kelley M, Rockwell S, Bermudes D, Low KB, Pawelek J. Eur J Cancer. 2000;36:2397–2402. doi: 10.1016/s0959-8049(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 116.Vaupel P. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 117.Evans SM, Hahn S, Pook DR, Jenkins WT, Chalian AA, Zhang P, Stevens C, Weber R, Weinstein G, Benjamin I, Mizra N, Morgan M, Rubin S, McKenna WG, Lord EM, Koch CJ. Cancer Res. 2000;60:2018–2024. [PubMed] [Google Scholar]
- 118.Aquino-Parsons C, Lim P, Green A, Minchinton AI. Gynecol Oncol. 1999;74:259–264. doi: 10.1006/gyno.1999.5443. [DOI] [PubMed] [Google Scholar]
- 119.Durand RE, Aquino-Parsons C. Radiother Oncol. 2006;80:138–142. doi: 10.1016/j.radonc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 120.Zhang L, Hill RP. Cancer Res. 2007;67:7789–7797. doi: 10.1158/0008-5472.CAN-06-4221. [DOI] [PubMed] [Google Scholar]
- 121.Le QT, Denko NC, Giaccia AJ. Cancer Metast Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 122.Yuan J, Narayanan L, Rockwell S, Glazer PM. Cancer Res. 2000;60:4372–4376. [PubMed] [Google Scholar]
- 123.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 124.Folkman J. Nature Rev. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 125.Cao Y, Li CY, Moeller BJ, Yu D, Zhao Yl, Dreher MR, Shan S, Dewhirst MW. Cancer Res. 2005;65:5498–5505. doi: 10.1158/0008-5472.CAN-04-4553. [DOI] [PubMed] [Google Scholar]
- 126.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue X, Pollard JW. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 127.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 128.Ansiaux R, Baudelet C, Jordan BF, Crokart N, Martinive P, DeWever J, Grégoire V, Feron O, Gallez B. Cancer Res. 2006;66:9698–9704. doi: 10.1158/0008-5472.CAN-06-1854. [DOI] [PubMed] [Google Scholar]
- 129.Nieder C, Wiedenmann N, Andratschke N, Molls M. Cancer Treat Rev. 2006;32:348–364. doi: 10.1016/j.ctrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 130.Oehler C, Dickinson DJ, Broggini-Tenzer A, Hofstetter B, Hollenstein A, Riesterer O, Vuong V, Pruschy M. Current Pharm Drugs. 2007;13:519–535. doi: 10.2174/138161207780162935. [DOI] [PubMed] [Google Scholar]
- 131.Wouters A, Pauwels B, Lardon F, Vermorken JB. Oncologist. 2007;12:690–712. doi: 10.1634/theoncologist.12-6-690. [DOI] [PubMed] [Google Scholar]
- 132.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, Giaccia AJ. Cancer Res. 2000;60:883–887. [PubMed] [Google Scholar]
- 133.Kaufman B, Scharf O, Arbeit J, Ashcroft M, Brown JM, Bruick RK, Chapman JD, Evans SM, Giaccia AJ, Harris AL, Huang E, Johnson R, Kaelin W, Koch CJ, Maxwell P, Mitchell J, Neckers L, Powis G, Rajendran J, Semenza GL, Simons J, Storkebaum E, Welch MJ, Whitelaw M, Melillo G, Ivy SP. Cancer Res. 2004;64:3350–3356. doi: 10.1158/0008-5472.can-03-2611. [DOI] [PubMed] [Google Scholar]
- 134.Melillo G. Cancer Metastasis Rev. 2007;26:341–352. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]
- 135.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson GB. Cell Metabol. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 136.Freiberg RA, Krieg AJ, Giaccia AJ, Hammond EM. Cell Cycle. 2006;5:1304–1307. doi: 10.4161/cc.5.12.2811. [DOI] [PubMed] [Google Scholar]
- 137.Martinive P, Defresne F, Bouzin C, Saliez J, Lair F, Grégoire V, Michiels C, Dessy C, Feron O. Cancer Res. 2006;66:11736–11744. doi: 10.1158/0008-5472.CAN-06-2056. [DOI] [PubMed] [Google Scholar]
- 138.Dennery PA. Birth Defects Res. 2007;81:155–162. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 139.Lin Q, Lee YJ, Yun Z. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 140.Tannock IF. Brit J Cancer. 1967;22:258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bedford JS, Phil D, Mitchell JB. Br J Radiol. 1974;47:687–696. doi: 10.1259/0007-1285-47-562-687. [DOI] [PubMed] [Google Scholar]
- 142.Kwok TT, Sutherland RM. Int J Radiat Oncol. 1992;22:1–4. doi: 10.1016/0360-3016(92)90843-7. [DOI] [PubMed] [Google Scholar]
- 143.Shrieve DC, Harris JW. Int J Radiat Biol. 1985;48:127–138. doi: 10.1080/09553008514551131. [DOI] [PubMed] [Google Scholar]
- 144.Bedford JS, Hall EJ. Br J Radiol. 1966;39:896–900. doi: 10.1259/0007-1285-39-468-896. [DOI] [PubMed] [Google Scholar]
- 145.Littbrand B, Révész L. Br J Radiol. 1969;42:914–924. doi: 10.1259/0007-1285-42-504-914. [DOI] [PubMed] [Google Scholar]