On the role of the two extracytoplasmic substrate-binding domains in the ABC transporter OpuA (original) (raw)
Abstract
Members of two transporter families of the ATP-binding cassette (ABC) superfamily use two or even four extracytoplasmic substrate-binding domains (SBDs) for transport. We report on the role of the two SBDs in the translocation cycle of the ABC transporter OpuA from Lactococcus lactis. Heterooligomeric OpuA complexes with only one SBD or one functional and one non-functional SBD (inactivated by covalent linkage of a substrate mimic) have been constructed, and the substrate binding and transport kinetics of the purified transporters, reconstituted in liposomes, have been determined. The data indicate that the two SBDs of OpuA interact in a cooperative manner in the translocation process by stimulating either the docking of the SBDs onto the translocator or the delivery of glycine betaine to the translocator. It appears that one of these initial steps, but not the later steps in translocation or resetting of the system to the initial state, is rate determining for transport. These new insights on the functional role of the extracytoplasmic SBDs are discussed in the light of the current knowledge of substrate-binding-protein-dependent ABC transporters.
Keywords: ABC-transporter/heterooligomers/rate-determining step/substrate-binding protein/translocation
Introduction
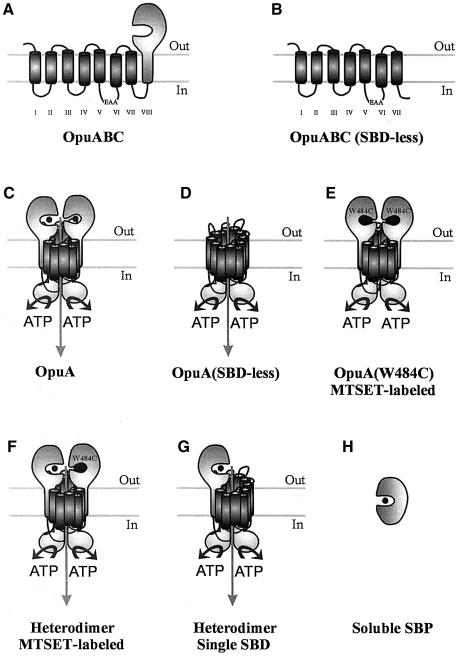
ABC transporters, found in all prokaryotic and eukaryotic species, use the hydrolysis of ATP to translocate solutes across cellular membranes. The translocator component of ABC transporters is composed of two multi-transmembrane and two intracellular ATP-binding subunits (Higgins et al., 1992). In addition to these ubiquitous components, prokaryotic ABC transporters involved in solute uptake into the cell employ a specific substrate-binding protein (SBP) to capture the substrate. These SBPs reside in the periplasmic space of Gram-negative bacteria. Gram-positive bacteria and Archaea, organisms without a periplasm, anchor the proteins to the outer surface of the cell membrane via an N-terminal lipid moiety (Sutcliffe and Russell, 1995) or, in the case of Archaea, can use an N-terminal transmembrane segment to anchor the protein to the cytoplasmic membrane (Albers et al., 1999). Recently, two families of ABC transporters in which one or two extracytoplasmic substrate-binding domains (SBDs) are fused to either the N- or C-terminus of the translocator protein have been detected (van der Heide and Poolman, 2002). This implies that two, or even four, substrate-binding sites may function in the ABC transporter complex. The ABC transporter described here, OpuA from Lactococcus lactis, is composed of two translocator subunits (OpuABC) each with a SBD at the C-terminal end (Figure 1A), and two ATPase subunits (OpuAA); the functional OpuA complex is depicted in Figure 1C.
Fig. 1. Schematic representation of the different protein(s) (complexes) used in this study. (A) OpuABC, substrate-binding/translocator protein; (B) OpuABC(SBD-less), OpuABC lacking the signal anchor sequence [transmembrane segment VIII in (A)] and the SBD; (C) OpuA, the wild-type transporter, which is composed of two copies of OpuABC and two copies of OpuAA (the ATPase subunit); (D) OpuA(SBD-less), transporter complex formed from two copies of OpuABC(SBD-less) and two copies of OpuAA; (E) MTSET-labeled OpuA(W484C), OpuA complex with Trp to Cys substitution at position 484 of OpuABC and labeled with a thiol-specific glycine betaine mimic; (F) Heterodimer composed of one OpuABC, one MTSET-labeled OpuABC(W484C) and two OpuAA subunits; (G) heterodimer composed of one OpuABC, one OpuABC(SBD-less) and two OpuAA subunits; (H) soluble SBP, SBD of OpuA expressed as soluble protein.
Translocation via SBP-dependent ABC transporters starts with the binding of the substrate to the SBP. Upon docking of the substrate-bound SBP onto the transmembrane domains (TMDs), a signal is transmitted to the nucleotide-binding domains (Davidson et al., 1992). This stimulates the ATPase activity, which is coupled mechanistically to critical rearrangements in the TMDs (Liu et al., 1999b; Davidson, 2002; Locher et al., 2002). As a result, the affinity for substrate is reduced by opening of the SBP, facilitating the transfer of the substrate to a binding site in the TMDs (Chen et al., 2001). This binding site reorients to the inside, the cytoplasm facing gate is opened and the substrate is released into the cell (Davidson, 2002; Locher et al., 2002) After the substrate has crossed the membrane, the SBP is dissociated and the transporter returns to its initial state through the dissociation of ADP and inorganic phosphate. In this model a single SBP is involved in substrate delivery to the translocator. The important question now is what is the role of the second (or third and fourth) SBD in systems with multiple SBDs?
In the present study, we investigated the role of the two SBDs in the translocation cycle of the osmoregulated ABC transporter OpuA from L.lactis (van der Heide and Poolman, 2000; van der Heide et al., 2001). We also determined whether the steps in translocation involving the SBDs are rate determining for the overall transport process. To approach these problems, we analyzed the binding and transport kinetics of glycine betaine by OpuA heterooligomers with only one SBD or one functional and one non-functional SBD.
Results
Binding and transport of glycine betaine
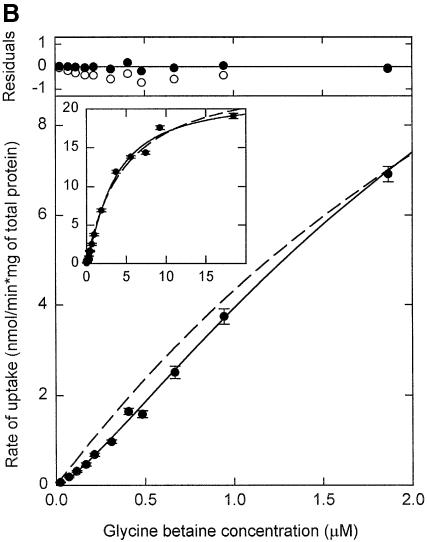
The OpuA system was amplified in L.lactis and purified by metal-affinity chromatography (Figure 2A, lanes 1 and 4). The purity of the protein samples was generally >95%, and the _n_-dodecyl-β-d-maltoside (DDM)-solubilized OpuA complex migrated on a size-exclusion column as a monodisperse species (Figure 2B). Although the majority of the experiments were carried out with the purified OpuA complex reconstituted in proteoliposomes, binding and transport of glycine betaine were initially studied in native membranes fused with liposomes. In order to effect the uptake of glycine betaine in these hybrid membranes, an ATP-regenerating system was included in the vesicle lumen. The ATP-dependent uptake of glycine betaine in these hybrid membranes is shown in Figure 3A (closed circles). The activity of right-side-in oriented OpuA decreased in time and ultimately stopped because of depletion of ATP and build up of ADP (Patzlaff and Poolman, 2003). If Mg-ATP was then added to the outside of these hybrid membranes, inside-out oriented OpuA was activated and glycine betaine was released (Figure 3A, open circles). From the initial rates of uptake and efflux, representing right-side-in and inside-out oriented OpuA, respectively, we estimate that the OpuA molecules are oriented randomly (47 ± 4% right-side-in). Transport via right-side-in oriented OpuA displayed a sigmoid dependence on the glycine betaine concentration (Figure 3B), from which an apparent affinity constant (_K_M) of 2.5 µM and a Hill coefficient of 1.34 ± 0.04 (n = 3) could be estimated. The apparent positive cooperativity is best observed from the analysis of the residuals (Figure 3B), which are non-random for the fit with the Michaelis–Menten equation and randomly distributed with the Hill equation. Non-Michaelis–Menten kinetics were also observed in proteoliposomes with purified OpuA (Table I, typical experiment), but the Hill coefficient was on average larger (1.7 ± 0.2, n = 3). The differences in the Hill coefficient for transport in hybrid membranes and proteoliposomes may reflect a somewhat different lipid environment, but this aspect has not been investigated further.
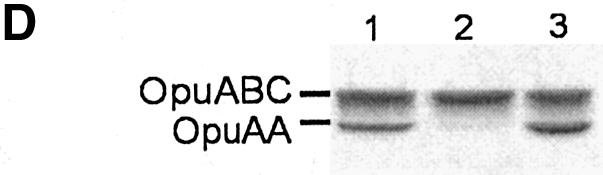
Fig. 2. Visualization of overexpression and purification of the proteins. (A) Coomassie Brilliant Blue stained SDS–PAGE gel (12.5% polyacrylamide) of membrane vesicles (6.5 µg of total protein was loaded per lane) containing OpuA (lane 1), OpuA(W484C) (lane 2) and SBD-less OpuA (lane 3). In lanes 4–7, 2.5 µg of purified protein was loaded per lane; OpuA (lane 4), OpuA(W484C) (lane 5), SBD-less OpuA (lane 6) and soluble SBP (lane 7). The OpuAA component of wild-type OpuA runs at a lower molecular weight than that of OpuA(W484C) or SBD-less OpuA, because it does not contain a his tag; the OpuAA subunits of the other complexes carry a N-terminal 10-his tag. Importantly, OpuAA without a his-tag copurifies with his-tagged OpuABC. (B) Size-exclusion chromatography of DDM-solubilized and Ni2+-NTA-purified OpuA. (C) Size-exclusion chromatography of Ni2+-NTA purified soluble SBP. (D) SDS–PAGE gel (12.5% polyacrylamide) showing the effect of glycerol on the stability of the OpuA complex (OpuAA: 47 kDa, OpuABC: 63 kDa). Purified OpuA (lane 1) was allowed to slowly dissociate by decreasing the glycerol concentration to 5% and repurified on Ni-NTA (lane 2). In the control sample (lane 3), the glycerol concentration was lowered to 5% (30 min at 4°C to allow the complex to dissociate) then increased to 20% (30 min at 4°C to allow the complex to reassemble), after which OpuA was repurified on Ni-NTA. Per lane, 2.5 μg of protein was loaded.
Fig. 3. Glycine betaine transport in hybrid membranes. (A) Uptake (filled circles) and efflux (open circles) of [14C]glycine betaine (final concentration 34 µM) in hybrid membranes (final protein concentration 0.4 mg/ml) was assayed in 400 mM KPi pH 7.0. Efflux of glycine betaine was initiated by the addition of 9 mM Mg-ATP after 20 min of uptake (indicated by arrow). (B) Kinetics of [3H]glycine betaine uptake. The data were analyzed with the Michaelis–Menten (dashed line) and the Hill equation (solid line). The error bars indicate the standard deviation from the mean of three measurements. The inset shows the high concentration range. The residuals, i.e. the difference between the experimental data and fitted line, are plotted for the Michaelis–Menten (open circles) and for the Hill (filled circles) equations.
Table I. Kinetic parameters of glycine betaine uptake in proteoliposomes.
| Protein | _K_M (µM) | _V_max (min–1) | Hill coefficient |
|---|---|---|---|
| Wild-type OpuA homodimer | 1.9 ± 0.1 | 39.4 ± 1.1 | 1.50 ± 0.15 |
| Wild-type/MTSET-labeled W484C heterodimer | 2.5 ± 0.4 | 35.6 ± 2.1 | 1.04 ± 0.09 |
| Wild-type/SBD-less heterodimer | 1.9 ± 0.6 | 17.2 ± 2.0 | 0.96 ± 0.11 |
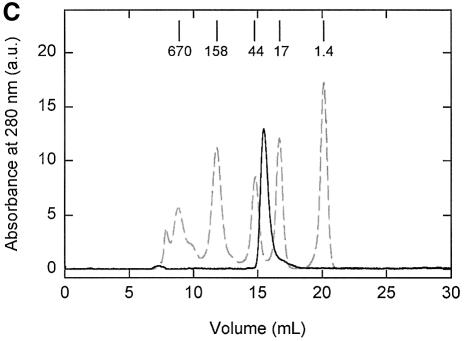
Since OpuA has two SBDs per functional complex; the sigmoid dependence on glycine betaine concentration could be at the level of substrate binding or a subsequent step, e.g. docking of the SBDs onto the translocator or substrate transfer. To discriminate between these possibilities, we analyzed the binding of glycine betaine to hybrid membranes with dimeric OpuA and to soluble SBD (hereafter referred to as substrate-binding protein, SBP). Soluble SBP was obtained by expressing the corresponding gene fragment in L.lactis and purification of the protein (Figures 1H and 2A, lane 7). Size-exclusion chromatography of purified SBP showed that the protein was monomeric (Figure 2C), i.e. in the 5–15 µM range analyzed on the column. The observed and calculated mol. wt of the unliganded SBP were 24 and 30 kDa, respectively. Figure 4 (filled circles) shows that membrane-embedded dimeric OpuA displayed glycine betaine binding with ‘normal’ hyperbolic saturation behavior. The same vesicles and glycine betaine solutions that yielded transport kinetics with a Hill coefficient of 1.34 were used for these binding experiments. Since the intrinsic fluorescence of the soluble SBP decreased in a substrate-concentration-dependent manner, this property was used to evaluate the binding of glycine betaine (Figure 4, open circles). The dissociation constant (_K_D) obtained for the soluble SBP was higher than for membrane-embedded OpuA (_K_D values of 4 and 0.5 µM, respectively; Figure 4), but, in both cases, the binding curves were hyperbolic. Taken together, these data suggest that the cooperativity observed in the transport results from a step subsequent to binding and involves interactions of the SBDs with the translocator.
Fig. 4. Binding of glycine betaine to membranes with amplified levels of OpuA and to soluble SBP. Binding of [3H]glycine betaine to hybrid membranes (filled circles) was measured in 400 mM KPi pH 7.0, 5 mM EDTA, using the ammonium sulfate precipitation method. The final OpuA concentration was ∼15 µg/ml. The error bars indicate the standard deviation from the mean of three measurements. After correction for background binding, the data were fitted to a hyperbola of the form B = _B_max * S/(K_D + S); the residuals are presented in the top panel. Binding of glycine betaine to soluble SBP (open circles) was measured in 50 mM KPi pH, 7.0, 200 mM KCl plus 10% (v/v) glycerol at a SBP concentration of 20 µg/ml by monitoring the change in protein fluorescence (Δ_F). The data are from three independent measurements and the standard deviation is indicated. Since the K_D value was at least five times larger than the protein concentration, the binding curve could be analyzed according to the equation Δ_F = Δ_F_max * S/(_K_D + S) (Lanfermeijer et al., 1999); S, glycine betaine concentration. The residuals are also presented in the upper panel.
Hybrid membranes, expressing OpuA at a level of 35 ± 3% of total membrane protein, displayed a maximal level of glycine betaine binding (_B_max) of 1.3 nmol/mg of total protein (Figure 4, closed circles). The expression level of OpuA was estimated from densitometric scanning of Coomassie Brilliant Blue stained SDS–PAGE gels and comparison with known concentrations of purified OpuA, using membranes of an opuA knockout strain as reference. In the membrane preparation in which the binding of glycine betaine was measured, the OpuA level was 35 ± 3%, but this level could be set from close to zero to ∼40%, depending on the nisin A concentration and induction time (Kunji et al., 2003). Thus, the _B_max of 1.3 nmol/mg of total protein corresponds to ∼3.7 ± 0.3 nmol/mg of OpuA, of which only 47 ± 4% has the SBD accessible for glycine betaine. Taking into account the random orientation of the protein complex, this yields a _B_max of 7.9 ± 0.8 nmol/mg of right-side-in OpuA or 1.8 ± 0.2 glycine betaine per OpuA (mol. wt of dimeric OpuA is 220 kDa).
Heterodimer formation: approach
To further dissect the role of the two SBDs in OpuA-mediated glycine betaine transport, we formed complexes with one functional and one non-functional SBD and complexes with only one (functional) SBD (Figure 1G and F). For these experiments, we took advantage of the observation that the DDM-solubilized OpuA complex, consisting of two molecules of OpuABC and two molecules of OpuAA, is stable in the presence of 20% (v/v) glycerol but slowly dissociates when the glycerol concentration is lowered to 5%. Evidence for this contention is presented in Figure 2D, where we show that OpuAA was no longer co-purified with his-tagged OpuABC on Ni-NTA when the glycerol concentration was lowered to 5% (lane 2). In the presence of 20% glycerol both proteins are co-purified in a one to one ratio, that is, not only the control sample (lane 1) but also the complex that had undergone a 20 to 5 to 20% glycerol treatment (lane 3). Importantly, lowering of the glycerol concentration from 20 to 5% for 30 min and then bringing the glycerol concentration back to 20% did not affect the activity of OpuA as determined after reconstitution in proteoliposomes. Thus, by mixing different species of OpuA and transiently lowering the glycerol concentration, one has the possibility to obtain heteroligomeric complexes with a composition that depends on the initial ratio of the species.
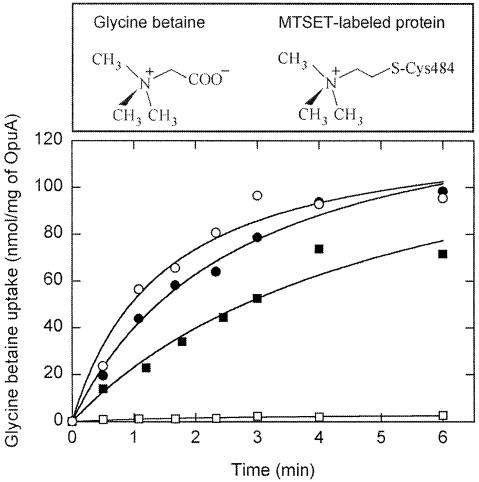
From the 3D structures of the ProX protein from Escherichia coli (E.Bremer and W.Welte, unpublished) and OpuAC from Bacillus subtilis (L.Schmitt, unpublished), homologs of the SBD in OpuABC, it is known that three Trp residues line the glycine betaine-binding site in the substrate-binding pocket. The equivalent of one of these residues in OpuABC, Trp484, was changed into Cys; OpuABC but also OpuAA of wild-type OpuA are both Cys-less. Compared with the wild-type OpuA system, OpuA(W484C) had a 10-fold increased _K_M for glycine betaine transport and a somewhat lowered _V_max (77 instead of 125 nmol/min × mg of OpuA). Importantly, labeling of this mutant with 2-[(trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET) completely abolished the transport activity, whereas the wild-type OpuA system was unaffected [Figure 5; the MTSET-labeled OpuA(W484C) is schematically depicted in Figure 1E]. This does not imply that the mutant is inactive in translocation; instead the occupation of the binding site by the substrate mimic (see Figure 5 for structures), resulting from labeling with MTSET, caused an inability to bind and therefore deliver glycine betaine to the translocator. The W484C mutation allowed us to specifically inactivate one of the SBDs in a heterodimeric complex consisting of one OpuABC(wild-type), one OpuABC(W484C) plus two molecules of OpuAA.
Fig. 5. The effect of MTSET on transport activity in proteoliposomes. Wild-type OpuA and OpuA(W484C) were incubated with freshly prepared 100 µM MTSET for 1 h at room temperature while bound to the Ni-NTA resin, which was followed by an additional 20 column volumes of washing with 50 mM KPi pH 8.0, 200 mM KCl, 20% glycerol, 0.05% DDM and 15 mM imidazole; the purification was otherwise the same as described in Materials and methods. Uptake of [14C]glycine betaine (final concentration 34 µM) in both untreated wild-type (filled circles) and OpuA(W484C) (filled squares) and MTSET-treated wild-type (open circles) and OpuA(W484C) (open squares) was assayed in 400 mM KPi pH 7.0.
Heterodimeric OpuA with one functional and one non-functional SBD
To establish whether both SBDs need to be functional for translocation activity of OpuA, heterodimeric complexes were allowed to form at different ratios of wild-type and W484C SBD. Experimentally, this involved purification of wild-type OpuA and OpuA(W484C), mixing of both complexes at different ratios, random dissociation–association of the subunits by lowering of the glycerol concentration from 20 to 5% and then increasing it back to 20%, membrane reconstitution of the protein complexes, and labeling with MTSET to inactivate the SBDs with the W484C mutation.
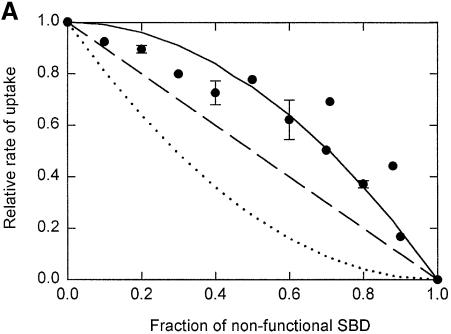
From the dependence of the transport activity on the fraction of active (a) over substrate-binding-defective protein (b), one can obtain information on the functional role of the two SBDs in the OpuA complex. Since two OpuABC subunits are present in OpuA, mixing of OpuA with two functional (AA) with OpuA with two non-functional (BB) SBDs will result in a population f(a,b) of homodimeric (AA and BB) and heterodimeric (AB) OpuA complexes according to f(a,b) = a2 + 2ab + b2. In our system the fraction (a2) with only wild-type protein (AA) is 100% active, whereas the fraction (b2) of double mutant protein (BB) has no activity. The observed activity will thus depend on the properties of the heterodimeric species AB. The following scenarios are possible: (i) If both SBDs are required for substrate binding or delivery (BB and AB are inactive), the activity (y) will decrease quadratically with decreasing fraction of wild-type protein (or increasing fraction of inactive mutant) and take the form y = 1 – 2b + b2 (Figure 6A; dotted line). (ii) If the two SBDs function independently and substrate binding or substrate delivery to the translocator is rate determining (AA is twice as active as AB), the activity will decrease linearly according to y = 1 – b (Figure 6A; dashed line). (iii) If, on the other hand, both SBDs function independently but the rate-determining step is in translocation, the activity as a function of the fraction of non-functional SBD will take the form y = 1 – b2 (Figure 6A; solid line). (iv) A similar dependence is expected when a single SBD is sufficient for substrate binding but the second SBD stimulates the delivery, that is, when both SBDs interact in a cooperative manner to deliver the substrate to the translocator. This would then be the rate-determining step in the overall transport.
Fig. 6. Glycine betaine transport by heterooligomeric complexes formed at different ratios of wild-type OpuA and MTSET-labeled OpuA(W484C). (A) The initial rate of uptake of [14C]glycine betaine (final concentration 34 µM) was measured in 400 mM KPi pH 7.0, from the linear increase in uptake over a period of 60 s. Some points show the average plus standard deviation of the data from duplicate measurements on three independent proteoliposome batches. Different dependences of the transport activity (= y) on the fraction of substrate-binding defective protein (= b) are indicated: y = 1 – 2_b_ + _b_2 (dotted line); y = 1 – b (dashed line); and y = 1 – _b_2 (solid line). (B) Wild-type OpuA and MTSET-labeled OpuA(W484C) were mixed in different ratios prior to membrane reconstitution, but the proteins were not allowed to form heterodimeric complexes. Uptake was performed as described in (A).
The experiments show that the transport activity decreased according to y = 1 – b2 (Figure 6A), clearly ruling out the first two scenarios but not discriminating between (iii) and (iv). To show that this outcome required true heterodimer formation, comparable amounts of wild-type and MTSET-labeled OpuA(W484C) were mixed prior to membrane reconstitution, i.e. without allowing them to form heterodimers, and handling steps identical to those in the heterodimer experiment were carried out. The transport activity decreased linearly with the fraction of mutant (MTSET-labeled) protein (Figure 6B), which is consistent with independent functioning of the two OpuA species and implies that the proteins do not dissociate–associate in the membrane on the time-scale of the experiment.
A heterodimeric complex in which one of the SBDs is absent
To discriminate between scenarios (iii) and (iv), that is, the rate-determining step in translocation versus cooperative effects between SBDs, heterooligomers were formed with only one SBD. For this purpose, the SBD together with the signal anchor sequence of OpuABC were deleted (Figure 1B) and SBD-less OpuA (Figure 1D) was purified. As expected the complex lacking SBD is completely inactive in transport (data not shown), but the expression, purification and complex stability were not affected (Figure 2, lanes 3 and 6). Importantly, transport of glycine betaine via SBD-less OpuA was observed upon addition of soluble SBP to the assay medium. At a final concentration of 40 µM of soluble SBP and SBD-less OpuA reconstituted at a protein to lipid ratio of 1:100 (wt/wt), the initial rate of glycine betaine uptake was 9 nmol/min × mg of SBD-less OpuA (data not shown). This rate corresponds to ∼7% of the activity of wild-type OpuA. However, the activity increased linearly with SBP concentration in the range 5–40 µM, indicating that the maximal activity had not been reached, most probably because of low affinity of the membrane-embedded translocator complex for the SBP.
If the steps involving the SBDs are not rate determining, but rather a subsequent step that we refer to as translocation/resetting of the system, one would expect dependence of the activity on the fraction of protein without SBD according to y = 1 – b2. Because SBD-less OpuA cannot transport and wild-type OpuA has two SBDs per translocator, the redistribution of the SBDs upon heterodimer formation now allows the translocator subunits from the SBD-less OpuA to partcipate in transport. Stated differently, the activity per mole of SBD would increase because the translocation path not operative in SBD-less OpuA would be recruited from this complex upon heterodimer formation with wild-type OpuA. It is important to note that the total molar protein concentration was kept constant in the heterodimer experiments. Thus, if translocation, and not substrate binding or SBD docking, would be rate determining the activity should decrease less than linearly with the fraction of SBD-less OpuA. However, the data clearly show linear dependence (Figure 7), ruling out scenario (iii). On the basis of these experiments, we conclude that (a) a step involving the SBDs, and not a later step in translocation or resetting of the system to the initial state, is rate determining; (b) the second SBD, W484C-MTSET labeled, in the wild-type/W484C-MTSET heterodimer facilitates the transfer of substrate from the first SBD to the translocator; and (c) cooperative interactions occur between the two SBDs or between the SBDs and the translocator.
Fig. 7. Glycine betaine transport by heterooligomeric complexes formed at different ratios of wild-type OpuA and SBD-less OpuA. Glycine betaine transport experiments were performed as described in the legend of Figure 6. Each point represents the average plus standard deviation of three independent experiments and measurements carried out in duplicate. The data points follow the line corresponding to y = 1 – b (see legend of Figure 6 for details).
Kinetics of transport by homo- and heterodimeric OpuA
If the sigmoid dependence of transport on glycine betaine concentration (Figure 3B) is related to the presence of two SBDs, acting in a cooperative manner in substrate delivery to the translocator, then a heterodimer with only one functional SBD should display a hyperbolic dependence on glycine betaine concentration. To address this question, wild-type and MTSET-labeled W484C OpuA or wild-type and SBD-less OpuA were mixed at a ratio of 1:9 and allowed to form heterooligomers (Figure 1F and G). Assuming random mixing of the proteins, the fraction of homodimeric wild-type OpuA is 0.01, the fraction of homodimeric MTSET-labeled W484C or SBD-less mutant is 0.81 and the fraction of heterodimers is 0.18. Since the homodimeric MTSET-labeled W484C and SBD-less mutant are completely inactive, the 18% of heterodimeric OpuA complexes will dominate the observed activity (∼95%). Indeed, and in contrast to wild-type OpuA that had undergone similar treatment, the wild-type/MTSET-labeled W484C heterodimer (Figure 1F) reconstituted in proteoliposomes displayed typical Michaelis–Menten kinetics (Figure 8 and Table I). The wild-type/MTSET-labeled W484C heterodimer showed comparable _K_M and _V_max for transport, when normalized to the fraction of active protein (0.18 from the heterodimer plus 0.01 from homodimeric wild-type). The reconstituted wild-type/SBD-less heterodimer (Figure 1G) also showed typical Michaelis–Menten kinetics and a comparable _K_M for transport, but the normalized _V_max was ∼2-fold lower than for the wild type (Figure 8 and Table I).
Fig. 8. Kinetics of glycine betaine transport in heterodimeric complexes. Proteoliposomes containing wild-type OpuA homodimers (filled circles), wild-type/MTSET-labeled W484C heterodimers (filled squares) and wild-type/SBD-less heterodimers (filled triangles) were used to assay the initial rate of uptake of [3H]glycine betaine in 400 mM KPi pH 7.0. The heterodimeric complexes were formed by mixing wild-type OpuA and OpuA(W484C) or SBD-less OpuA at ratios of 1:9 as described in Materials and methods. The activities of the heterodimeric complexes were normalized for protein content by ignoring the fraction (0.81) of homodimeric OpuA(W484C) or SBD-less OpuA complex, as these species have no detectable activity. In these experiments, the activity is dominated by the fraction (0.18, which is 95% of all active species) of heterodimeric complexes. Each point represents the average plus standard deviation of two measurements. The best fit of the data was obtained with the Hill (filled circles, wild-type OpuA) or Michaelis–Menten (filled squares and triangles, heterodimeric complexes) equations, which yielded random distribution of the residuals (not shown).
In conclusion, the second SBD contributes to the transport of glycine betaine by stimulating the initial step(s) of translocation, e.g. via docking of the SBDs onto the translocator or substrate delivery to the translocator. To obtain the maximal rate of transport, the second SBD does not need to be competent in substrate delivery itself as the wild-type/MTSET-labeled W484C heterodimer, with the label on Cys484, possibly mimicking the substrate-bound state, elicits a similar _V_max to that of a protein complex with two wild-type SBDs.
Discussion
In this paper we describe the functional role of the two extracytoplasmic SBDs of OpuA from L.lactis, using purified and membrane-reconstuted OpuA with either zero, one or two SBDs or one functional and one non-functional SBD per translocator complex (Figure 1C–G). The data clearly indicate that a single SBD per dimeric translocator complex is sufficient and minimally needed for transport. Within the complex with two SBDs, the two domains interact in cooperative manner and enhance the transport capacity. The cooperative interactions are observed not only in the wild-type system (Figure 1C), but also in the complex with two SBDs of which one of the binding sites has been ‘inactivated’ via covalent coupling of a substrate mimic. Our data also show that a step involving the SBDs, i.e. docking of the SBDs onto the translocator or substrate delivery to the translocator, is rate determining and not a later step in translocation or resetting of the system for another transport cycle.
What is the evidence for SBD-dependent cooperative interactions in transport? First, the rate of transport increased sigmoidally (positive cooperativity) with the glycine betaine concentration, whereas binding of glycine betaine displayed a hyperbolic (non-cooperative) concentration dependence with a stoichiometry of 1.8 ± 0.2 glycine betaine bound per OpuA. Secondly, the rate of transport increased hyperbolically with the glycine betaine concentration in the heterodimeric single SBD (Figure 1G) and MTSET-labeled (Figure 1F) complexes. Thirdly, the heterodimeric MTSET-labeled complex was approximately twice as active as the complex with only one SBD (based on data presented in Figures 6A, 7 and 8), even though in both systems only one SBD was capable of binding glycine betaine. This suggests that the MTSET-labeled SBD enhances transport by stimulating glycine betaine transfer from wild-type SBD to the translocator. Although a substrate mimic has been used to label the OpuA(W484C) protein and prevent glycine betaine from binding to the SBD, we do not know whether the MTSET-labeled subunit is in a closed-liganded-like or open-liganded-like conformation. Importantly, the turnover number of MTSET-labeled heterodimer and wild-type OpuA are comparable and significantly higher than that of the single SBD heterodimer. This strongly suggests that the MTSET-labeled subunit in the heterodimeric complex is in a functionally relevant conformation and capable of exerting cooperative interactions. Although wild-type and heterodimeric MTSET-labeled OpuA have a similar maximal rate of uptake, the cooperativity is no longer a function of the glycine betaine in the heterodimer (Figure 8 and Table I). The MTSET-labeled SBD of the heterodimer is trapped in a state in which it is no longer capable of responding to the glycine betaine concentration, but, otherwise, this substrate-mimic-modified OpuA complex is functional.
The translocation cycle of a substrate-binding-protein-dependent ABC transporter can be subdivided into (i) substrate binding to the SBD, (ii) docking of the SBD onto the translocator, (iii) substrate donation to the translocator, (iv) translocation of the substrate, and (v) resetting of the system to the initial state. As far as we are aware, the step that is rate determining is not known for (substrate-binding-protein-dependent) ABC transporters, but, by analogy with many (secondary) transport systems that do not use a SBD but directly bind the substrate in the translocator (Poolman and Konings, 1993), one could anticipate that step (iv) or (v) is the slowest. Substrate-binding is generally fast compared with the overall translocation, which for ABC transporters, but also for secondary transporters, is in the range 0.1–10 per second. From the present study it is evident that multiple SBDs enhance the transport capacity of OpuA and that step (ii) or (iii) rather than translocation per se or resetting are rate determining.
What is the evidence that substrate delivery to the translocator or related step may be rate determining? The association rate constants (_k_on) for ligand-binding to substrate-binding proteins are in the range 1–100 × 106 M–1 s–1 (Miller et al., 1980; Lanfermeijer et al., 1999, 2000), and, even at micromolar concentrations of glycine betaine and using the lower limit for _k_on of 106 M–1 s–1, the binding of glycine betaine will be much faster than the turnover of the system (∼0.6–0.7 s–1). Thus, it is unlikely that binding of glycine betaine is limiting translocation via OpuA. What about dissociation of the substrate from the SBD, which is related to the _K_D via the association rate constant (_k_on)? The _K_D for glycine betaine binding is ∼0.5 µM and given a _k_on of 1–100 × 106 M–1 s–1, the dissociation rate constant (_k_off) is 0.5–50 s–1 and also this will give rise to a rate that is faster than the turnover of the OpuA. We do not know the _k_off under translocation conditions; however, it is unlikely that the _k_off under conditions of ATP hydrolysis will be lower than in the absence of ATP (see also Lanfermeijer et al., 1999). Both the heterodimer titration experiments (Figure 6) and the kinetic data of the heterodimeric complexes (Figure 8) indicate that the second SBD enhances the transport activity. Ruling out glycine betaine association with, or dissociation from, the SBD as a limiting factor, this stimulation by the second SBD may occur at the level of docking of the SBD onto the translocator or substrate donation to the translocator The second SBD does not necessarily have to be proficient in substrate binding and donation, rather the cooperative interaction is sufficient for the stimulation of transport. This is consistent with the idea that a single SBD docks onto the translocator and donates its substrate. The second SBD may stimulate this docking or perhaps the dissociation of the complex after the substrate has been transferred, by interacting with either the first SBD or some regulatory site on the translocator.
Although we cannot rule out the possibility that two SBDs dock onto the translocator simultaneously, we consider this unlikely given what is known of other ABC transporters. For instance, Amy Davidson and colleagues have shown that the vanadate-trapped transition state complex contains a single maltose-binding protein (MBP) associated with the translocator (Chen et al., 2001). Also, in the recently published BtuCD structure there is no space to dock two vitamin B12-binding proteins onto the proposed docking site (Borths et al., 2002; Locher et al., 2002). On the basis of second-site suppressor analysis and further biochemical studies, there is good evidence that one lobe of MBP interacts with MalF and the other with MalG of the maltose system (Hor and Sherman, 1993). A similar conclusion has been reached for the histidine transport system (Liu et al., 1999a), and, given the likelihood of conservation of protein structure and transport mechanism, we thus assume that at a given time a single SBD donates its substrate to the translocator.
How general are the findings of cooperative interactions between the two SBDs of OpuA? We have recently shown that ABC transporters with multiple SBDs can be found within the OTCN (e.g. OpuA) and PAO families of the ABC superfamily, and both families contain members with two or four SBDs per ‘dimeric’ complex (van der Heide and Poolman, 2002). Recently, we have purified a ‘four-SBD’ ABC transporter from the PAO family, and preliminary experiments show that this system also displays cooperative transport kinetics (G.K.Schuurman-Wolters and B.Poolman, unpublished). What about ABC transporters with a soluble, either periplasmic or lipid-anchored, substrate-binding protein? There is no biochemical evidence for cooperative interactions involving multiple SBDs, but, by genetically manipulating the periplasmic concentration of MBP, it has been observed that the rate of transport increases sigmoidally with the MBP concentration (Manson and Boos, 1985). These preliminary observations are consistent with our view of the role of multiple SBDs in OpuA and other systems under investigation. Importantly, from the published papers of substrate-binding-protein-dependent ABC transporters that we have studied, there is no evidence against a mechanism involving multiple SBPs acting in a cooperative manner in the translocation process. The interactions may occur at submillimolar to millimolar concentrations of SBP, typical for SBPs in the periplasm (Ames et al., 1996), and involve relatively low affinities for protein–protein association (_K_D values > 50 µM). Such interactions are difficult to study under ‘well-defined’ in vitro conditions, simply because purified binding proteins are not available for these experiments at (sub)millimolar concentrations. For instance, the highest concentrations of maltose- and histidine-binding proteins used in combination with proteoliposome-embedded translocator complex are 40 µM for the histidine-binding protein (Ames et al., 1996; Liu and Ames, 1997) and 10–100 µM for the MBP (Davidson and Nikaido, 1990; Dean et al., 1992), which is well below their periplasmic concentrations. We have succeeded in measuring glycine betaine transport via purified and membrane-reconstituted OpuA(SBD-less) (Figure 1D) in the presence of soluble SBP (Figure 1H), but even at a 40 µM concentration of SBP the system was not saturated with glycine-betaine-binding protein.
In conclusion, we show for the first time that two SBDs of an ABC transporter act in a cooperative manner in the translocation process. We present evidence that the cooperative interactions influence either the docking of the SBDs onto or the donation of glycine betaine to the translocator. Moreover, we show that either one of these steps and not a later step in translocation or resetting of the system is rate determining for transport. Future work is aimed at elucidating the generalities of these observations by comparative studies of ABC transporters with soluble and covalently linked SBDs.
Materials and methods
Bacterial strains, plasmids and growth conditions
OpuA(W484C) was constructed through site-directed mutagenesis by using pNZopuA2His, bearing the wild-type opuAA and opuABC genes (van der Heide and Poolman, 2000), and the W484C forward primer 5′-CATAAAGATATTGTGATCACTGGTTGTTCTCCTCATTGG-3′, yielding pNZOpuA-(W484C)2His. The same plasmid was used to construct OpuA without a SBD, SBD-less OpuA; this construct also lacks the signal anchor sequence that precedes the SBD (van der Heide and Poolman, 2002). In this case, a _Bam_HI restriction site was engineered at the predicted end of the translocator domain at nucleotide 2169 and counted from the start of the opuAA gene. After digestion with _Nco_I and _Bam_HI, the opuAA and SBD-less opuABC genes (encoding for ATPase and transmembrane subunits, respectively) were exchanged for the wild-type genes of pNZopuA2His, yielding pNZopuA(ΔSBD)2His. Each of the constructs harbors a sequence at the 5′ end of the first gene and the 3′ end of the second gene that specifies a N-terminal 10-his tag and C-terminal 6-his tag, respectively. A plasmid bearing a gene fragment that specifies the SBD as soluble protein was constructed by engineering a _Nco_I restriction site at the start (starting at nucleotide 2163, counted from the start of the opuAA gene) of the substrate-binding protein gene. After digestion with _Nco_I and _Bam_HI, the wild-type genes of pNZopuAHis were exchanged for the substrate-binding protein gene, containing a C-terminal 6-his tag; this yielded pNZopuCHis.
Lactococcus lactis strain NZ9000 was cultivated semi-anaerobically at 30°C in M17 broth at pH 6.5, supplemented with 1.0% (w/v) glucose and 5 µg/ml chloramphenicol when carrying pNZopuAHis or derivatives. For isolation of membrane vesicles, cells were grown in a 10 l pH-regulated fermentor to an OD600 of 2, after which transcription from the nisA promoter was switched on by the addition of 0.2% (v/v) culture supernatant of the nisin A producing strain NZ9700 (de Ruyter et al., 1996). The cells were harvested 1 h after the start of induction.
Isolation of membrane vesicles
Membrane vesicles were prepared by passage of the cells (2 l of cells, resuspended to OD600 ∼100 in 100 ml of 50 mM KPi pH 7.0) through a pressure cell (Kindler type NN2002; double passage at 1000 bar), following (partial) digestion of the cell wall with 10 mg/ml lysozyme for 30 min at 30°C. The membrane preparations were stored in liquid nitrogen.
Purification of OpuA and heterooligomer formation
Membrane vesicles bearing wild-type or mutant OpuA were resuspended in buffer A (50 mM KPi pH 8.0, 200 mM KCl, 20% glycerol) to a final concentration of 5 mg/ml of protein and solubilized with 0.5% DDM for 30 min on ice. Following centrifugation, the solubilized material was incubated with Ni2+-NTA resin (0.5 ml of resin per 10 mg of membrane protein) for 1–2 h at 4°C in the presence of 15 mM imidazole. Next the resin was washed with 20 column volumes of buffer A supplemented with 0.05% DDM and 15 mM imidazole. The his-tagged proteins were eluted from the column with 3 column volumes of buffer A supplemented with 0.05% DDM and 200 mM imidazole.
Purified OpuA wild-type and/or mutant complexes were mixed at different ratios (total protein concentration of 0.4 mg/ml) and, subsequently, allowed to slowly dissociate by decreasing the glycerol concentration to 5% (v/v). For this purpose the protein mixture was diluted with buffer A without glycerol but supplemented with 0.05% DDM. After 30 min incubation at 4°C, the glycerol concentration was increased again to 20% by adding buffer A containing 0.05% DDM and 60% (v/v) glycerol. Reassembly of the complexes was allowed to continue for 30 min at 4°C, after which the proteins were incorporated into liposomes.
Size-exclusion chromatography
Ni2+-NTA-purified OpuA complex was concentrated to ∼1 mg/ml with an Amicon Ultra-15 centrifugal 100 kDa filter (Millipore). This procedure concentrated OpuA without noticeable loss of protein or concentration of the detergent. Approximately 100 µl of protein sample was loaded onto an analytical size-exclusion chromatography column (Shodex KW804; 0.8 × 30 cm) equilibrated with 50 mM KPi pH 8.0, 200 mM NaCl, 20% (v/v) glycerol, 0.05% DDM and run at a flow rate of 0.5 ml/min at 4°C. For size-exclusion chromatography of the soluble SBP, 200 µl of 0.3 mg/ml of Ni2+-NTA-purified protein was loaded onto a Superdex 200 column (1 × 30 cm), equilibrated with 50 mM KPi pH 7.0, 200 mM KCl, 10% (v/v) glycerol and run at a flow rate of 0.5 ml/min at room temperature.
Membrane reconstitution and membrane fusion
OpuA and heterooligomeric complexes were reconstituted in liposomes composed of 50 mol% synthetic dioleoyl-phosphatidylethanolamine (DOPE) and 50 mol% dioleoyl-phosphatidylglycerol (DOPG), essentially as described (van der Heide and Poolman, 2000). The final protein to lipid ratio was 1:100 (w/w), corresponding to ∼1 mol of OpuA per 30 000 mol of lipids. For some experiments, membrane vesicles were fused with liposomes composed of 50 mol% DOPE and 50 mol% DOPG, using 10 mg DOPE/DOPG mixture per mg of membrane vesicle protein (∼1 mg of endogenous lipid). The hybrid membranes were obtained by two freeze/thaw cycles, followed by extrusion through a polycarbonate filter (200 nm pore size).
Transport assays
ATP-driven uptake of glycine betaine in proteoliposomes was performed as described before (van der Heide and Poolman, 2000), with some modifications. Briefly, proteoliposomes were loaded with an ATP-regenerating system, consisting of 2.4 mg/ml creatine kinase, 9 mM Mg-ATP plus 24 mM creatine phosphate (disodium salt). After extrusion of the proteoliposomes through a polycarbonate filter (200 nm pore size), the proteoliposomes were washed twice and resuspended in 100 mM KPi, pH 7.0 (iso-osmotic with luminal contents, osmolality of ∼190 mosmol/kg) to a concentration of 125 mg of lipids per ml. For osmotically activated transport, the proteoliposomes were diluted to a lipid concentration of 12 mg/ml into 400 mM KPi pH 7.0 (final osmolality of ∼750 mosmol/kg). Following incubation for 1 min at 30°C, the transport reaction was initiated by the addition of radiolabeled substrate ([14C]- or [3H]glycine betaine at final concentrations ranging from 0.024 to 34 µM). At given time intervals, 40 µl samples were taken and diluted with 2 ml ice-cold stop buffer, 400 mM KPi pH 7.0. The samples were filtered rapidly through 0.45 µm pore-size cellulose nitrate filters (Schleicher & Schuell) and washed twice with 2 ml of stop buffer. The radioactivity on the filters was determined by liquid scintillation counting. For the initial rate measurements, uptake was followed for 60 s by taking several time points, but only the linear ‘initial’ part of the curve was used to determine the rate.
For the ATP-driven uptake of glycine betaine in the hybrid membranes, the ATP-regenerating system was enclosed during the fusion of membrane vesicles with liposomes. The fused and loaded membranes were washed twice with 100 mM KPi pH 7.0 and resuspended in 100 mM KPi pH 7.0, to a concentration of 33 mg of lipids/ml. After dilution of the hybrid membranes to a lipid concentration of 4.5 mg/ml into 400 mM KPi pH 7.0, the transport assay was performed as described above.
All transport activity measurements were performed at least in duplicate and carried out with different batches of proteoliposomes. Kinetic data were analysed with the equation: V = _V_max * _S_n/(_K_Mn + _S_n), in which V is the initial rate of uptake, _V_max is the maximal initial rate of uptake, S is the glycine betaine concentration, _K_M is the apparent affinity constant, and n is the Hill coefficient. If n is 1, the equation takes the form of a Michaelis–Menten equation.
Ligand binding assays
Glycine betaine binding to OpuA was measured using the ammonium sulfate precipitation method (Richarme and Kepes, 1983). Hybrid membranes, without ATP-regenerating system, were washed and resuspended in 100 mM KPi pH 7.0 to a concentration of 33 mg of lipids/ml. After dilution of the membranes to 1.3 mg lipids/ml into 100 mM or 400 mM KPi pH7.0 plus 5 mM EDTA (total volume 100 µl), [3H]glycine betaine, at final concentrations ranging from 0.024 to 18 µM, was added and binding was allowed to proceed for 2 min at 30°C. The hyperosmotic conditions had no effect on binding but were used in most cases to obtain comparable conditions for the binding and transport measurements. The reaction was stopped by adding 2 ml ice-cold 70% saturated ammonium sulfate and rapid filtration of the mixture through 0.45 µm pore-size cellulose nitrate filters. The filters were washed twice with 2 ml ice-cold 70% saturated ammonium sulfate and radioactivity was counted with a liquid scintillation spectrometer. Glycine betaine binding to the soluble SBP of OpuA was measured by the same precipitation method but also by monitoring changes in intrinsic protein fluorescence upon substrate binding. Fluorescence spectra were obtained with an Aminco SPF-500 spectrofluorometer. A quartz cuvette contained 1 ml of a soluble SBP solution (0.2–0.5 µM) in filtered 50 mM KPi pH 7.0, supplemented with 200 mM KCl and 10% glycerol, and the mixture was continuously stirred and kept at 20°C with a circulating water bath. The effects of glycine betaine additions on the intrinsic protein fluorescence were measured at an excitation wavelength of 295 nm (slit width of 2 nm) and the emission at 365 nm (slit width of 5 nm). Analysis of the spectral data and the appropriate controls were carried out as described previously (Lanfermeijer et al., 1999, 2000).
Materials
M17 broth was obtained from Difco, Kansas City, KS, USA; Ni2+-NTA resin from Qiagen Inc.; Biobeads SM-2 from Bio-Rad; and DDM and MTSET) from Anatrace Inc, Maurnee, OH. Synthetic lipids were obtained from Avanti Polar Lipids, Alabaster, AL. The molecular weight markers for the size exclusion experiments with detergent-solubilized OpuA and soluble SBP were obtained from Sigma and Bio-Rad, respectively. Radiolabeled [_N_-methyl-14C] and [_N_-methyl-3H]choline chloride (55 mCi/mmol and 80 mCi/mmol, respectively) were from Amersham Biosciences, Piscataway, NJ, and these precursors were used to synthesize [_N_-methyl-14C] and [_N_-methyl-3H]glycine betaine as described (Landfald and Strom, 1986). Creatine kinase and creatine phosphate were obtained from Sigma Chemical Co. All other chemicals were of reagent grade and purchased from commercial sources.
Acknowledgments
Acknowledgements
We thank our colleagues Mark Doeven, Sietse Henstra, Jason Patzlaff and Gea Schuurman-Wolters for stimulating discussions. This work was supported by a ‘Top-subsidie’ grant (NWO-CW, grant number 700-50-302) to B.P.
References
- Albers S.V., Elferink,M.G., Charlebois,R.L., Sensen,C.W., Driessen,A.J. and Konings,W.N. (1999) Glucose transport in the extremely thermoacidophilic Sulfolobus solfataricus involves a high-affinity membrane-integrated binding protein. J. Bacteriol., 181, 4285–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G.F., Liu,C.E., Joshi,A.K. and Nikaido,K. (1996) Liganded and unliganded receptors interact with equal affinity with the membrane complex of periplasmic permeases, a subfamily of traffic ATPases. J. Biol. Chem., 271, 14264–14270. [DOI] [PubMed] [Google Scholar]
- Borths E.L., Locher,K.P., Lee,A.T. and Rees,D.C. (2002) The structure of Escherichia coli BtuF and binding to its cognate ATP binding cassette transporter. Proc. Natl Acad. Sci. USA, 99, 16642–16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sharma,S., Quiocho,F.A. and Davidson,A.L. (2001) Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl Acad. Sci. USA, 98, 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A.L. (2002) Mechanism of coupling of transport to hydrolysis in bacterial ATP-binding cassette transporters. J. Bacteriol., 184, 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A.L. and Nikaido,H. (1990) Overproduction, solubilization and reconstitution of the maltose transport system from Escherichia coli. J. Biol. Chem., 265, 4254–4260. [PubMed] [Google Scholar]
- Davidson A.L., Shuman,H.A. and Nikaido,H. (1992) Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc. Natl Acad. Sci. USA, 89, 2360–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D.A., Hor,L.I., Shuman,H.A. and Nikaido,H. (1992) Interaction between maltose-binding protein and the membrane-associated maltose transporter complex in _Escherichia coli._Mol. Microbiol., 6, 2033–2040. [DOI] [PubMed] [Google Scholar]
- de Ruyter P.G., Kuipers,O.P. and de Vos,W.M. (1996) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol., 62, 3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C.F. (1992) ABC transporters: from microorganisms to man. Annu. Rev. Cell. Biol., 8, 67–113. [DOI] [PubMed] [Google Scholar]
- Hor L.I. and Shuman,H.A. (1993) Genetic analysis of periplasmic binding protein dependent transport in Escherichia coli. Each lobe of maltose-binding protein interacts with a different subunit of the MalFGK2 membrane transport complex. J. Mol. Biol., 233, 659–670. [DOI] [PubMed] [Google Scholar]
- Kunji E.R., Slotboom,D.J. and Poolman,B. (2003) Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta, 1610, 97–108. [DOI] [PubMed] [Google Scholar]
- Landfald B. and Strom,A.R. (1986) Choline–glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J. Bacteriol., 165, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfermeijer F.C., Picon,A., Konings,W.N. and Poolman,B. (1999) Kinetics and consequences of binding of nona- and dodecapeptides to the oligopeptide binding protein (OppA) of Lactococcus lactis. Biochemistry, 38, 14440–14450. [DOI] [PubMed] [Google Scholar]
- Lanfermeijer F.C., Detmers,F.J., Konings,W.N. and Poolman,B. (2000) On the binding mechanism of the peptide receptor of the oligopeptide transport system of Lactococcus lactis. EMBO J., 19, 3649–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.E. and Ames,G.F. (1997) Characterization of transport through the periplasmic histidine permease using proteoliposomes reconstituted by dialysis. J. Biol. Chem., 272, 859–866. [DOI] [PubMed] [Google Scholar]
- Liu C.E., Liu,P.Q., Wolf,A., Lin,E. and Ames,G.F. (1999a) Both lobes of the soluble receptor of the periplasmic histidine permease, an ABC transporter (traffic ATPase), interact with the membrane-bound complex. Effect of different ligands and consequences for the mechanism of action. J. Biol. Chem., 274, 739–747. [DOI] [PubMed] [Google Scholar]
- Liu P.Q., Liu,C.E. and Ames,G.F. (1999b) Modulation of ATPase activity by physical disengagement of the ATP-binding domains of an ABC transporter, the histidine permease. J. Biol. Chem., 274, 18310–18318. [DOI] [PubMed] [Google Scholar]
- Locher K.P., Lee,A.T. and Rees,D.C. (2002) The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science, 296, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Manson M.D. and Boos,W. (1985) Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J. Biol. Chem., 260, 9727–9733. [PubMed] [Google Scholar]
- Miller D.M., Olson,J.S. and Quiocho,F.A. (1980) The mechanism of sugar binding to the periplasmic receptor for galactose chemotaxis and transport in Escherichia coli. J. Biol. Chem., 255, 2465–2471. [PubMed] [Google Scholar]
- Patzlaff J.S. and Poolman,B. (2003) The ATP/substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. J. Biol. Chem., 278, 29546–29551. [DOI] [PubMed] [Google Scholar]
- Poolman B. and Konings,W.N. (1993) Secondary solute transport in bacteria. Biochim. Biophys. Acta, 1183, 5–39. [DOI] [PubMed] [Google Scholar]
- Richarme G. and Kepes,A. (1983) Study of binding protein–ligand interaction by ammonium sulfate-assisted adsorption on cellulose esters filters. Biochim. Biophys. Acta, 742, 16–24. [DOI] [PubMed] [Google Scholar]
- Sutcliffe I.C. and Russell,R.R. (1995) Lipoproteins of Gram-positive bacteria. J. Bacteriol., 177, 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide T. and Poolman,B. (2000) Osmoregulated ABC transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl Acad. Sci. USA, 97, 7102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide T. and Poolman,B. (2002) ABC transporter: one, two or four substrate-binding domains? EMBO rep., 3, 938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide T., Stuart,M.C. and Poolman,B. (2001) On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J., 20, 7022–7032. [DOI] [PMC free article] [PubMed] [Google Scholar]