NANOG is a Direct Target of TGFβ/Activin Mediated SMAD Signaling in Human ES Cells (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 6.
Published in final edited form as: Cell Stem Cell. 2008 Aug 7;3(2):196–206. doi: 10.1016/j.stem.2008.07.001
SUMMARY
Self-renewal of human embryonic stem (ES) cells is promoted by FGF and TGFβ/Activin signaling, and differentiation is promoted by BMP signaling, but how these signals regulate genes critical to the maintenance of pluripotency has been unclear. Using a defined medium, we show here that both TGFβ and FGF signals synergize to inhibit BMP signaling, sustain expression of pluripotency-associated genes such as NANOG, OCT4, and SOX2, and promote long-term undifferentiated proliferation of human ES cells. We also show that both TGFβ- and BMP-responsive SMADs can bind with the NANOG proximal promoter. NANOG promoter activity is enhanced by TGFβ/Activin and FGF signaling, and is decreased by BMP signaling. Mutation of putative SMAD binding elements reduces NANOG promoter activity to basal levels, and makes NANOG unresponsive to BMP and TGFβ signaling. These results suggest that direct binding of TGFβ/Activin-responsive SMADs to the NANOG promoter plays an essential role in sustaining human ES cell self-renewal.
Keywords: Human embryonic stem cells, FGF, TGFβ, BMP, SMAD, NANOG
INTRODUCTION
Although the signaling pathways that contribute to the sustained undifferentiated proliferation of human embryonic stem (ES) cells remain to be completely elucidated, it is clear that several developmentally important signals are involved. Among them, fibroblast growth factor (FGF) family members and transforming growth factor β (TGFβ)/Activin promote self-renewal (Amit et al., 2004; Beattie et al., 2005; Besser, 2004; Greber et al., 2006; James et al., 2005; Levenstein et al., 2005; Li et al., 2005; Lu et al., 2006; Ludwig et al., 2006b; Sato et al., 2004; Vallier et al., 2005; Wang et al., 2005; Xiao et al., 2006; Xu et al., 2005a; Xu et al., 2005b; Yao et al., 2006) and bone morphogenetic proteins (BMPs) promote differentiation (Pera et al., 2004; Xu et al., 2002; Xu et al., 2005b). Clonal growth of human ES cells is supported by basic FGF (bFGF) on fibroblasts in the presence of a commercially available serum substitute (Amit et al., 2000), and the same medium supports human ES cells in the absence of direct contact with feeder layers if it is conditioned on fibroblasts prior to use (Xu et al., 2001). Higher concentrations of bFGF support human ES cells in the absence of fibroblasts or fibroblast conditioned medium, and these higher concentrations are in part needed because of the instability of bFGF in those conditions (Levenstein et al., 2005). These initial studies were performed in medium including a commercially available, poorly defined serum replacement, that contains BMP activity at sufficient levels to induce differentiation of human ES cells in the absence of fibroblasts (Xu et al., 2005b). Under these conditions, inhibition of BMP activity is beneficial to human ES cell self-renewal, and at high concentrations, bFGF itself has an ability to inhibit BMP signaling (Xu et al., 2005b). However, inhibition of BMP activity by itself is not sufficient for the long-term maintenance of human ES cells, indicating additional roles of FGF signaling (Xu et al., 2005b).
Undifferentiated human ES cells are characterized by activation of SMAD2/3 (TGFβ/Activin pathway) and by repression of SMAD1/5/8 (BMP pathway) (Beattie et al., 2005; Besser, 2004; Greber et al., 2006; James et al., 2005; Vallier et al., 2005; Xiao et al., 2006; Xu et al., 2005b). The TGFβ super-family of secreted factors include TGFβ, Activins, Nodal, and BMPs, several of which are expressed by human ES cells (Beattie et al., 2005; Besser, 2004; Greber et al., 2006; James et al., 2005; Sperger et al., 2003; Vallier et al., 2005; Xiao et al., 2006; Xu et al., 2005b). TGFβ super-family members signal by bringing together type I and type II receptors on the cell surface. The type II receptor phosphorylates the type I receptor, which then propagates the signal by phosphorylating receptor-activated SMAD (R-SMAD) proteins (Shi and Massague, 2003). TGFβ/Activin signaling occurs through SMAD2/3, and BMP signaling occurs through SMAD1/5/8. Both groups of R-SMADs, once activated, bind the same co-SMAD (SMAD4) and the resulting complex assembles with other transcriptional factors in the nucleus, which then regulate specific gene expression. Receptors for Activin (Act-IB/ALK4 and ActR-IIB) and BMP (BMPRIA/ALK3 and ActRIIB) are both expressed by human ES cells (Sperger et al., 2003). Human ES cells treated with TGFβ/Activin inhibitor SB431542 rapidly differentiate when cultured on fibroblasts or in fibroblast conditioned medium (Beattie et al., 2005; James et al., 2005; Vallier et al., 2005). At low or modest concentrations of FGFs, both Activin and TGFβ have strong positive effects on undifferentiated proliferation of human ES cells, and based on the SB431542 inhibitor studies, it has been suggested that TGFβ/Activin signaling is essential for human ES cell self-renewal. However, when TGFβ/Activin signaling is inhibited with SB431542, there is a concomitant rise in BMP signaling activity, evidenced by an increased phosphorylation of SMAD1/5/8 (Beattie et al., 2005; James et al., 2005; Vallier et al., 2005), so it has been somewhat unclear whether signaling through SMAD2/3 is merely acting to inhibit the sister BMP pathway, or whether TGFβ/Activin signaling has other, independent roles.
Recent studies have revealed transcriptional interaction between FGF, TGFβ, and BMP pathways in human ES cells. For example, Activin induces bFGF expression, and bFGF induces Tgfβ1/TGFβ1 and Grem1/GREM1 (a BMP antagonist) expression and inhibits Bmp4/BMP4 expression in both mouse embryonic fibroblast (feeder cells for human ES cells) and human ES cells (Greber et al., 2006). This reciprocity of induction between the FGF and TGFβ/Activin pathways may explain why at high doses of bFGF, exogenous TGFβ has very modest effects on undifferentiated human ES cell proliferation in defined conditions (Ludwig et al, 2006), and similarly, at sufficient doses of Activin, the beneficial dose of exogenous FGF is greatly reduced (Vallier et al., 2005; Xiao et al., 2006). Taken together, these studies raise the question whether both FGF and TGFβ/Activin signals are required, or whether either alone is sufficient to sustain human ES cell self-renewal.
How the extrinsic factors that sustain human ES cells intersect with the intrinsic transcriptional networks essential to pluripotency remains largely unexplored. The core transcription factors essential to maintaining both mouse and human ES cells include the homeodomain transcription factors NANOG and OCT4 (POU5F1) and the HMG-box transcription factor SOX2 (Chambers et al., 2003; Hay et al., 2004; Matin et al., 2004; Mitsui et al., 2003; Nichols et al., 1998; Zaehres et al., 2005). Disruption of Oct4 or Nanog leads to mouse ES cell differentiation to trophectoderm and extra-embryonic endoderm, respectively (Chambers et al., 2003; Mitsui et al., 2003; Nichols et al., 1998). Inhibition of NANOG gene expression also causes human ES cell differentiation to extraembryonic cell lineages (Hyslop et al., 2005; Zaehres et al., 2005), and overexpression allows feeder-independent proliferation of human ES cells (Darr et al., 2006). NANOG, OCT4, and SOX2 co-occupy and regulate many developmentally important homeodomain genes and collaborate to form an extensive regulatory circuitry including autoregulatory and feedforward loops (Boyer et al., 2005; Kuroda et al., 2005; Lee et al., 2006; Rodda et al., 2005).
Here we further dissect the requirements of FGF and TGFβ/Activin signaling in human ES cell self-renewal, and explore the links between extrinsic TGFβ/Activin signaling and the intrinsic transcriptional regulators of human ES cell self-renewal and pluripotency. We find that either FGF or TGFβ signaling alone cannot sustain the long-term undifferentiated proliferation of human ES cells in our specific culture conditions. We also find that SMADs bind with the NANOG promoter and that SMAD2/3 activity enhances NANOG promoter activity. These results establish a direct link between an extrinsic factor that maintains human ES cell self-renewal and the transcriptional regulation of this key pluripotency gene NANOG.
RESULTS
Both FGF and TGFβ signals are required to sustain human ES cell self-renewal
For these studies, we used a defined medium TeSR1 (T1) that includes bFGF and TGFβ1 (Ludwig et al., 2006b). Using a luciferase reporter assay (Lopez-Rovira et al., 2002) that we have previously shown responds to BMP activity in human ES cells in a dose dependent manner (Xu et al., 2005b), FGF and TGFβ signaling synergistically repressed BMP signaling in H9 human ES cells (Fig. 1A and Fig. S1). We have previously shown that BMP4 treated human ES cells differentiate to trophoblast in conditioned medium, and that human chorionic gonadotrophin (HCG) is dramatically upregulated during this differentiation (Xu et al., 2002). The antagonism between FGF and BMP signaling in human ES cells cultured in T1 was also apparent in a dose-dependent inhibition of HCG secretion by bFGF in BMP4 treated human ES cells (Fig. 1B).
Fig. 1. Either FGF or TGFβ signaling antagonizes BMP signaling.
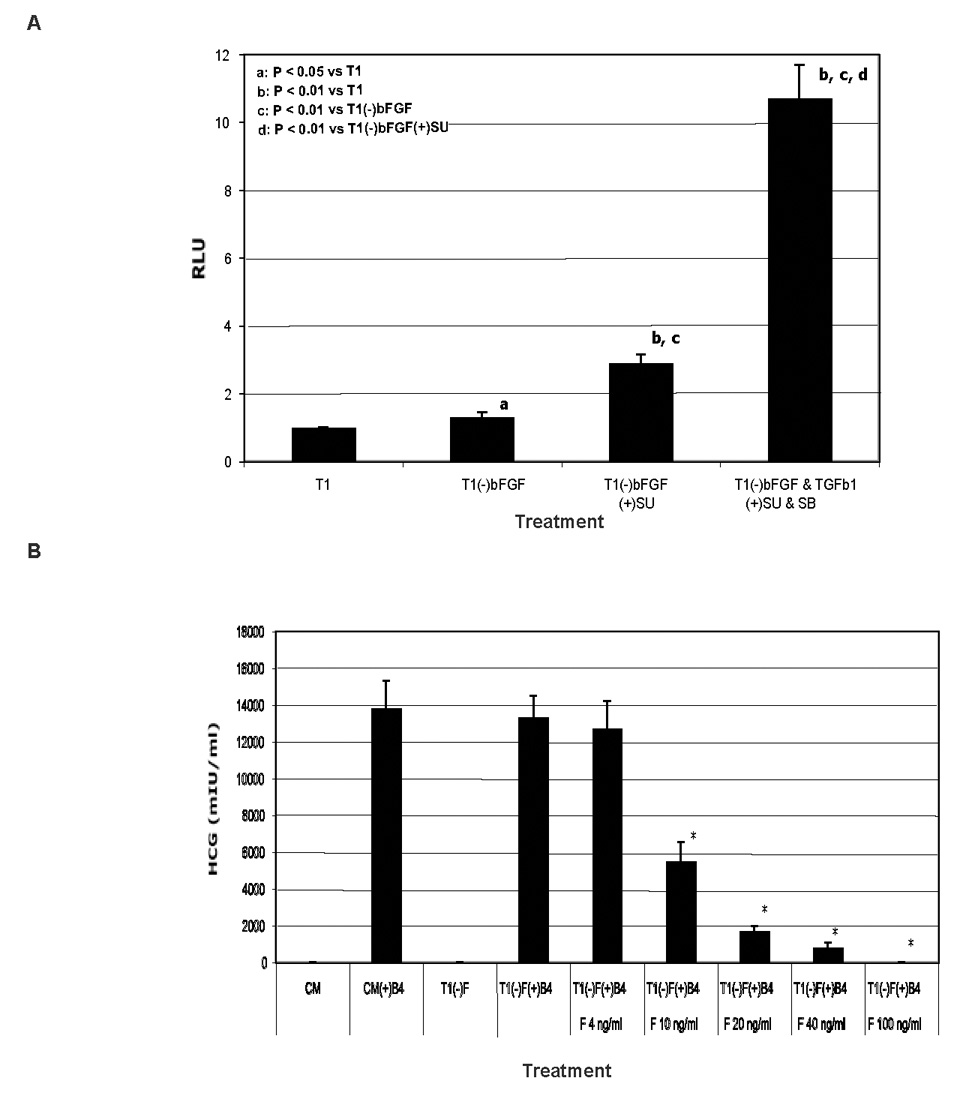
A. Both FGF and TGFβ signals synergistically repress BMP signal in human ES cells in luciferase reporter assays. H9 cells were transfected with pID120-Luc and a trace amount of pRL-tk (for internal control) on day 1. Treatments of the cells started on day 2 with T1 medium (T1) or T1 minus or plus 100 ng/ml bFGF, 0.6 ng/ml TGFβ1, 10 µM SU5402 (SU, inhibitor of FGF receptors), and 10 µM SB431542 (SB, inhibitor of TGFβ receptors). The cells were lysed on day 3 for analysis of luciferase activity. B. bFGF inhibits HCG secretion from trophoblast differentiated from BMP4-treated human ES cells. H14 cells cultured in fibroblast-conditioned medium (CM) were rinsed with DMEM/F12 basal medium and switched to CM or T1 medium with or without various concentrations of bFGF (F) and 100 ng/ml BMP4 (B4) for 7 days with daily refreshment of the media. The spent media were collected on day 7, and assayed for HCG. Results are shown as mean ± standard deviation. *P < 0.01 compared to T1(−)bFGF(+)BMP4. SB has been previously been shown to inhibit the levels of phosphorylated Smad2 and Smad3 in human ES cells (James et al., 2005).
The antagonism between FGF or TGFβ signaling and BMP signaling was analyzed in human ES cells cultured in T1 medium by using an inhibitor of FGF signaling, SU5402, and an inhibitor of TGFβ signaling, SB431542 (SB) (Fig. S2). SU5402 alone or together with BMP4 reduced the level of phosphorylated SMAD2/3, and SU5402 also enhanced BMP4 expression. SB431542 synergized with BMP4 to elevate the level of phosphorylated SMAD1/5/8 and BMP4 production. These data indicate that FGF and TGFβ signaling antagonize BMP signaling either directly by repressing SMAD1/5/8 phosphorylation or BMP4 production, or indirectly by promoting SMAD2/3 phosphorylation.
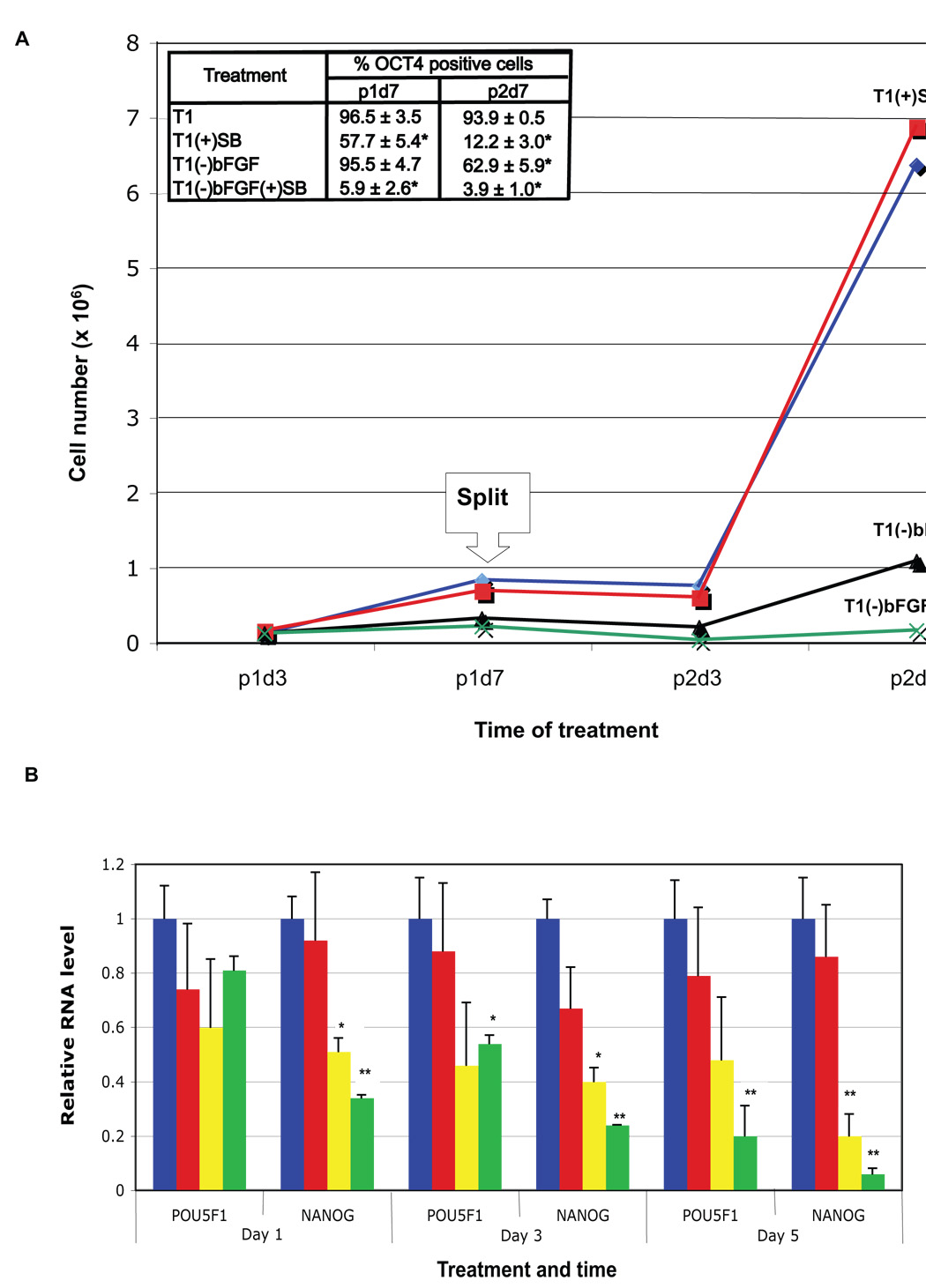
We next tested the long-term requirements of FGF and TGFβ signaling for human ES cell self-renewal by culturing H1 cells in T1 medium without bFGF [T1(−)bFGF] or with 10 µM SB [T1(+)SB]. After 7 days of culture, almost all the cells cultured in T1(−)bFGF remained OCT4+, about half of the cells in T1(+)SB remained OCT4+, while almost all of the cells in T1(−)bFGF(+)SB become OCT4− (Fig. S3 and Fig. 2A). Continuous treatment of the cells for 14 days (cells split at 7 days) led to a further decline in the OCT4+ percentage in each of the treatment groups (62.9%, 12.2%, and 3.9% for T1(−)bFGF, T1(+)SB, and T1(−)bFGF(+)SB, respectively). Cell numbers in T1(+)SB remained similar to that of T1 control, but were greatly reduced in T1(−)bFGF (Fig. 2A). Similar results were obtained from H9 cells (data not shown). These data suggest that both FGF and TGFβ signaling are required to maintain long-term human ES cell self-renewal in T1, and that FGF signaling also sustains cell proliferation, independent of TGFβ signaling. Microarray gene expression profiles (Table S1), QPCR (Fig. 2B), and western blotting (Fig. S4) of human ES cells subjected to inhibition of FGF and TGFβ signaling paralleled these results, i.e., inhibition of either FGF or TGFβ signaling alone led to a modest initial decline in the expression levels of pluripotency-associated genes such as OCT4, NANOG, and NODAL, and inhibition of both signals led to dramatic, rapid decline in expression of these genes.
Fig. 2. FGF and TGFβ synergize to sustain human ES cell self-renewal.
A. bFGF and TGFβ effects on H1 cell growth and maintenance of OCT4 expression. 3.5 105 H1 cells were seeded in individual wells of 6-well plates in triplicate and cultured in T1 medium, T1 plus 10 µM SB431542 [T1(+)SB], T1 minus 100 ng/ml bFGF [T1(−)bFGF], or T1 minus 100 ng/ml bFGF plus 10 µM SB [T1(−)bFGF(+)SB]. The cells were split 1:3 on day 7 into the corresponding media and cultured for an additional 7 days. Total cell number per group was counted on days 3 and 7 of each passage (labeled as p1d3, p1d7, p2d3, and p2d7, respectively), and OCT4+ cell percentage determined by flow cytometry on p1d7 and p2d7. Results are shown as mean ± standard deviation. *P < 0.01 compared to T1. B. Expression of pluripotency genes in human ES cells treated with various growth factors or inhibitors. H9 cells were cultured in T1, T1(−)bFGF, T1(+)SB, or T1(−)bFGF(+) SB medium for 1, 3 or 5 days and followed by QPCR for expression of OCT4 and NANOG. *P < 0.05, **P < 0.01 compared to T1.
SMADs directly bind to the NANOG proximal promoter
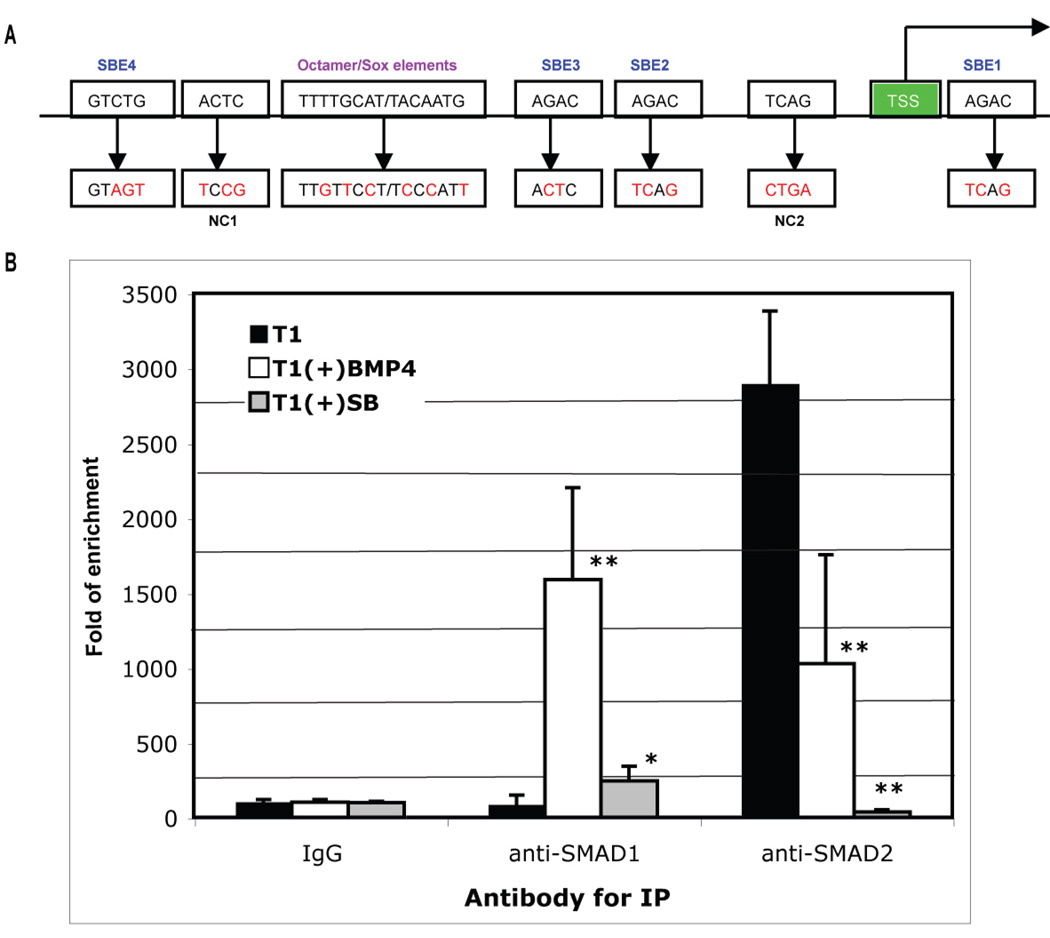
Among the key transcription factors known to control pluripotency, NANOG expression was repressed the most by inhibition of TGFβ signaling, either in the presence or absence of bFGF (Table S1 and Fig. 2B). We therefore decided to examine whether NANOG itself is a direct target of SMAD-mediated signaling pathways. Both TGFβ- and BMP-responsive SMADs bind to DNA via their MAD homolog 1 (MH1) domain (Kim et al., 1997; Shi et al., 1998), and SMAD binding elements (SBEs) have been identified in genes responsive to TGFβ and BMP signaling, including a consensus sequence (G)AGAC and its complement GTCT(C) (Massague and Wotton, 2000). Regulatory elements essential for NANOG expression in human ES cells have previously been mapped to an upstream 404-bp proximate promoter region (Kuroda et al., 2005). We noticed that this region also contains four putative SBEs surrounding the Octamer/Sox elements (Fig. 3A and Fig. S5).
Fig. 3. ChIP for SMADs binding to the NANOG proximal promoter.
A. Schematic representation of NANOG proximal promoter region (not to scale) and mutagenesis strategy (see Fig. S4 for its complete sequence). TSS: transcription start site; SBE: SMAD binding element; NC: sequence mutated for negative control mutation; and red letters for mutated nucleotide residues. B. ChIP assay for SMAD binding to the NANOG proximal promoter. H1 cells were cultured in T1 medium, T1 plus 10 µM SB431542 [T1(+)SB], or T1 plus 100 ng/ml BMP4 [T1(+)BMP4] for 24 hours. The cells were harvested and processed for ChIP with isogenic IgG, anti-SMAD1/5/8, or anti-SMAD2/3 antibodies. The enrichment of the precipitated DNA by each of the antibodies versus the IgG was analyzed by QPCR using primers flanking the proximal promoter region. Results from two duplicated experiments are shown as fold of DNA enrichment. *P < 0.05, **P < 0.01 compared to T1.
By using chromatin immunoprecipitation (ChIP) and quantitative polymerase chain reaction (QPCR), we analyzed enrichment of the NANOG promoter region from the nuclear lysates of H1 cells with anti-SMAD1/5/8 or anti-SMAD2/3 antibodies (Fig. 3B). Enrichment of the promoter region by anti-SMAD2/3 antibody was high for control human ES cells cultured in T1, but reduced dramatically for SB431542-treated cells and reduced modestly (but significantly) for BMP4-treated cells. In contrast, enrichment of the promoter region by anti-SMAD1/5/8 antibody was low for control human ES cells, was increased markedly for BMP4-treated cells, and was increased modestly (but significantly) for SB431542-treated cells. These results suggest that both BMP-responsive SMADs (SMAD1, -5, and -8) and TGFβ-responsive SMADs (SMAD2 and - 3) can interact with the NANOG proximal promoter. The binding by SMAD2/3 is dominant in undifferentiated human ES cells, and is sustained by TGFβ signaling, whereas the binding by SMAD1/5/8 is low in human ES cells, but increases with BMP signaling.
Electrophoretic mobility shift assays (EMSA) was performed with biotin-labeled 80 bp DNA amplified from the proximal NANOG promoter region, which contained the four putative SBEs surrounding the Octamer/Sox elements (Fig. 3A and Fig. S5). This probe allowed us to pull down SMAD2/3 from human ES cells, as verified by western blotting (Fig. 4A). By using this probe we observed a clear shift after incubation with the nuclear extract of H9 cells and a super-shift when an antibody against SMAD2/3, but not SMAD1/5/8, was present in the incubation (Fig. 4B). The super-shift disappeared when the four SBEs were mutated in the probe (Fig. 4B). The shift and super-shift were also present with another human ES cell line H1, but absent with IMR90 fetal fibroblasts (data not shown), suggesting that the SMAD binding occurs in human ES cells but not the fibroblasts. H9 cells treated with BMP4 for 3 hours caused a super-shift with the anti-SMAD1/5/8 antibody, while the super-shift with the anti-SMAD2/3 antibody remained; on the other hand, only a super-shift with the anti-SMAD1/5/8 antibody occurred on H9 cells treated with 10 µM SB431542 for 3 hours (Fig. 4C). These data agree with the biological effects of BMP4 that induces nuclear translocation of SMAD1/5/8, and SB431542 that inhibits nuclear translocation of SMAD2/3. Similar results were obtained with H9 cells treated with BMP4 or SB431542 in the presence of 10 µg/ml cycloheximide, a protein synthesis inhibitor (Fig. 4D), suggesting direct binding of the SMADs to the NANOG promoter.
Fig. 4. EMSA for SMAD binding to the NANOG proximal promoter.
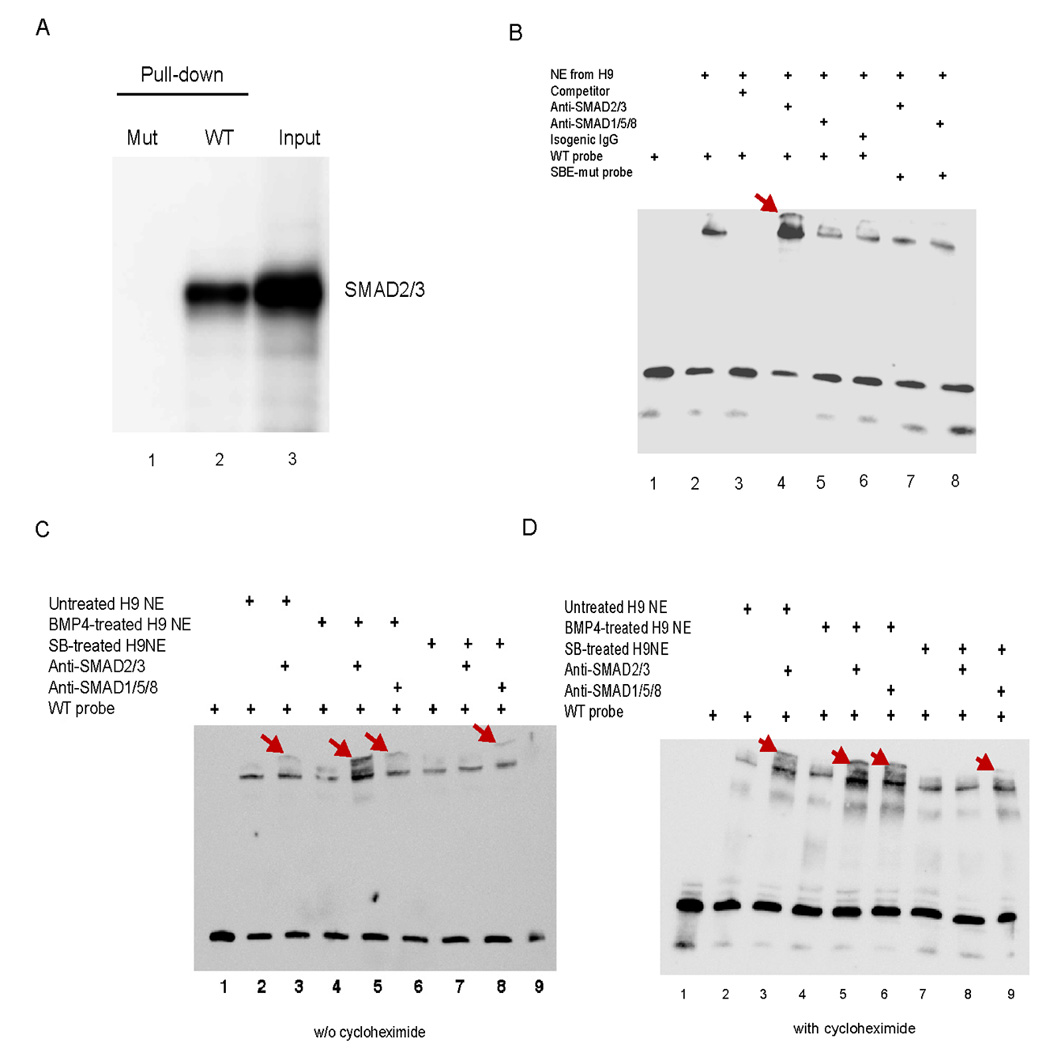
A. DNA pull-down to verify probes for EMSA. A biotin-labeled probe of 80 bp was synthesized by PCR from the NANOG proximal promoter in the plasmid pNANOG-Luc for a wild-type probe (WT) or from the mutated promoter in pNANOG(mSBEs)-Luc for a mutated probe (SBE mut). Only the WT probe was able to pull down SMAD2/3 from the extract of H9 cells as verified by western blotting. B–D. EMSA on the nuclear extract (NE) from H9 cells untreated (B) or treated with 100 ng/ml BMP4 or 10 µM SB431542 for 3 h in the absence (C) or presence (D) of 10 µg/ml cycloheximide (Biomol, Plymouth Meeting, PA), a protein synthesis inhibitor. An unlabeled probe at 200-fold concentration of the labeled probe was used as a competitor, anti-SMAD2/3 or anti-SMAD1/5/8 antibody for super-shift, and isogenic IgG as a control.
FGF, TGFβ, and BMP signaling regulate NANOG promoter activity
By constructing luciferase reporter vectors driven by various regions of the NANOG promoter, we first confirmed the essential role of the proximal region (Fig. S6) as previously reported (Kuroda et al., 2005). We used the reporter vector driven by this region (pNANOG-Luc) to test the effect of various signals on NANOG promoter activity in the human ES cell line H9. The reporter activity was reduced by 10 µM SB431542 or by TGFβ1 withdrawal from T1 [T1(−)TGFβ1], and greatly enhanced by the addition of Activin (Fig. 5A). Interestingly, either BMP4 addition to T1 or bFGF withdrawal from T1 alone did not reduce reporter activity, but both of these treatments did augment the decline of the activity caused by TGFβ1 withdrawal or inhibition (Fig. 5A). These data suggest that TGFβ/Activin plays a central role in sustaining NANOG promoter activity in undifferentiated human ES cells. Overexpression of NANOG sustained OCT4 expression in H9 cells in the absence of FGF and TGFβ signaling (Fig. 6). This data indicates that both FGF and TGFβ signaling support the pluripotency of human ES cell by sustaining NANOG expression.
Fig. 5. NANOG proximal promoter activity in luciferase reporter assay.
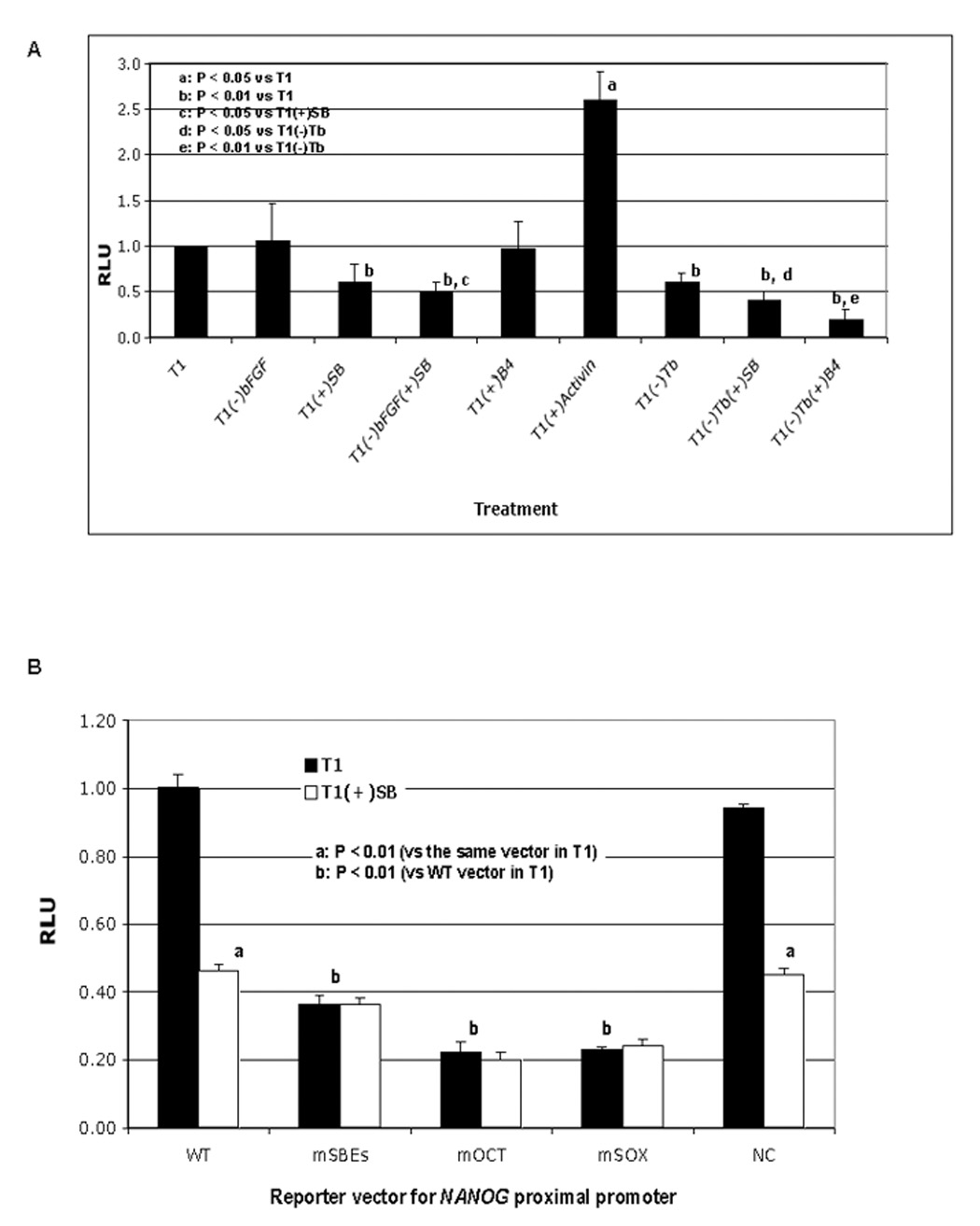
A. Regulation of NANOG promoter reporter activity by FGF, TGFβ, and BMP signaling. H9 cells were transfected with pNANOG-Luc and a trace amount of pGL4.70 (as an internal control) on day 1. Treatments of the cells started on day 2 with T1 medium, T1 minus a specific signal stimulator 100 ng/ml bFGF or 0.6 ng/ml TGFβ1 (Tb), plus 10 ng/ml Activin, 100 ng/ml BMP4 (B4) or 10 µM SB431542 (SB). After 24 hours of treatment, cells were harvested for analysis of luciferase activity. B. Effect of SBE mutations on pNANOG-Luc activity. H9 cells were transfected with wild-type pNANOG-Luc (WT), pNANOG(mSBEs)-Luc (mSBEs), pNANOG(mOCT)-Luc (mOCT), pNANOG(mSOX)-Luc (mSOX) or pNANOG(NC)-Luc (NC) on day 1 (all with pGL4.70), cultured in T1 alone or T1 plus SB starting on day 2, and harvested after 24 hours of treatment for analysis of luciferase activity.
Fig. 6. NANOG overexpression bypasses the need for both TGFβ and FGF signaling to sustain human ES cell self-renewal.
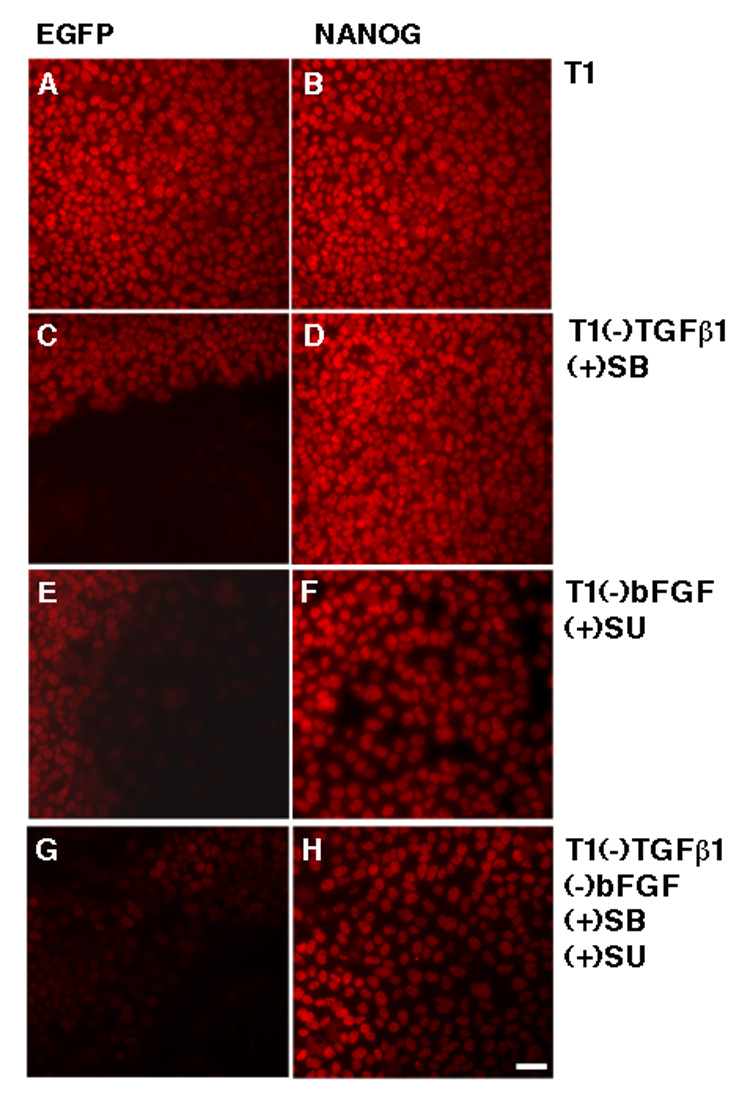
H1 cells transduced with lentiviral particles containing NANOG or EGFP and selected by puromycin were split to plates with T1 medium. Next day, the medium was replaced with T1, T1 minus TGFβ1 plus 10 µM SB431542 [T1(−)TGFβ1(+)SB], T1 minus bFGF plus 10 µM SU5402 [T1(−)bFGF(+)SU], or T1(−)TGFβ1(+)SB(−)bFGF(+)SU. After 5-day treatment with these media, the cells were fixed and processed for immunostaining for OCT4 expression. SU5402 has previously been shown to inhibit the level of phosphorylated ERK in human ES cells (Kang et al., 2005).
Putative SBE sites in the NANOG proximal promoter are required for its activity
We mutated each of the putative SBE sites to generate a mutant reporter vector pNANOG(mSBEs)-Luc (Fig. 3A) and tested the effect of these mutations on the NANOG promoter activity in H9 cells. Compared to human ES cells transfected with the wild-type reporter vector, cells transfected with pNANOG(mSBEs)-Luc had dramatically reduced reporter activity and SB431542 failed to cause further reduction (Fig. 5B). Similar reduction of the reporter activity was seen with the vectors with mutated Octamer- and Sox elements in the NANOG promoter, pNANOG(mOCT)-Luc and pNANOG(mSOX)-Luc, respectively (Fig. 4B), consistent with a previous report (Kuroda et al., 2005). To set up a negative control (NC) for the above binding site-specific mutations, we generated a vector named pNANOG(NC)-Luc with two mutated sequences located downstream of the first SBE and upstream of the fourth SBE, respectively, in the NANOG proximal promoter (Fig. 3A). The reporter activity of this vector was not significantly different from that of the wild-type vector pNANOG-Luc (Fig. 5B). Taken together, these data suggest that TGFβ-responsive SMADs may bind the putative SBEs and sustain NANOG promoter activity, and that the effect of TGFβ signaling on NANOG promoter activity relies on the integrity of the putative SBEs and the Octamer/Sox elements in the promoter.
DISCUSSION
Recently developed feeder-free human ES cell media generally include an FGF family member and an agonist of TGFβ/Activin signaling (Amit et al., 2004; Beattie et al., 2005; Levenstein et al., 2005; Li et al., 2005; Lu et al., 2006; Ludwig et al., 2006b; Sato et al., 2004; Vallier et al., 2005; Wang et al., 2005; Xu et al., 2005a; Xu et al., 2005b; Yao et al., 2006). At the high concentrations of bFGF (100 ng/ml) included in the defined media we used for the current studies, removal of the exogenous TGFβ has only very minor effects on culture performance, observable only when the cells are carefully monitored over several passages (Ludwig et al., 2006b). However, inhibition of endogenous TGFβ/Activin signaling in cells grown in T1 resulted in a significant decline in the percentage of cells expressing ES cell markers after only a week of culture, indicating that autocrine TGFβ/Activin signaling is important in these specific culture conditions (Fig. 2A). We also found that either FGF or TGFβ signaling repressed BMP signaling in human ES cells in these conditions (Fig. 1). However, although maintaining BMP signaling below some threshold that could cause differentiation is undoubtedly important, suppression of BMP signaling alone is not sufficient to sustain prolonged culture of human ES cells (Xu et al., 2005b), implying that FGF and TGFβ play additional roles. Both FGF and TGFβ/Activin signaling are required to maintain a high percentage of OCT4+ cells in prolonged human ES cell culture in our current culture conditions, and FGF signaling is also important for promoting proliferation (Fig. 2A).
Among the core transcription factors involved in the control of pluripotency, NANOG is downregulated the most in human ES cells after TGFβ/Activin signaling is blocked for three days (Table S1), which led us to examine whether NANOG itself is a target of SMAD signaling. The presence of repetitive SBEs in the NANOG proximal promoter region suggested the potential involvement of direct SMAD binding in the regulation of NANOG expression. ChIP experiments using an antibody to SMAD2/3 documented enrichment for the NANOG promoter region in undifferentiated human ES cells. A gel shift assay suggested binding of SMAD2/3 or SMAD1/5/8 to the NANOG promoter DNA depending on activation of the TGFβ or BMP signaling and integrity of the SBEs in the promoter, and a luciferase NANOG reporter promoter assay demonstrated an increase of activity correlated with conditions where SMAD2/3 signaling is active. An antibody for SMAD1/5/8 demonstrated enrichment of the same NANOG promoter region in BMP4-treated human ES cells. However, although addition of BMP4 or withdrawal of bFGF reduced NANOG expression after 3 days, neither repressed the shorter term NANOG reporter assays unless TGFβ/Activin signaling was already absent. This suggests that the TGFβ signal dominates in determining the initial level of NANOG promoter activity, and that FGF and BMP signals may ultimately regulate NANOG expression through more indirect mechanisms. The fact that overexpression of NANOG bypassed the need for both TGFβ and FGF signaling to sustain human ES cell self-renewal further suggest that these signaling pathways act via regulation of NANOG expression.
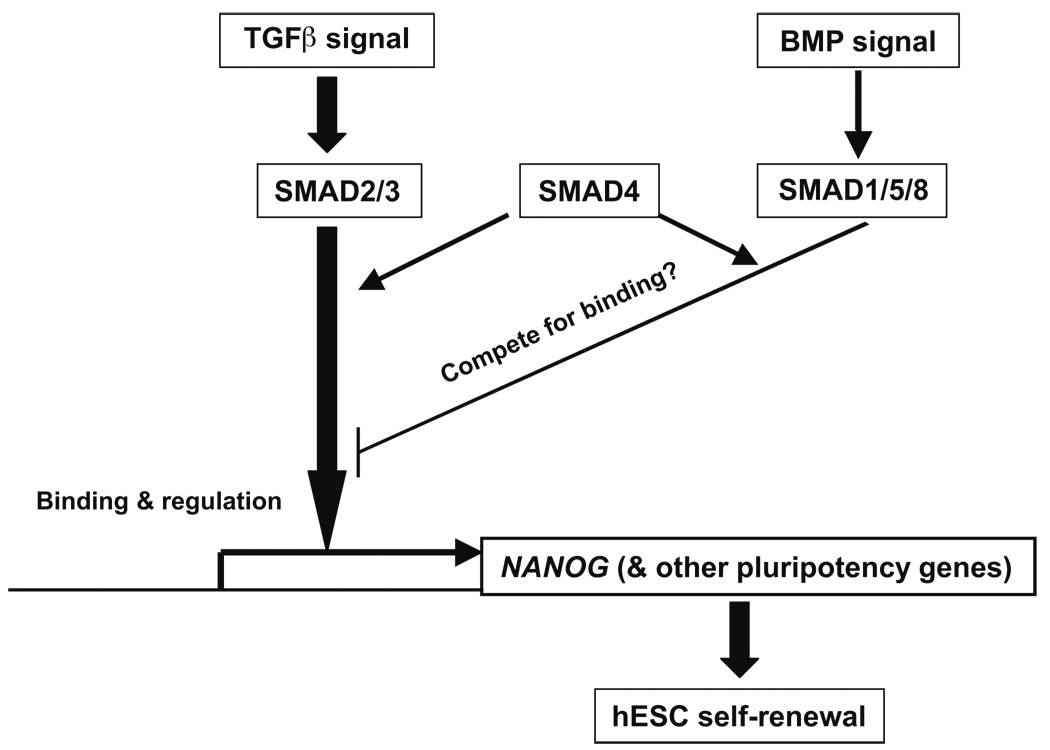
It has been reported that Nanog can physically interact with Smad1 in mouse ES cells, interfere with the recruitment of co-activators to active Smad transcriptional complexes, and repress the expression of BMP-responsive genes (Suzuki et al., 2006). Our data suggest that SMADs bind the NANOG promoter and regulate NANOG expression, and both observations suggest a regulatory loop between NANOG and SMADs. Figure 7 is a model for SMAD-NANOG regulation that incorporates these findings. Further studies are necessary to test whether SMADs form complexes with other transcription factors to bind target genes, and confirm any functional significance of a competition between SMAD1/5/8 and SMAD2/3 in the binding of the cofactor SMAD4. How FGF signaling regulates the pluripotency genes also remains to be elucidated.
Fig. 7. Model of SMAD regulation of NANOG transcription in human ES cells.
Arrows represent induction and hammer-ended lines represent inhibition.
EXPERIMENTAL PROCEDURES
Reagents
TGFβ1 (R&D Systems, Minneapolis, MN), bFGF (Invitrogen, Carlsbad, CA), and LiCl (Sigma, St. Louis, MO) were components in T1 medium (Ludwig et al., 2006b). These components were withdrawn or added back to the medium to test the gain- or loss-of-function of TGFβ, FGF, and WNT pathways, respectively. In addition, 10 ng/ml Activin A or 10 µM SB431542 (Tocris, Ellisville, MO), 100 ng/ml BMP4 or 100 ng/ml Noggin (R&D Systems), and 100 ng/ml bFGF or 10 µM SU5402 (Pharmacia & Upjohn Co., Kalamazoo, MI) were used to activate or inhibit TGFβ, BMP, and FGF signaling, respectively.
Medium and cell culture
Human ES cell lines H1, H9, and H14 were used in this study. Human ES cells were maintained on plates coated with growth factor-depleted matrigel (BD Biosciences, San Jose, CA) either in serum free, defined medium (Ludwig et al., 2006a), or in fibroblast conditioned medium (Xu et al., 2001). Cell cultures in each of these treatments were evaluated for OCT4 expression after 14 days (the cells were split once after 7 days). Growth curves over two passages and marker expression following 14 days of culture were also obtained as previously described (Ludwig et al., 2006b). A minimum of three replicates in triplicate was performed. Statistical analysis was performed using ANOVA, and percentage data was arcsine transformed prior to analysis.
Luciferase reporter assay
A BMP-responsive firefly luciferase reporter plasmid, pID120-Luc (Lopez-Rovira et al., 2002) was used to test BMP signaling levels in human ES cells (Xu et al., 2005b). Luciferase reporter plasmids driven by various regions of the NANOG promoter (Kuroda et al., 2005) were constructed by inserting these promoter regions, the longest (−1942 to 24 in reference to the transcription start site), the proximal (−380 to 24), and the truncated (−1942 to −380) into the promoterless pGL4.10 plasmid (Promega, Madison, WI). After confirmation of the essential role of the proximal promoter region (Kuroda et al., 2005), the reporter plasmid driven by this region, named pNANOG-Luc, was used throughout this study. To test the role of SMAD binding in regulation of the NANOG promoter activity, we generated a mutated reporter vector, pNANOG(mSBEs)-Luc, by mutating the four putative SBEs in the proximal promoter of the reporter plasmid using the Site- Directed Mutagenesis Kit (Stratagene). Mutations were also made on the Octamer and Sox elements in the proximal promoter generating two mutated reporter vectors, pNANOG(mOCT)-Luc and pNANOG(mSOX)-Luc, respectively, as described (Kuroda et al., 2005). Mutations of two nonspecific areas in the promoter region were made to generate another mutated reporter vector, pNANOG(NC)-Luc, as a negative control (Fig. 3A). All the mutations were confirmed by sequencing.
The luciferase assay was performed by transfecting human ES cells on day 1 with each of the reporter vectors together with a trace amount (1/20 of the DNA amount for the test vector) of pRL-tk or pGL4.70 plasmid (Promega) to express Renilla luciferase as an internal control. FuGENE 6 (Roche Applied Science, Indianapolis, IN) was used for all the transfections. The cells were treated with various media on day 2 and harvested on day 3 for luciferase assay. Both the firefly and Renilla luciferase activities in the lysates were tested by using the Dual-Luciferase Reporter Assay System (Promega) on a 3010 Luminometer (BD Biosciences). The ratio between firefly luciferase activity and the Renilla luciferase activity was obtained for each sample. Relative luciferase unit (RLU) was calculated via normalization of each of the ration for all groups by the average ratio for the control group.
Flow cytometry
Human ES cells cultured in various media were processed for flow cytometry analysis to detect OCT4+ cells as described (Xu et al., 2002). Mouse anti-human OCT4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 2 mg/ml and AlexaFluor 488-labeled rabbit anti-mouse IgG (Invitrogen) at 1:1000 dilution were used.
Immunoassay of HCG in spent culture medium
Human ES cells cultured in conditioned medium were rinsed with DMEM/F12 basal medium and switched to T1 with or without various concentrations of bFGF and 100 ng/ml BMP4 for 7 days with daily refreshment of the media. The spent media were collected on day 7, and assayed for HCG as described (Xu et al., 2002).
Reverse transcription-QPCR
Total cellular RNA was extracted by Trizol Reagent (Invitrogen), and treated with RQ1 RNase-free Dnase (Promega) according to the manufacturer’s instructions. Total RNA was reverse transcribed to generate cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The cDNA pool was subjected to QPCR by using 2X TaqMan® Universal PCR Master Mix (Applied Biosystems) on the AB 7300 Real Time PCR System (Applied Biosystems). The following conditions were used in QPCR: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 15 sec at 95 °C and 1 min at 60 °C. TaqMan® Gene Expression Assays (Table S2) were used and the β2M assay was tested as an endogenous reference to calculate the relative expression levels of target genes according to Applied Biosystems’ instructions.
ChIP-QPCR
Human ES cells were grown to a final count of about 1 108 cells for each reaction. Cells were chemically cross-linked by the addition of one-tenth volume of fresh 11% formaldehyde solution for 15 min at room temperature. Cells were rinsed twice with 1 PBS and harvested using a silicon scraper and flash frozen in liquid nitrogen and stored at −80 °C prior to use. Upon thawing, the cells were lysed using lysis buffer, and sonicated to solubilize and shear crosslinked DNA. We used a Sonicator 3000 (Misonix, Farmingdale, NY) and sonicated at power 7 for 10 30 sec pulses (90 sec pause between pulses) at 4 °C while samples were immersed in an ice bath. The resulting whole-cell extract was incubated overnight at 4 °C with 100 Protein G magnetic beads that had been preincubated with the appropriate antibodies against SMAD2/3 (Cell Signaling Technology, Danvers, MA) or SMAD1/5/8 (Santa Cruz Biotechnology) or an isogenic immunoglobin. Beads were washed five times with RIPA buffer and one time with TE containing 50 mM sodium chloride. Bound complexes were eluted from the beads by heating at 65 °C with occasional vortexing, and crosslinking was reversed by overnight incubation at 65 °C. Whole-cell extract DNA (reserved from the sonication step) was also treated for crosslink reversal. Immunoprecipitated DNA and whole-cell extract DNA were then purified by treatment with RNaseA, proteinase K, and multiple phenol:chloroform:isoamyl alcohol extractions. Purified DNA was used as template for QPCR to amplify the proximal promoter of NANOG with the forward primer sequence 5′-CTT CAG GTT CTG TTG CTC GGT TTT C and reverse primer sequence 5′-TCC CGT CTA CCA GTC TCA CCA. The PCR product size was 101 bp. The 2X iQ SYBR Green Supermix was used for QPCR on the AB 7300 Real Time PCR System. Following PCR conditions were used: 10 min at 95 °C and 40 cycles of 30 sec at 95 °C, and 1 min at 60 °C.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from human ES cell line using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL) based on the manufacturer’s instructions, and stored at −80 °C. A DNA probe of 80 bp was synthesized by PCR from the proximal NANOG promoter in the pNANOG-Luc plasmid using a forward primer (labeled with biotin at the 5′ end) 5′-CCC ACC TAG TCT GGG TTA CTC TGC-3′, and reverse primer 5′- TCT CAG TTA ATC CCG TCT ACC AGT C -3′. An unlabeled wild-type competitor was synthesized the same way except that an unlabeled forward primer was used. A probe mutated at the four SBEs was synthesized by PCR from the plasmid pNANOG(mSBEs)-Luc. The DNA probes were gel purified and used in the EMSA and DNA pull-down assays.
The EMSA was performed by using the LightShift Chemiluminescent EMSA Kit (Pierce) based on the manufacturer’s instructions. One µg of the nuclear extract and 1 ng of the biotin-labeled probe were mixed in the EMSA Binding Buffer (Pierce). Where specified, 200 ng unlabeled competitor, 1:10 dilution of the anti-SMAD2/3 or anti-SMAD1/5/8 antibody or isogenic IgG was added and incubated on ice for 1 h prior to the addition of the labeled probe. The binding reactions were incubated for 20 min. at room temperature, and resolved on a pre-run 6% PAGE gel in 0.5 M Tris-borate-EDTA for 1.5 h at 80 V. The resolved reactions on the gel were transferred to a Zeta Probe membrane (BioRad, Hercules, CA). The membrane was air-dried, UV-crosslinked, blocked in the Blocking Solution, and then incubated with streptavidin conjugated with horseradish peroxidase followed by substrates of the peroxidase (all from Pierce). Locations of the biotin-labeled probe on the membrane were visualized and recorded by FujiImager (Japan).
DNA pull-down and western blotting
Human ES cells cultured in 6-well plates were harvested and lysed in the DP buffer composed of 50 mM Hepes (pH 7.4), 150 mM sodium chloride, 15 mM sodium fluoride, 2 mM ethylene diamine tetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 10% glycerol, 1% Triton X-100, 20 mM β-glycerophosphate, 1 mM sodium pyrophosphate, 0.5% bovine serum albumin, and a cocktail of freshly added protease inhibitors including 10 µg/ml aprtinin, 10 µg/ml leupeptin, and 1 mM orthovanadate. The protein concentration of the cell lysate was determined. 300 µl of streptavidin-agarose beads (Pierce) were pelleted, washed, and pre-adsorbed with 500 µl of 1 mg/ml BSA, 50 µg Poly(dI:dC), and 50 µg sheared salmon sperm DNA for 30 min. at room temperature for pre-blocking. The beads were washed 5 times and re-suspended in 300 µl of the DP buffer. One µg of the biotin-labeled DNA probe (wild-type or mutated) (see above) was incubated with 300 µg of the cellular proteins in the EMSA Binding Buffer (Pierce) for 20 min. at room temperature. 30 µl of the pre-blocked streptavidin-agarose beads were added to the probe-lysate reaction and incubated at 4 °C overnight for biotin-mediated binding. The bound beads were washed 5 times with the DP buffer and re-suspended in 20 µl of Alaemli Buffer (BioRad). The suspension was boiled for 5 min. 15 µl of the supernatant were loaded onto a SDS gel for western blotting to detect SMAD2/3 with the anti-SMAD2/3 antibody.
Lentiviral transduction of NANOG to human ES cells
The cDNA for the open reading frame of human NANOG (accession: NM_024865) was obtained by PCR amplification from a cDNA pool of human ES cells. A cassette containing the NANOG cDNA, the internal ribosomal entry site, and the puromycin-resistant gene was cloned into a lentiviral vector modified from those as previously described by us (Ma et al., 2003). The 293FT cell line (Invitrogen) was used to produce transgene-expressing lentivirus. For packaging, 293FT cells were cotransfected with the _NANOG_-expressing lentiviral vector and two helper plasmids pMD.G and psPAX2 with Superfect Transfection Reagent (Qiagen, Valencia, CA). Three days posttransfection, the viral particles were harvested and concentrated from the supernatant by ultracentrifugation. The titer obtained generally ranged between107 to 108 viral particles/ml.
For NANOG transduction, the human ES cells that had been partially trypsinized were plated at 2 105 cells/well in fibroblast-conditioned medium on matrigel-coated plates. Next day the lentiviral particles containing NANOG or an enhanced green fluorescent protein (EGFP, as a negative control) were added to the cell cultures at multiplicity of infection of ~20 per well in the presence of polybrene at 6 µg/ml (Sigma). Following overnight incubation, the lentivirus-containing medium was replaced with fresh conditioned medium. The transduced cells were propagated as non-transduced cells, and selection with puromycin at 0.5 µg/ml started 5 days posttransduction. The expression of the NANOG transgene or EGFP expression in human ES cells following selection and was confirmed by RT-PCR (data not shown). The cells were then split to plates with T1 medium. Next day, the cells were treated with various media to inhibit either TGFβ or FGF signaling or both. After 5-day treatment, the cells were fixed and processed for immunostaining for OCT4 expression.
Immunohistochemistry
Cells were immunostained as described (Xu et al., 2005b). Rabbit anti-human OCT4 antibody (Santa Cruz Biotechnology) at 1:400 and Alexa Fluor 594-labeled anti-rabbit IgG secondary antibodies (Molecular Probes) at 1:1000 dilution were used to detect OCT4 in the cells. The anti-human SMAD2/3 antibody at 0.2 µg/ml and Alexa Fluor 488-labeled anti-mouse IgG secondary antibodies (Molecular Probes) at 1:1000 dilution were used to detect SMAD2/3 in the cells. DAPI-coated slides were used to stain the nuclei of the cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tenneille Ludwig and Jenny Frane for providing TeSR1 media, Marian Piekarczyk, Leann Crandall, and Tiwanna Compton for laboratory support, and Deborah Faupel for editing the manuscript. We also thank Gudrun Jonsdottir and Karen Heidarsdottir from the WiCell Research Institute in Iceland for conducting the microarray experiments, and Mark Kronenberg for helpful discussion on the EMSA. This work was supported by funding from private donations, National Institutes of Health grants P51 RR000167 and P20 GM069981, the W. M. Keck Foundation to J.A.T., and the Connecticut Stem Cell Research Grants #06SCB14 and 06SCD02 to R.X. The contents in this work are solely the responsibility of the authors and do not necessarily represent the official views of the State of Connecticut. The authors declare competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2002;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- Besser D. Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3. J Biol Chem. 2004;279:45076–45084. doi: 10.1074/jbc.M404979200. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Darr H, Mayshar Y, Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- Greber B, Lehrach H, Adjaye J. FGF2 Modulates TGF{beta} Signaling in MEFs and Human ES cells to Support hESC Self-renewal. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0476. [DOI] [PubMed] [Google Scholar]
- Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Kang HB, Kim JS, Kwon HJ, Nam KH, Youn HS, Sok DE, Lee Y. Basic fibroblast growth factor activates ERK and induces c-fos in human embryonic stem cell line MizhES1. Stem Cells Dev. 2005;14:395–401. doi: 10.1089/scd.2005.14.395. [DOI] [PubMed] [Google Scholar]
- Kim J, Johnson K, Chen HJ, Carroll S, Laughon A. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature. 1997;388:304–308. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, Vandenheuvel-Kramer K, Manning D, Thomson JA. Basic FGF Support of Human Embryonic Stem Cell Self-Renewal. Stem Cells. 2005 doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006a;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006b;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. Embo J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Oostwaard DW, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang YF, Jayaraman L, Yang H, Massague J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Raya A, Kawakami Y, Morita M, Matsui T, Nakashima K, Gage FH, Rodriguez-Esteban C, Izpisua Belmonte JC. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:10294–10299. doi: 10.1073/pnas.0506945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005 doi: 10.1182/blood-2004-10-4065. [DOI] [PubMed] [Google Scholar]
- Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates FGF, Wnt and BMP pathways in human embryonic stem cells. Stem Cells. 2006 doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O'Sullivan C, Delavan-Boorsma K, Mok M, Bronstein A, Carpenter MK. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005a;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005b;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.