Leukocytes are primed in peripheral blood for activation during term and preterm labour (original) (raw)
Abstract
We hypothesized that the priming and activation of maternal leukocytes in peripheral blood is a key component of parturition, and that inappropriate preterm priming of leukocytes might initiate preterm labour and delivery. The purpose of this study was to characterize peripheral blood leukocyte activation during human term and preterm labour. We obtained blood samples from pregnant women at term and preterm, both in labour and not in labour. Leukocytes were characterized according to cell subtype and cell surface marker expression. Additionally, we quantified leukocyte cytokine mRNA production, migratory ability and reactive oxygen species production of neutrophils and macrophages. We found that both term and preterm labour were associated with an increase in monocyte and neutrophil proportion or number—neutrophil migratory ability and cell surface marker expression indicating activation. Messenger RNA expression of IL-1β and IL-8, MCP-1 and TLR-2 was also increased. We conclude that leukocytes in peripheral blood are primed in preparation for activation during term and preterm labour, and that this may contribute to the pathophysiological events of parturition. These data may lead to novel therapies and diagnostic tools for the prevention and/or diagnosis of preterm birth.
Keywords: parturition, pregnancy, leukocyte, cytokine, immunology
Introduction
Human parturition is increasingly recognized to be an inflammatory event. We and others have shown a massive influx of both neutrophils and macrophages into two of the major effector tissues of parturition: the myometrium and cervix, simultaneously with term labour (Thomson et al., 1999; Osman et al., 2003). Leukocyte migration during parturition appears to be actively promoted by factors released by the myometrium and cervix during labour (Ledingham et al., 2001) and culminates in tissue leukocytes releasing inflammatory cytokines (Athayde et al., 1999; Ledingham et al., 2001; Ietta et al., 2002; Young et al., 2002; Tornblom et al., 2003). In contrast, maternal inflammatory responses (including the activation status of leukocytes) are inhibited during pregnancy, with both neutrophils (Crouch et al., 1995; Kindzelskii et al., 2002) and monocytes (Luft and Remington, 1984) displaying reduced chemotaxis, adherence, reactive oxygen metabolite release, phagocytosis and microbial killing in pregnant compared with non-pregnant women. This may allow the fetal allograft to survive in a potentially immunologically hostile environment. The timing of the ‘switch’ whereby leukocytes of pregnant women are restored to their non-pregnant activation status is unknown.
We hypothesized that the ‘priming and activation’ (specifically increased ability for chemotaxis, increased reactive oxygen species (ROS) and cytokine production and the expression of cell surface activation markers) of maternal leukocytes in peripheral blood is a key component of parturition, and that preterm delivery would be associated with inappropriate preterm priming of leukocytes. The purpose of the study presented here was to characterize and quantify peripheral blood leukocyte (PBL) priming and activation in women in term and preterm labour, compared with gestation-matched controls in order to further understand the molecular and biochemical events accompanying labour. If our hypothesis is correct, then agents which inhibit priming and activation of leukocytes in peripheral blood may be novel therapies for the prevention of preterm birth. Additionally, measurement of leukocyte priming and activation might be a novel diagnostic tool for identifying imminent or actual preterm labour.
Materials and Methods
Subjects
We recruited pregnant women attending the Princess Royal Maternity Hospital, Glasgow. Women with clinical evidence of infection or abruption or suffering from any pregnancy complications such as pregnancy-induced hypertension, systemic disease such as diabetes, or any other medical problem that would predispose them to an alteration in their immune response, and women with multiple pregnancies were all excluded. Written informed consent was obtained from each woman prior to recruitment and the study was approved by the Local Research Ethics Committee (RN05OB013).
Heparinized peripheral blood samples were taken from pregnant women in four different clinical groups as follows: preterm pregnant women at 28 to <37 weeks of gestation who were not in labour (PTNL); preterm pregnant women at 28 to <37 weeks of gestation who were in labour (PTL); term pregnant women at 37–42 weeks of gestation who were not in labour (TNL); and term pregnant women at 37–42 weeks of gestation who were in labour (TL). No woman was sampled more than once, or included in any more than one of these groups. The diagnosis of labour was made in women who had signs and symptoms of labour (regular painful contractions). Additionally, to avoid including women with these signs and symptoms but who were not actually in labour [a common scenario preterm (Kenyon et al., 2001)], we excluded those who did not deliver within 14 days of sampling. These women were deemed not to have been in labour, their results were not included in the analysis and their details are not presented here. Non-labouring women were recruited from antenatal clinics, and labouring women were recruited from the labour ward. Gestational age was estimated in all women from an ultrasound scan which had been performed in the first trimester. All women in the preterm labouring group had been given betamethasone 12 mg as part of their routine clinical management prior to sampling.
Analysis of leukocyte subpopulations
The absolute cell numbers of leukocyte subpopulations from whole heparinized peripheral blood were determined using a Sysmex KX-21 Haematology Analyzer which categorizes leukocytes according to cell size.
Fluorescence-activated cell sorting analysis
Fluorescence-activated cell sorting (FACS) with a FACSCalibur machine (Becton Dickinson, Heidelberg, Germany) was used to identify and, subsequently, quantify cell surface marker expression according to standard protocols. Briefly, 50 µl of whole blood from each subject was incubated with the appropriate monoclonal antibodies and isotype controls and 2 ml 1× Pharm Lyse lysing buffer (BD Pharmingen) for 15 min in the dark at room temperature. Samples were then centrifuged at 200 g for 5 min and washed in 2 ml FACS buffer containing phosphate-buffered saline (PBS) (Invitrogen Life Technologies, UK), 1% fetal bovine serum (Invitrogen) and 0.1% sodium azide (Sigma-Aldrich), fixed in 2% paraformaldehyde and stored at 4°C until measured by flow cytometry within 24 h. Cells were then washed and suspended in FACS flow buffer (Becton Dickinson) before analysis by flow cytometry. At least 10 000 cells were acquired for analysis for each antibody combination.
We used fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)- or allophycocyanin (APC)-conjugated antihuman monoclonal antibodies to surface antigens: -CD3, -CD4, -CD8, -CD11a, -CD11b, -CD14, -CD15, -CD16, -CD19, -CD26, -CD28, -CD45, -CD54, -CD56, -CD62L and -CD64 (BD Pharmingen) and FITC-, PE- or APC-conjugated immunoglobulins of the relevant isotypes (BD Pharmingen) as controls. Each monoclonal antibody was used at saturating concentrations optimized by initial titrations for flow cytometric staining. The negative control panels were comprised of mixtures of isotype (IgG1, IgG2a and IgM) (BD Pharmingen). The data were saved for later analysis using the CellQuest software (Becton Dickinson).
Calibration of FACS analysis
We used recommended controls for the settings (BD Biosciences, 2002). The FITC- or PE- or APC-conjugated isotypes were used as negative controls. Anti-CD45, -CD3, -CD4 or -CD8 was used for the settings for the leukocytes and lymphocytes, respectively, whereas CD14 and CD15 were used for the settings for monocytes and neutrophils, respectively. The same batch of these isotype controls and specific antibodies were used in every experiment. The FACSCalibur instrument was calibrated weekly using BD calibration beads (with multi-peaks). The beads were used to adjust instrument settings, set fluorescence compensation and check instrument sensitivity, as it has been shown that such bead-adjusted settings reduced day-to-day variability of measurements of fluorescence intensity (Waxdal et al., 1998; BD Biosciences, 2002; Korpi-Steiner et al., 2008). The assays were optimized at the beginning of this study, and thereafter, we did not change the major settings.
Neutrophil isolation
Neutrophils were separated from whole blood using dextran and ficoll sedimentation. Briefly, heparinized whole blood was transferred to 15 ml conical tubes (BD), and 6% dextran T500 (Sigma-Aldrich) was added to a final concentration of 1% and gently mixed. The tubes were left standing to allow the red blood cells (RBCs) to settle for 1 h at room temperature. The cell-rich upper phase of the mixture was carefully aspirated. Hypotonic lysis was briefly applied to remove the remaining RBCs. The cells were carefully layered over Ficoll-Hypaque (Amersham Biosciences 1.077 density), then centrifuged at 400_g_ for 30 min at room temperature. Neutrophils settled to the bottom of the Ficoll, with mononuclear cells remaining at the ficoll interface. The supernatants were discarded and the neutrophils were suspended in 15 ml of PBS buffer (Invitrogen, UK), and then centrifuged at 300_g_ for 5 min. The cells were resuspended in PBS containing 0.05% BSA (Sigma) at a concentration of 2 × 106 cells/ml and kept on ice until the cell migration assay.
Analysis of cell migration
Neutrophil migratory capacity was studied in response to _N_-formyl-methionyl-leucyl-phenylalanine (fMLP) in a 96-well disposable chemotaxis/cell migration chamber (ChemoTx, 106-5, Neuro Probe). fMLP (Sigma-Aldrich) was used at concentrations optimized by initial titrations at a range from10−5 to 10−8M. fMLP concentration at 10−7M was selected for final experimental protocols. The stock solution of fMLP was prepared at 1 × 10−2 M in DMSO, and aliquots were stored at −70°C.
The chemotactic cell migration assay was according to the manufacturer's instructions (ChemoTx, Neuro Probe) with minor modification. Briefly, the ChemoTx microplate wells were filled with 29 µl test solutions such as positive (fMLP, 10−7M) and negative controls (absence of fMLP). The framed membrane filter was positioned over the filled ChemoTx microplate. Fifty microlitres of cell suspension was loaded on the filter top. All the test samples and negative controls were prepared in triplicates. The ChemoTx microplate and filter were covered with the lid and incubated at 37°C in humidified air with 5% CO2 for 1 h. After the incubation, the cells on the membrane filter top were gently wiped off using cotton wool swabs. The ChemoTx microplate and filter with lid was centrifuged at 400 g for 1 min in order to remove any migrated neutrophils from the membrane filter to the wells. The migrated cells in the ChemoTx microplate wells were counted using trypan blue exclusion test. The number of cells migrating towards the fMLP (10−7M) was expressed as a ratio of those randomly migrating in the absence of fMLP.
Analysis of ROS production
ROS production was measured using flow cytometry. Stock solution of 2′,7′-dichlorofluorescin diacetate (DCFH-DA) (Sigma-Aldrich) was prepared at 10−2M in absolute ethanol, and aliquots were stored at −20°C. A concentration at 5 × 10−6 M of DCFH-DA (Sigma-Aldrich) was used for the ROS production assay.
First, 100 µl of the heparinized peripheral blood was added to 2 ml of RBC lysis buffer (containing 0.83% NH4Cl, .0.1% KHCO3 and 0.009% EDTA). The tube was allowed to stand for 5 min at room temperature to enable the RBCs to lyse. The cells were then centrifuged at 200_g_ for 5 min at room temperature and washed in PBS (Invitrogen). The cells were resuspended in 1 ml of PBS containing 0.1% BSA (Sigma Aldrich) and 5 × 10−6 M of DCFH-DA and incubated at 37°C for 15 min for the basal ROS production. The samples with 100 ng/ml of PMA (phorbol-12-myristate-13-acetate, Sigma-Aldrich) for stimulation were further incubated at 37°C for 20 min. The test samples were kept on ice until flow cytometric analysis. Propidium iodide dye was used to gate for live cells and was added to each sample at a final concentration of 5 µg per ml.
ROS in the cells oxidize the membrane-permeable form of the probe 2′,7′ dichlorofluorescein (DCFH), changing the non-fluorescent intracellular DCFH into highly fluorescent dichloroflurescein (DCF). The non-polar and non-fluorescent form of DCFH is DCFH-DA. DCF data were collected in the FL1 channel with a FACSCalibur machine using the data acquisition software CellQuest (Becton Dickinson). At least 10 000 cells were examined in each sample. DCF data (from non-stimulated cells) were expressed as mean fluorescence intensity as basal ROS production, or expressed as the ratio of mean channel fluorescence intensity (MFI) data from non-stimulation/MFI data from stimulation, with gating on the appropriate cell subtype.
Immunoassay
Human plasma from heparinized peripheral blood was isolated by centrifugation and stored at −70°C. Circulating cortisol (CORT), progesterone (P4) and estradiol (E2) levels in the plasma samples were measured according to the manufacturer's instructions using an Immulite Immunoassay Analyzer [Immulite 1000 cortisol (LKCO1), Immulite 1000 progesterone (LKPG1) and Immulite 1000 estradiol (LKE21) from Diagnostic Products Corporation (DPC, CA, USA)].
Real-time quantification of mRNA
The leukocytes were isolated using dextran sedimentation (as detailed earlier), and total RNA was isolated according to the manufacturer's instructions using ABI PRISM 6100 Nucleic Acid PrepStation (Applied Biosystems, UK). RNA was reverse-transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems, UK) according to the manufacturer's instructions. cDNA was quantified using TaqMan technology on an ABI Prism 7900HT (Applied Biosystems). Briefly, 1.25 µl of 20× target assay or control assay mix was added to 12.5 µl of 2× Mastermix (Applied Biosystems), 10.25 µl deionized distilled water and 1 µl cDNA. The target assay mix and the 18S rRNA (4310893E) control probe were purchased from Applied Biosystems. Primers and fluorogenic probes for MCP-1 (CCL-2), ICAM-1, IL-1β, IL-6, IL-8, TLR-2 and TLR-4 were purchased from Applied Biosystems (Hs00234140_m1; Hs00277001_m1; Hs00174097_m1; Hs00174131_m1, Hs00174103_m1; Hs00152932_m1; and Hs00152939_m1). The thermal cycling conditions comprised an initial step at 50°C for 2 min and 95°C for 10 min, and then 40 cycles at 95°C for 15 s and 60°C for 1 min. Data analysis was performed using the Applied Biosystems Sequence Detection Software 2.2, which calculated the threshold cycle (CT) values. The samples tested were normalized to the housekeeping gene 18S rRNA. The relative gene expressions of the samples from labouring women at term or preterm were compared with the samples from non-labouring women at term or preterm, respectively. The expression of the target assays was normalized by subtracting the CT value of the 18S control from the CT value of the relevant target assay. The fold increase relative to the 18S control was obtained by using the formula 2-deltaCt.
Statistical analysis
A two-way between-group analysis of variance was conducted to explore the impact of each of gestation and of labour status on the dependent variables (cell surface marker expression, cell migration, ROS production, etc). Two-way between-group analysis of variance is appropriate where there are two independent factors specifying the nature of the measurements (Altman, 1991). In this case, the factors were ‘gestation’ (term or preterm) and ‘labour status’ (in labour or not in labour). Although there is no requirement for data to be normally distributed in order to test analysis of variance (Altman, 1991), samples should be taken from populations of equal variances. We used Levene's test to determine equality of error variances. Where Levene's test indicated that variances of the two groups were not equal, we used a more stringent level of statistical significance as recommended (Pallant, 2007).
Two-way analysis of variance was used to determine whether there was any interaction between the two independent variables (gestation and labour status). Where there was no significant interaction between gestation and labour, we report the main effects of these variables as recommended. Where there was a significant interaction between gestation and labour, we separately analysed and reported the effects of labour in the preterm and term gestation groups using either a two-sided _t_-test (for the majority of data which were normally distributed) or a Mann–Whitney U test (for data which were not normally distributed). For consistency, data are expressed as means ± SEM throughout.
Results
Maternal characteristics are shown in Table I, and (with the obvious exception of gestation) were similar in all groups.
Table I.
Characteristics of study group
| Preterm not in labour (PTNL) | Preterm in labour (PTL) | Term not in labour (TNL) | Term in labour (TL) | |
|---|---|---|---|---|
| Number | 28 | 7 | 31 | 24 |
| Age in years (mean, range) | 29 (17–37) | 26 (17–38) | 28 (17–42) | 29 (20–38) |
| Gestational age in days (mean, range) | 210 (196–238) | 224 (196–245) | 276 (260–285) | 280 (261–291) |
| Parity (mean, range) | 1 (0–4) | 1 (0–2) | 1 (0–4) | 1 (0–3) |
| History of previous abortion or miscarriage (number) | 7 | 1 | 14 | 8 |
| Smoking status (number) | 3 | 1 | 4 | 3 |
Absolute cell numbers of subpopulations of maternal circulating leukocytes
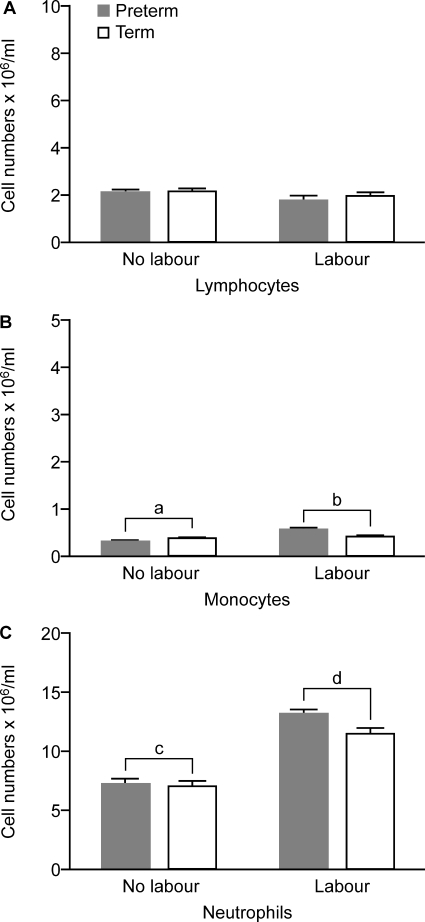
Analysis of the subsets of circulating leukocytes (Fig. 1) showed a greater absolute number of monocytes and neutrophils in labouring compared with non-labouring group women (P < 0.01, P < 0.001, respectively) (Fig. 2; quantified using the Sysmex KX-21 Haematology Analyzer). There were no differences in lymphocyte numbers between labouring and non-labouring women, nor were there any differences between term and preterm groups for any of the leukocyte subset counts.
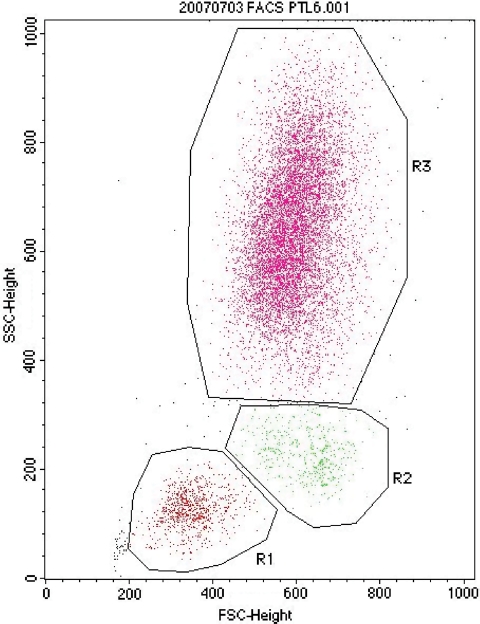
Figure 1.
Leukocyte subpopulations identified in whole blood by FACS analysis of gating on forward (FSC) and side (SSC) scatter. Groups R1, lymphocytes; R2, monocytes; R3, neutrophils.
Figure 2.
Absolute numbers of circulating leukocytes in preterm labouring (PTL, n = 7), term labouring (TL, n = 24), preterm non-labouring (PTNL, n = 28) and term non-labouring (TNL, n = 31) women. Data are given as mean ± SEM. a < b, P < 0.01; c < d, P < 0.001.
Populations of circulating leukocytes during pregnancy
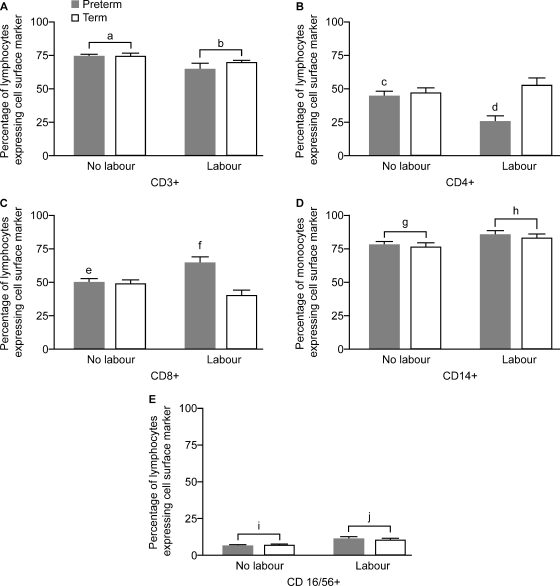
When we analysed the frequencies of the different subsets of peripheral blood lymphocytes and monocytes in gestation-matched women, we observed significantly lower proportions of CD3+ T cells (P < 0.01) and significantly greater proportions of CD14+ monocytes (P < 0.05) and CD16/CD56+ NK cells (P < 0.05) in labouring compared with not labouring women (representative plots in Fig. 3, graph of frequencies in Fig. 4A, D and E). We did not observe any significant differences in the percentage distribution of CD19+ B cells with labour (data not shown). Analysis of CD4+ T cells showed a significant interaction between labour and gestation, further exploration of the differences showing a significantly lower proportion of CD4+ T cells and a greater proportion of CD8+ T cells in preterm labouring versus non-labouring women (P < 0.01 and 0.05, respectively, _t_-test) but no differences between labouring and non-labouring women at term (Fig. 4B and C). There were no significant effects of gestation on CD3+ T cells, CD14+ monocytes or CD16/CD56+ NK cells (Fig. 4).
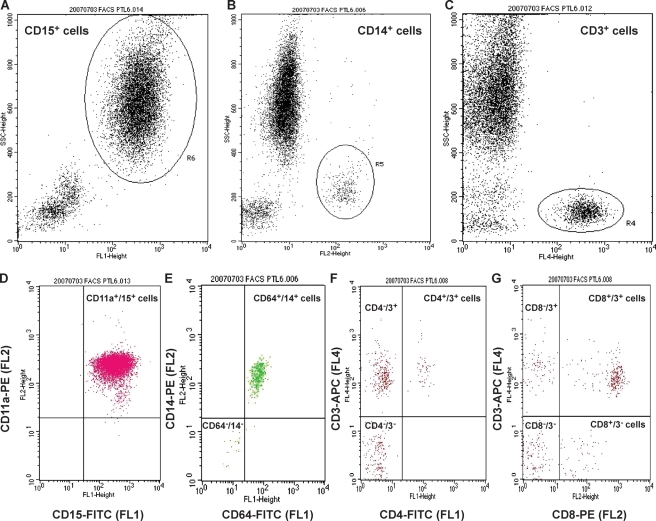
Figure 3.
FACS analysis of leukocyte populations. Gates were drawn for each leukocyte population on the basis of side-scatter (SSC) characteristics versus the positive cells for each antibody to specific leukocyte surface markers: anti-CD15-FITC (FL1) (A) was used to identify neutrophils (R6), anti-CD14-PE (FL2) was used for monocytes (R5) (B) and anti-CD3-APC (FL4) was used for T lymphocytes (R4) (C).
The dot-plot profiles represent acquisitions of different numbers of cells. (D) _X_-axis represents staining for neutrophils (R3 in Fig. 1) (anti-CD15) and _y_-axis represents staining for CD11a receptor (anti-CD11a). The upper right quadrant of the plot indicates cells positive for both fluorescent markers (CD11a+/15+ cells). (E) _X_-axis represents staining for CD64 receptor (anti-CD64) (the Fc-gamma receptor 1 (FcγRI) that binds monomeric IgG-type antibodies with high affinity), and _y_-axis represents staining for monocytes (R2 in Fig. 1) (anti-CD14). The upper right quadrant of the plot indicates CD64+/14+ cells, and the lower left quadrant of the plot indicates cells negative for both fluorescent markers (CD64−/14− cells). (F) _X_-axis represents staining for CD4 receptor (anti-CD4), and _y_-axis represents staining for lymphocytes (R1 in Fig. 1) (anti-CD3). The upper right quadrant of the plot indicates CD4+/3+ cells, the upper left quadrant of the plot indicates CD4−/3+ cells and the lower left quadrant of the plot indicates CD4−/3− cells. (G) _X_-axis represents staining for CD8 receptor (anti-CD8), and _y_-axis represents staining for lymphocytes (R1 in Fig. 1) (anti-CD3). The upper right quadrant of the plot indicates CD8+/3+ cells, the upper left quadrant of the plot indicates CD8−/3+ cells, the lower right quadrant of the plot indicates CD8+/3− cells and the lower left quadrant of the plot indicates CD8−/3− cells.
Figure 4.
Percentage of lymphocytes (A and E), T lymphocytes (B and C), or monocytes (D) expressing cell surface markers as identified by FACS analysis (data presentation and subject groups as for Fig. 2). a > b, P < 0.01; c > d, P < 0.01 (_t_-test); e < f, P < 0.05 (_t_-test); g < h, P < 0.05; i < j, P < 0.05. Cells identified by each marker are as follows: CD3+ (T cells), CD14+ (monocytes), CD16/56+ [natural killer (NK) cells], CD4+ (T helper cells), CD8+ (cytotoxic T cells) (PTL, n = 7; PTNL, n = 10; TL, n = 10 and TNL, n = 10, abbreviations as for Fig. 2).
Neutrophil migratory activity
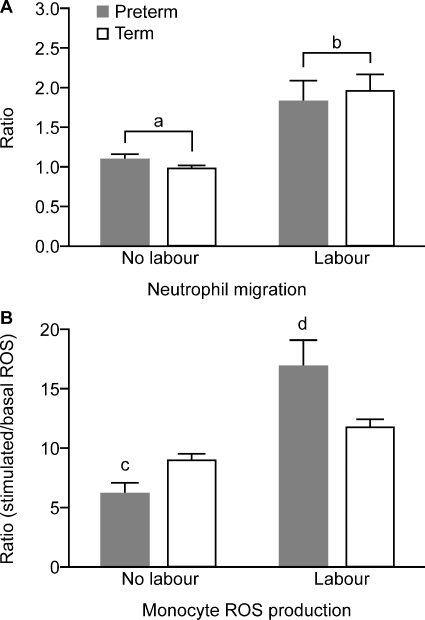
After incubation at 37°C for 1 h, neutrophil migration in response to fMLP (10−7 μM) expressed as a ratio of those randomly migrating in the absence of fMLP was significantly greater in labouring compared with non-labouring women (P < 0.001) (Fig. 5A).
Figure 5.
(A) Migratory ability of circulating neutrophils expressed as a ratio of number of cells migrating in response to fMLP and those randomly migrating in the absence of fMLP. (B) Monocyte oxidative burst in response to phorbol-12-myristate-13-acetate stimulation expressed as a ratio of basal ROS production (data presentation and numbers in subject groups as for Fig. 2). a < b, P < 0.001; c < d, P < 0.001 (_t_-test) (PTL, n = 7; PTNL, n = 10; TL, n = 10 and TNL, n = 10, abbreviations as for Fig. 2).
ROS production
The basal ROS values in selectively gated neutrophils or monocytes were not significantly different in labouring compared with non-labouring women (data not shown). There were no effects of labour or gestation on stimulated ROS (oxidative burst generated in response to 100 ng/ml PMA stimulation) in neutrophils. In contrast, in monocytes, there was an interaction between gestation and labour on oxidative burst, with PMA stimulation being significantly greater in preterm, but not in term, labour, compared with gestation-matched women (P < 0.001, _t_-test) (Fig. 5B).
Cell surface marker expression
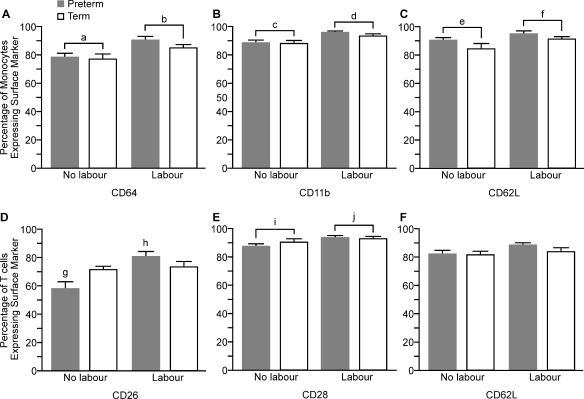
In labouring women, we observed a significant increase in the proportion of monocytes expressing each of the cell surface markers CD64 (P < 0.01), CD11b (P < 0.001) and CD62L (P < 0.05) compared with monocytes from non-labouring women (Fig. 6A, B and C). There were no differences in the percentage of monocytes expressing CD54 (data not shown).
Figure 6.
Percentage of cells expressing activation markers or adhesion molecules on the surface of circulating monocytes and T lymphocytes (data presentation and subject groups as for Fig. 2). a < b, P < 0.01; c < d, P < 0.001; e < f, P < 0.05; g < h, P = 0.001 (_t_-test); i < j, P < 0.01. CD 62L, l-selectin; www.hcdm.org (PTL, n = 7; PTNL, n = 10; TL, n = 10 and TNL, n = 10, abbreviations as for Fig. 2).
There was no effect of labour on the percentage of T cells expressing CD54 (data not shown) or CD62L (Fig. 6F). A significantly greater number of T cells from labouring women expressed CD28 (P < 0.01). There was an interaction between the effect of labour and gestation in the proportion of T cells expressing CD26: further analysis showed an increase in CD26 in preterm, but not in term, labouring women compared with gestation-matched non-labouring women (P = 0.001, PTL/PTNL, _t_-test) (Fig. 6D).
Over 90% of neutrophils expressed each of the cell surface markers CD11a, CD 11b and CD62L, and there were no significant differences in the numbers of expressing cells between labouring and non-labouring women (data not shown).
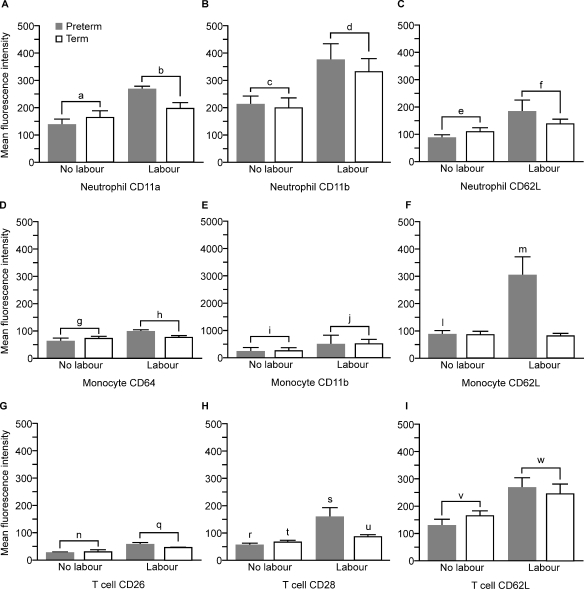
Analysis of the MFI of surface activation markers and adhesion molecules is shown in Fig. 7. Neutrophils from labouring women showed a significantly higher surface expression of CD11a and CD11b (P = 0.001) and CD62L (P < 0.02) compared with the non-labouring women. Monocytes from labouring women showed significantly higher surface expression of CD11b (P = 0.001) and CD64 (P < 0.05) when compared with monocytes from non-labouring women. There was an interaction between the effect of labour and gestation in MFI of CD62L on the surface of monocytes: monocytes from preterm, but not from term, labouring women showed significantly higher expression of CD62L compared with gestation-matched non-labouring women (P < 0.05, PTL/PTNL, _t_-test). T lymphocytes from labouring women showed significantly higher expression of CD26 (P < 0.001) and CD62L (P < 0.01) compared with non-labouring women. There was a significant interaction between the effect of labour and gestation in MFI of CD28 on the surface of T cells with preterm, but not with term, labouring women showing significantly higher expression. However, analysis within each gestation group also found higher expression of CD28 in both preterm P < 0.01 (Mann–Whitney U) and term P < 0.05 (_t_-test) labouring women versus gestation-matched controls.
Figure 7.
Mean fluorescence intensity of expression of activation markers and adhesion molecules on the surface of circulating neutrophils, monocytes and T lymphocytes (data presentation and subject groups as for Fig. 2). a < b, c < d, P < 0.001; e < f, P < 0.02; g < h P < 0.05; i < j, P = 0.001; l < m, P < 0.05, _t_-test; n < q, P < 0.001; r < s, P < 0.01 (Mann–Whitney U); t < u, p, P < 0.05 (_t_-test); v < w, P < 0.01. (PTL, n = 7; PTNL, n = 10; TL, n = 10 and TNL, n = 10, abbreviations as for Fig. 2).
Steroid levels in plasma
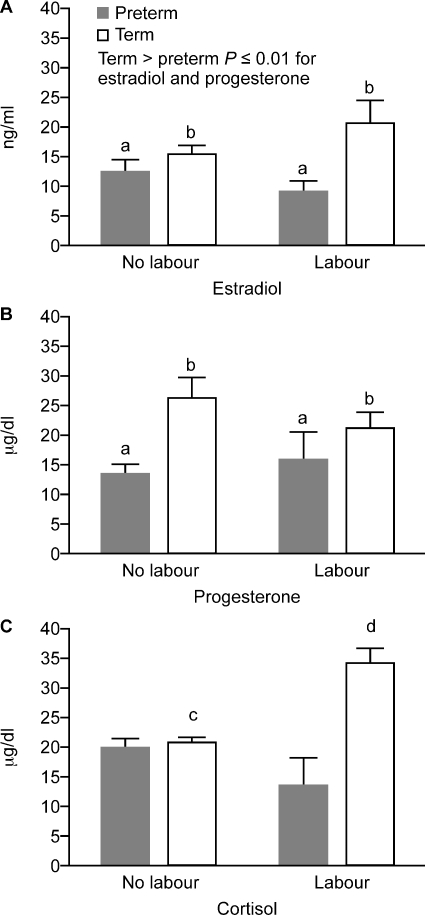
Levels of estradiol and progesterone were significantly greater in term versus preterm pregnant women (P = 0.01) (Fig. 8A and B). There was an interaction between the effect of labour and gestation in plasma cortisol levels: further analysis showed a significant increase in cortisol in labour at term (_t_-test P < 0.001) but not at preterm. There was no effect of labour on progesterone or estradiol levels.
Figure 8.
Cortisol (CORT), progesterone (P4) and estradiol (E2) levels in plasma (data presentation and subject groups as for Fig. 2). Term > preterm, b > a, P ≤ 0.01 for each of estradiol and progesterone; c < d, P < 0.01 ( PTL, n = 7; PTNL, n = 12; TL, n = 15 and TNL, n = 14).
Gene expression
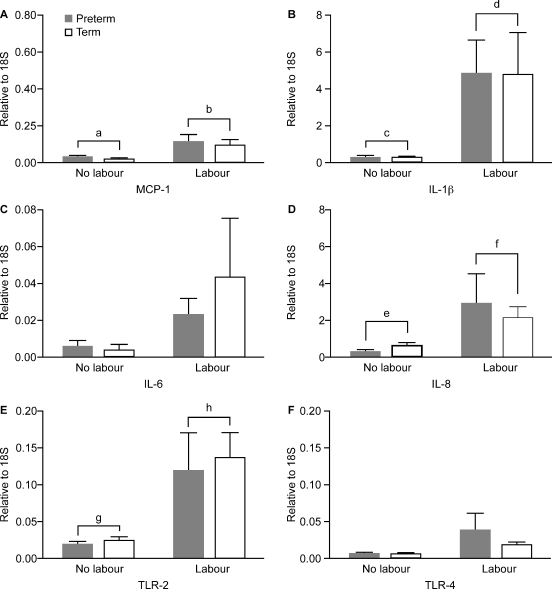
The quantification of mRNA gene expression in maternal circulating leukocytes is shown in Fig. 9. Expression levels of MCP-1 (CCL-2), IL-1β and IL-8 (CXCL8) were significantly greater in labouring women compared with non-labouring women (P < 0.01) (Fig. 9A, B and D). There was no effect of labour on IL-6 or ICAM-1 expression (data not shown). Messenger RNA expression of the innate immune receptor TLR2 was significantly greater in labouring compared with non-labouring women (P = 0.001) (Fig. 9E). There was no effect of gestation on any expression of any of these genes.
Figure 9.
Expression of proinflammatory cytokines MCP-1 (CCL2), interleukin (IL)-1β, IL-6, IL-8 (CXCL8), toll-like receptor (TLR)2 and TLR4 in circulating leukocytes (data presentation and subject groups as for Fig. 2). a < b, c < d, e < f, P < 0.01; g < h, P < 0.001 (PTL, n = 7; PTNL, n = 10; TL, n = 10 and TNL, n = 10).
Discussion
Pregnancy is associated with a reduction in neutrophil metabolic activity via retrograde transport of glucose-6-phosphate dehydrogenase (Kindzelskii et al., 2002). Although labour is known to be associated with a neutrophilia (Siegel and Gleicher, 1981), few previous studies have investigated the composition and activation of maternal PBLs during human parturition. Gervasi et al. (2001) showed that preterm labour was associated with a significant increase in the expression (as indicated by the median mean channel brightness) of CD11b, CD15 and CD66b on neutrophils and CD11b and CD15 on monocytes. Additionally, an increased ratio of oxidative burst over basal intracellular oxygen radical species production was demonstrated both in neutrophils and in monocytes in preterm labour (Gervasi et al., 2001). Women in labour at term were not studied. Gervasi hypothesized that this intravascular inflammation might arise from the insult leading to preterm labour/delivery, or that maternal intravascular in vivo inflammation may be a phenomenon associated with parturition. In contrast, Lloyd et al. (2007) showed a decrease in MHC (major histocompatability complex) II expression in monocytes in women in labour at term and preterm and a decrease in lipopolysaccharide-induced cytokine expression, suggesting that term and preterm labour are associated with immune paresis, possibly as a consequence of inflammatory events. Our own reported work supports the hypothesis of an association between maternal intravascular inflammation and parturition, in both term and preterm labour.
In this study, we showed an increase in either absolute or percentage numbers of circulating neutrophils, monocytes and NK cells during term and preterm parturition. Crucially, neutrophils and monocytes are the very cells that we have shown to invade uterine tissues during parturition at term (Thomson et al., 1999; Osman et al., 2003). It seems likely therefore that neutrophils and monocytes increase in number and are activated during labour. The site of this proliferative activity and activation is unknown: immature leukocytes may proliferate in bone marrow before circulating in blood and infiltrating the uterus, or they may proliferate within the uterine tissues, leave the uterus via draining blood or lymph vessels and re-enter the bloodstream in increased numbers. Support for this latter hypothesis comes from a study of pre-eclampsia, where leukocytes leaving the uterine vein showed greater activation status than circulating leukocytes (Mellembakken et al., 2002). Wherever the site of activation, it appears that increased peripheral blood and uterine tissue neutrophil, monocyte/macrophage and NK cell numbers are a consistent event in both term and preterm labour, implying a functional role.
In parallel with this increase in neutrophil, monocyte and NK cell numbers, we showed that the migratory response of neutrophils to chemotactic stimuli is significantly greater in labouring compared with non-labouring women. Myometrium and cervix both generate more of the chemotactic agent IL-8 during parturition (Osman et al., 2003); thus, we hypothesize that increased neutrophil homing is ensured by increased neutrophil count, increased chemotactic response of the circulating neutrophils and increased uterine chemoattractant production during parturition. We further hypothesize that leukocyte emigration from peripheral blood into the uterus is additionally encouraged by the expression of CD62L [l-selectin, which mediates tethering and rolling of leukocytes onto endothelium (one of the first steps in leukocyte emigration)] which we show here is increased in neutrophils, T cells and monocytes during term and preterm labour. Neutrophil and monocyte adhesion to myometrial and cervical endothelium will be promoted during parturition by the increased CD11a and CD11b expression which we demonstrated: CD11a and b mediate binding to ICAM-1, which we previously showed was up-regulated in endothelium of human cervix and myometrium during labour (Ledingham et al., 2001). Once leukocytes emigrate to the myometrium and cervix, chemotaxis of more neutrophils and monocytes is mediated by their own increased expression of IL-8 and MCP-1, respectively, again as shown here.
We also showed that the generation of the proinflammatory cytokine IL-1β (possibly mediated via increased cell surface CD64 density) is greater in peripheral blood in labouring women, and this mirrors what we have seen in the myometrium and cervix (Young et al., 2002; Osman et al., 2003). IL-1β likely amplifies myometrial contractions by increasing both basal and store-operated calcium entry into myometrial cells (Tribe et al., 2003) and by stimulating cyclooxgenase 2 and hence prostaglandin production (Rauk and Chiao, 2000). Further evidence of the role of IL-1β and IL-8 in parturition is underlined by data showing that subcutaneous IL-1β injections stimulate preterm labour in a mouse model (Romero and Tartakovsky, 1992) and that exogenous IL-8 production stimulates cervical ripening in a guinea pig model (Chwalisz et al., 1994). Another neutrophil product, MMP 8 (collagenase), likely contributes to the cervical remodelling which occurs in the early phases of parturition (Barclay et al., 1993).
The role of TLR4 in mediating parturition has been demonstrated by others in mice (Wang and Hirsch, 2003); here we have shown increased mRNA expression of TLR2 by leukocytes during parturition. The role of TLR2 in this situation is unknown, although recent data from our group showing that it down-regulates inflammatory chemokine responses (McInnes et al., submitted for publication) suggest that it may put a ‘brake’ on the inflammatory events of labour.
Most, but not all, leukocyte activation was common to both term and preterm labour. The major exception to this was T cell activation: the proportion of CD8 (cytotoxic T cells) was greater in preterm, but not in term, labour; and the percentage of T cells expressing CD26 (adenosine de-aminase complexing protein 2, www.hcdm.org) and the absolute T cell expression of CD28 antigen (www.hcdm.org, Tp44) (which mediates co-stimulatory signals, amplifying interleukin production) was greater in preterm, but not in term, labour. Additionally, the stimulated monocyte oxidative burst was greater in preterm compared with term labour. These data potentially imply different mechanisms of term and preterm labour and may relate to the greater involvement of micro-organisms in the pathophysiology of preterm parturition. An important caveat is that women in the preterm group had all received betamethasone to improve fetal lung maturation: this may explain the lack of rise of endogenous cortisol in association with labour in the preterm group. However, we believe that any effects of steroids are likely to be minor, given that they had no effect on MHC II expression in a previous study (Lloyd et al., 2007).
In summary, we have shown that leukocytes are primed for initiating the processes of migration and extravasation in peripheral blood during term and preterm labour. Agents which inhibit priming of leukocytes in peripheral blood may provide novel therapies for the prevention of preterm birth. Additionally, measurement of leukocyte priming might be a novel diagnostic tool for identifying imminent or actual labour.
Funding
This work was supported by a grant from Action Medical Research (Ref No. SP4068) and the Wellcome ‘Value in People’ award.
Acknowledgements
We thank William McNally (Glasgow) for help on measuring circulating cortisol, progesterone and estradiol levels and Ronnie Grant (Edinburgh) for help in preparing the figures. We thank the staff of the Princess Royal Maternity and Glasgow Royal Infirmary for their help in obtaining blood samples. This work was carried out largely in the Section of Reproductive and Maternal Medicine, University of Glasgow.
References
- Altman D. Relation between Several Variables. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. pp. 325–364. [Google Scholar]
- Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon BH. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- Barclay CG, Brennand JE, Kelly RW, Calder AA. Interleukin-8 production by the human cervix. Am J Obstet Gynecol. 1993;169:625–632. doi: 10.1016/0002-9378(93)90634-u. [DOI] [PubMed] [Google Scholar]
- BD Biosciences. Introduction to Flow Cytometry: A Learning Guide. 2002 [Google Scholar]
- Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum Reprod. 1994;9:2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- Crouch SP, Crocker IP, Fletcher J. The effect of pregnancy on polymorphonuclear leukocyte function. J Immunol. 1995;155:5436–5443. [PubMed] [Google Scholar]
- Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–1129. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- Ietta F, Todros T, Ticconi C, Piccoli E, Zicari A, Piccione E, Paulesu L. Macrophage migration inhibitory factor in human pregnancy and labor. Am J Reprod Immunol. 2002;48:404–409. doi: 10.1034/j.1600-0897.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:989–994. doi: 10.1016/s0140-6736(00)04234-3. [DOI] [PubMed] [Google Scholar]
- Kindzelskii AL, Huang JB, Chaiworapongsa T, Fahmy RM, Kim YM, Romero R, Petty HR. Pregnancy alters glucose-6-phosphate dehydrogenase trafficking, cell metabolism, and oxidant release of maternal neutrophils. J Clin Invest. 2002;110:1801–1811. doi: 10.1172/JCI200215973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi-Steiner NL, Sheerar D, Puffer EB, Urben C, Boyd J, Guadarrama A, Schell K, Denlinger LC. Standardized method to minimize variability in a functional P2X(7) flow cytometric assay for a multi-center clinical trial. Cytometry B Clin Cytom. 2008;74:319–329. doi: 10.1002/cyto.b.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledingham M, Thomson A, Jordan F, Young A, Crawford M, Norman J. Cell adhesion molecule expression in cervix and myometrium during pregnancy and parturition. Obstet Gynecol. 2001;97:235–242. doi: 10.1016/s0029-7844(00)01126-1. [DOI] [PubMed] [Google Scholar]
- Lloyd J, Allen M, Azizia M, Klein N, Peebles D. Monocyte major histocompatibility complex class II expression in term and preterm labor. Obstet Gynecol. 2007;110:1335–1342. doi: 10.1097/01.AOG.0000289226.08442.e1. [DOI] [PubMed] [Google Scholar]
- Luft BJ, Remington JS. The adverse effect of pregnancy on macrophage activation. Cell Immunol. 1984;85:94–99. doi: 10.1016/0008-8749(84)90281-8. [DOI] [PubMed] [Google Scholar]
- Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–160. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham M, Thomson A, Jordan F, Greer I, Norman J. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- Pallant J. Two-Way Between Groups ANOVA. SPSS. Berkshire: McGraw Hill Open University Press; 2007. [Google Scholar]
- Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43:152–159. doi: 10.1111/j.8755-8920.2000.430304.x. [DOI] [PubMed] [Google Scholar]
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- Siegel I, Gleicher N. Peripheral white blood cell alterations in early labor. Diagn Gynecol Obstet. 1981;3:123–126. [PubMed] [Google Scholar]
- Thomson A, Telfer J, Young A, Campbell S, Stewart C, Cameron I, Greer I, Norman J. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- Tornblom S, Bystrom B, Sennstrom M, Chromek M, Maul H, Brauner A, Ekman-Ordeberg G. Non infected preterm parturition is related to increased cytokine levels in human cervix. J Soc Gynecol Investig. 2003;10:202A. [Google Scholar]
- Tribe RM, Moriarty P, Dalrymple A, Hassoni AA, Poston L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod. 2003;68:1842–1849. doi: 10.1095/biolreprod.102.011403. [DOI] [PubMed] [Google Scholar]
- Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- Waxdal MJ, Monical MC, Palini AG. Inter-laboratory relative fluorescence intensity measurements using FlowCal 575 calibration beads: a baseline study. Cytometry. 1998;33:213–218. [PubMed] [Google Scholar]
- Young A, Thomson A, Ledingham M, Jordan F, Greer I, Norman J. Immunolocalization of pro-inflammatory cytokines in myometrium, cervix and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]