Highly specialized microbial diversity in hyper-arid polar desert (original) (raw)
Abstract
The McMurdo Dry Valleys in Antarctica are a cold hyperarid polar desert that present extreme challenges to life. Here, we report a culture-independent survey of multidomain microbial biodiversity in McKelvey Valley, a pristine example of the coldest desert on Earth. We demonstrate that life has adapted to form highly-specialized communities in distinct lithic niches occurring concomitantly within this terrain. Endoliths and chasmoliths in sandstone displayed greatest diversity, whereas soil was relatively depauperate and lacked a significant photoautotrophic component, apart from isolated islands of hypolithic cyanobacterial colonization on quartz rocks in soil contact. Communities supported previously unreported polar bacteria and fungi, but archaea were absent from all niches. Lithic community structure did not vary significantly on a landscape scale and stochastic moisture input due to snowmelt resulted in increases in colonization frequency without significantly affecting diversity. The findings show that biodiversity near the cold-arid limit for life is more complex than previously appreciated, but communities lack variability probably due to the high selective pressures of this extreme environment.
Keywords: Antarctica, biodiversity, endolith, extremophile, hypolith
The McMurdo Dry Valleys located in southern Victoria Land, Antarctica are a unique biome comprising the largest ice-free region on the Antarctic continent. They are designated by international treaty as an Antarctic Special Managed Area (1) to reflect their environmental significance. Classified as a hyperarid desert, the Dry Valleys are among the most threatened environments from climate change due to their polar location (2), and environmental stress is more pronounced in the higher inland valleys (3). They are characterized by extreme cold and dry conditions, resulting in sublimation of snowfall with minimal localized liquid-water input to soils (4–6). The Dry Valleys floor comprises dry permafrost (7) that commonly supports a polygonal surface terrain characterized by a desert pavement of sandstone, quartz, and granitic rocks embedded in mineral soils. Higher plants and animals are absent (8), although microbial colonization has been recorded for some Dry Valleys locations. This desert can be viewed as nearing the cold-arid limit for life, because evidence for microbial activity in inland snow is questionable (see ref. 20).
Endolithic colonization of pore spaces within rocks has been recorded in isolated high Dry Valleys sites (9, 10). Colonization as chasmoliths in cracks and fissures was reported as relatively infrequent (9, 10). In lower valleys experiencing less environmental stress, hypolithic communities have been recorded beneath quartz rocks in soil contact (11, 12) in addition to soil microbial communities (13–17). To date, a comprehensive multidomain diversity assessment comparing these refuge communities has been lacking, and so, community structure remains unresolved, and uncertainty exists as to whether distinct taxa colonize niches near the cold arid limit for life or if they represent an extension of autochthonous soil or aquatic communities from less extreme locations.
An understanding of biodiversity in such communities advances our knowledge of microbial ecology under extreme stress and also informs potential effects of environmental change to endangered polar landscapes. Here, we present findings from a polyphasic molecular study targeting all domains of life to fully characterize microbial diversity in four distinct microbial niches occurring concomitantly in the dry permafrost of the high inland McKelvey Valley. We identify highly-specialized communities that supported previously unreported polar bacteria and fungi, and demonstrate that landscape-scales and stochastic moisture input had little impact on community structure.
Results
Chasmoliths and endoliths occurred exclusively in above-ground sandstone with a mean frequency of 1 and 3%, respectively (n = 100). Hypoliths occurred exclusively on quartz in soil-contact with a mean frequency of 4.9% (n = 1,260) in typical polygons. A single snowmelt-influenced polygon supported a near 5-fold higher frequency of colonization (22%), and there was no significant difference in available quartz substrate between “dry” and snowmelt-influenced polygons. Microscopy of colonized rock surfaces revealed _Chroococcidiopsis_-like cyanobacterial morphotypes dominated sandstone substrates, whereas oscillatorian cyanobacterial morphotypes dominated quartz. Copious extracellular polymeric substance was present around colonized areas.
We used real-time quantitative (q)PCR to estimate the absolute and relative abundance of recoverable phylotypes for archaea, bacteria, and eukarya as a proxy for relative biomass (Table 1). Whereas each lithic niche supported both eukaryal and bacterial phylotypes, soil supported bacterial phylotypes only. Presence of a soil-based inhibitor to eukaryal PCR amplification was discounted after successful recovery of amplicons from artificially “spiked” soil samples. Eukaryal phylotypes in lithic niches accounted for a relatively low abundance (<5%) of total recoverable phylotypes. The hypolithon supported greatest overall abundance of recoverable phylotypes, with values for surrounding soil several orders of magnitude lower.
Table 1.
Diversity statistics and recovery of microbial phyla from soils, sandstone chasmoliths, sandstone endoliths, and quartz hypoliths in McKelvey Valley, McMurdo Dry Valleys, Antarctica
| Soil | Hypolith | Chasmolith | Endolith | |
|---|---|---|---|---|
| Diversity indices | ||||
| Alpha diversity | ||||
| Shannon's Index | 3.3 | 2.3 | 2.8 | 2.8 |
| Simpson Diversity Index | 1 | 0.8 | 0.9 | 0.9 |
| Pielou's Evenness | 0.9 | 0.7 | 0.7 | 0.7 |
| Omega diversity | ||||
| FST bacteria | 0.299 | 0.305 | 0.301 | 0.301 |
| FST eukarya | — | 0.105 | 0.083 | 0.083 |
| AvTD | 77 | 68 | 84 | 83 |
| VarTD | 255 | 1293 | 425 | 393 |
| qPCR (SSU rRNA copy no.) | ||||
| Archaea | 0 | 0 | 0 | 0 |
| Bacteria | 0.06 × 108 | 55 × 108 | 0.8 × 108 | 2.4 × 108 |
| Eukarya | 0 | 6.4 × 106 | 1.5 × 106 | 0.3 × 106 |
| Clone libraries | ||||
| Bacteria | ||||
| No. clones | 180 | 169 | 144 | 172 |
| No. RFLP-defined phylotypes | 72 | 24 | 41 | 43 |
| No. O.T.U. (97% cutoff) | 48 | 11 | 25 | 27 |
| Chao 1 richness | 44.2 | 8.8 | 23.4 | 25.4 |
| Coverage, % | 87 | 95 | 91 | 95 |
| Average similarity to known phylotypes using BLAST | 94 | 97 | 95 | 96 |
| Eukarya | ||||
| No. clones | — | 58 | 55 | 55 |
| No. RFLP-defined phylotypes | — | 10 | 10 | 8 |
| No. O.T.U. (97% cutoff) | — | 1 | 4 | 5 |
| Chao 1 richness | — | 1.8 | 3.1 | 4.1 |
| Coverage, % | — | 100 | 98 | 98 |
| Average similarity to known phylotypes using BLAST | — | 99 | 99 | 98 |
| Phylum abundance, % | ||||
| Cyanobacteria | 0 | 95 | 64 | 56 |
| [Chroococcidiopsis sp.] | — | [0] | [57] | [51] |
| [Nostocales] | — | [0] | [1] | [5] |
| [Oscillatoriales] | — | [95] | [0] | [0] |
| [Unidentified cyanobacterium] | — | [0] | [6] | [0] |
| Acidobacteria | 31 | 2 | 2 | 5 |
| Actinobacteria | 33 | 2 | 5 | 4 |
| Bacteriodetes | 2 | 0 | 7 | 16 |
| Chloroflexi | 3 | 0 | 0 | 0 |
| _Deinococcus_-Thermus | 6 | 0 | 0 | 2 |
| Gemmatimonadetes | 8 | 0 | 0 | 0 |
| Planctomycetes | <1 | 0 | 0 | 2 |
| Alpha proteobacteria | 0 | 1 | 4 | 4 |
| Beta proteobacteria | 4 | 0 | 0 | 0 |
| Gamma proteobacteria | 0 | 0 | 8 | 4 |
| Unidentified bacteria | 13 | 0 | 8 | 2 |
| Ascomycota | 0 | 0 | <1 | <1 |
| Basidiomycota | 0 | 0 | <1 | <1 |
| Chlorophyta | 0 | <1 | 2 | 4 |
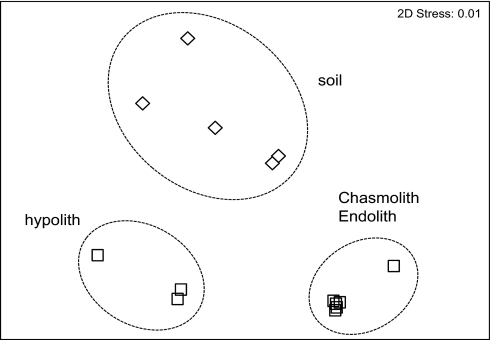
Variation in multidomain community structure among all colonized rocks and soil samples was assessed using terminal restriction fragment length polymorphism (t-RFLP) (Fig. 1). The relative contribution of each domain-specific t-RFLP profile to overall community diversity/abundance in any given sample was calculated based on relative abundance obtained from qPCR data. Differences between soil and rock substrates were significant (ANOSIM, Global R = 0.719, P < 0.001). Multiple rank correlations (BEST analysis) of abiotic (Table S1 and S2) and community diversity/abundance data (t-RFLP) revealed that the combination of factors most important in influencing community diversity in soils were soluble salts, K and C (ρ_w_ 0.274). Combinations including sodium and moisture content resulted in significant but weaker correlations. For rock substrates, the soluble salts, organic carbon, and moisture content were below detectable limits, and the most important variables determining community structure among quartz and sandstone were Cl (ρ_w_ 0.794) and Cl, K, and porosity (ρ_w_ 0.688).
Fig. 1.
Nonmetric multidimensional scaling plot of Bray Curtis similarities for bacterial and eukaryal rRNA gene phylotypes recovered from soils, sandstone chasmoliths and endoliths, and quartz hypoliths in McKelvey Valley. Dashed line represents statistically significant groupings (ANOSIM, Global R = 0.719, P < 0.1, n = 14).
We were able to assign putative identification to a relatively high percentage (92%) of t-RFLP peaks. These data revealed some general trends among substrates. Chamsoliths and endoliths were dominated by Chroococcidiopsis phylotypes, whereas _Leptolyngbya_-like phylotypes dominated hypoliths. Cyanobacterial signals were absent from all but three soil samples, and comprised a very low fraction (<10%) of overall t-RFLP signal. Soil t-RFLP profiles indicated communities dominated by Acidobacteria, Alpha-proteobacteria and Actinobacteria. Chamsoliths and endoliths supported fungal and algal phylotypes, whereas hypoliths supported only algal eukaryotes. No eukaryal signals were recorded for any soils.
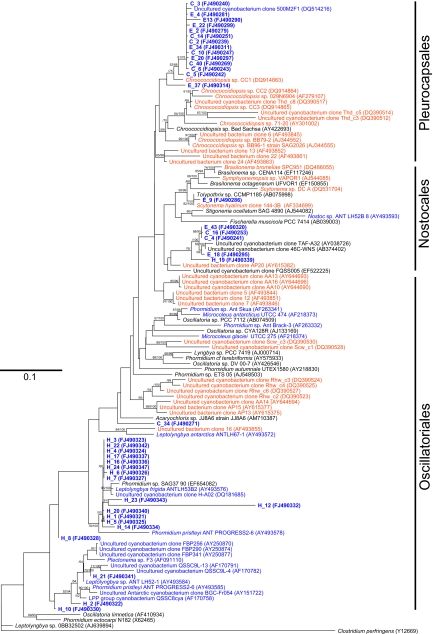
To further characterize community structure, we constructed clone libraries based on near full-length 16S and 18S rRNA gene sequences to determine phylogenetic identity for all recoverable phylotypes. Estimates of sampling effort revealed high coverage for bacterial and eukaryal libraries (Table 1). All operational taxonomic units (O.T.U.) were assigned a phylogenetic identity based on near full-length rRNA sequence (Fig. 2; Fig. S1–S3).
Fig. 2.
Phylogenetic relationships among cyanobacterial 16S rRNA phylotypes recovered from sandstone chasmoliths and endoliths and quartz hypoliths in McKelvey Valley. Phylotypes recovered during this study are shown in bold type. Sequence code prefix denotes location. H, hypolith; C, chasmolith, E, endolith. Blue font denotes Antarctic and glacial phylotypes, orange font denotes phylotypes from nonpolar deserts, black font denotes nondesert phylotypes. Tree topologies are supported by Bayesian posterior probabilities (first number) and bootstrap values for 1,000 replications (second number). (Scale bar, 0.1-nt changes per position.)
Archaea-specific primers failed to generate archaeal sequences, despite attempts with varying PCR stringency and alternative sets of archaea-specific PCR primers. Therefore, it was concluded that there were no recoverable archaeal phylotypes in our Dry Valleys samples.
Interpolation of clone library sequence data with qPCR data allowed an estimate of relative abundance for all phylotypes across all domains in the community (Table 1). The lithic substrates were all dominated by cyanobacteria; for chasmoliths and endoliths, these cyanobacteria comprised diazotrophic Chroococcidiopsis and Nostocales phylotypes, whereas for hypoliths, they were exclusively Oscillatoriales (Fig. 2). An additional source of bacterial phototrophy was indicated by Chloroflexi in soil and endolithon. Other bacterial phylotypes were phylogenetically diverse, and overall, bacterial diversity spanned 14 phyla (Fig. S1), although in lithic niches, they comprised relatively few of the total recoverable phylotypes. The only ubiquitous phyla in the Dry Valleys terrain were the acidobacteria and actinobacteria, although cyanobacteria were ubiquitous among lithic niches.
The eukaryal phylotypes resolved phylogenetically within the Chlorophyta (Algae), Ascomycota (Fungi), and Basidiomycota (Fungi). Whereas chlorophytes were indicated for all lithic niches, the fungi were present only in endolithic and chamsolithic communities. A relatively restricted phylogenetic diversity was indicated for the chlorophytes with two closely related groups within the Trebouxiophyceae and a single Bracteococcus phylotype (Fig. S2). Among the fungi, four genera were indicated by phylotypes affiliated within the Dothideomycetes (Ascomycota), Sordariomycetes (Ascomycota), and Cystobasidiomycetes (Basidiomycota) (Fig. S3). We recovered a single endolithic fungal phylotype (FJ490293) that displayed low phylogenetic affinity with any known taxon. This taxon accounted for ≈10% of recoverable eukaryal phylotypes, and so may represent a previously uncharacterized lichen mycobiont or free-living fungus. Similarly unidentified bacteria with little phylogenetic affiliation to any known phylum accounted for 2–13% of phylotypes and were most common in soil.
Our statistical analyses included approaches designed to assess both alpha diversity (number of taxa) and omega diversity (phylogenetic diversity) (Table 1). On the basis of Shannon and Simpson, estimates soils appeared most biodiverse; however, this estimate is misleading, because soils supported a relatively large number of closely related acidobacterial and actinobacterial phylotypes. Estimates of evenness indicated that soil communities were markedly more even in terms of taxon contribution to overall abundance than any lithic community. We exploited the sequence-based nature of our data to estimate phylogenetic diversity in the different communities. The FST statistic was used to show that bacterial assemblages in soil, hypoliths, chasmoliths, and endoliths were significantly different from each other (FST = 0.302, P < 0.00001; AMOVA). Eukaryal assemblages in sandstone and quartz were also significantly different (FST = 0.088, P < 0.00001; AMOVA), although chamsolith and endolith eukaryal assemblages were not significantly different. Overall, community was significantly different at the phylogenetic level between each niche. Taxonomic distinctness (TD) estimates were used to illustrate the phylogenetic basis of community composition. Average (Av) TD reflects the frequency of phylogenetically defined species, and here, was used to show that soil and hypoliths were significantly less diverse than chamsoliths and endoliths. Variation (Var) in TD reflects the level of phylogenetic species differentiation within a community, and clearly showed that soils were depauperate compared with lithic niches.
To address the question of how variable lithic communities are on a landscape scale in the Dry Valleys, we selected hypolithic colonization as an indicator, because this colonization occurred most frequently at all locations and there was no significant difference in colonization frequency for typical polygons within or between locations (P > 0.05). Because bacterial phylotypes accounted for >98% of recoverable phylotypes, we assessed variation in bacterial community t-RFLP profiles for the hypervariable 16S-23S ITS region (n = 61). No significant difference in community structure within or between locations could be delineated based on this highly variable marker (Fig. S4). The inclusion of samples in a polygon atypically experiencing moisture sufficiency (and significantly greater colonization frequency) due to localized snowmelt revealed that, although 14% of rocks displayed apparent differences in diversity/abundance profiles, this pattern was not significantly different from overall community structure on a landscape scale (Fig. S4).
Discussion
Our study represents a complete assessment of terrestrial microbial biodiversity across surface niches at the cold-arid limit for life, and provides insights into ecosystem complexity under extreme stress. These highly-specialized communities face unpredictable effects from climate change. They may be out-competed by invasive species should warming occur, or encounter catastrophic ecostystem shift (18) should aridity increase with further cooling. Given that current global climate-warming trends are most pronounced in polar regions (19) but that increased localized cooling in the Dry Valleys region may also be occurring (5), it is timely to document this endangered biome.
We identified four distinct microbial communities as chasmoliths, endoliths, hypoliths, and in bulk soil that occurred concomitantly in polygonal terrain of McKelvey Valley. Lithic communities were dominated by different cyanobacteria, and overall, diversity spanned 16 phyla. This level of diversity suggests a greater ecosystem complexity in the high inland Dry Valleys than previously appreciated. Our data counters the view based on soil studies that cyanobacteria are restricted to wetter, more productive polar locations (16, 20); rather, we demonstrate that, under severe xeric stress, soil becomes too extreme, and the last refuge for life is in lithic niches where distinct communities develop.
The soil biota of McKelvey Valley was dominated by known radiation and desiccation tolerant taxa such as Deinococcus and Rubrobacter, and such adaptation has also been observed for moisture-impacted Dry Valley soils (13, 16). Nonpolar hyperarid soils in the Atacama Desert were instead dominated by _Frankia_-like actinobacteria at depths of 20–200 mm (21). The relatively low number of microorganisms and taxonomic evenness of the community, plus absence of primary producers, indicate that soil “communities” in McKelvey Valley may possibly represent a transient soil-bourne inoculum rather than a stable community. Conversely, the specific occurrence of Chloroflexi in soils, a phylum known to be important in tundra soils (22), suggests a true soil community may indeed exist. We did not investigate whether the deeper subsurface permafrost supported microorganisms.
The absence of Chroococcidiopsis in molecular and morphological examinations from hypolithon and most soils is surprising given that this cyanobacterium was the dominant taxon in sandstone substrates in our study and has been recorded as the main component of hypolithic communities in nonpolar deserts (23–25). A study focusing on cyanobacteria in soils of Beacon Valley also failed to generate amplicons using cyanobacteria-specific PCR primers, whereas they were commonly retrieved for soils of lower valleys supporting lakes with a presumed aquatic origin for phylotypes (11). The absence is unlikely to be a result of UV or desiccation stress, because it is one of the most radiation- and desiccation-tolerant organisms known (26). Rather, we suggest, in light of the fact that soils were also the only substrate that did not support algae, that this inland soil presents an environment that somehow precludes significant photoautotrophic colonization. This finding has major implications for productivity in such inland valleys, because it makes the isolated “islands” of lithic colonization the only significant source of primary production. The reasons for this absence are unclear. We did not identify levels of any soil abiotic variable likely to inhibit photosynthesis per se. We suggest that, in addition to increased exposure to incident light, highly xeric conditions, and lack of thermal buffering in soils, the relative instability of the soil substrate may also hinder colonization given the slow colonization rates for Antarctic microorganisms (27). Therefore, an upward revision of standing biomass and productivity in the Dry Valleys is warranted, because previous estimates have been based largely on soil and aquatic biota (2).
It has been previously shown that Antarctic lakes are a significant source of inoculum in lower lake-bearing valleys, with soils, hypoliths, and aquatic niches supporting the same cyanobacteria (11). There may also be an aquatic origin for oscillatorian hypoliths in our study, because they are common aquatic Antarctic cyanobacteria, although there are no lakes in the immediate vicinity of the study site (the closest, Lake Vashka, is >10 km distant). This finding suggests that lake dispersal reaches deep inland to McKelvey Valley, so why are they not also colonists of soil and sandstone niches? This absence may be explained by viewing these cyanobacteria as opportunists. They were absent from soil and sandstone, yet occurred on quartz in soil where entrapment of wind-dispersed lake-derived organic matter containing cyanobacterial and algal incoculum can be envisaged, and the microenvironment is known to be more favorable than surrounding soil (24, 28). Therefore, relatively rapid colonization under favorable conditions could occur, whereas for sandstone ingress on an exposed substrate and the microclimate may be too challenging (29).
The lichens recorded in our study displayed little similarity in terms of community diversity with sandstone endoliths from Beacon sandstone recovered 24 years ago (30). Both mycobiont and phycobiont phylotypes in our study affiliated with different genera as did cyanobacteria. This identification may indicate the existence of multiple lichen associations in the Dry Valleys; or more speculatively, our study may indicate Antarctic lichens reflect climatic warming trends and are becoming less unique, because the morphology and cyanobacterial composition of our lichens more closely resembled those recorded for alpine regions of Europe (31).
Nitrogen fixation has been demonstrated by _Chroococcidiopsis_-dominated communities from alpine endoliths of gypsum (32), whereas others conclude that polar and other endoliths largely used abiotic combined nitrogen sources (27). Data also indicated phylotypes indicating diazotrophic cyanobacterial, alpha proteobacterial and actinobacterial taxa. This can be viewed as a useful adaptation in this nitrogen-poor Dry Valleys location. All niches in McKelvey Valley supported a putative heterotrophic bacterial component presumably supported by microbial carbon and nitrogen input.
The lack of significant community variation among samples for a given substrate and between locations reflects the high selective pressure in these high inland sites where environmental stresses are exacerbated compared with lower valleys (6). It also illustrates that our study may be broadly applicable to the inland Dry Valleys in general due to reduced heterogeneity. Across all substrates, salinity-related factors were influential to community structure. It has been suggested that salinity may act as a stressor in certain dry valleys (33), although in our study, soils did not display inhibitory levels of soluble salts. Our multivariate analysis points to a complex interaction of salinity, carbon, moisture, and other variables in this ecosystem.
We used multiple approaches to attempt recovery of archaeal signatures from soils, quartz and sandstone, but all proved unsuccessful. It has been suggested that archaea are unable to tolerate the environmental stress in extreme xeric environments (34), and this inability may explain their absence. Other Dry Valleys studies have not generally focused on archaea, although they were also concluded to be absent from an endolith recovered from the Asgard Range (30). Given the high degree of environmental heterogeneity observed in Antarctic soils (2), their occurrence may yet be recorded elsewhere in the Dry Valleys biome where less moisture stress is experienced.
It has been estimated that polar endoliths are exceptionally persistent over geological time periods (35). Endoliths supported the greatest diversity of phylotypes, shared the greatest number of phylotypes with other niches, and they are very long-lived. Therefore, we propose that they act as a reservoir for terrestrial microbiota. This notion is supported by recent isotopic evidence indicating that some organic matter in Dry Valleys soils remote from sources of liquid water has an endolithic origin (36). Weathering of sandstone is accelerated by endolithic colonization (10), and therefore, can be envisaged to disperse endolithic taxa, although in a relatively slow manner. The relatively rare occurrence of endoliths may restrict the volume of inoculum released, but this dispersal may nonetheless represent an important source over time in high inland valleys where hydroterrestrial taxa may also be dispersed but are unable to proliferate. The occurrence of algae from the usually lichenized endolithic Trebouxiophyceae in McKelvey Valley hypolithon without a mycobiont may further indicate local dispersal of endolithic taxa.
Recovery of cyanobacterial phylotypes, including first records for these substrates, allowed comparison with several other deserts worldwide due to ubiquity of this phylum in desert lithic niches. A clear separation among phylogenetic lineages from Antarctic and nonpolar desert locations within the Pleurocapsales, Nostocales, and Oscillatoriales was evident. This pattern may indicate isolated and regionally seeded cyanobacterial populations occur. This idea supports the view that global occurrence among terrestrial soil bacteria is determined primarily by localized factors (37, 38), although the Antarctic is exposed to globally dispersed aerosols that likely disseminate microbial propagules in a near-ubiquitous manner (39). We suggest that extreme xeric low nutrient environments such as deserts minimize the possible interference of abiotic variables in biogeographic studies, and thus, lithic communities offer a useful model for further testing hypotheses related to biogeography in microbial ecology.
We recovered a limited number of bacterial and fungal phylotypes with low phylogenetic affiliation to any known taxon from other deserts or substrates worldwide. This observation indicates novel diversity at a high taxonomic level in inland Dry Valleys despite the environmental stresses exceeding maritime and lake-influenced Antarctic locations where similar claims for fungi (17) and cyanobacteria (11) have been made. Some records also point to a physiological plasticity among Antarctic taxa, for example, we identified basidiomycetous yeasts in endolithic niches yet these taxa also occurred as colonists of archaeological wood in the Antarctic (17).
We have characterized highly-specialized communities inhabiting multiple lithic and soil niches in the inland region of the most extreme cold desert on Earth. This diversity is in contrast to apparently more homogenous communities in the lower, wetter Dry Valleys. The occurrence of location-specific cyanobacterial lineages may be a result of lithic niches acting as local reservoirs for dispersal of microbial biomass. The specialized communities and possible endemism for certain phyla, together with climate-change related threats, emphasize the conservation value of the inland Dry Valleys ecosystem. Ironically, the frequency of hypolithic colonization may have potential as a bioindicator of climate change given that a comparison of colonization frequency in this study with maritime polar (40) and nonpolar deserts (24, 41) suggests that landscape-scale patterns may be closely related to climatic variables. Additional relevance lies with environmental similarities between polar regions on Earth and Mars during recent history and the implications for habitability of Mars (42).
Materials and Methods
Field Sampling.
The high inland McKelvey Valley (central valley coordinates 77°26′ S, 161°33′ E) was surveyed during Antarctica New Zealand event K021B in January 2008. Frost polygons of 40 m2 average area were used as in situ quadrats. All sandstone and quartz substrate was surveyed, and frequency of colonization for chasmoliths, endoliths, and hypoliths was recorded. Epilithic lichens were not observed. Soil samples were taken after removing the topmost 2.5 cm of loose soil to minimize transient particles in sampling and be consistent with the average depth of hypolithic colonization. A total of 10 randomly-selected and nonadjacent polygons were surveyed (five on a south-facing slope used in rRNA and ITS studies, plus five additional polygons from a north-facing slope for ITS studies). A single polygon from the southern slope that supported multiple chasmolithic, edolithic, and hypolithic colonization was selected for diversity comparison between niches. Soil and rock samples were sampled aseptically and stored in sterilized plastic containers with no headspace at −80 °C until processed.
Abiotic Variables.
A suite of 18 abiotic variables, including moisture content, porosity, pH, Soluble salts, total organic carbon, total nitrogen, and metals, were measured for each substrate. Ambient temperatures remained below freezing throughout the sampling period in January 2008 although solar heating of ground created isolated patches of snowmelt. Long-term climate data are available at the following link: www.scar.org. Moisture content and total organic content in soils and rocks were measured gravimetrically after heating to 100 and 450 °C, respectively. Rock porosity was measured by vacuum displacement. Soluble salts and pH were measured by potentiometric determination. Total carbon and nitrogen were determined using a thermal conductivity detector at 900 °C. All elemental tests were conducted after air-drying and nitric/hydrochloric acid digestion using ICP-MS according to the Environmental Protection Agency 200.2 for soils or using the EDX elemental scanning function during scanning electron microscopy of rock surfaces.
Recovery of Environmental DNA and Target Loci.
Recovery of environmental DNA used a protocol optimized for lithic microorganisms (24). PCR amplification of rRNA genes was carried out using domain-specific forward primers for bacteria (43), eukarya (44), and archaea (22), and universal reverse primers (43, 45). Alternative sets of archaea-specific primers were also tested (46). The ITS region was amplified using rRNA gene-specific primers from flanking regions (47, 48).
Real-Time Quantitative PCR.
PCR amplification was quantified in real-time (Prism 7000; Applied Biosystems) by flourometric monitoring with SYBR Green 1 dye (Invitrogen). All standard curves were constructed using plasmids from cloned rRNA genes (Qiagen) separately for archaea, bacteria, and eukarya.
Terminal RFLP.
Restriction digests (MspI for 16S/18S rRNA, HaeIII for ITS) of FAM-labeled PCR amplicons were subjected to fragment analysis by capillary electrophoresis (3730 Genetic Analyzer; Applied Biosystems). The software Perl and R were used to identify true peaks and bin fragments of similar size (49). A virtual digest using HaeIII and MspI was carried out on the sequences retrieved from the bacterial and eukaryal clone libraries. This analysis allowed the assignment of phylogenetic identity to individual peaks.
Clone Library Construction and Sequencing.
Samples were selected for clone library construction (PCR Cloningplus kit; Qiagen) based on those with t-RFLP profiles most similar to other samples for a given substrate. Transformants were screened using RFLP (MspI, HaeIII, and Cfo I) before automated sequencing (3730 Genetic Analyzer; Applied Biosystems). Phylotypes were delineated on the basis of 97% sequence similarity using the freeware DOTUR (50). All sequences generated by this study have been deposited in the National Center for Biotechnology Information GenBank database under accession numbers FJ490210-490344 and FJ895042-FJ895089. Screening for possible chimeric sequences was made using Chimera_Check (http://rdp.cme.msu.edu). Approximate phylogentic affiliations were then determined by BLAST searches of the National Center for Biotechnology Information GenBank database (http://www.ncbi.nlm.nih.gov). Estimates of clone library sampling effort were made using the freeware EstimateS (51). Sampling effort was assessed by calculation of Coverage and Rarefaction curves, estimates of library richness were made using the nonparametric estimators ACE and Chao 1.
Phylogenetic Analyses.
Multiple alignments were created with reference to selected GenBank sequences using BioEdit v7.0.9.0 (52). The alignments were tested against prescript models of evolution using the softwares PAUP* 4.0b10 (53) and Modeltest v3.0 (54). The criteria described by the most appropriate evolutionary model were input for maximum likelihood analysis using Genetic Algorithm for Rapid Likelihood Inference (GARLI) Version 0.96 Beta (55). Robustness of furcated branches was supported by both bootstrap values (1,000 replicates) determined using PAUP* 4.0b10 and Bayesian posterior probabilities (56) calculated using Bayes v3.0b4 (57). Values (in percentage) were shown on all branch nodes supported by >50% of the trees.
Statistical Analyses.
Alpha diversity indices (Shannon's Index, Simpsons Diversity Index, and Pielou's Evenness) were calculated using untransformed data. Phylogenetic data were used to calculate the FST statistic, AvTD and VarTD. Quantification of the degree of phylogenetic differentiation between communities was expressed by the FST statistic (58) using the software Arlequin v3.0 (59). TD indices were calculated to reflect phylogenetic diversity within populations (60). Differences stated as significant were tested using one-way and two-way ANOVA, AMOVA, or analysis of similarity (ANOSIM). Multivariate analysis of diversity data were performed on square-root transformed diversity data, and on nontransformed normalized data for environmental variables. Nonmetric multidimensional scaling ordinations (NMDS) were used to visualize Bray Curtis Similarities (diversity data) and Euclidean Distances (environmental data). In BEST analyses, the BIO-ENV procedure was used to maximize the rank correlation between biotic and environmental data; thereby, establishing a ranking (ρ_w_) for the effects of environmental variables on diversity. All analyses were performed using Primer v6.1.6 (61). All results stated as significant have a confidence level of P < 0.05 unless stated otherwise.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Brett Arenz (University of Minnesota) for field assistance, and Robert Blanchette (University of Minnesota) and Donald Cowan (University of the Western Cape, Cape Town, South Africa) for useful discussions and advice on defining the research topic. Logistical and field support was provided by Antarctica New Zealand. This work was funded in part by the University of Waikato Vice Chancellor's Fund, the Hong Kong Research Grants Council Grant No. 7733/08M, and the United States National Science Foundation Grant No. 0537143.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. FJ490210-490344 and FJ895042-FJ895089).
See Commentary on page 19749.
References
- 1.Scientific Committee on Antarctic Research. SCAR Bulletin No 155. 2004. [Accessed January 7, 2009]. Available at http://www.scar.org.
- 2.Cowan D, Ah Tow L. Endangered Antarctic Environments. Annu Rev Microbiol. 2004;58:649–690. doi: 10.1146/annurev.micro.57.030502.090811. [DOI] [PubMed] [Google Scholar]
- 3.Doran PT, et al. Valley floor climate observations from the McMurdo Dry Valleys, Antarctica, 1986–2000. J Geophys Res. 2002;107:4772. doi: 10.1029/2001JD002045. [DOI] [Google Scholar]
- 4.Horowitz NH, Cameron RE, Hubbard JS. Microbiology of the Dry Valleys of Antarctica. Antarctic Sci. 1972;176:242–245. doi: 10.1126/science.176.4032.242. [DOI] [PubMed] [Google Scholar]
- 5.Wynn-Williams DD. Ecological aspects of Antarctic microbiology. Adv Microbial Ecol. 1990;11:71–146. [Google Scholar]
- 6.Doran PT, et al. Antarctic climate cooling and terrestrial ecosystem response. Nature. 2002;415:517–520. doi: 10.1038/nature710. [DOI] [PubMed] [Google Scholar]
- 7.Van Everdingen RO. Geocryological terminology. Can J Earth Sci. 1976;13:862–867. [Google Scholar]
- 8.Boyd WL, Rothenberg I, Boyd JW. Soil microorganisms at Paradise Harbour, Antarctica. Ecology. 1970;51:1040–1045. [Google Scholar]
- 9.Friedmann EI, Ocampo R. Endolithic blue-green algae in the Dry Valleys: Primary producers in the Antarctic ecosystem. Science. 1976;193:1247–1249. doi: 10.1126/science.193.4259.1247. [DOI] [PubMed] [Google Scholar]
- 10.Friedmann EI. Endolithic microorganisms in the Antarctic cold desert. Science. 1982;215:68–74. doi: 10.1126/science.215.4536.1045. [DOI] [PubMed] [Google Scholar]
- 11.Wood SA, Rueckert A, Cowan DA, Cary SC. Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. ISME J. 2008;2:308–320. doi: 10.1038/ismej.2007.104. [DOI] [PubMed] [Google Scholar]
- 12.Broady PA. The distribution of terrestrial and hydro-terrestrial algal associations at thre contrasting locations in southern Victoria Land, Antarctica. Algalogical Studies. 2005;118:95–112. [Google Scholar]
- 13.Aislabie JM, et al. Dominant bacteria in soils of Marble Point and Wright Valley, Victoria Land, Antarctica. Soil Biol Biochem. 2006;38:3041–3056. [Google Scholar]
- 14.Smith JJ, Ah Tow L, Stafford W, Cary C, Cowan DA. Bacterial diversity in three different Antarctic cold desert mineral soils. Microb Ecol. 2006;51:413–421. doi: 10.1007/s00248-006-9022-3. [DOI] [PubMed] [Google Scholar]
- 15.Barrett JE, et al. Co-variation in soil biodiversity and biogeochemistry in northern and southern Victoria land, Antarctica. Antarctic Sci. 2006;18:535–548. [Google Scholar]
- 16.Neiderberger TD, et al. Microbial community composition in soils of northern Victoria Land, Antarctica. Environ Microbiol. 2008;10:1713–1724. doi: 10.1111/j.1462-2920.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 17.Arenz BE, Held BW, Jurgens JA, Farrell RL, Blanchette RA. Fungal diversity in soils and historic wood from the Ross Sea region of Antarctica. Soil Biol Biochem. 2006;38:3057–3064. [Google Scholar]
- 18.Rietkirk M, Dekker SC, de Ruiter PC, van de Koppel J. Self-organized patchiness and catastrophic shifts in ecosystems. Science. 2004;305:1926–1929. doi: 10.1126/science.1101867. [DOI] [PubMed] [Google Scholar]
- 19.Hansen J, Ruedy R, Glasgoe J, Sato M. GISS analysis of surface temperature change. J Geophys Res. 1999;104:30997–31022. [Google Scholar]
- 20.De los Rios A, Wierzchos J, Sancho LG, Ascaso C. Exploring the physiological state of continental Antarctic endolithic microorganisms by microscopy. FEMS Microbiol Ecol. 2004;50:143–152. doi: 10.1016/j.femsec.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Connon SA, Lester ED, Shafaat HS, Obenhuber DC, Ponce A. Bacterial diversity in hyperarid Atacama Desert soils. J Geophys Res. 2007;112:G04S17. doi: 10.1029/2006JG000311. [DOI] [Google Scholar]
- 22.Costello EK, Schmidt SK. Microbial diversity in alpine tundra wet meadow soil: Novel Chloroflexi from a cold, water-saturated environment. Environ Microbiol. 2006;8:1471–1486. doi: 10.1111/j.1462-2920.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger WH, et al. Communit composition and photosynthesis by photoautotrophs under quartz pebbles, southern Mojave Desert. Ecology. 2003;84:3222–3231. [Google Scholar]
- 24.Warren-Rhodes KA, et al. Hypolithic bacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microbial Ecol. 2006;52:389–398. doi: 10.1007/s00248-006-9055-7. [DOI] [PubMed] [Google Scholar]
- 25.Pointing SB, Warren-Rhodes K, Lacap DC, Rhodes KL, McKay CP. Hypolithic community shifts occur as a result of liquid water availability along environmental gradients in China's hot and cold hyperarid deserts. Environ Microbiol. 2007;9:414–424. doi: 10.1111/j.1462-2920.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- 26.Billi D, Friedmann EI, Hofer K, Grilli-Caiola M, Ocampo-Friedmann R. Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl Environ Microbiol. 2000;66:1489–1492. doi: 10.1128/aem.66.4.1489-1492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedmann EI, Kibler AP. Nitrogen economy of endolithic microbial communities in hot and cold deserts. Microbial Ecol. 1980;6:95–108. doi: 10.1007/BF02010548. [DOI] [PubMed] [Google Scholar]
- 28.Smith MC, Bowman JP, Scott FJ, Line MA. Sublithic bacteria associated with Antarctic quartz stones. Antarctic Sci. 2000;12:177–184. [Google Scholar]
- 29.McKay CP, Neinow JA, Meyer MA, Friedmann EI. Continuous nanoclimate data (1985–1988) from the Ross Desert (McMurdo Dry Valleys) cryptoendolithic microbial ecosystem. Antarctic Res Ser. 1993;61:201–207. [Google Scholar]
- 30.De la Torre JR, Goebel BM, Friedmann EI, Pace NR. Microbial diversity of the cryptoendolithic communities from the McMurdo Dry Valleys, Antarctica. Appl Environ Microbiol. 2003;69:3858–3867. doi: 10.1128/AEM.69.7.3858-3867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigler WV, Bachofen R, Zeyer J. Molecular characterization of endolithic bacteria inhabiting exposed dolomite in central Switzerland. Environ Microbiol. 2003;5:618–627. doi: 10.1046/j.1462-2920.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 32.Boison G, Mergel A, Jolkver H, Bothe H. Bacterial life and dinitrogen fixation at a gypsum rock. Appl Environ Microbiol. 2004;70:7070–7077. doi: 10.1128/AEM.70.12.7070-7077.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moorhead DL, et al. Ecological legacies: Impacts on ecosystems of the McMurdo Dry Valleys. Bioscience. 1999;49:1009–1019. [Google Scholar]
- 34.Rothrock MJ, Jr, Garcia-Pichel F. Microbial diversity of benthic mats along a tidal desiccation gradient. Environ Microbiol. 2005;7:593–601. doi: 10.1111/j.1462-2920.2005.00728.x. [DOI] [PubMed] [Google Scholar]
- 35.Sun HJ, Friedmann EI. Growth on geological time scales in the Antarctic cryptoendolithic microbial community. Geomicrobiol J. 1999;16:193–202. [Google Scholar]
- 36.Hopkins DW, et al. Isotopic evidence for the provenance and turnover of organic carbon by soil microorganisms in the Antarctic Dry Valleys. Environ Microbiol. 2008;11:597–608. doi: 10.1111/j.1462-2920.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 37.Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lozupone CA, Knight R. Global patterns in bacterial diversity. Proc Natl Acad Sci USA. 2007;104:11436–11440. doi: 10.1073/pnas.0611525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce DA, et al. Microorganisms in the atmosphere over Antarctica. FEMS Microbiol Ecol. 2009;69:143–157. doi: 10.1111/j.1574-6941.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 40.Cockell CS, Stokes DM. Hypolithic colonization of opaque rocks in the Arctic and Antarctic polar desert. Arct Antarct Alp Res. 2004;38:335–342. [Google Scholar]
- 41.Warren-Rhodes KA, et al. Cyanobacterial ecology across environmental gradients and spatial scales in China's hot and cold deserts. FEMS Microbiol Ecol. 2007;61:470–482. doi: 10.1111/j.1574-6941.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 42.Jakosky BM, Nealson KH, Bakermans C, Ley RE, Mellon MT. Subfreezing activity of microorganisms and the potential habitability of Mars' Polar regions. Astrobiology. 2003;3:343–350. doi: 10.1089/153110703769016433. [DOI] [PubMed] [Google Scholar]
- 43.Lane DJ. In: Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt E, Goodfellow M, editors. Chichester, UK: John Wiley & Sons; 1991. pp. 115–148. [Google Scholar]
- 44.White TJ, Burns T, Lee S, Taylor JW. In: PCR Protocols: A Guide to Methods and Applications. Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. New York: Academic; 1990. pp. 315–322. [Google Scholar]
- 45.Harris JK, Kelley ST, Pace NR. New perspectives on uncultured bacterial phylogenetic division OP11. Appl Environ Microbiol. 2004;70:845–849. doi: 10.1128/AEM.70.2.845-849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker GC, Smith JJ, Cowan DA. Review and re-analysis of domain-specific 16S primers. J Microbial Methods. 2003;55:541–555. doi: 10.1016/j.mimet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Frank DN, et al. Culture-independent molecular analysis of microbial constituents of the healthy human outer ear. J Clin Microbiol. 2003;41:295–303. doi: 10.1128/JCM.41.1.295-303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt DE, et al. Evaluation of 23S rRNA PCR primers for use in phylogenetic studies of bacterial diversity. Appl Environ Microbiol. 2006;72:2221–2225. doi: 10.1128/AEM.72.3.2221-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdo Z, et al. Statistical method for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environ Microbiol. 2006;8:929–938. doi: 10.1111/j.1462-2920.2005.00959.x. [DOI] [PubMed] [Google Scholar]
- 50.Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cowell RK. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7.7. 2005. [Accessed January 7, 2009]. Available at http://www.purl.oclc.org/estimates.
- 52.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 53.Swofford DL. Version 4.0b10. Sunderland, MA: Sinauer Associates; 2002. PAUP*: Phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- 54.Posada D, Crandall KA. Modeltest: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 55.Zwickl DJ. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Austin, TX: University of Texas; 2006. PhD dissertation. [Google Scholar]
- 56.Rannala B, Yang Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J Mol Evol. 1996;43:304–311. doi: 10.1007/BF02338839. [DOI] [PubMed] [Google Scholar]
- 57.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 58.Martin AP. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl Environ Microbiol. 2002;68:3673–3682. doi: 10.1128/AEM.68.8.3673-3682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Excoffier LGL, Scheider S. Arlequin v3.0: An integrated software package for population genetics data analysis. Evol Bioinformat Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 60.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Australian J Ecol. 1993;18:117–143. [Google Scholar]
- 61.Clarke KR, Gorley RN. PRIMER v6: User Manual/Tutorial. Plymouth, UK: PRIMER-E; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information