LACTB is a filament-forming protein localized in mitochondria (original) (raw)
Abstract
LACTB is a mammalian active-site serine protein that has evolved from a bacterial penicillin-binding protein. Penicillin-binding proteins are involved in the metabolism of peptidoglycan, the major bacterial cell wall constituent, implying that LACTB has been endowed with novel biochemical properties during eukaryote evolution. Here we demonstrate that LACTB is localized in the mitochondrial intermembrane space, where it is polymerized into stable filaments with a length extending more than a hundred nanometers. We infer that LACTB, through polymerization, promotes intramitochondrial membrane organization and micro-compartmentalization. These findings have implications for our understanding of mitochondrial evolution and function.
Mitochondria descend from ancient Gram-negative bacteria that, through endosymbiosis, became permanent residents of eukaryotic cells (1–3). As a consequence, mitochondria and Gram-negative bacteria share several biochemical features, including DNA organization, core metabolism, and a double-membrane architecture. In Gram-negative bacteria, but not in mitochondria, a mesh-like layer of peptidoglycan is deposited between the outer and inner membrane, offering protection against mechanical stress. Following endosymbiosis, the peptidoglycan layer lost its structural importance, and was subsequently eliminated from the early eukaryotic cell. Although eukaryotes lack peptidoglycan, proteins deriving from the penicillin-binding protein (PBP) family (4) are found in all major eukaryotic lineages, including vertebrates (5).
The bacterial PBPs constitute a large family of serine proteases that is distinguished by 3 conserved amino acid motifs that contribute to the formation of the catalytic site. The -SXXK- motif contains the catalytic serine residue, which undergoes reversible acylation through substrate binding, whereas the -[SY]X[NT]- and the -[KH][ST]G- motifs contribute to substrate docking (4). PBPs catalyze the hydrolysis or transpeptidation of the terminal D-alanyl-D-alanine moiety in peptidoglycan stem peptides. The acceptor for the transpeptidation reaction is the ε-amino group of lysine or diaminopimelate in an adjacent stem peptide. Through these reactions, PBPs contribute to modulate the extent of peptidoglycan cross-linking during bacterial cell division and cell wall elongation (4).
In contrast, the function of PBP homologues in eukaryotic organisms remains largely unexplored. Amino acid sequence analyses show that the 3 conserved amino acid motifs required for catalytic activity are conserved in all eukaryotic PBP homologues (5), suggesting that they can function as active-site serine enzymes. Within the metazoan division, nematodes harbor the largest number of PBP homologues (5), and in Caenorhabditis elegans the PBP homologue LACT-1 may be involved in pathogen recognition (6). LACTB is the only PBP homologue of mammals, and LACTB has been identified in all mammalian genomes sequenced to date (5, 7, 8). Recently, a causative link between LACTB and obesity was detected through gene co-expression analysis based on data integrated from multiple sources (9). This finding was subsequently validated in vivo through LACTB overexpression in transgenic mice, which resulted in an obese phenotype (9). Although the biochemical mechanism for the obesity-promoting effect of LACTB remains unclear, it is evident that LACTB can affect whole-organism energy homeostasis and therefore that LACTB is directly or indirectly involved in the regulation of the metabolic circuitry.
In this study we have performed a molecular dissection to elucidate the biochemical function of LACTB. We show that LACTB, unlike any known bacterial PBP family protein, can polymerize into stable filaments occupying the mitochondrial intermembrane space. We speculate that LACTB filaments may play a role in submitochondrial organization and therefore possibly affect mitochondrial metabolon organization.
Results and Discussion
LACTB Is Localized in the Mitochondrial Intermembrane Space.
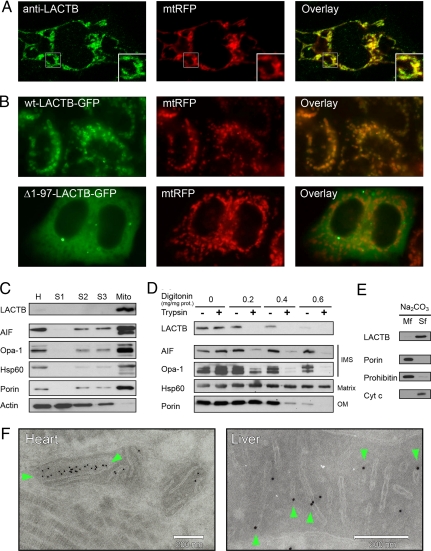
LACTB is widely expressed in different mammalian tissues (7, 10–13). Although proteome survey studies show that LACTB is associated with mitochondria (10–13), other studies suggest that LACTB is localized in non-mitochondrial compartments (14, 15). Therefore, we used a set of complementary experimental techniques to determine the subcellular localization of LACTB. First, we used HeLa cells genetically modified to express red fluorescent protein (RFP) exclusively in mitochondria (mtRFP). Endogenous LACTB of mtRFP HeLa cells was detected with an anti-LACTB antibody (Fig. 1A). LACTB and mtRFP displayed a similar intracellular distribution, confirming that LACTB is associated with mitochondria. The N-terminal 97 aa segment of LACTB does not form part of the conserved PBP domain and may therefore be responsible for organelle targeting (5, 7). To investigate if the N-terminal amino acid segment of LACTB influences its subcellular localization, we fused GFP to the C-terminus of WT LACTB and also to a truncated LACTB lacking the first 97 aa. Expression of these fusion proteins in mtRFP HeLa cells confirmed that the N-terminal segment of LACTB is necessary for mitochondrial localization (Fig. 1B). These results indicate that WT LACTB resides exclusively inside mitochondria.
Fig. 1.
Subcellular localization of LACTB in HeLa cells and rat tissues. (A and B) Fluorescence microscopy images of mtRFP. (A) Endogenous LACTB visualized with an anti-LACTB antibody and an Alexa 488-coupled secondary antibody. The region marked with a square is enlarged (Inset). (B) Transfection with plasmid constructs encoding WT LACTB or an N-terminally truncated LACTB fused to a C-terminal GFP (wt-LACTB-GFP, and Δ_1–97-LACTB-GFP_). (C) Immunoblotting of proteins from rat liver homogenate (H), cytosolic fractions (S1-S3), and mitochondrial fraction (Mito). (D) Immunoblotting of LACTB from rat liver mitochondria incubated with digitonin and trypsin compared with compartment-specific markers for intermembrane space (IMS), matrix, and outer membrane (OM). (E) Soluble mitochondrial proteins were separated from integral membrane proteins through extraction with Na2CO3. The membrane fraction (Mf) and soluble fraction (Sf) were applied on SDS/PAGE gels and analyzed by immunoblotting. (F) Rat tissue cryo-sections immuno-labeled with an anti-LACTB antibody displayed by nanogold particles. The particle densities were 5.9 μm−2 over mitochondria and 0.8 μm−2 elsewhere (n = 3,820). Particles located over cristae are marked with green arrows.
Next, we prepared tissue fractions from rat livers. Proteins extracted from the different tissue fractions were analyzed by immunoblotting using antibodies against LACTB and compartment-specific marker proteins. In crude rat liver extracts, the anti-LACTB antibody revealed a single 55 kDa band, which, as predicted, had segregated with the mitochondrial fraction (Fig. 1C). To determine in which intra-mitochondrial compartment LACTB resides, we incubated the mitochondria with trypsin and sufficient digitonin to permeabilize the outer, but not the inner, mitochondrial membrane. Immunoblotting revealed that LACTB, like other intermembrane space proteins such as apoptosis-inducing factor (AIF) (16) and optic atrophy protein 1 (Opa-1) (17), became accessible to trypsin only upon outer membrane solubilization (Fig. 1D). To determine if LACTB is a soluble protein or an integral membrane protein, mitochondria were treated with sodium carbonate followed by centrifugation to precipitate the membranes. Immunoblotting of the resulting fractions showed that LACTB was completely separated from the membrane marker proteins porin and prohibitin, indicating that LACTB is a soluble protein (Fig. 1E). Last, we prepared samples of cryo-sectioned rat tissues for immuno-electron microscopy. LACTB was visualized by nanogold particles coupled to the anti-LACTB antibody. The results revealed individual nanogold particles, or, less frequently, large particle clusters over the intermembrane space of mitochondria (Fig. 1F). We conclude that LACTB resides in the mitochondrial intermembrane space.
LACTB Forms a Soluble High Molecular Weight Homopolymer.
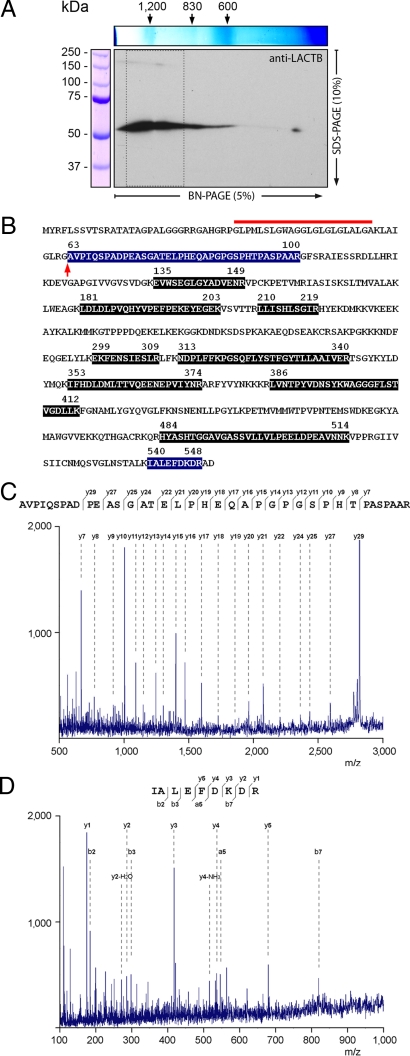
The molecular machineries for several essential metabolic processes are localized in the mitochondrial intermembrane space. To investigate if LACTB is associated with any of these processes, we extracted intermembrane space proteins using non-denaturing conditions. Proteins were separated by 2D blue native SDS/PAGE for identification by immunoblotting and MS. Immunoblotting with the anti-LACTB antibody revealed a band ranging from 600 kDa to several MDa in the native direction of the gel (Fig. 2A). However, in the denaturing direction of the gel, this band separated into a major 55 kDa component and a minor 200 kDa component [Fig. 2A and supporting information (SI) Fig. S1]. This finding suggests that LACTB is part of a multi-protein complex or exists as a homopolymer. Analyses of the major immunoreactive band by MS yielded high-scoring MS/MS spectra from peptides covering almost half of LACTB's amino acid sequence (Fig. 2 B-D and Fig. S2). Notably, we obtained an MS/MS spectrum of a 3,676.3 Da peptide that could be assigned to a 38 aa segment starting from alanine 63 (Fig. 2C). However, the amino acid sequence of the N-terminal cleavage site of this peptide shows that it could not have been formed by the trypsin used for sample preparation. This implies that the 3,676.3 Da peptide is located at LACTB's N-terminus and indicates that mature LACTB is formed from the preprotein by removal of 62 aa (Fig. 2B). This cleavage site is preceded by a segment of hydrophobic amino acids (Fig. 2B), suggesting that LACTB is imported to the intermembrane space through a bipartite pre-sequence cleaved first by the mitochondrial processing peptidase followed by a second cleavage by a protease at the outer surface of the inner membrane (19). Having defined the mature 55 kDa LACTB protein, we proceeded with the MS analysis of the remaining region (indicated in Fig. 2A) of the 2D blue native SDS gel. This analysis resulted in the identification of LACTB in the minor 200 kDa immunoreactive band as well; however, it did not yield any protein that could be a possible LACTB binding partner (Table S1). Therefore, these findings support the hypothesis that LACTB is forming a homopolymer.
Fig. 2.
Analysis of LACTB by MS. (A) Soluble mitochondrial intermembrane space proteins separated by 2D blue native SDS/PAGE followed by immunoblotting with an anti-LACTB antibody. The region boxed by dashed lines was analyzed by MS. (B) Amino acid sequence of rat LACTB (RefSeq:XP_217181). Peptides identified by MS/MS are highlighted. The deduced cleavage site for the mitochondrial import pre-sequence, marked with an arrow, displays a tetrapeptide motif (_AVPI_-) shared by several mitochondrial intermembrane space proteins (18). The hydrophobic amino acid segment located adjacent to the cleavage site is marked with a red line. (C and D) MS/MS spectra, amino acid sequence, and fragment ions from peptides assigned to the N-terminal (C) and the C-terminal (D) segments of LACTB.
LACTB Is Polymerized into Ordered Filaments.
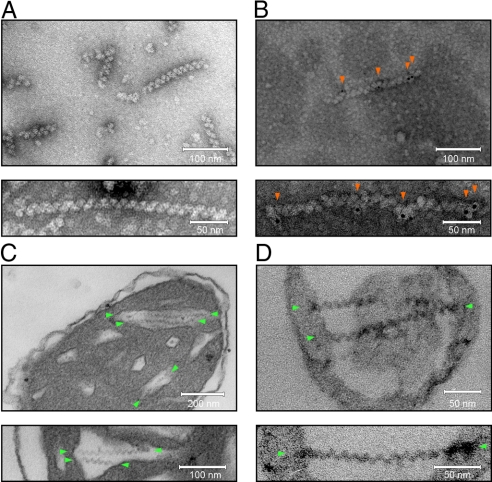
To investigate the structure of the high molecular mass form of LACTB, we used EM. Extracted intermembrane space proteins were separated by centrifugation in a CsCl-density gradient and the resulting fractions were examined after negative staining. At a gradient density of 1.25 to 1.28 g/cm3, we observed characteristic filaments of various lengths composed of globular subunits (Fig. 3A). We noted that, as a result of their heterogeneous size, these filaments would probably migrate over a broad molecular mass range upon electrophoretic separation, suggesting that they represented LACTB polymers. Subsequent MS analyses confirmed that the 1.25 to 1.28 g/cm3 CsCl-gradient fraction contained LACTB. Using whole-mount immuno-electron microscopy, we showed that the anti-LACTB antibody labeled the filaments (Fig. 3B). These findings demonstrate that LACTB can polymerize and form filaments with an open symmetry. To investigate the organization of the LACTB polymer, we aligned and averaged images of 128 subunits (Fig. S3). Using tilting and averaging, the subunit volume was calculated to be 360 nm3. This volume could, depending on packing constraints, accommodate 3 to 5 molecules of LACTB, implying a subunit mass of 150 to 250 kDa. This size is in agreement with the molecular mass of 200 kDa observed for the minor LACTB band in the 2D gels (Fig. 2A and Fig. S1), suggesting that the LACTB polymer is composed of tetrameric subunits.
Fig. 3.
Visualization of LACTB by transmission EM. (A and B) Proteins from CsCl-gradient fractions visualized through negative staining with uranyl acetate. (C and D) Thin sections of mitochondria and submitochondrial particles after chemical fixation, embedding, and staining with uranyl acetate and lead citrate. (A) Naked filaments and (B) filaments decorated with anti-LACTB antibodies and nanogold particles (red arrows). The particle densities were 540 μm−2 over filaments and 3 μm−2 elsewhere (n = 148). (C) Isolated rat liver mitochondria with filaments in the cristal part of the intermembrane space (green arrows). (D) SMPs containing entrapped filaments (green arrows; see also Fig. S5). Previous investigators have described intramitochondrial filaments in various tissues from several different species, including humans. Reports on filaments that, based on their location and geometry, may represent LACTB are listed in Table S3.
Stable LACTB Filaments Are Formed Inside Mitochondria.
To investigate if LACTB polymers occur in situ, we prepared mitochondria for immuno-electron microscopy. Although cryo-sections labeled with the anti-LACTB antibody confirmed that LACTB formed clusters in the intermembrane space (Fig. S4), this approach yielded no further ultrastructural information. In contrast, chemical fixation of mitochondria under conditions optimized for ultrastructural preservation revealed organized filaments (Fig. 3C and Movie S1) occupying distinct regions of the intermembrane space. Like polymeric LACTB, these filaments were composed of globular subunits arranged in straight or slightly curved strands. Measurements showed that these filaments and LACTB polymers shared a similar thickness [13.7 nm ± 0.2 vs. 15.5 nm ± 0.1 (SEM), n = 25] and repeat distance [15.4 nm ± 0.1 vs. 14.4 nm ± 0.1 (SEM), n = 150]. These findings demonstrate that LACTB polymers can form in situ, suggesting that the polymerization is an ordered process with a specific physiological function.
We noted that LACTB filaments were confined to the intra-cristal region of the intermembrane space, i.e., to the region formed by infoldings of the inner membrane, and that the filament ends appeared to be tethered to the inner membrane (Fig. 3C and Movie S1). The uniform organization of the LACTB filaments relative to the inner membrane suggested that they interact in a specific way. To investigate this issue, we used submitochondrial inner membrane vesicles (SMPs). During SMP preparation, mitochondria are disrupted and freely diffusing molecules are lost. As a consequence, SMPs contain only membrane components and membrane-bound macromolecules (Fig. S5). Chemical fixation of SMPs followed by EM analysis revealed that they harbored intact LACTB filaments (Fig. 3D). The filaments spanned across the entire vesicle interior and were tethered to the vesicle membrane at the ends. We conclude that LACTB filaments are spatially organized structures, forming a stable module via interactions with inner membrane components. Importantly, these findings point to the possibility that LACTB has a structural function.
We estimated the amount of LACTB in mitochondria by using quantitative immunoblotting. We found that a liver mitochondrion contained, on average, 1,500 molecules of LACTB (Fig. S6 and Table S2 for a comparison with other mitochondrial proteins). We conclude that LACTB is an abundant mitochondrial protein and therefore has the potential to impact mitochondrial ultrastructure.
Polymerization Is Probably a Unique Feature of LACTB and Its Metazoan Orthologues.
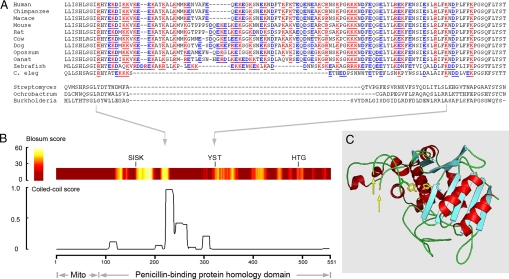
To gain insight into the molecular basis for LACTB's polymerization, we analyzed the amino acid sequence for secondary structure motifs predicted to mediate protein-protein interactions. The analysis revealed a segment enriched in charged and hydrophobic amino acids showing a high propensity for coiled-coil motif formation (Fig. 4 A and B). To assess if this region could be positioned for protein-protein interactions, we performed homology modeling using the crystal structure of the Streptomyces R61 D-alanyl-D-alanine carboxypeptidase (20), an extensively studied bacterial homologue of LACTB. According to our model, LACTB exhibits a characteristic PBP fold consisting of an α/β region and an all-helical region (Fig. 4C). The predicted coiled-coil segment is positioned on LACTB's surface, permitting formation of a flexible loop (Fig. 4C). The loop may contribute to the formation of complementary patches, thereby promoting self-assembly of the LACTB polymer.
Fig. 4.
Structural model of LACTB. (A) Amino acid alignment of predicted coiled-coil segment in metazoan LACTB orthologues and the corresponding regions from 3 bacterial penicillin-binding proteins (protein data bank accession numbers are listed in ref. 5). (B) Amino acid similarity scores for LACTB and Streptomyces R61 D-alanyl-D-alanine carboxypeptidase (colored band) and probability of coiled-coil formation for LACTB (trace). The 3 catalytic site signature motifs -SISK-, -YST-, and -HTG-, universally conserved in penicillin-binding proteins (4), are indicated. (C) Three-dimensional model of LACTB shows the position of the predicted coiled-coil segment (yellow arrows), and the side chains of the catalytic site residues (yellow).
Concluding Remarks.
Proteins forming polymers with an open symmetry typically have structural functions that are conserved over long evolutionary distances. This is exemplified by the 3 main cytoskeletal elements of eukaryotic cells; actin, tubulin, the intermediate filament proteins, and their respective bacterial homologues; MreB, FtsZ, and crescentin. In contrast, PBPs are not known to assemble into filaments but instead promote subcellular structure formation through enzymatic mechanisms. The difference between a possible structural function of LACTB exerted through filament formation and the overtly enzymatic function of PBPs is very large. This raises intriguing questions as to what advantage intramitochondrial filaments conferred to the eukaryotic cell and why LACTB was endowed with the property to self-assemble into filaments. One may hypothesize that, after the loss of the peptidoglycan layer, novel molecular mechanisms for mitochondrial intermembrane space organization had to be developed, and that gene regulatory mechanisms favored evolutionary tinkering of proteins previously functioning in that context.
Recent results from gene network analyses and a transgenic mouse model indicate that LACTB can affect the energy balance leading to an increased fat storage (9). We speculate that LACTB has a physiological role in promoting submitochondrial organization, thereby affecting metabolite flux through specific loci in the metabolic web.
Experimental Procedures
LACTB Expression Plasmids.
Plasmids for LACTB expression were generated using the Gateway cloning system (Invitrogen), as previously described (8).
Cell Culture.
HeLa cells were stably transfected with a plasmid encoding for a mitochondria-targeted RFP (mtRFP). HeLa cells were transiently transfected with LACTB constructs cloned into the pcDNA-DEST47 plasmid (Invitrogen) using the FuGENE HD Transfection Reagent (Roche) according to the manufacturer's instructions. Cells were fixed with 4% paraformaldehyde, washed, and embedded for fluorescence microscopy.
Isolated Mitochondria.
Mitochondria were isolated from livers of male Wistar rats, as previously described (21). Proteins were extracted from the mitochondrial intermembrane space by using a hypo-osmolar medium to break the mitochondrial outer membrane. Extracted mitochondrial intermembrane space proteins were separated on a CsCl-gradient and then prepared for electron microscopy as detailed (22), with minor modifications. SMPs were prepared using sonication.
Gel Electrophoresis.
One-dimensional SDS/PAGE and 2D blue native SDS/PAGE were performed using the BioRad MiniPROTEAN and PROTEAN II systems. 2D blue native PAGE was performed as detailed (23), with minor modifications.
MS.
Proteins separated by gel electrophoresis were prepared for MS as previously described (8). MALDI-TOF analyses were performed using a Bruker Autoflex III mass spectrometer. Liquid chromatography-MS analyses were performed using an HPLC system from LC Packings connected to a Bruker Esquire 3000 plus ion trap mass spectrometer.
EM.
Samples in negative staining were viewed using a Philips Tecnai F20 electron microscope. Embedded and thin-sectioned samples were viewed with a JEOL 1200 EX II electron microscope.
Antibodies.
Anti-LACTB antibody was raised, purified, and characterized as previously described (8). Anti-actin antibody was from MP Biomedicals, anti-AIF antibody and anti-cytochrome c antibody were from Millipore, anti-Hsp60 antibody and anti-prohibitin antibody were from Santa Cruz Biotechnology, anti-Opa-1 antibody was from BD Biosciences, anti-porin/VDAC antibody was from Calbiochem, peroxidase-conjugated anti-rabbit IgG antibody and peroxidase conjugated anti-mouse IgG antibody were from Sigma, and Alexa 488-conjugated anti-rabbit antibody was from Invitrogen.
Complete details about the experimental procedures can be found in the SI Text.
Supplementary Material
Supporting Information
Acknowledgments.
We thank E. Jokitalo and P. Laurinmäki for help with electron microscopy at the Institute of Biotechnology; and P. Bernardi for his advice and encouragement. This study was funded by grants from the Academy of Finland, the University of Helsinki Research Foundation, the Sigrid Juselius Foundation, the Finska Läkaresällskapet, the Magnus Ehrnrooth foundation, the K. Albin Johansson foundation, and the Svenska Kulturfonden. J.S. was supported by the National Graduate School in Informational and Structural Biology.
Footnotes
The authors declare no conflicts of interest.
This article is a PNAS Direct Submission.
References
- 1.Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–630. doi: 10.1038/nature04546. [DOI] [PubMed] [Google Scholar]
- 2.Cavalier-Smith T. Origin of mitochondria by intracellular enslavement of a photosynthetic purple bacterium. Proc R Soc B. 2006;273:1943–1952. doi: 10.1098/rspb.2006.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Duve C. The origin of eukaryotes: A reappraisal. Nat Rev Genet. 2007;8:395–403. doi: 10.1038/nrg2071. [DOI] [PubMed] [Google Scholar]
- 4.Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 5.Peitsaro N, et al. Evolution of a family of metazoan active-site serine enzymes from penicillin-binding proteins: A novel facet of the bacterial legacy. BMC Evol Biol. 2008;8:e26. doi: 10.1186/1471-2148-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pujol N, et al. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008;4:e1000105. doi: 10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TS, et al. Identification, genomic organization, and mRNA expression of LACTB, encoding a serine β-lactamase-like protein with an amino-terminal transmembrane domain. Genomics. 2001;78:12–14. doi: 10.1006/geno.2001.6643. [DOI] [PubMed] [Google Scholar]
- 8.Liobikas J, et al. Expression and purification of the mitochondrial serine protease LACTB as an N-terminal GST fusion protein in Escherichia coli. Prot Exp Pur. 2006;45:335–342. doi: 10.1016/j.pep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452:429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koc EC, et al. The large subunit of the mammalian mitochondrial ribosome. Analysis of the complement of ribosomal proteins present. J Biol Chem. 2001;276:43958–43969. doi: 10.1074/jbc.M106510200. [DOI] [PubMed] [Google Scholar]
- 11.Rome S, et al. Microarray profiling of human skeletal muscle reveals that insulin regulates ≈800 genes during a hyperinsulinemic clamp. J Biol Chem. 2003;278:18063–18068. doi: 10.1074/jbc.M300293200. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SW, et al. Characterization of the human heart mitochondrial proteome. Nat Biotechnol. 2003;21:281–286. doi: 10.1038/nbt793. [DOI] [PubMed] [Google Scholar]
- 13.Pagliarini DJ, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark HF, et al. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 2003;13:2265–2270. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, et al. Systematic characterization of the murine mitochondrial proteome using functionally validated cardiac mitochondria. Proteomics. 2008;8:1564–1575. doi: 10.1002/pmic.200700851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otera H, Ohsakaya S, Nagaura Z-I, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005;24:1375–1386. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olichon A, et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- 18.Verhagen AM, et al. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ. 2007;14:348–357. doi: 10.1038/sj.cdd.4402001. [DOI] [PubMed] [Google Scholar]
- 19.Chacinska A, Koehler CM, Milenkovic D, Lithgow, Pfanner N. Importing mitochondrial proteins: Machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly JA, Kuzin AP. The refined crystallographic structure of a DD-peptidase penicillin-target enzyme at 1.6 Å resolution. J Mol Biol. 1995;254:223–236. doi: 10.1006/jmbi.1995.0613. [DOI] [PubMed] [Google Scholar]
- 21.Johans M, et al. Modification of permeability transition pore arginine(s) by phenylglyoxal derivatives in isolated mitochondria and mammalian cells. Structure-function relationship of arginine ligands. J Biol Chem. 2005;280:12130–12136. doi: 10.1074/jbc.M413454200. [DOI] [PubMed] [Google Scholar]
- 22.Kastner B, et al. GraFix: Sample preparation for single-particle electron cryomicroscopy. Nat Methods. 2008;5:53–55. doi: 10.1038/nmeth1139. [DOI] [PubMed] [Google Scholar]
- 23.Wittig I, Braun HP, Schägger H. Blue native PAGE. Nat Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information