Cocaine Esterase Prevents Cocaine-Induced Toxicity and the Ongoing Intravenous Self-Administration of Cocaine in Rats (original) (raw)
Abstract
Cocaine esterase (CocE) is a naturally occurring bacterial enzyme, is a very efficient protein catalyst for the hydrolysis of cocaine, and has previously been shown to protect rodents from the lethal effects of cocaine. The current studies were aimed at evaluating the capacity of a longer acting mutant form (CocE T172R/G173Q; DM CocE) of CocE to protect against the lethal effects of cocaine, and alter ongoing intravenous cocaine self-administration in rats. A dose-response analysis revealed a dose-dependent suppression of cocaine-reinforced responding with 1.0 mg of CocE T172R/G173Q producing saline-like rates of responding. The effects of 1.0 mg of CocE T172R/G173Q on cocaine-reinforced responding were then compared with responding when saline was available for injection, whereas the selectivity of CocE T172R/G173Q's effects was assessed by evaluating the effects of 1.0 mg of CocE T172R/G173Q on (−)-2β-carbomethoxy-3β-phenyltropane (WIN-35065-2)- and food-reinforced responding. Although 1.0 mg of CocE T172R/G173Q suppressed responding maintained by 0.1 mg/kg/injection cocaine, a significant increase in responding was observed when responding was maintained by 1.0 mg/kg/injection cocaine, resulting in a 10-fold rightward shift in the dose-response curve for cocaine self-administration at a dose that did not significantly alter responding maintained by either WIN-35065-2 or food. These findings demonstrate that a long-acting form of CocE is effective at abruptly reducing the ongoing self-administration of low doses of cocaine, and provides a robust antagonism of cocaine's reinforcing effects. Furthermore, these studies provide strong evidence for the potential usefulness of a suitable, stable, and long-acting form of CocE as a pharmacotherapy for cocaine abuse in humans.
Cocaine abuse remains a significant public health problem with 2006 estimates of 2.4 million current users, and approximately 1.7 million individuals identified as dependent on, or abusers of cocaine in the United States alone (Substance Abuse and Mental Health Services Administration, 2007). Despite longstanding efforts, there are currently no approved pharmacological therapies for the treatment of cocaine abuse. Difficulties in identifying compounds capable of selectively antagonizing cocaine's reinforcing effects are due, at least in part, to cocaine's primary mechanism of action as a monoamine uptake blocker and the inherent difficulties in antagonizing a blocker. Three main approaches have been taken toward the development of pharmacotherapies for cocaine abuse: 1) “agonist” therapeutics, aimed at providing a replacement drug for cocaine (e.g., Grabowski et al., 2004); 2) cocaine antagonists, aimed at blocking cocaine at its site(s) of action (e.g., Newman et al., 2005; Rothman et al., 2008); and 3) modulators of cocaine, aimed at altering the effects of cocaine by acting at sites other than monoamine transporters (e.g., Mello, 1990; Roberts and Brebner, 2000; Platt et al., 2002; Dackis and O'Brien, 2003). Although these strategies are capable of decreasing cocaine self-administration in laboratory animals and humans, each has been met with difficulties including poor behavioral selectivity and/or increased abuse liability.
Alternatively, a smaller, but significant effort has been made toward the development of protein-based pharmacotherapies aimed at altering the pharmacokinetics of cocaine, thus reducing the amount of cocaine that is able to reach its central site(s) of action. Two main pharmacokinetic strategies are currently being investigated: 1) sequestration of cocaine in the periphery with cocaine-specific antibodies (e.g., Fox et al., 1996; Martell et al., 2005), and 2) enhancement of the clearance of cocaine through cocaine-specific enzymes or catalytic antibodies (e.g., Landry and Yang, 1997; Turner et al., 2002). Despite the fact that significant and selective decreases in cocaine self-administration have been reported in rats (Fox et al., 1996; Mets et al., 1998; Baird et al., 2000), significant hurdles remain for both pharmacokinetic approaches.
With respect to cocaine-specific antibodies, although the passive administration of cocaine-specific antibodies has resulted in significant decreases in cocaine self-administration in rats (Fox et al., 1996; Carrera et al., 2000), active immunization has only been shown to inhibit the reinstatement of responding after acute cocaine challenges, but not the ongoing self-administration of cocaine (Carrera et al., 2000; Kantak et al., 2000). In fact, active immunization of rats against cocaine has been shown to result in significant increases in cocaine intake, suggesting that cocaine-specific antibodies are easily surmounted by increasing cocaine intake when antibody titer levels are low (Carrera et al., 2000). Despite these potential problems, a cocaine-specific vaccine has been moved into human studies with similarly promising results (Kosten et al., 2002; Martell et al., 2005). In a 14-week, dose-escalation study, increases in antibody titer levels were reported to be well tolerated and corresponded to modest decreases in cocaine use, and a prolonged attenuation of cocaine's subjective effects (Martell et al., 2005). Although significant decreases in cocaine-positive urine samples have been reported during vaccination when antibody titers are high, a 10-fold increase in cocaine-positive urine samples was observed during the 8-week period after vaccination when antibody levels were decreasing (Kosten et al., 2008), further suggesting that cocaine-specific antibodies are surmountable when titer levels are low.
One approach to overcome this requirement for high antibody levels has been the development of catalytic antibodies that catalyze the breakdown of cocaine to ecgonine methyl ester and benzoic acid, thus, allowing for the antibodies to circumvent the stoichiometric limitations of traditional antibodies. Although the catalytic cocaine antibody, mAb 15A10, has been shown to produce significant and selective decreases in cocaine-reinforced responding in rats (Mets et al., 1998; Baird et al., 2000), this effect was only observed with high doses (30 mg/kg) of mAb 15A10, probably because of the relatively poor catalytic efficiencies of mAb 15A10 [_K_cat/_K_m; ∼26-fold lower than native butyrylcholinesterase (BChE)]. Alternatively, increased clearance of cocaine by cocaine-specific enzymes has also been investigated. Recent studies (Pan et al., 2005) have identified a mutant butyrylcholinesterase with an improved catalytic efficiency (∼456-fold greater than native BChE) sufficient to allow for the selective inhibition of the reinstatement of responding after an acute cocaine challenge after pretreatment with a low dose (2 mg/kg) of butyrylcholinesterase (Brimijoin et al., 2008); however, this enzyme has yet to be evaluated in ongoing cocaine self-administration procedures.
A bacterial cocaine esterase (CocE) has been identified with catalytic efficiencies ∼800-fold greater than BChE (Larsen et al., 2002; Turner et al., 2002). WT CocE has been shown to protect, and reduce, the toxic effects of cocaine in rats (Cooper et al., 2006) and mice (Ko et al., 2007), and the cardiovascular (J. H. Wood et al., unpublished observations) and seizurogenic (Jutkiewicz et al., 2009) effects of cocaine in rats, suggesting that WT CocE can rapidly clear large amounts of cocaine from the body. Although WT CocE has proven to be highly effective at protecting rodents against acute cocaine toxicity, its relatively short half-life in vivo (_t_½, 15 min; Cooper et al., 2006) probably limits its usefulness in the treatment of cocaine abuse. Recent attempts to improve CocE's duration of action through site-directed mutagenesis have identified a double mutant (DM; T172R/G173Q) form of CocE with a significantly improved in vivo half-life (_t_1/2, 4.5 h in mice) without significantly altering the enzyme's catalytic efficiency (Gao et al., 2009).
Self-administration procedures in laboratory animals have proven to be a valuable preclinical model for the assessment of reinforcing properties of drug and nondrug reinforcers, as well as the identification of therapeutics with the potential of decreasing drug use in humans (e.g., Carroll et al., 2006). The current studies were aimed at evaluating the capacity of a longer acting CocE T172R/G173Q to protect rats from the lethal effects of cocaine, and to alter the ongoing self-administration of cocaine in rats. Rats were trained to respond for 0.1 mg/kg/injection cocaine under a fixed ratio (FR) 5 schedule of reinforcement, and a dose-response analysis was conducted to determine the dose of CocE T172R/G173Q required to inhibit cocaine-reinforced responding. After this analysis, the effective dose of CocE T172R/G173Q (1.0 mg/rat), and an equivalent dose of WT CocE were further evaluated for their capacity to alter responding reinforced by 0.1 mg/kg/injection cocaine, and compared with the rates and patterns of responding emitted when saline was substituted for cocaine. The capacity of 1.0 mg/rat CocE T172R/G173Q to alter responding maintained by higher doses of cocaine was also evaluated in rats trained to respond for 0.32 and 1.0 mg/kg/injection cocaine. In addition, the selectivity of the effects of CocE T172R/G173Q on cocaine-reinforced responding were assessed by evaluating the capacity of 1.0 mg/rat CocE T172R/G173Q to alter responding that was reinforced by a nonhydrolyzable cocaine analog, WIN-35065-2 (e.g., Cooper et al., 2006), and liquid food. These studies demonstrate the capacity of low doses of a long-acting form of CocE to selectively and robustly antagonize the ongoing self-administration of cocaine in rats while having no effect on responding that was reinforced by other drug or nondrug reinforcers, and they suggest that a suitable, long-acting form of CocE could prove to be a valuable tool in the treatment of cocaine abuse in humans.
Materials and Methods
Subjects.
Male Sprague-Dawley rats (275–350 g) were obtained from Harlan (Indianapolis, IN) and maintained in a temperature- and humidity-controlled environment, on a 12-h dark/light cycle with lights on at 7:00 AM. Rats that were used to determine in vivo duration of action were free-fed, whereas rats that were involved in self-administration experiments were maintained with ∼20g of food per day from 7 days after surgery until the end of the experiment. All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health, and all experimental procedures were approved by the University of Michigan Committee on the Use and Care of Animals.
Prevention of Cocaine-Induced Convulsion and Lethality by CocE T172R/G173Q.
The capacity of CocE T172R/G173Q to prevent the convulsant and lethal effect of cocaine was assessed to provide a measure of the in vivo duration of CocE T172R/G173Q's actions. Rats (n = 8 per group) were placed in a test chamber (49 cm long × 23 cm wide × 21 cm high clear shoe box rodent cage with standard cob bedding) 30 min before pretreatment with one of four doses of CocE T172R/G173Q administered on a milligram per kilogram basis (0.0, 1.0, 3.0, and 10.0 mg/kg) via tail vein injection. Pretreatments were administered at one of four times (1, 60, 120, and 240 min) before administration of an LD100 dose of cocaine (180.0 mg/kg i.p.). After cocaine administration, all rats were observed for 60 min with the presence or absence of convulsion and/or lethality recorded. Cocaine-induced convulsion was defined as the loss of righting posture, combined with clonic limb movements, whereas cocaine-induced lethality was defined as the cessation of observable movement and respiration.
Surgery.
Rats were surgically prepared with chronic indwelling femoral catheters in the left femoral vein under ketamine/xylazine (90:10 mg/kg i.p.) anesthesia. Catheters were tunneled under the skin and attached to stainless steel tubing, exiting the back through a metal tether button, which was sutured to the muscle between the scapula. Rats were allowed 5 to 7 days to recover from surgery before the start of any experiments. Catheters were flushed with 0.2 ml of heparinized saline (100 U/ml) before the start of self-administration sessions and after the completion of the session to prolong the duration of catheter patency.
Apparatus.
All experimental sessions were conducted in operant conditioning chambers (30.5 cm wide × 24 cm deep × 21 cm high; Med Associates Inc., St. Albans, VT) placed inside sound-attenuating cubicles. Each chamber was equipped with a single nose-poke device (ENV-114BM; Med Associates Inc.) positioned 6 cm above the stainless steel grid floor, and illuminated with a yellow stimulus light. Each chamber was also equipped a green LED stimulus light, located above the nose poke, and a white house light. An air-driven pneumatic syringe pump (IITC Life Science, Woodland Hills, CA) allowed for drug delivery through Tygon tubing connected to a fluid swivel (Instech Laboratories Inc., Plymouth Meeting, PA) and spring tether that was held in place by a counterbalanced arm. For food self-administration sessions, chambers were also equipped with a liquid-food dipper with a 50-μl dipper cup (model E14-05; Coulbourn Instruments, Allentown, PA).
Acquisition of Responding.
All rats were initially trained to nose poke for 0.56 mg/kg/injection cocaine during daily, 60-min sessions under a FR1 schedule of reinforcement. Illumination of the yellow nose-poke light signaled drug, food, or saline availability, and subsequent nose pokes resulted in an injection (100 μl/kg/0.5 s) accompanied by the illumination of a green LED above the nose poke. For food self-administration sessions, chambers were also equipped with a liquid-food dipper with a 50-μl dipper cup (Model E14-05; Coulbourn Instruments). A 5-s timeout (TO) followed injections, or dipper presentations during which time the house light was illuminated and responses were recorded but had no scheduled consequence. During operant training the schedule requirements were progressively increased (FR1, FR2, FR3, and FR5) until responding for 0.56 mg/kg/injection cocaine stabilized under the final schedule of FR5TO5, defined as three consecutive sessions with less than 20% difference and no increasing or decreasing trend in responding. Upon stabilization of responding, the dose of cocaine, or reinforcer available for injection/presentation (i.e., WIN-35065-2/liquid food) was changed, and responding was again allowed to stabilize before any experimental manipulations.
Dose-Response Analysis of CocE T172R/G173Q on Cocaine-Reinforced Responding.
Upon acquisition of stable responding for 0.1 mg/kg/injection cocaine, the capacity of CocE T172R/G173Q (0.032, 0.1, 0.32, and 1.0 mg/rat i.v.) to alter responding that was reinforced with 0.1 mg/kg/injection cocaine was evaluated. Twelve rats (n = 6 rats/dose) were exposed to two different doses of CocE T172R/G173Q with doses randomly administered on a milligram per rat basis immediately before the start of the session, with six rats also exposed to a single saline-substitution session. Doses of CocE T172R/G173Q and the single-session saline substitutions were presented in random order, and separated by at least three sessions (or until responding stabilized).
Comparison of the Effects of WT CocE and CocE T172R/G173Q on Cocaine-Reinforced Responding.
Upon acquisition of stable responding for 0.1 mg/kg/injection cocaine, comparisons of the effects of CocE T172R/G173Q and WT CocE on 0.1 mg/kg/injection cocaine-reinforced responding were performed in six rats. Each rat was exposed to each of three pretreatments (saline substitution, 1.0 mg/rat WT CocE, and 1.0 mg/rat CocE T172R/G173Q), with manipulations presented in random order, and separated by at least three sessions (or until responding stabilized). The effects of 1.0 mg of CocE T172R/G173Q (administered on a milligram per rat basis) and saline substitution on responding maintained by 0.32, and 1.0 mg/kg/injection cocaine were assessed in a second group of six rats, with each rat exposed to both 1.0 mg/rat CocE T172R/G173Q and saline at both doses of cocaine, with the doses of cocaine and order of manipulations randomly assigned.
Effect of CocE T172R/G173Q on WIN-35065-2- and Liquid-Food-Reinforced Responding.
The effects of 1.0 mg/rat CocE T172R/G173Q on responding reinforced by 0.01 mg/kg/injection WIN-35065-2 or 50 μl of liquid food (Ensure; Abbott Laboratories, Abbott Park, IL) were assessed in separate groups (n = 6/group). The effects of 1.0 mg/rat CocE T172R/G173Q on WIN-35065-2-reinforced responding were compared with responding maintained during a single-session saline substitution with manipulations made in random order and separated by at least three sessions (or until responding stabilized). Likewise, the effects of 1.0 mg/rat CocE T172R/G173Q on food-reinforced responding were compared with responding maintained during a session in which rats were allowed to respond for empty-dipper presentations with manipulations made in random order and separated by at least three sessions (or until responding stabilized).
Drugs.
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Bethesda, MD), WIN-35065-2 was provided by Dr. Ivy Carroll (National Institute on Drug Abuse, Research Triangle Institute, NC). All drugs were dissolved in physiological saline, and administered in a volume of 0.1 ml/kg i.v. over a period of 0.5 s. WT CocE and DM were prepared by Drs. Diwa Narasimhan and Roger Sunahara (University of Michigan) and administered via intravenous infusion.
Data Analysis.
Data from the cocaine-induced convulsion and lethality studies are expressed as percentage of occurrence, with significant differences in the percentage of occurrence of cocaine-induced convulsion or lethality between the DM CocE-treated rats and the PBS-treated rats determined by use of Fisher's exact test (GraphPad Prism; GraphPad Software Inc., San Diego, CA). Cumulative records (Figs. 4 and 5) depict representative temporal patterns of responses (steps) and injections (pips) during the 60-min sessions, but do not include responses made during the timeout portions of the sessions. Responses represent the mean ± S.E.M., n = 6, number of responses that resulted in an injection or food delivery, but do not include responses made during reinforcement or the subsequent timeout period. Response stability was defined as three consecutive sessions with less than 20% difference in responding and no increasing or decreasing trend in the number of injections earned. Responding during sessions in which rats were pretreated with WT CocE, or CocE T172R/G173Q, were compared with responding during single-session saline substitutions (or when responding resulted in empty dippers), and responding maintained by the baseline reinforcers (0.1 mg/kg/injection cocaine, 0.01 mg/kg/injection WIN-35065-2, or 50 μl of liquid food); significant differences were determined by one-way ANOVA with post hoc Bonferroni tests (GraphPad Prism). Quarter-life (QL) calculations were used as a measure of intrasession response patterning, and were calculated by dividing the time required for 25% of the total responses to be made by the total session length (60 min). QL values represent the mean ± S.E.M. (n = 6/group) as determined for sessions in which rats were pretreated with CocE, saline was substituted, or when food was removed, and compared with the QL values for the baseline sessions immediately preceding these sessions. Significant differences in QL values between baseline and treatment sessions were determined by two-tailed, paired _t_-tests (GraphPad Prism).
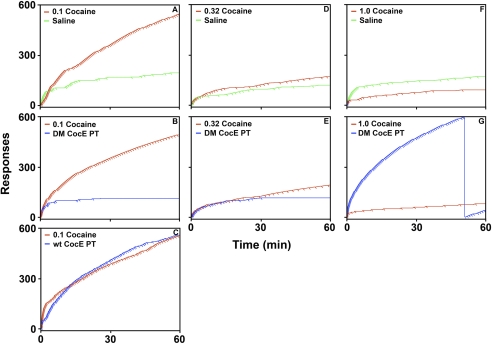
Fig. 4.
Effects of pretreatment with 1.0 mg/rat WT CocE, 1.0 mg/rat CocE T172R/G173Q (DM CocE), or saline substitution on the intrasession pattern of responses (steps) and injections (pips) maintained by cocaine as shown by representative cumulative-response records. Left, cumulative-response records from a representative rat maintained on 0.1 mg/kg/injection cocaine (red trace), as well as the corresponding saline substitution (green trace) (A), 1.0 mg/rat (2.89 mg/kg) DM CocE (blue trace) (B), and 1.0 mg/rat WT (2.87 mg/kg) CocE test sessions (blue trace) (C). Center, cumulative-response records from a representative rat maintained on 0.32 mg/kg/injection cocaine (red trace), as well as the corresponding saline substitution (green trace) (D), and 1.0 mg/rat (2.83 mg/kg) DM CocE (blue trace) (E) test sessions. Right, cumulative-response records from a representative rat maintained on 1.0 mg/kg/injection cocaine (red trace), as well as the corresponding saline substitution (green trace) (F), and 1.0 mg/rat (2.81 mg/kg) DM CocE (blue trace) (G) test sessions.
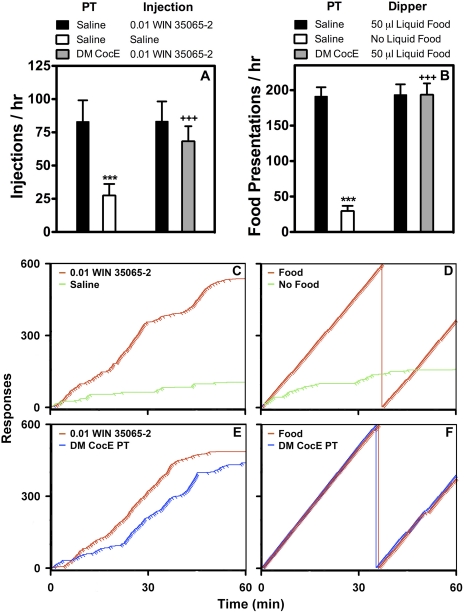
Fig. 5.
Effects of pretreatment with WT CocE and CocE T172R/G173Q (DM CocE) on the ongoing self-administration of 0.01 mg/kg/injection WIN-35065-2 or liquid food in rats (n = 6/group). A, rats were maintained on 0.01 mg/kg/injection WIN-35065-2 during daily 60-min sessions (■) under a FR5TO5 schedule of reinforcement, and randomly exposed to a single-session saline substitution (□), and pretreatment with 1.0 mg/rat (2.88 ± 0.02 mg/kg) DM CocE ( ) before sessions in which responding was reinforced by 0.01 mg/kg/injection WIN-35065-2. B, rats were maintained on 50 μl of liquid food during daily 60-min sessions (■) under a FR5TO5 schedule of reinforcement, and randomly exposed to a single session in which responding resulted in the presentation of an empty dipper cup (□), and pretreatment with 1.0 mg/rat (2.86 ± 0.02 mg/kg) DM CocE (
) before sessions in which responding was reinforced by 0.01 mg/kg/injection WIN-35065-2. B, rats were maintained on 50 μl of liquid food during daily 60-min sessions (■) under a FR5TO5 schedule of reinforcement, and randomly exposed to a single session in which responding resulted in the presentation of an empty dipper cup (□), and pretreatment with 1.0 mg/rat (2.86 ± 0.02 mg/kg) DM CocE ( ) before sessions in which responding was reinforced by liquid food. Data represent the mean (±S.E.M.) number of injections or food presentations earned during 60-min sessions. Effects of pretreatment with 1.0 mg/rat CocE DM CocE, or saline substitution (no food) on the intrasession pattern of responses (steps) and injections (pips) maintained by WIN-35065-2 (C and E) or liquid food (D and F) as shown by representative cumulative-response records. Cumulative-response records from a representative rat maintained on 0.01 mg/kg/injection WIN-35065-2 (red trace), as well as the corresponding saline substitution (green trace) (C), and 1.0 mg/rat (2.86 mg/kg) DM CocE (blue trace) (E) test sessions. Cumulative-response records from a representative rat maintained on 50 μl of Ensure (red trace), as well as the corresponding empty-dipper presentation (green trace) (D) and1.0 mg/rat (3.10 mg/kg) DM CocE (blue trace) (F) test sessions. ***, p < 0.001. Significant differences in the number of injections or food presentations earned during the treatment and baseline sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests. +++, p < 0.001. Significant difference from the number of injections or food presentations earned during baseline and single-session saline substitutions or empty-dipper presentations were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests.
) before sessions in which responding was reinforced by liquid food. Data represent the mean (±S.E.M.) number of injections or food presentations earned during 60-min sessions. Effects of pretreatment with 1.0 mg/rat CocE DM CocE, or saline substitution (no food) on the intrasession pattern of responses (steps) and injections (pips) maintained by WIN-35065-2 (C and E) or liquid food (D and F) as shown by representative cumulative-response records. Cumulative-response records from a representative rat maintained on 0.01 mg/kg/injection WIN-35065-2 (red trace), as well as the corresponding saline substitution (green trace) (C), and 1.0 mg/rat (2.86 mg/kg) DM CocE (blue trace) (E) test sessions. Cumulative-response records from a representative rat maintained on 50 μl of Ensure (red trace), as well as the corresponding empty-dipper presentation (green trace) (D) and1.0 mg/rat (3.10 mg/kg) DM CocE (blue trace) (F) test sessions. ***, p < 0.001. Significant differences in the number of injections or food presentations earned during the treatment and baseline sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests. +++, p < 0.001. Significant difference from the number of injections or food presentations earned during baseline and single-session saline substitutions or empty-dipper presentations were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests.
Results
Prevention of Cocaine-Induced Convulsion and Lethality by CocE T172R/G173Q.
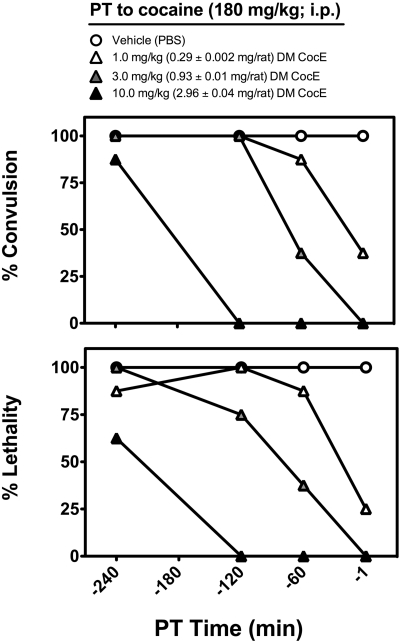
Cocaine (180.0 mg/kg i.p.) induced convulsion, followed by lethality in 100% of the PBS-pretreated rats at all pretreatment times (1, 60, 120, and 240 min). Pretreatment with CocE T172R/G173Q, administered on a milligram per kilogram basis, produced a dose-dependent inhibition of the occurrence of both convulsion and lethality after an LD100 dose of cocaine, with significant inhibition of both endpoints observed after 1-min pretreatments of 1.0 mg/kg (0.29 ± 0.002 mg/rat) CocE T172R/G173Q, 1- and 60-min pretreatments of 3.0 mg/kg (0.93 ± 0.01 mg/rat) CocE T172R/G173Q, and 1-, 60-, and 120 min pretreatments of 10.0 mg/kg (2.96 ± 0.04 mg/rat) CocE T172R/G173Q (Fig. 1). The durations of protection from cocaine-induced convulsion (pretreatment time sufficient to result in a 50% reduction in the occurrence of convulsion) were ∼15 min for 1.0 mg/kg, ∼75 min for 3.0 mg/kg, and ∼190 min for 10.0 mg/kg CocE T172R/G173Q, whereas the durations of protection from cocaine-induced lethality (pretreatment time sufficient to result in a 50% reduction in lethality after an LD100 dose of cocaine) were ∼25 min for 1.0 mg/kg, ∼80 min for 3.0 mg/kg, and ∼220 min for 10.0 mg/kg CocE T172R/G173Q.
Fig. 1.
Time course of the protective effects of CocE T172R/G173Q (DM CocE) against the convulsive and lethal effects of cocaine (180.0 mg/kg i.p.). Rats (n = 8/group) were pretreated with one of three doses (1.0, 3.0, or 10.0 mg/kg i.v.) of CocE T172R/G173Q (DM CocE), or vehicle (PBS) at one of four time points (1, 60, 120, or 240 min) before a LD100 dose of cocaine (180.0 mg/kg i.p.) and observed for 60 min thereafter. Top, dose-response analysis of the protective effects of CocE T172R/G173Q (DM CocE) against the convulsive effect of 180 mg/kg cocaine. Bottom, dose-response analysis of the protective effects of CocE T172R/G173Q (DM CocE) against the lethal effect of 180 mg/kg cocaine. Data represent the percentage of rats exhibiting cocaine-induced convulsion or lethality. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences in the percentage of convulsion or lethality compared with PBS-treated rats were determined by Fisher's exact tests.
Dose-Response Analysis of CocE T172R/G173Q on Cocaine-Reinforced Responding.
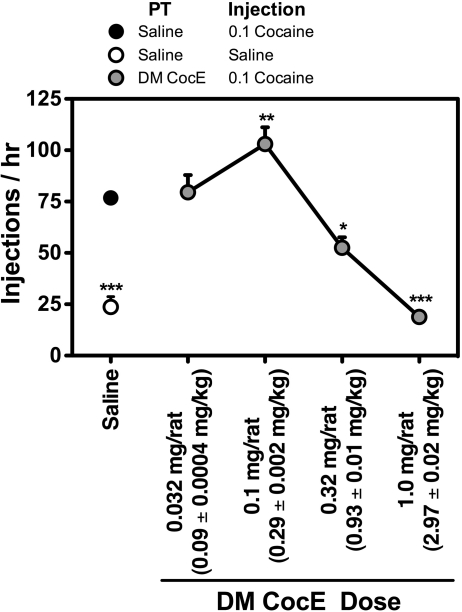
The effects of various doses of CocE T172R/G173Q, administered on a milligram per rat basis, on the ongoing self-administration of cocaine were assessed in rats trained to respond for 0.1 mg/kg/injection cocaine, and compared with sessions in which saline was substituted for cocaine. As shown in Fig. 2, responding occurred at high rates when reinforced with 0.1 mg/kg/injection cocaine, with rats earning approximately 80 injections during the 60-min session. Conversely, low rates of responding were observed during single-session saline substitutions, with rats generally completing only 20 fixed ratios per session. Pretreatment with CocE T172R/G173Q had biphasic effects on cocaine-reinforced responding with an increase in responding observed at a dose of 0.1 mg/rat (0.09 ± 0.0004 mg/kg; p < 0.01), and dose-dependent decreases in responding observed when doses of 0.32 mg/rat (0.93 ± 0.01 mg/kg; p < 0.05), and 1.0 mg/rat (2.97 ± 0.02 mg/kg; p < 0.001) CocE T172R/G173Q were administered. Not only were significant decreases in cocaine-reinforced responding observed during sessions in which rats were pretreated with either 0.32 and 1.0 mg of CocE T172R/G173Q, but these low levels of responding were similar to those observed during the single-session saline substitution tests. QL values for the baseline sessions, and the sessions in which saline was substituted, or pretreatments of CocE T172R/G173Q administered are shown in Table 1. There were no significant differences in the mean QL values for the five baseline sessions (0.20 ± 0.01 − 0.24 ± 0.02), suggesting that 0.1 mg/kg/injection cocaine maintained similar patterns of responding throughout the course of the experiments. Moreover, the patterns of responding during sessions in which rats were pretreated with 1.0 mg/rat CocE T172R/G173Q were similar to those observed during saline substitution (QL values = 0.10 ± 0.02; p < 0.001 and 0.10 ± 0.03; p < 0.05, respectively), with QL values significantly lower than the corresponding baseline sessions.
Fig. 2.
Dose-response analysis of the capacity of CocE T172R/G173Q (DM CocE) to alter responding reinforced by 0.1 mg/kg/injection cocaine. Rats were maintained on 0.1 mg/kg/injection cocaine during daily 60-min sessions in which responding was reinforced under a FR5TO5 schedule of reinforcement. Data represent the mean (±S.E.M.) number of injections earned during 60-min sessions. ●, responding under baseline conditions; ○, responding during a single-session saline substitution;  , responding during sessions in which rats were pretreated on a milligram per rat basis with 0.032, 0.1, 0.32, or 1.0 mg i.v. DM CocE and allowed to respond for 0.1 mg/kg/injection cocaine. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences in responding compared with baseline conditions were determined by one-way ANOVA with post hoc Bonferroni tests.
, responding during sessions in which rats were pretreated on a milligram per rat basis with 0.032, 0.1, 0.32, or 1.0 mg i.v. DM CocE and allowed to respond for 0.1 mg/kg/injection cocaine. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences in responding compared with baseline conditions were determined by one-way ANOVA with post hoc Bonferroni tests.
Table 1.
Comparison of the effect of CocE T172R/G173Q pretreatment and saline substitution on quarter-life values for responding maintained by 0.1 mg/kg/injection cocaine
| Treatment | Quarter-Life (± S.E.M.) | |
|---|---|---|
| 0.1 mg/kg/Injection Cocainea | Saline/DM CocEb | |
| Saline substitution | 0.24 (0.02) | 0.10 (0.03)* |
| 0.032 mg DM CocE | 0.22 (0.03) | 0.18 (0.02) |
| 0.1 mg DM CocE | 0.20 (0.01) | 0.20 (0.01) |
| 0.32 mg DM CocE | 0.20 (0.01) | 0.33 (0.07) |
| 1.0 mg DM CocE | 0.23 (0.01) | 0.10 (0.02)*** |
Effects of WT CocE and CocE T172R/G173Q on Cocaine-Reinforced Responding.
To examine the relationship between the duration of enzymatic activity and the capacity of CocE to alter the ongoing self-administration of cocaine, rats were pretreated with equal doses of 1.0 mg/rat of the short-acting WT CocE (2.83 ± 0.01 mg/kg), and the longer acting CocE T172R/G173Q (2.85 ± 0.02 mg/kg) immediately before the start of sessions in which responding was reinforced with 0.1 mg/kg/injection cocaine, and the overall rates and patterns of responding were compared with sessions in which either 0.1 mg/kg/injection cocaine or saline were available for injection. As shown in Fig. 3, high rates of responding were maintained during the baseline condition with approximately 80 injections of 0.1 mg/kg cocaine earned per 60-min session. Substitution of saline for cocaine resulted in a significant decrease in the overall rate of responding, with rats earning approximately 20 injections during these single-session substitutions. Although pretreatment with 1.0 mg/rat WT CocE did not affect the overall rate of responding for 0.1 mg/kg/injection cocaine, significant decreases in cocaine-reinforced responding were observed during sessions in which rats were pretreated with 1.0 mg/rat CocE T172R/G173Q with rates of responding no different from those that were maintained by saline, and rats, in general, earning fewer than 20 injections per session.
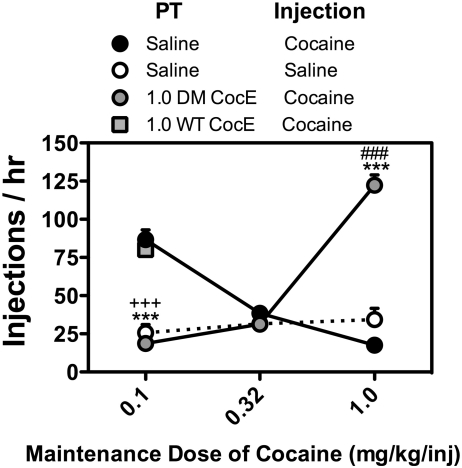
Fig. 3.
Effects of pretreatment with WT CocE and CocE T172R/G173Q (DM CocE) on the ongoing self-administration of cocaine. Rats (n = 6) were maintained on 0.1 mg/kg/injection cocaine during daily 60-min sessions in which responding was reinforced on an FR5TO5, and randomly exposed to pretreatments with 1.0 mg/rat (2.83 ± 0.01 mg/kg) WT CocE ( ), and 1.0 mg/rat (2.82 ± 0.01 mg/kg) DM CocE (
), and 1.0 mg/rat (2.82 ± 0.01 mg/kg) DM CocE ( ) before sessions in which responding was reinforced by 0.1 mg/kg/injection cocaine single session, as well as a single-session saline substitution (○). A second group of rats (n = 6) were maintained on 0.32 and 1.0 mg/kg/injection cocaine (in random order) during daily 60-min sessions in which responding was reinforced on an FR5TO5, and randomly exposed to a pretreatment with 1.0 mg/rat (2.85 ± 0.01 mg/kg) DM CocE (
) before sessions in which responding was reinforced by 0.1 mg/kg/injection cocaine single session, as well as a single-session saline substitution (○). A second group of rats (n = 6) were maintained on 0.32 and 1.0 mg/kg/injection cocaine (in random order) during daily 60-min sessions in which responding was reinforced on an FR5TO5, and randomly exposed to a pretreatment with 1.0 mg/rat (2.85 ± 0.01 mg/kg) DM CocE ( ) before sessions in which responding was reinforced by the baseline dose of cocaine single session, and a single-session saline substitution (○). Data represent the mean (±S.E.M.) number of injections earned during 60-min sessions. ***, p < 0.001. Significant differences in the number of injections earned during treatment and baseline sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests. +++, p < 0.001. Significant differences in the number of injections earned during baseline and saline-substitution sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests. ###, p < 0.001. Significant differences in the number of injections earned during treatment and saline-substitution sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests.
) before sessions in which responding was reinforced by the baseline dose of cocaine single session, and a single-session saline substitution (○). Data represent the mean (±S.E.M.) number of injections earned during 60-min sessions. ***, p < 0.001. Significant differences in the number of injections earned during treatment and baseline sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests. +++, p < 0.001. Significant differences in the number of injections earned during baseline and saline-substitution sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests. ###, p < 0.001. Significant differences in the number of injections earned during treatment and saline-substitution sessions were determined by one-way repeated measures ANOVA with post hoc Bonferroni tests.
To further evaluate the effectiveness of CocE T172R/G173Q to alter cocaine-reinforced responding, rats were pretreated on a milligram per rat basis with 1.0 mg of CocE T172R/G173Q immediately before sessions in which rats were allowed to respond for cocaine (0.32 or 1.0 mg/kg/injection), and the overall rates and patterns of responding were compared with sessions in which either cocaine or saline were available (Fig. 3). When 0.32 mg/kg cocaine was available for injection responding occurred at rates lower than those maintained by 0.1 mg/kg/injection cocaine, with rats, in general, earning approximately 40 injections. Rates of responding during saline substitutions were not significantly different from baseline sessions, or sessions in which rats were pretreated with 1.0 mg/rat (2.87 ± 0.03 mg/kg) CocE T172R/G173Q. Yet lower, but stable rates of responding were obtained when responding was reinforced with 1.0 mg/kg/injection cocaine with rats, in general, earning approximately 20 injections per session. Pretreatment with 1.0 mg/rat (2.86 ± 0.02 mg/kg) CocE T172R/G173Q resulted in a significant increase in responding for 1.0 mg/kg/injection cocaine with rats earning on average 122 injections of per session (Fig. 3); none of these rats died. Increases in the number of injections earned were also observed after saline substitution (∼35 injections; Fig. 3); however, rates of saline-maintained responding were not significantly different from when responding was reinforced with 1.0 mg/kg/injection cocaine.
A representative set of cumulative records for baseline sessions in which responding was reinforced by 0.1, 0.32, or 1.0 mg/kg/injection cocaine and the three experimental conditions for an individual rat are shown in Fig. 4. When responding was reinforced with 0.1 mg/kg/injection cocaine, responding was initiated early in the session and maintained at relatively stable rates throughout the 60-min session (Fig. 4, A–C), and there were no significant differences in the mean QL values for the three baseline cocaine sessions (0.20 ± 0.02 − 0.26 ± 0.03; Table 2). As shown in Fig. 4A, when saline was substituted for cocaine, responding occurred at rates roughly equivalent to when cocaine was available for injection during the initial portion of the session, and then slowed for the remainder of the session with mean QL values (0.06 ± 0.01; p < 0.01) significantly lower than the QL values when responding was reinforced by 0.1 mg/kg/injection cocaine. Although pretreatment with 1.0 mg/rat (2.83 ± 0.01 mg/kg) WT CocE did not alter the overall rates of responding for cocaine (Fig. 3), significant differences in intrasession responding were observed. As shown in Fig. 4C, when 1.0 mg/rat WT CocE was administered before the session, responding for 0.1 mg/kg/injection cocaine slowed early in the session, but rapidly recovered to rates approximately equivalent to those observed in baseline sessions resulting in significantly higher QL values (0.34 ± 0.04; p < 0.01) compared with sessions in which either 0.1 mg/kg/injection cocaine (QL value = 0.24 ± 0.04) or saline (QL value = 0.12 ± 0.03) were available for injection. In contrast with WT CocE, pretreatment with 1.0 mg/rat (2.85 ± 0.03 mg/kg) CocE T172R/G173Q resulted in a significant decrease in cocaine-reinforced responding. Similar to the pattern of responding observed when saline was substituted for cocaine, pretreatment with 1.0 mg/rat CocE T172R/G173Q resulted in rates of 0.1 mg/kg/injection cocaine-reinforced responding that were approximately equivalent to those observed in baseline sessions for the first 5 to 15 min with lower rates of responding for the remainder of the session (Fig. 4B). Although QL values for sessions in which rats were pretreated with 1.0 mg/rat CocE T172R/G173Q (0.07 ± 0.02; p < 0.05) were significantly lower than baseline sessions in which responding was reinforced by 0.1 mg/kg/injection, these QL values did not differ from those obtained during saline substitution.
Table 2.
Comparison of the effects of pretreatment with WT CocE, CocE T172R/G173Q, and saline substitution on quarter-life values for responding maintained by cocaine, WIN-35065-2, or liquid food
| Baseline Reinforcer | Quarter-life (± S.E.M.) | |||||
|---|---|---|---|---|---|---|
| Baseline | Salinea | Baseline | DM CocEb | Baseline | WT CocEc | |
| Cocaine | ||||||
| 0.1 mg/kg/injection | 0.26 (0.03) | 0.12 (0.03)*** | 0.20 (0.02) | 0.08 (0.02)*** | 0.24 (0.04) | 0.37 (0.05)**,††† |
| 0.32 mg/kg/injection | 0.11 (0.02) | 0.08 (0.01) | 0.11 (0.01) | 0.08 (0.01) | ||
| 1.0 mg/kg/injection | 0.05 (0.01) | 0.09 (0.02) | 0.05 (0.01) | 0.23 (0.05)**,† | ||
| WIN-35065-2 | ||||||
| 0.01 mg/kg/injection | 0.25 (0.04) | 0.15 (0.03)* | 0.23 (0.02) | 0.35 (0.02)*,†† | ||
| Liquid food | ||||||
| 50 μl of Ensure | 0.24 (0.01) | 0.08 (0.02)*** | 0.25 (0.01) | 0.24 (0.01)††† |
In contrast with when responding was reinforced with 0.1 mg/kg/injection cocaine, when 0.32 mg/kg/injection cocaine was available, responding occurred at high rates during the initial ∼5 min of sessions, then slowed and occurred at a lower, but stable rate throughout the remainder of the session (Fig. 4, D and E) with mean QL values of 0.11 ± 0.02 (Table 2). Although there were no significant differences in the patterns of responding maintained by 0.32 mg/kg/injection cocaine and saline, there was a statistical trend toward lower QL values when rats were pretreated with 1.0 mg/rat (2.87 ± 0.03 mg/kg) CocE T172R/G173Q compared with baseline patterns of responding for 0.32 mg/kg/injection cocaine (QL values = 0.11 ± 0.02, and 0.08 ± 0.01 for cocaine alone, and after CocE pretreatment, respectively; p = 0.07; Fig. 4, D and E). Similar to when 0.32 mg/kg/injection cocaine was available, high rates of responding were observed during the initial few minutes of the sessions in which 1.0 mg/kg/injection cocaine was available, with lower, but stable rates of responding occurring for the remainder of the session (Fig. 4, F and G), and mean QL values of 0.05 ± 0.01. Although rats actually earned more injections during the single-session saline substitutions compared with when responding was reinforced with 1.0 mg/kg/injection cocaine, the majority of the responding still occurred within the initial 5 min of the session, with responding slowing throughout the session as observed during saline substitutions from the two lower doses of cocaine (Fig. 4, A, D, and F), with a mean QL value of 0.09 ± 0.02. Conversely, 1.0 mg/kg/injection cocaine maintained high rates of responding throughout the 60-min session when rats were pretreated with 1.0 mg/rat (2.86 ± 0.02 mg/kg) CocE T172R/G173Q, with mean QL values significantly greater than those observed during baseline sessions in which responding was reinforced by 1.0 mg/kg/injection cocaine (QL value = 0.23 ± 0.05; p < 0.001).
Effects of CocE T172R/G173Q on WIN-35065-2- and Liquid-Food-Reinforced Responding.
To determine whether the suppression of cocaine-reinforced responding by CocE T172R/G173Q was a selective effect of the esterase's action on cocaine, or a nonselective suppression of responding, the effective dose of 1.0 mg/rat CocE T172R/G173Q was evaluated for its capacity to alter responding maintained by the nonhydrolyzable cocaine analog, WIN-35065-2 (0.01 mg/kg/injection), and a 10-s access to liquid food (50 μl of Ensure), and compared with responding maintained during single-session extinction probes (saline injection or empty-dipper presentation). As shown in Fig. 5, high rates of responding were maintained throughout the experimental protocol when responding was reinforced by either 0.01 mg/kg/injection WIN-35065-2 (∼80 injections per session), or liquid food (∼190 presentations per session). Similar to the effects of substituting saline for cocaine (Figs. 3 and 4), significant decreases in the overall rates of responding were observed when saline was substituted for WIN-35065-2 (Fig. 5A; p < 0.001) and when responding resulted in the presentation of an empty dipper cup (Fig. 5B; p < 0.001). However, in contrast with 0.1 mg/kg/injection cocaine, pretreatment with 1.0 mg/rat (WIN: 2.88 ± 0.02 mg/kg; food: 2.86 ± 0.02 mg/kg) CocE T172R/G173Q failed to significantly alter responding that was reinforced by either WIN-35065-2 or liquid food; however, a slight decrease in WIN-35065-2-reinforced responding was observed in all rats after pretreatment with 1.0 mg/rat CocE T172R/G173Q.
A representative set of cumulative records for one rat maintained on WIN-35065-2, and another rat maintained on liquid food are shown in Fig. 5. As shown in Fig. 5, C and E, 0.01 mg/kg/injection WIN-35065-2 maintained high rates of responding with mean QL values (0.22 ± 0.02 − 0.24 ± 0.04) similar to those observed for cocaine; however, responding occasionally slowed toward the end of the 60-min session. Substitution of saline for WIN-35065-2 (Fig. 5C) resulted in pattern of responding that was similar to that observed when saline was substituted for 0.1 mg/kg/injection cocaine, and resulted in a significant decrease in the mean QL value (0.13 ± 0.02; p < 0.05) compared with baseline sessions in which responding was maintained by WIN-35065-2. Although 1.0 mg/rat (2.88 ± 0.02 mg/kg) CocE T172R/G173Q did not affect the overall rate of responding for WIN-35065-2, an effect on the pattern of intrasession responding with a significant increase in the mean QL value (0.34 ± 0.02; p < 0.05) was observed after pretreatments with CocE T172R/G173Q (Fig. 5E). Similar to 0.1 mg/kg/injection cocaine and WIN-35065-2, liquid food maintained high rates of responding that were stable throughout the experimental protocol, with mean QL values (0.24 ± 0.01 − 0.25 ± 0.01) similar to those observed for drug reinforcers (Fig. 5, D and F). Likewise, these high rates of responding were sensitive to the reinforcer because responding occurred at significantly lower levels when the reinforcer was omitted, with responding slowing over the course of the 60-min session, and mean QL values (0.08 ± 0.02; p < 0.001) significantly lower than those observed when responding was maintained by food presentation (Fig. 5D). However, as shown in Fig. 5F, pretreatment with 1.0 mg/rat (2.88 ± 0.02 mg/kg) CocE T172R/G173Q did not alter the intrasession pattern of responding that was maintained by liquid food because there was no difference in the mean QL values obtained during baseline conditions (0.25 ± 0.01) and sessions in which rats were pretreated with CocE T172R/G173Q (0.24 ± 0.01).
Discussion
The current studies were aimed at evaluating the capacity of a longer acting mutant form of CocE (CocE T172R/G173Q) to protect against the lethal effects of cocaine, and to alter the reinforcing effects of cocaine, WIN-35065-2, and food in the rat. Similar to reports in mice (Gao et al., 2009), CocE T172R/G173Q produced a dose-dependent inhibition of the cocaine-induced convulsion and lethality, with observed durations of action of approximately 25, 80, and 220 min for doses of 1.0 mg/kg (0.29 ± 0.002 mg/rat), 3.0 mg/kg (0.93 ± 0.01 mg/rat), and 10.0 mg/kg (2.96 ± 0.04 mg/rat) CocE T172R/G173Q, respectively. CocE T172R/G173Q produced a similar dose-dependent decrease in 0.1 mg/kg/injection cocaine-reinforced responding with significant decreases observed after doses of 0.32 and 1.0 mg/rat (0.93 ± 0.01 and 2.97 ± 0.02 mg/kg, respectively). Moreover, pretreatment with 1.0 mg/rat (2.86 ± 0.02 mg/kg) CocE T172R/G173Q produced a 10-fold rightward shift of the dose-response curve for cocaine self-administration without affecting responding for other drug or nondrug reinforcers. Taken together, these studies provide strong evidence that the thermostable mutant form of the cocaine-hydrolyzing enzyme, CocE T172R/G173Q, is capable of providing not only long-lasting protection against the toxic effects of cocaine, but that it is also capable of inhibiting the reinforcing effects of cocaine in rats.
Similar to previous reports with WT CocE in rats and mice (Cooper et al., 2006; Ko et al., 2007), CocE T172R/G173Q produced a dose-dependent protection against cocaine-induced convulsion and lethality. However, unlike WT CocE, which had an in vivo duration of action of approximately 15 min (Cooper et al., 2006), a similar dose of 3.0 mg/kg (0.93 ± 0.01 mg/rat) CocE T172R/G173Q was capable of protecting rats for approximately 80 min. Although the duration of WT CocE's protective effects is sufficient to inhibit the lethal effects of cocaine (Cooper et al., 2006), this relatively short duration of action was insufficient to alter the reinforcing effects of even a low dose of cocaine over a 60-min period, with only a transient suppression of cocaine-reinforced responding observed after pretreatment with 1.0 mg/rat (2.83 ± 0.01 mg/kg) WT CocE. Conversely, the prolonged in vivo duration of action of CocE T172R/G173Q was sufficient to affect the ongoing self-administration cocaine with 0.1 mg/kg/injection cocaine maintaining saline-like rates and patterns of responding throughout the 60 min after pretreatment with 1.0 mg/rat (2.85 ± 0.02 mg/kg) CocE T172R/G173Q. Not only do these findings suggest that the longer acting CocE T172R/G173Q is capable of suppressing the ongoing self-administration of cocaine, but also that the duration of suppression is correlated with the in vivo duration of action of the cocaine-hydrolyzing enzymes.
In the current studies, cocaine maintained dose-dependent levels of responding with high rates of responding observed when 0.1 mg/kg/injection cocaine was available, and low, but stable rates of responding observed when 1.0 mg/kg/injection was available. Intermediate rates of responding were observed during the single-session saline substitutions regardless of the maintenance dose of cocaine. Based on the fact that cocaine- and saline-maintained rates and intrasession patterns of responding were easily differentiated when responding was maintained by a relatively low dose of cocaine, the capacities of different doses of CocE T172R/G173Q to alter cocaine-reinforced responding were evaluated in rats responding for 0.1 mg/kg/injection cocaine. CocE T172R/G173Q had biphasic effects on cocaine-reinforced responding, with the lowest active dose of CocE T172R/G173Q (0.1 mg/rat = 0.29 ± 0.02 mg/kg) resulting in an increased rate of cocaine-maintained responding, and dose-dependent and significant decreases in the number of cocaine injections earned after higher doses of 0.32 mg/rat (0.93 ± 0.01 mg/kg) and 1.0 mg/rat (2.97 ± 0.02 mg/kg) CocE T172R/G173Q. Not only did pretreatment with 1.0 mg/rat (2.97 ± 0.02 mg/kg) CocE T172R/G173Q decrease the total number of cocaine injections earned to an extent that was equal to or greater than that which was observed during saline substitution, but the patterns of cocaine-reinforced responding also were more similar to those maintained by saline [QL values = (0.08 ± 0.02 − 0.10 ± 0.02) and (0.10 ± 0.03 − 0.12 ± 0.03) for CocE T172R/G173Q and saline, respectively] than when responding was reinforced by 0.1 mg/kg/injection cocaine (QL values = 0.20 ± 0.02 − 0.23 ± 0.01), suggesting that 1.0 mg/rat (2.97 ± 0.02 mg/kg) CocE T172R/G173Q was capable of completely antagonizing the reinforcing effects of 0.1 mg/kg/injection cocaine.
Although the high rates of responding maintained by 0.1 mg/kg/injection cocaine provided an ideal baseline to determine whether a sufficiently high dose of CocE T172R/G173Q could completely antagonize the reinforcing effects of lower doses of cocaine, stoichiometric limitations of enzymatic approaches to treat drug abuse necessitated the evaluation of CocE T172R/G173Q against larger unit-doses of cocaine. Despite the fact that 1.0 mg/rat (2.85 ± 0.02 mg/kg) CocE T172R/G173Q inhibited responding maintained by 0.1 mg/kg/injection cocaine to saline-like levels, CocE Tr17R/G173Q (1.0 mg/rat = 2.87 ± 0.03 mg/kg) did not significantly alter the overall rates of responding for a moderate dose of 0.32 mg/kg/injection cocaine. However, it should be noted that, although the overall rates of responding were not different, there was a trend toward lower QL values when rats were pretreated with 1.0 mg/rat CocE T172R/G173Q, suggesting that CocE T172R/G173Q slowed responding for 0.32 mg/kg/injection cocaine in the later portion of the session, as is seen with saline substitution. Conversely, when rats were allowed to respond for 1.0 mg/kg/injection cocaine, pretreatment with 1.0 mg/rat (2.86 ± 0.02 mg/kg) CocE T172R/G173Q resulted in large increases in responding, with an average cocaine intake during the CocE condition of greater than 122 mg/kg/h. Not only did all of these rats survive, but the rates and intrasession patterns of responding for 1.0 mg/kg/injection cocaine also were similar to those maintained by a 10-fold lower dose of cocaine (0.1 mg/kg/injection), suggesting that 1.0 mg/rat CocE T172R/G173Q and effectively reducing the functional dose of cocaine available for injection resulted in a 10-fold rightward shift in the reinforcing effects of cocaine.
It is noteworthy that these effects of CocE T172R/G173Q on cocaine-reinforced responding were observed at a dose that did not significantly alter rates of responding for the nonhydrolyzable cocaine analog, WIN-35065-2, or liquid food. This behavioral selectivity is further supported by the fact that CocE T172R/G173Q (1.0 mg/rat = 2.86 ± 0.02 mg/kg) failed to affect the intrasession patterns of responding for liquid food; however, pretreatment with 1.0 mg/rat (2.88 ± 0.02 mg/kg) CocE T172R/G173Q did alter the patterns of responding for WIN-35065-2. Although the rates of WIN-35065-2-reinforced responding were slowed during the initial portion of the session after pretreatment with CocE T172R/G173Q, responding during later portions of the session typically occurred at near baseline rates. These effects of CocE T172R/G173Q on the patterns of WIN-35065-2-reinforced responding were more similar to those observed with the short-acting WT CocE on 0.1 mg/kg/injection cocaine-reinforced responding than the longer acting CocE T172R/G173Q, suggesting that the effects of CocE T172R/G173Q were surmounted as the session progressed. Because WIN-35065-2 is a cocaine analog that lacks the ester bond that is hydrolyzed by CocE, it is likely that the initial suppression of responding resulted from a sequestration of WIN-35065-2 by CocE T172R/G173Q, which was later surmounted as might be predicted once blood levels of the nonhydrolyzable cocaine analog WIN-35065-2 were sufficiently high to saturate the cocaine binding site of CocE T172R/G173Q.
Although these are not the first studies to evaluate the protein-based pharmacokinetic approach to altering reinforcing properties of cocaine, they do suggest that longer acting cocaine-hydrolyzing enzymes, such as CocE T172R/G173Q, may have distinct advantages compared with other protein-based approaches (i.e., cocaine-specific vaccines, catalytic antibodies, and other hydrolyzing enzymes). For instance, although cocaine-specific vaccines have provided promising results in both rodents (Fox et al., 1996; Carrera et al., 2000; Kantak et al., 2000) and humans (Martell et al., 2005; Kosten et al., 2008), their capacity to decrease the subjective and reinforcing effects of cocaine, in general, has been limited by the need for high levels of circulating antibodies. Moreover, significant increases in cocaine intake have been observed in both rats and humans when antibody levels are too low (Carrera et al., 2000; Kantak et al., 2000; Martell et al., 2005; Kosten et al., 2008), an effect that may result in an increased risk for cocaine toxicity as the antibodies simply sequester the cocaine in the periphery, but do not increase its clearance. One approach to circumvent these problems has been the development of cocaine-specific, catalytic antibodies, which not only bind cocaine in the periphery, but also catalyze its degradation to inactive metabolites (Landry et al., 1993), a strategy that has been shown to inhibit cocaine self-administration at doses that did not alter responding reinforced by other drug and nondrug reinforcers (Mets et al., 1998; Baird et al., 2000). However, because of the relatively poor catalytic efficiency of mAb 1510A (∼26-fold lower than native BChE) relatively large doses (12–30 mg/kg) were required to affect cocaine-reinforced responding. A mutant butyrylcholinesterase with significantly improved catalytic efficiency (∼456-fold greater than native BChE; Pan et al., 2005) has recently been reported to selectively inhibit the reinstatement of responding by acute cocaine challenge (Brimijoin et al., 2008), but it is unclear whether this enzyme would have similar effects on cocaine self-administration.
Although CocE T172R/G173Q exhibits some of the same limitations as cocaine-specific vaccines (i.e., the inhibitory effects of CocE T172R/G173Q are surmountable by larger unit-doses of cocaine), CocE T172R/G173Q possesses a distinct advantage compared with cocaine-specific vaccines in that it continues to metabolize cocaine as long as it is active, thus reducing the potentially toxic effects of higher levels of cocaine intake. In addition, CocE T172R/G173Q has advantages over other cocaine-hydrolyzing proteins because of its superior catalytic efficiency (∼800-fold greater than native BChE; Larsen et al., 2002; Turner et al., 2002; Gao et al., 2009), resulting in significant improvements in potency, and substantially larger rightward shifts of the cocaine dose-response curve compared with mAb 1510A. Despite these promising effects of CocE T172/G173Q on cocaine's toxic and reinforcing properties, it is likely that further improvements to CocE will be required for the successful treatment of a chronic relapsing disease such as cocaine abuse. For instance, although CocE T172/G173Q represents a significant advancement toward extending the in vivo duration of action (∼15 times longer than WT CocE), significantly longer acting enzymes would be required to reduce the need for frequent dosing. Moreover, although repeated exposure to CocE T172R/G173Q did not alter the effectiveness of CocE T172R/G173Q to affect cocaine self-administration in the current studies, previous studies have reported a modest loss of function after repeated exposure to the WT CocE in mice with high anti-CocE antibody titers (Ko et al., 2007). Mutagenesis studies are currently underway to identify other mutant forms of CocE with even longer durations of action and reduced immunogenicity.
In summary, these studies describe a series of experiments that demonstrate the capacity of the longer acting mutant, CocE T172R/G173Q, to provide a long-lasting protection against cocaine-induced convulsion and lethality, and a dose-dependent and selective inhibition the ongoing self-administration of cocaine in rats. Not only did increasing doses of CocE T172R/G173Q decrease responding maintained by 0.1 mg/kg/injection cocaine to saline-like levels, but 1.0 mg/rat (2.87 ± 0.01 mg/kg) CocE T172R/G173Q produced a 10-fold rightward shift in the dose-response curve for cocaine self-administration without affecting responding for other drug and nondrug reinforcers. These studies provide strong evidence to support the notion that cocaine-hydrolyzing enzymes, such as CocE T172R/G173Q, with high catalytic efficiencies and prolonged durations of action are capable of selectively inhibiting the ongoing self-administration of cocaine, and may prove to be a valuable strategy for the treatment of cocaine abuse in humans.
Acknowledgments
We thank Davina Barron and Nhu Truong for excellent technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA021416, DA023213, F013771].
ABBREVIATIONS:
BChE
butyrylcholinesterase
ANOVA
analysis of variance
CocE
cocaine esterase
DM
double mutant
FR
fixed ratio
mAb
monoclonal antibody
PBS
phosphate-buffered saline
TO
timeout
QL
quarter-life
WT
wild type
WIN-35065-2
(−)-2β-carbomethoxy-3β-phenyltropane.
References
- Baird et al., 2000.Baird TJ, Deng SX, Landry DW, Winger G, Woods JH. (2000) Natural and artificial enzymes against cocaine. I. Monoclonal antibody 15A10 and the reinforcing effects of cocaine in rats. J Pharmacol Exp Ther 295:1127–1134 [PubMed] [Google Scholar]
- Brimijoin et al., 2008.Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. (2008) A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera et al., 2000.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. (2000) Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci U S A 97:6202–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll et al., 2006.Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. (2006) Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse. AAPS J 8:E196–E203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper et al., 2006.Cooper ZD, Narasimhan D, Sunahara RK, Mierzejewski P, Jutkiewicz EM, Larsen NA, Wilson IA, Landry DW, Woods JH. (2006) Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol Pharmacol 70:1885–1891 [DOI] [PubMed] [Google Scholar]
- Dackis and O'Brien, 2003.Dackis C, O'Brien C. (2003) Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci 1003:328–345 [DOI] [PubMed] [Google Scholar]
- Fox et al., 1996.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, et al. (1996) Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med 2:1129–1132 [DOI] [PubMed] [Google Scholar]
- Gao et al., 2009.Gao D, Narashimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara R, Zhan CG. (2009) Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol 75:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski et al., 2004.Grabowski J, Shearer J, Merrill J, Negus SS. (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, 1996.Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC: [Google Scholar]
- Jutkiewicz et al., 2009.Jutkiewicz EM, Baladi MG, Cooper ZD, Narasimhan D, Sunahara RK, Woods JH. (2009) A bacterial cocaine esterase protects against cocaine-induced epileptogenic activity and lethality. Ann Emerg Med 54:409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak et al., 2000.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. (2000) Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 148:251–262 [DOI] [PubMed] [Google Scholar]
- Ko et al., 2007.Ko MC, Bowen LD, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Cooper ZD, Woods JH. (2007) Cocaine esterase: interactions with cocaine and immune responses in mice. J Pharmacol Exp Ther 320:926–933 [DOI] [PubMed] [Google Scholar]
- Kosten et al., 2008.Kosten T, Martell B, Poling J, Gardner T. (2008) Cocaine vaccine: 6-month follow-up during drop in antibody levels (abstract 394); College on Problems of Drug Dependence Annual Meeting; 2008 June 14-18; San Juan, Puerto Rico: [Google Scholar]
- Kosten et al., 2002.Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, Fox B. (2002) Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine 20:1196–1204 [DOI] [PubMed] [Google Scholar]
- Landry and Yang, 1997.Landry DW, Yang GX. (1997) Anti-cocaine catalytic antibodies–a novel approach to the problem of addiction. J Addict Dis 16:1–17 [DOI] [PubMed] [Google Scholar]
- Landry et al., 1993.Landry DW, Zhao K, Yang GX, Glickman M, Georgiadis TM. (1993) Antibody-catalyzed degradation of cocaine. Science 259:1899–1901 [DOI] [PubMed] [Google Scholar]
- Larsen et al., 2002.Larsen NA, Turner JM, Stevens J, Rosser SJ, Basran A, Lerner RA, Bruce NC, Wilson IA. (2002) Crystal structure of a bacterial cocaine esterase. Nat Struct Biol 9:17–21 [DOI] [PubMed] [Google Scholar]
- Martell et al., 2005.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. (2005) Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry 58:158–164 [DOI] [PubMed] [Google Scholar]
- Mello, 1990.Mello NK. (1990) Preclinical evaluation of the effects of buprenorphine, naltrexone and desipramine on cocaine self-administration. NIDA Res Monogr 105:189–195 [PubMed] [Google Scholar]
- Mets et al., 1998.Mets B, Winger G, Cabrera C, Seo S, Jamdar S, Yang G, Zhao K, Briscoe RJ, Almonte R, Woods JH, et al. (1998) A catalytic antibody against cocaine prevents cocaine's reinforcing and toxic effects in rats. Proc Natl Acad Sci U S A 95:10176–10181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman et al., 2005.Newman AH, Grundt P, Nader MA. (2005) Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem 48:3663–3679 [DOI] [PubMed] [Google Scholar]
- Pan et al., 2005.Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. (2005) Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci U S A 102:16656–16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt et al., 2002.Platt DM, Rowlett JK, Spealman RD. (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163:265–282 [DOI] [PubMed] [Google Scholar]
- Roberts and Brebner, 2000.Roberts DC, Brebner K. (2000) GABA modulation of cocaine self-administration. Ann N Y Acad Sci 909:145–158 [DOI] [PubMed] [Google Scholar]
- Rothman et al., 2008.Rothman RB, Baumann MH, Prisinzano TE, Newman AH. (2008) Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol 75:2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2007.Substance Abuse and Mental Health Services Administration (SAMHSA) (2007) Results from the 2006 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07–4293. SAMHSA, Rockville, MD: [Google Scholar]
- Turner et al., 2002.Turner JM, Larsen NA, Basran A, Barbas CF, III, Bruce NC, Wilson IA, Lerner RA. (2002) Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry 41:12297–12307 [DOI] [PubMed] [Google Scholar]