Osmolality-induced tuning of action potentials in trigeminal ganglion neurons (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 6.
Abstract
The present study explored the effect of anisotonicity on action potential (AP) in cultured trigeminal ganglion (TG) neurons. We demonstrate that the number of evoked APs was increased by both hypo- and hypertonic treatment. Transient Receptor Potential Vanilloid 4 receptor (TRPV4) activator increased the number of APs, but only hypotonic-response was markedly blocked in TRPV4−/− mice. Additionally, inhibition of PKC attenuated hypotonicity-induced increase, whereas antagonism of PKA attenuated hypertonicity-response. We conclude that anisotonicity increases excitability of nociceptors, which might be involved in anisotonicity-induced nociception. The increase of APs by hypo- and hypertonicity is mediated through different receptor and intracellular signaling pathways.
Keywords: osmolality, action potential, TRPV4, intracellular signaling pathway, nociception
Maintenance of osmotic balance is of particular significance for nervous systems of metazoan animals. Osmolality plays an important role in the regulation of neurons excitability [1]. For instance, changes in osmolality have been reported to affect the excitability in neocortical slices [2]. Recent studies report that both hypo- and hypertonicity can stimulate C-fiber afferent and consequently produce pain-related behavior [3, 4]. Besides this, anisotonicity is also involved in neuropathic pain and the nociceptive signal transductions induced by pH, mechanical stimuli and inflammators [3, 5, 6, 7]. Tonicity-induced modulations of ion channels or receptors, such as Transient Receptor Potential Vanilloid 1 receptor (TRPV1), voltage-gated calcium (VGCCs) and potassium channels (VGPCs) [8, 9, 10] have been recently reported. Additionally, APs in rat sensory myelinated fibers are selectively blocked by hyerosmolar solutions [11]. However, there is lack of evidence concerning the direct effect of anisotonicity on the action potential (AP) in primary sensory neurons. Transient Receptor Potential Vanilloid 4 (TRPV4), an important osmotic cellular sensor, is present in sensory neurons that express properties of nociceptors [5, 12, 13, 18]. Accumulating data support an involvement of TRPV4 in anisotonicity-induced nociception [3, 4]. The present study examined the effect of hypo- and hypertonicity on AP in cultured TG neurons and further explored whether TRPV4 receptor and some intracellular signal pathways were selectively involved in this process.
All experiments were performed in accordance with the standards established by the National Institutes of Health and with the approval of the Duke University Institutional Animal Care and Use Committee. Trigeminal ganglions from male Sprague-Dawley rats (180–200 g) and mice (C57BL/6 wild type and TRPV4 knockout) were dissected aseptically, diced into small pieces, and then incubated in 3 ml modified Hank’s balanced salt solution (mHBSS) with 0.1% collagenase (Type XI-S) for 20–40 min at 37 °C. The mHBSS solution contains (in mM): NaCl 130, KCl 5, KH2PO4 0.3, NaHCO3 4, Na H2PO4 0.3, D-glucose 5.6, and EGTA 10, HEPES 10 at pH 7.4. Individual cells were dissociated by triturating them, followed by a 10 min incubation at 37 °C with 10 μg/ml DNase I (Type IV) in F-12 medium (Life Technologies, Gaithersburg, MD) and centrifuged for 3 times at 5×1500 rpm/min. The cells were cultured in F-12 supplemented with 10% fetal bovine serum for 24 hr at 37 °C in a water saturated atmosphere with 5% CO2.
Whole-cell path clamp experiments were performed at room temperature. Recordings were obtained using an Axopatch-200B patch clamp amplifier (Axon Instruments, Foster City, CA) and the output was digitized with a Digidata 1322A converter (Axon Instruments). The sampling rate was 10 kHz. Current-clamp configuration was used to record APs. The resistance of glass pipettes was 1–2 MΩ when filled with pipette solution composed of (in mM): K-Asp 118, KCl 20, MgCl2 2, CaCl2 1, EGTA10, HEPES 10, Na2ATP 5 at pH 7.2 and osmolality 300mOsm. TG neurons were continuously perfused with extracellular solution composed of (mM): NaCl 88, KCl 5, MgCl2 1, CaCl2 2, HEPES 10, D-mannitol 106 at pH 7.4 and osmolality 300mOsm. Hypo- and hypertonic solutions were obtained by adjusting the concentration of D-Mannitol. After studying the effect of anisotonic stimuli on evoked APs, capsaicin- and 4α-PDD-induced currents were recorded by switching to voltage-clamp configuration. The currents were recorded in “standard external solution”, composed of (in mM): NaCl 147, KCl 5, MgCl2 1, CaCl2 2, D-glucose 10, HEPES 10 at pH 7.4 and osmolality 300mOsm, with the holding potential being −60 mV. The capacitance and series resistance (<90%) was compensated. Data obtained from neurons in which uncompensated series resistance resulted in voltage-clamp errors > 5mV were not taken in further analysis. The osmolality was measured using the Advanced Micro Osmometer, model 3300 (Advanced instruments Inc, Norwood, Massachusetts).
Data are expressed as means±S.E.M. Recorded data were analyzed with pClamp (Axon Instruments, Foster City, CA) and SigmaPlot (SPSS Inc., Chicago IL) software. Paired or unpaired t test was used for statistical analysis with the significance level set at P < 0.05.
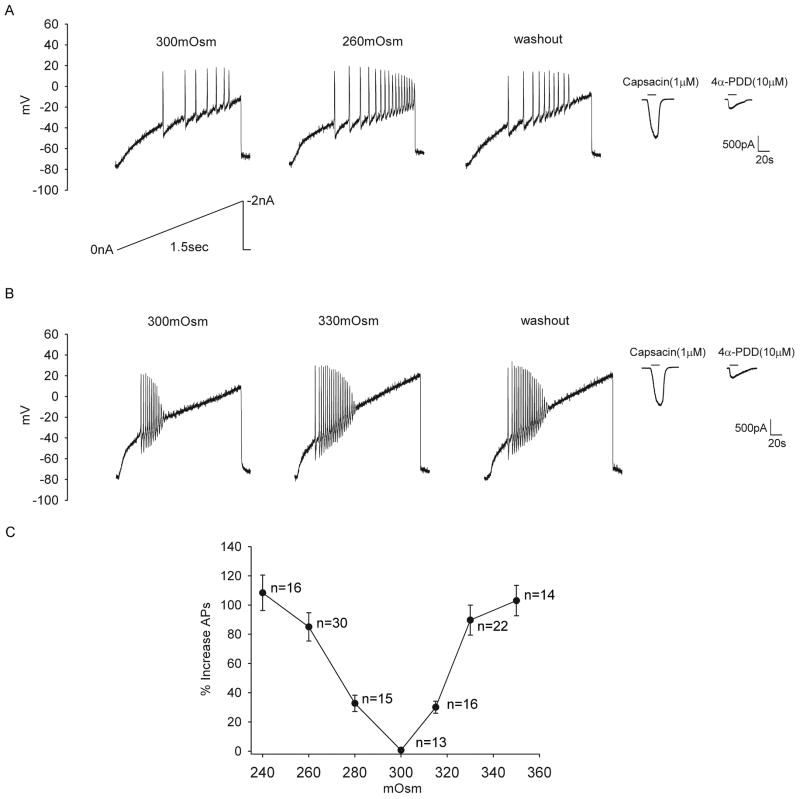
The resting membrane potential was adjusted to −80 mV at the beginning of the experiment. In order to examine the effect of anisotonicity on the number of evoked AP, APs were trigged by ramps ranging from 0 to −2nA in 1.5 seconds [14]. Hypo- and hypertonic stimuli were applied in different TG neurons. It was found that when the extracellular solution was changed from istonic (300mOsm) to hypotonic solution (260mOsm), the number of APs was increased by 85.1±9.7% (n=30, paired t test, P<0.01). After hypotonicity was washed out for 5 min, the number of APs was partially recoverable (Fig. 1A). Similarly, after exposed to hypertonic solution (330mOsm), the number of APs was increased by 89.7±10.3% (n=22, paired t test, P<0.01). After a 5 min washout, the number of APs was not markedly affected (Fig. 1B). Here it is noted that modulation of APs by hypotonicity was partially reversible (about 50%), whereas hypertonicity-induced modulation not. The dose–response curve of the inhibition by anisotonic stimuli was presented in Fig. 1C. Since 260mOsm and 330mOsm exhibited marked modulation on APs and these changes in osmolality were modest, these concentrations were used in all subsequent experiments.
Figure 1. Effect of anisotonicity on APs in TG neurons.
A. The number of evoked APs was increased from 9.3±3.1 to 17.2±3.4 (n=30, paired t test, P<0.01) by hypotonic treatment (260mOsm) and recovered to 13.5±3.0 after washout. Typical recordings show that the number of APs triggered by ramp protocol was 7, 17 and 10 before, during and after hypotonic treatment. Capsacin- and 4α-PDD-induced currents were recorded in the same TG neuron. B. The number of evoked APs was increased from 8.8±4.1 to 16.7±2.1 (n=22, paired t test, P<0.01) by hypertonic treatment (330mOsm). After washout, the number of APs was 15.7±1.3. Typical recordings show that the number of APs was 10, 19 and 18 before, during and after hypertonic treatment. Capsacin- and 4α-PDD-induced currents were recorded in the same TG neuron. C. Plot of the percentage in increased APs as a function of osmolality shows that more effect is produced with larger osmotic gradient.
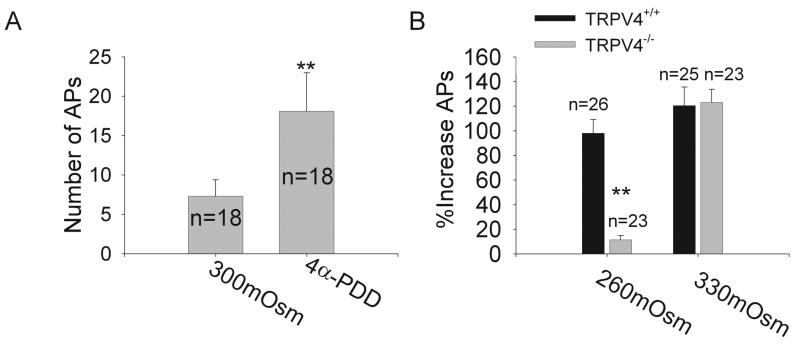
We continued to explore whether TRPV4 was involved in the modulation of APs induced by anisotonicity. Here, we firstly tested the effect of TRPV4 receptor agonist 4α-PDD on APs. After exposed to 10 μM 4α-PDD for 3 min, the number of APs was increased by 140±2.5% (n=18, paired t test, P<0.01) (Fig. 2A). After 4α-PDD was washed out, the number of APs was partially (about 70%) recoverable (data not shown). We further tested the effect of anisotonicity on APs in TG neurons from TRPV4+/+ and TRPV4−/− mice. Similar with the results obtained from rat TG neurons, in TRPV4+/+ mice TG neurons, the number of APs was increased 98.0±11.3% (n=26, paired t test, P<0.01) and 120.5±15.2% (n=25, paired t test, P<0.01) by hypo- and hypertonic treatment, respectively. Here it is noted that the excitability of TRPV4−/− mice TG neurons was not changed by hypotonic treatment. However, in TRPV4−/− mice TG neurons the number of APs was irreversibly increased by 123.0±10.8% (n=23, paired t test, P<0.01) after exposed to hypertonicity (Fig. 2B).
Figure 2. Involvement of TRPV4 receptor in hypotonic modulation.
A. After exposed to 10 μM 4α-PDD, the number of APs was markedly increased from 7.3±2.1 to 18.1±4.9. **P<0.01 _vs._ 300mOsm, paired t test. **B.** After hypotonic treatment, the number of APs was reversibly increased by 98.0±11.3% (from 5.4±1.5 to 10.7±2.0, n=26, paired t test, _P_<0.01) in TRPV4+/+ mice TG neurons, but only by 11.1±3.4% (from 6.2±1.5 and 6.9±2.0, n=23, paired t test, _P_>0.05) in TRPV4−/− mice TG neurons. However, the number of APs was irreversibly increased by 120.5±15.2% (from 6.1±2.8 to 13.5±3.4, n=25, paired t test, P<0.01) and 123.0±10.8% (from 5.9±2.2 to 13.1±1.4, n=23, paired t test, P<0.01) in TRPV4+/+ and TRPV4−/− mice TG neurons, respectively. **P<0.01 vs. TRPV4+/+ mice, unpaired t test.
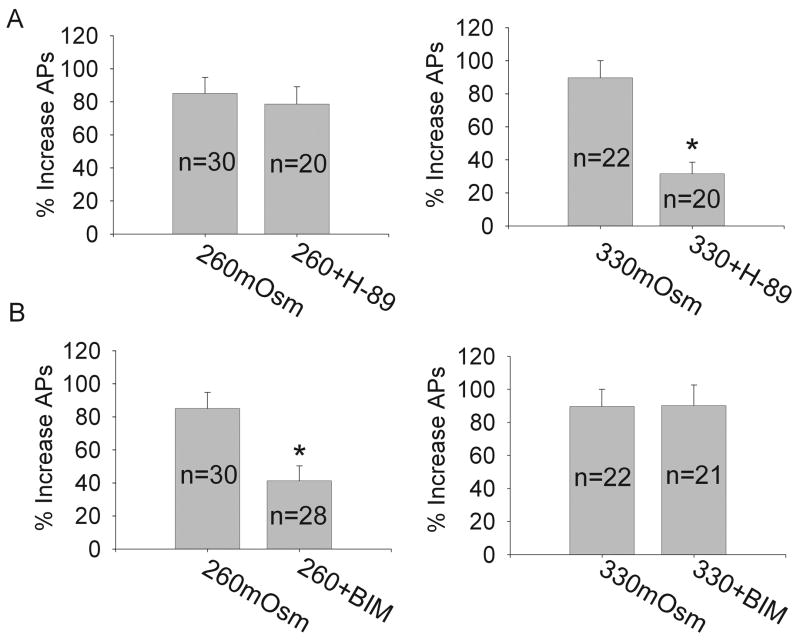
It is known that the excitability of neurons can be modulated by intracellular signaling pathways. In the present study we also tested some intracellular signaling pathways to determine whether they are involved in anistonicity-induced modulation of the firing pattern of APs. We firstly tested PKA system for previous studies report that activation of PKA can enhance the excitability of neurons [15]. It was found that after exposed to 1 mM pCPT-cAMP, APs was increased from 9.0±0.7 to 12.4±2.1 (n=8, paired t test, P<0.05), while after pre-incubation of PKA antagonist 10 μM H-89 for 10 min, APs was decreased from 8.6±1.6 to 6.1±0.8 (n=8, paired t test, P<0.05) in isotonic solution (data not shown). Here, after pre-treatment with H-89, APs was increased 78.7±10.3% (n=20, paired t test, P<0.01) and 31.5±7.1% (n=20, paired t test, P<0.05) by hypo- and hypertonic treatment, respectively (Fig. 3A).
Figure 3. Involvement of intracellular signaling pathway in hypo- versus hypertonic modulation.
A. For PKA system, hypotonicity-induced increase of APs was not affected by pre-incubation of H-89 and the number of APs was increased by 78.7±10.3%from 8.5±1.6 to 15.1±2.1 after exposed to hypotonicity (n=20, paired t test, P<0.01). By contrast, hypertonicity-induced modulation was markedly attenuated and the number of APs was increased only by 31.5±7.1% from 9.3±1.5 to 12.1±1.0 after hypertonic treatment (n=20, paired t test, P<0.05). *P<0.01 vs. 330mOsm, unpaired t test. B. For PKC system, hypotonicity-induced increase of APs was selectively attenuated in the presence of BIM and the number of APs was increased only by 41.3±9.0% from 9.1±1.6 to 13.0±0.8 after exposed to hypotonicity (n=28, paired t test, P<0.05). However, hypertonic-response was not unaffected by BIM and the number of APs was increased by 90.3±12.3% from 8.7±2.0 to 15.6±1.3 after hypertonic treatment (n=21, paired t test, P<0.01). *P<0.01 vs. 260mOsm, unpaired t test.
We then tested PKC system and found that after pre-incubation of 1 μM PMA, the agonist of PKC, the number of evoked APs was increased from 8.3±2.4 and 13.5±3.0 (n=9, paired t test, P<0.05) in the presence of isotonic extracellular solution. APs was reduced from 9.4±1.5 to 6.0±1.3 (n=8, paired t test, P<0.05) by pre-treatment with 1 μM BIM, the antagonist of PKC, in isotonic solution. After exposed to BIM, the number of APs was increased by 41.3±9.0% (n=28, paired t test, P<0.05) in the presence of hypotonic solution. However, hypertonicity-induced increase of APs was not markedly affected by pre-incubation of BIM (Fig. 3B).
In this study, we explored the effect of osmolality on APs in cultured small- to medium-sized TG neurons for this type of TG and DRG neurons are shown to have characteristics of nociceptors [16, 17]. After testing the effect of anisotonicity, 1 μM capsaicin and 10 μM 4α-PDD were applied in the isotonic extracellular solution respectively to test whether the neuron was capsaicin-sensitive and whether it had TRPV4 receptors. The data presented here were obtained from the neurons in which inward currents could be induced by both capsaicin and 4α-PDD (except those from TRPV4−/− mice TG neurons). By using depolarizing pulses, it was found that the threshold of TG neurons was not markedly affected by either hypo- (300mOsm: −52.7±1.5, 260mOsm: −54.9±3.1; n=30, paired t test, _P_>0.05) or hypertonic treatment (300mOsm: −53.0±2.7, 330mOsm: −55.9±2.4; n=22, paired t test, _P_>0.05) (data not shown). So the present study mainly focused on the effect of anisotonicity on the number of evoked APs. We demonstrate that after the treatment with hypo- and hypertonicity, the number of evoked APs was increased greatly, indicative of hyperexcitability of TG neurons (Fig. 1A & 1B). Additionally, anisotonicity produced more effect in the presence of larger osmotic gradient (Fig. 1C). These results provide the direct evidence which might be responsible for anisotonicity-induced nociception.
TRPV4 receptor can function as a transducer of osmotic and mechanical stimuli in the presence of inflammatory mediator [3, 6]. To test whether TRPV4 was involved in osmolality-induced modulation of APs, TRPV4 agonist (4α-PDD) and TRPV4−/− mice were used. It was found that 4α-PDD reversibly increased the number of APs (Fig. 2A), indicating that it mimicked the modulation induced by hypotonicity. In addition, the effect of hypotonicity was markedly blocked in TRPV4−/− mice TG neurons, whereas the effect of hypertonicity was unaffected (Fig 2B). Collectively, these results indicate that TRPV4 receptor is selectively responsible for hyponicity-induced hyperexcitability of TG neurons. Similarly, TRPV4 receptor is selectively involved in hypotonicity-induced modulation of VGCCs and VGPCs [9, 10] as well as GABA-activated currents and ATP-activated currents (Liu C et al., unpublished data). The present study also demonstrates that PKA inhibitor selectively attenuated the increase of APs induced by hypertonicity, while PKC inhibitor selectively attenuated hypotonicity-induced increase, indicating that intracellular signaling pathways are selective for hypo- versus hypertonic response. Here it is noted that the increase of APs by hypotonicity was reversible, whereas that by hypertonicity not. Combined with the above discussion, one of the possible reasons is that the effective time of different signaling pathway modulated by hypo- versus hypertonicity varies.
The electrical excitability of nociceptive neurons is an important component of the pain response, which is determined by the function and expression of a diverse group of ligand- and voltage-gated ion channels. Anisotonicity-induced modulation of ion channels is recently reported and through this modulation anisotonicity can produce algesic effect (for example, by increasing capsaicin-induced currents and decreasing VGPCs) as well as analgesic effect (for example, by inhibiting VGCCs) [8, 9, 10]. In this study, after exposed to anisotonicity, the number of evoked APs was markedly increased but the threshold was not significantly affected. Therefore, it is suggested that anisotonicity-induced increase of APs is likely due to the integrative modulations of ion channels on the membrane, that is, anisotonicity produces more algesia than analgesia. Besides this, the increased APs number might not be due to the direct modulation of sodium channels but result from the tuning of other ion channels (such as VGPC and Ca-activated potassium channels which can modulate the repetitive firing pattern [19]). Combined with previous reports, it is also suggested that different mechanisms underlie the nociception induced by hypo- versus hypertonicity. This conclusion is deduced mainly from the followings: first, hypo- and hypertonicity exhibit different effect on VGPCs [9]; second, although hypo- and hypertonicity produce similar effect on APs as well as some ion channels, intracellular pathways are selective for hypo- and hypertonicity-induced modulation [8, 9, 10]; third, although TRPV4 receptor is reported to mediate mild hypertonicity-induced pain-related behavior in vivo, there is still lack of evidence supporting the role of TRPV4 receptor in hypertonic-induced nociception in vitro [3]. However, the present study did not strive to reveal the effect of anisotonicity on the morphology of APs and more experiments need to be performed to further analyze the effect of anisotonicity on intrinsic electrical properties underlying nociceptors excitability. Anisotonic stimulus has been well known to be pro-nociceptive, illustrated by the universal understanding of “pouring salt into one’s wounds”. The present framework demonstrates that hypo- and hypertonic stimuli can lead to hyperexcitability of TG neurons, showing the direct evidence underlying the nociception induced by anisotonicity.
Figure A.
Acknowledgments
We thank Dr. Wolfgang Liedtke for TRPV4−/− mice. This work was supported by National Institute of General Medical Sciences Grant GM-63577 and by grants from Philip Morris Inc. USA and Philip Morris International and National Natural Science Foundation of China (30571537).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32:275–285. doi: 10.1016/s0920-1211(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosen AS, Andrew RD. Osmotic effects upon excitability in rat neocortical slices. Neuroscience. 1990;38:579–590. doi: 10.1016/0306-4522(90)90052-6. [DOI] [PubMed] [Google Scholar]
- 3.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 5.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamamoto DT, Forkey MW, Davis WL, Kajander KC, Simone DA. The role of pH and osmolality in evoking the acetic acid-induced wiping response in a model of nociception in frogs. Brain Res. 2000;862:217–229. doi: 10.1016/s0006-8993(00)02138-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Liu C, Liu L. Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res. 2008;1208:56–66. doi: 10.1016/j.brainres.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Liu C, Liu L. The modulation of voltage-gated potassium channels by anisotonicity in trigeminal ganglion neurons. Neuroscience. 2008;154:482–495. doi: 10.1016/j.neuroscience.2008.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Chen L, Liedtke W, Simon SA. Changes in Osmolality Sensitize the Response to Capsaicin in Trigeminal Sensory Neurons. J Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- 11.Matsuka Y, Spigelman I. Hyperosmolar solutions selectively block action potentials in rat myelinated sensory fibers: implications for diabetic neuropathy. J Neurophysiol. 2004;91:48–56. doi: 10.1152/jn.00689.2003. [DOI] [PubMed] [Google Scholar]
- 12.Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62:2985–3001. doi: 10.1007/s00018-005-5181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M, Watanabe Y, Oyama Y, Mizuno A, Kusano E, Hirao A, Ookawara S. Localization of mechanosensitive channel TRPV4 in mouse skin. Neurosci Lett. 2003;353:189–192. doi: 10.1016/j.neulet.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Yang T, Bruno MJ, Andersen OS, Simon SA. Voltage-gated ion channels in nociceptors: modulation by cGMP. J Neurophysiol. 2004;92:2323–2332. doi: 10.1152/jn.00355.2004. [DOI] [PubMed] [Google Scholar]
- 15.Song XJ, Wang ZB, Gan Q, Walters ET. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J Neurophysiol. 2006;95:479–492. doi: 10.1152/jn.00503.2005. [DOI] [PubMed] [Google Scholar]
- 16.Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium currents in four types of rat sensory neurons differentiated by membranes properties. J Neurophysiol. 1995;74:1870–1879. doi: 10.1152/jn.1995.74.5.1870. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Oortgiesen M, Li L, Simon SA. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J Neurophysiol. 2001;85:745–758. doi: 10.1152/jn.2001.85.2.745. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 19.Scholz A, Gruß M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurons of rat dorsal root ganglion studied in a thin slice preparation. J Physiol (London) 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]