Human RAP1 inhibits non-homologous end joining at telomeres (original) (raw)
Abstract
Telomeres, the nucleoprotein structures at the ends of linear chromosomes, promote genome stability by distinguishing chromosome termini from DNA double-strand breaks (DSBs). Cells possess two principal pathways for DSB repair: homologous recombination and non-homologous end joining (NHEJ). Several studies have implicated TRF2 in the protection of telomeres from NHEJ, but the underlying mechanism remains poorly understood. Here, we show that TRF2 inhibits NHEJ, in part, by recruiting human RAP1 to telomeres. Heterologous targeting of hRAP1 to telomeric DNA was sufficient to bypass the need for TRF2 in protecting telomeric DNA from NHEJ in vitro. On expanding these studies in cells, we find that recruitment of hRAP1 to telomeres prevents chromosome fusions caused by the loss of TRF2/hRAP1 from chromosome ends despite activation of a DNA damage response. These results provide the first evidence that hRAP1 inhibits NHEJ at mammalian telomeres and identify hRAP1 as a mediator of genome stability.
Keywords: cancer, DNA repair, genome instability, telomeres
Introduction
The prescient work of Barbara McClintock in the 1940s demonstrated that an essential function of telomeres is to prevent the fusion of chromosome ends to each other or to DNA breaks (McClintock, 1941). To fulfil this role, telomeres must locally inhibit the DNA damage response; a feat that involves TRF2 and POT1 repressing DNA damage signalling through ATM and ATR kinases (Denchi and de Lange, 2007). Telomere dysfunction can result from a variety of events, including structural changes at telomeres, loss of a telomere binding protein, or the gradual shortening of the telomeric repeat tract. Proteins that specifically bind to telomeric repeats have a critical role in chromosome end protection, and their deletion results in telomere fusions in a broad range of species (Baumann and Cech, 2001; Heacock et al, 2004; Celli and de Lange, 2005; Miller et al, 2005; Pardo and Marcand, 2005).
Early models predicted that telomere-binding proteins outcompete the non-sequence specific binding of DNA repair factors near chromosome ends. However, it has become apparent that key factors involved in DNA double-strand break (DSB) repair are present at chromosome termini without triggering end-to-end fusions (d'Adda di Fagagna et al, 2004; Longhese, 2008). The molecular mechanism underlying this phenomenon has remained elusive, but may relate to the t-loop, a structure in which the 3′ overhang of the telomere loops back and invades internal telomeric repeats on the same chromosome arm (Griffith et al, 1999). Such structures have been visualized by electron microscopy in DNA samples from a variety of species (Murti and Prescott, 1999; Munoz-Jordan et al, 2001; Nikitina and Woodcock, 2004; Tomaska et al, 2004).
A number of proteins have been identified that specifically localize to mammalian telomeres, including three factors that directly bind telomeric DNA: POT1, TRF1, and TRF2, and three associated proteins TIN2, RAP1 and TPP1 (Palm and de Lange, 2008). A truncated version of TRF2 (TRF2ΔBΔM) acts as a dominant-negative mutant by forming heterodimers with the endogenous protein that are unable to bind to DNA (van Steensel et al, 1998). Cells expressing TRF2ΔBΔM show reduced TRF2 at telomeres and chromosome ends are subject to non-homologous end joining (NHEJ; Smogorzewska et al, 2002). A requirement for TRF2 in chromosome capping is further supported by the marked telomere fusion phenotype observed in mouse embryonic fibroblasts after deletion of the TRF2 gene (Celli and de Lange, 2005).
Using an in vitro assay for telomere capping, we have previously shown that both hRAP1 and TRF2 are required to protect telomeric DNA ends from NHEJ (Bae and Baumann, 2007). By targeting hRAP1 to telomeric DNA in the absence of TRF2, we now demonstrate that protection from NHEJ can be mediated by hRAP1 alone. Our results indicate that hRAP1 blocks NHEJ at telomeres with TRF2 serving to recruit hRAP1 to chromosome ends. Consistent with these biochemical studies, targeting hRAP1 to telomeres in human cells expressing dominant negative TRF2 provides protection from telomere fusions.
Results
RAP1 inhibits NHEJ in vitro
In an attempt to define the specific functions of hRAP1 and TRF2 in the protection of telomeric DNA ends from NHEJ, we sought to bestow hRAP1 with the ability to bind vertebrate telomeric DNA independently of TRF2. In this context, we examined the DNA-binding domain of the Schizosaccharomyces pombe Teb1 protein (also known as SpX). Teb1 was initially identified through computational approaches as a possible telomere-binding protein (Blue et al, 1997), but biochemical experiments failed to demonstrate high-affinity binding to fission yeast telomeric repeats, and no function in telomere maintenance was reported (Vassetzky et al, 1999; Spink et al, 2000). Instead, Teb1 preferentially binds TTAGGG repeats and may function as a transcription factor for numerous S. pombe genes containing this sequence motif in their promoters (Vassetzky et al, 1999). As TTAGGG corresponds to the vertebrate telomeric repeat, we were intrigued by the possibility of utilizing Teb1 to target proteins of interest to human telomeres.
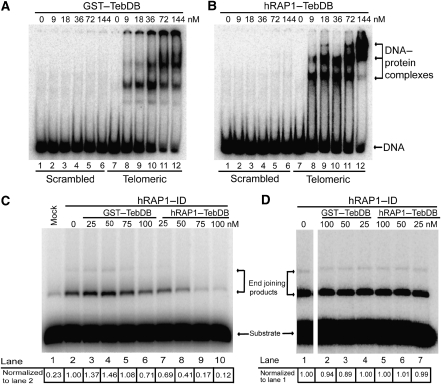
To further characterize the Teb1 DNA-binding domain (from hereon referred to as TebDB), we expressed and purified a 195-amino-acid fragment of Teb1 fused to glutathione-_S_-transferase (GST). TebDB showed robust and specific binding to vertebrate telomeric repeats (Figure 1A) with an apparent binding constant of 25 nM, which is similar to the reported _K_d value for TRF2 (Hanaoka et al, 2005). Fusing hRAP1 to the N-terminus of TebDB did not diminish its affinity or specificity for TTAGGG repeats (Figure 1B; _K_d[app]=15 nM).
Figure 1.
RAP1–TebDB binds telomeric DNA and inhibits NHEJ. (A) Electrophoretic mobility shift assay (EMSA) of double-stranded scrambled and telomeric DNA oligonucleotides incubated with indicated amounts of GST–TebDB. No protein was added in lanes 1 and 7. (B) EMSA of DNA substrates incubated with hRAP1–TebDB. (C) Inhibition of end joining by hRAP1–TebDB at telomeric DNA ends. Linear plasmid DNA containing twelve 5′-TTAGGG-3′ repeats at one end was incubated with GM00558 cell-free extract that was either mock depleted (lane 1) or immunodepleted (ID) of hRAP1 and TRF2 with anti-hRAP1 (lanes 2–10). GST–TebDB (lanes 3–6) and hRAP1–TebDB (lanes 7–10) were added at the indicated concentrations before incubation with DNA substrates. As each DNA substrate contains one telomeric (head) and one non-telomeric (tail) end, the presence of tail-to-tail fusions serves as an internal control for the presence of NHEJ activity in the extract (lane 1). End joining products were quantified by densitometry and were normalized to hRAP1-ID extract (lane 2). (D) Linear plasmid DNA containing twelve scrambled telomeric repeats (5′-TGAGTG-3′) at one end was incubated with GM00558 cell-free extract that was ID of hRAP1 and TRF2 with anti-hRAP1 (lanes 1–7). GST–TebDB (lanes 2–4) and hRAP1–TebDB (lanes 5–7) were added at the indicated concentrations before incubation with DNA substrates. End joining products were quantified by densitometry and were normalized to hRAP1-ID extract (lane 1). Gel was spliced to remove intervening lanes that were not pertinent to this experiment.
Having shown that TebDB fusions to GST and hRAP1 bind human telomeric DNA in gel mobility shift assays, we tested the ability of these proteins to protect telomeric DNA from NHEJ in vitro. We have previously shown that telomeric DNA ends are protected from double-strand break repair activities when incubated with NHEJ-competent human lymphocyte extract (Bae and Baumann, 2007). After immunodepleting the extract of TRF2 and hRAP1, end protection was lost but could be restored by adding back recombinant TRF2 and hRAP1, whereas addition of either protein alone was insufficient (Bae and Baumann, 2007). We now wanted to test whether TebDB-mediated recruitment of hRAP1 to telomeric DNA bypasses the need for TRF2. Addition of GST–TebDB to TRF2/hRAP1-immunodepleted extract had little effect on NHEJ at telomeric ends, indicating that high-affinity binding of this exogenous protein does not bestow end protection (Figure 1C, lanes 3–6). Instead, a modest increase in end joining activity was observed at lower concentrations of GST–TebDB (lanes 3 and 4). In contrast, hRAP1–TebDB inhibited end joining in a concentration-dependent manner (Figure 1C, lanes 7–10) with a sixfold reduction in end joining products being observed at a concentration at which GST–TebDB had no effect (Figure 1C, compare lanes 5 and 9). For comparison, the addition of recombinant hRAP1 and TRF2 to TRF2/hRAP1-immunodepleted extract resulted in a fivefold reduction in end joining of telomeric DNA ends (Bae and Baumann, 2007).
To verify that end protection by hRAP1–TebDB is due to TebDB targeting hRAP1 specifically to telomeric DNA ends, TebDB fusion proteins were added to a control substrate in which the telomeric sequence was scrambled. Neither GST–TebDB nor hRAP1–TebDB inhibited NHEJ at these ends (Figure 1D), demonstrating that both the telomeric DNA binding ability of TebDB and the NHEJ-inhibiting activity of hRAP1 are required for telomeric DNA end protection. As immunodepletion had removed most of TRF2 (Supplementary Figure 1), these results indicate that TRF2 normally contributes to NHEJ inhibition at telomeres by recruiting hRAP1 that in turn blocks end joining. To test this model in vivo, we proceeded to evaluate ways of TRF2-independent recruitment of hRAP1 to telomeres in cells.
Localization of TebDB to telomeres in vivo
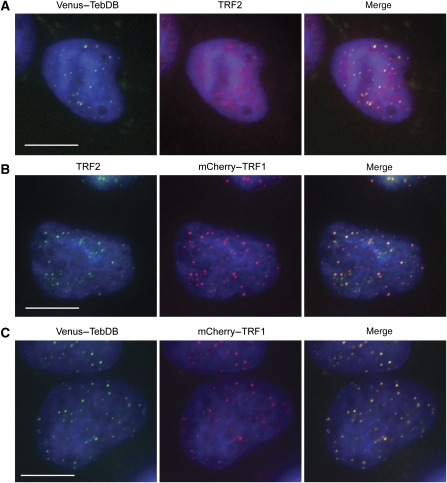
Encouraged by the high affinity and specificity of TebDB for vertebrate telomeric DNA (Figure 1A and B), we examined the subcellular localization of TebDB fused to the GFP variant Venus. To ensure efficient nuclear import, the nuclear localization signal from SV40 large T antigen was included in Venus–TebDB and all other TebDB-containing fusion constructs used in this study. Fluorescence microscopic analysis of HeLa S3 cells expressing Venus–TebDB revealed punctate nuclear staining that largely co-localized with endogenous TRF2 (Figure 2A). However, a minor fraction of Venus–TebDB foci lacked a corresponding TRF2 signal. This could reflect Venus–TebDB localization to non-telomeric sites in addition to telomeres. Alternatively, the fluorescent fusion protein may simply visualize telomeric loci more efficiently than the TRF2 antibody. To distinguish between these possibilities, we generated a fusion of TRF1 to the fluorescent protein mCherry and examined the extent of co-localization with endogenous TRF2. Consistent with fluorescent fusion proteins labelling telomeres more efficiently, all TRF2 foci co-localized with mCherry–TRF1, with a few additional mCherry–TRF1 foci in places with weak or non-detectable TRF2 signal (Figure 2B). When mCherry–TRF1 and Venus–TebDB were co-expressed widespread co-localization of the two proteins was observed (Figure 2C). We concluded that TebDB is capable of mediating the localization of fusion proteins to human telomeres in vivo.
Figure 2.
Telomeric localization of TebDB in human cells. (A) HeLa S3 cells transfected with Venus–TebDB (green) were stained with a mouse monoclonal antibody for TRF2 followed by an AlexaFluor 594-conjugated secondary antibody (red). Nuclei were visualized by counterstaining with DAPI (blue). Cells were subjected to nucleoplasmic extraction so that only chromatin-associated proteins remain within nuclei. (B) Cells expressing mCherry–TRF1 (red) were stained with a mouse monoclonal antibody for TRF2 and AlexaFluor 488 secondary antibody (green). (C) Visualization of Venus–TebDB (green) and mCherry–TRF1 (red) in co-transfected cells. All scale bars correspond to 10 μm.
Expression of TRF2_Δ_B_Δ_M results in preferential loss of hRAP1 from telomeres
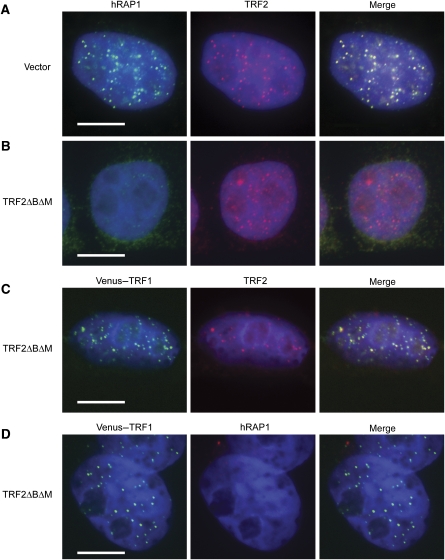
High-level expression of TRF2ΔBΔM is thought to drive endogenous TRF2 into heterodimeric complexes that fail to bind telomeric DNA, thereby reducing the association of endogenous TRF2 with telomeres (van Steensel et al, 1998; Fairall et al, 2001). Interestingly, several studies have indicated that telomeric TRF2 foci remain detectable in cells expressing TRF2ΔBΔM (van Steensel et al, 1998; d'Adda di Fagagna et al, 2003; Kim et al, 2009). In addition, ChIP analysis from cells expressing TRF2ΔBΔM showed a 14-fold increase in 53BP1 at telomeres, whereas TRF2 was reduced by only 50% (d'Adda di Fagagna et al, 2003). In light of the absence of haploinsufficiency in TRF2+/− heterozygous murine cells (Denchi and de Lange, 2007) and modest phenotypes observed in TRF2 knockdown experiments (Takai et al, 2003; Xu and Blackburn, 2004; NSB, JSF and PB, unpublished data), these observations suggest that TRF2ΔBΔM may mediate telomere uncapping by acting on other targets in addition to sequestering endogenous TRF2. A probable candidate for such a target is hRAP1, which interacts with a region of TRF2 present in the dominant-negative fragment (Li et al, 2000). As TRF2ΔBΔM is expressed at much higher levels than endogenous TRF2, the majority of TRF2ΔBΔM will form homodimers, which lack the ability to bind telomeric DNA or endogenous TRF2. However, endogenous hRAP1 may be sequestered by such TRF2ΔBΔM homodimers, thus preventing its recruitment to telomeres. To investigate the possibility that preferential loss of hRAP1 contributes to telomere fusions when TRF2ΔBΔM is expressed, we introduced TRF2ΔBΔM into HeLa S3 cells and analysed the localization of endogenous hRAP1 and TRF2. A nucleoplasm extraction procedure (Li et al, 2000) was used to ameliorate the high nucleoplasmic background associated with TRF2ΔBΔM expression when probed using an antibody against TRF2. Although TRF2 and hRAP1 foci were prominent in cells transfected with empty vectors (Figure 3A), no telomeric hRAP1 was detected in TRF2ΔBΔM-expressing cells (Figure 3B and D). In contrast, and consistent with previous results (van Steensel et al, 1998; Li et al, 2000; Kim et al, 2009), TRF2 foci were reduced but readily detectable in these cells (Figure 3B). We verified that the remaining TRF2 foci were telomeric in origin by co-expressing TRF2ΔBΔM and fluorescently tagged TRF1 as a telomeric marker that is unaffected by TRF2ΔBΔM expression (van Steensel et al, 1998). After antibiotic selection to eliminate untransfected cells, telomeric TRF2 was observed in the majority of cells (147 out of 211 cells had more than five telomeric TRF2 foci), whereas hRAP1 was not detected (Figure 3C and D). Despite the presence of telomeric TRF2 in these cells, metaphase spreads confirmed widespread uncapping as 29.3% of telomeres had participated in fusions (see below). In summary, these experiments revealed extensive telomere uncapping under conditions in which TRF2, but not hRAP1, was detectable at telomeres. We cannot rule out the possibility that differences in antibody affinity contribute to the apparent loss of hRAP1 but retention of telomeric TRF2. However, together with the in vitro study described above, these results further support that loss of hRAP1 directly contributes to telomere uncapping. A corollary to this hypothesis predicts that hRAP1 inhibits NHEJ at telomeres.
Figure 3.
Dominant-negative TRF2 (TRF2ΔBΔM) preferentially removes hRAP1 from telomeres. (A) Immunostaining of hRAP1 (AlexaFluor 488, green) and TRF2 (AlexaFluor 594, red) in HeLa S3 cells transfected with and selected for the presence of the vector controls. DNA was stained with DAPI (blue). (B) Visualization of TRF2 and hRAP1, as shown in (A), in cells expressing TRF2ΔBΔM. (C) Cells expressing TRF2ΔBΔM and Venus–TRF1 (green) were stained with anti-TRF2 (AlexaFluor 594, red) and DAPI (blue). (D) Cells, as shown in (C), were stained with anti-hRAP1 (AlexaFluor 594, red). All scale bars correspond to 10 μm.
TebDB neither uncaps nor protects human telomeres
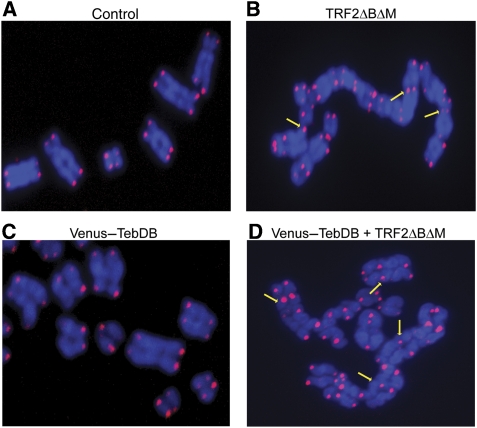
Telomeric localization of TebDB allowed us to target hRAP1 to chromosome ends independent of TRF2 and analyse whether such recruitment would ameliorate the effects of expressing TRF2ΔBΔM. Before proceeding with this experiment we had to examine whether TebDB binding alone affected telomere capping. To address this issue, we carried out telomere FISH on metaphase spreads prepared from cells transfected with empty vector, Venus–TebDB or TRF2ΔBΔM. Although chromosome structure was normal in cells harbouring the vector control (Figure 4A), abundant chromosome fusions were observed in cells expressing TRF2ΔBΔM, giving rise to long trains of fused chromosomes (Figure 4B). Chromosome fusions were not observed in cells expressing TebDB (Figure 4C).
Figure 4.
TebDB neither induces nor protects against NHEJ-dependent telomere fusions. Telomere FISH was carried out on metaphase spreads from cells transfected with and selected for (A) vector control, (B) TRF2ΔBΔM, (C) Venus-TebDB, (D) or Venus-TebDB and TRF2ΔBΔM. Telomeres were visualized with an AlexaFluor 543-labelled locked nucleic acid probe complementary to the G-rich strand (red). Chromosomes were counterstained with DAPI (blue). Representative chromosomes from the respective samples are shown. Some telomere–telomere fusions are highlighted with yellow arrows.
Although TebDB alone was insufficient to protect telomeric DNA ends from NHEJ-mediated fusions in vitro (Figure 1C), it was critical to test whether TebDB expression would partially or completely negate the effect of TRF2ΔBΔM in vivo. Analysis of metaphase spreads from cells co-expressing TebDB and TRF2ΔBΔM revealed that 46% of telomeres were fused (Figures 4D and 5E), indicating that co-expression with TebDB exacerbated the TRF2ΔBΔM phenotype. This synergistic effect may be related to TebDB displacing some TRF2 from telomeres and thus acting synergistically with TRF2ΔBΔM. However, as TebDB expression alone neither induced nor inhibited NHEJ-mediated telomere fusions, we proceeded to use this telomere-binding domain to target hRAP1 to chromosome ends independent of TRF2.
Figure 5.
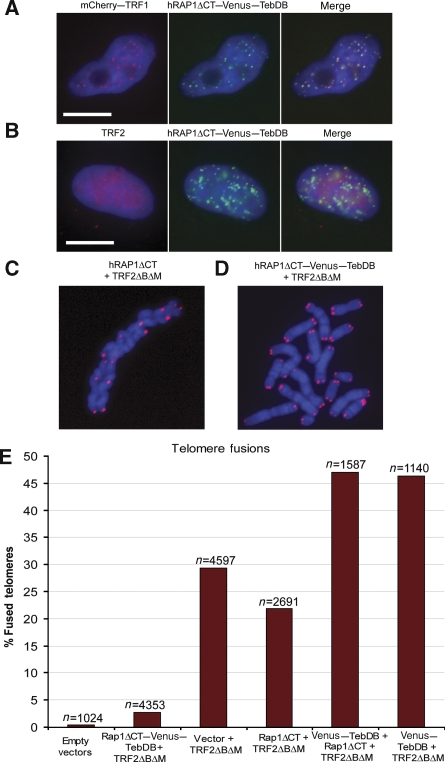
RAP1ΔCT–Venus–TebDB localizes to and protects telomeres in the absence of TRF2. (A) Co-localization of mCherry–TRF1 and hRAP1ΔCT–Venus–TebDB at telomeres in cells expressing TRF2ΔBΔM. (B) Cells expressing TRF2ΔBΔM and hRAP1ΔCT–Venus–TebDB were stained with anti-TRF2 and AlexaFluor 594-conjugated secondary antibody (red). Nucleoplasmic extraction was used to limit the visualization to chromatin-associated TRF2. hRAP1ΔCT–Venus–TebDB was visualized because of Venus fluorescence. Scale bars correspond to 10 μm. (C, D) Telomere FISH carried out on metaphase chromosomes transfected with and selected for expression of the indicated proteins. Telomeres were detected with an AlexaFluor 543-labelled locked nucleic acid probe that detects the G-rich strand (red). Chromosomes were stained with DAPI (blue). (E) Quantification of telomere fusions in metaphase spreads of cells transfected with and selected for the indicated constructs. Telomere fusions were quantified in images of metaphases from cells collected 72 h after transfection.
Telomeric hRAP1 counteracts uncapping by TRF2_Δ_B_Δ_M
To restore hRAP1 at telomeres after loss of TRF2, we fused Venus–TebDB to the previously characterized hRAP1ΔCT truncation that lacks the homodimerization and TRF2 interaction domains (Li et al, 2000; Li and de Lange, 2003). The hRAP1ΔCT fragment was chosen as it cannot interact with endogenous TRF2 or TRF2ΔBΔM, and will therefore neither be recruited to telomeres by TRF2, nor will it interfere with the ability of TRF2ΔBΔM to remove endogenous hRAP1 and TRF2 from telomeres (O'Connor et al, 2004). In contrast, we found that a full-length hRAP1–TebDB fusion efficiently recruited TRF2ΔBΔM to telomeres, thereby complicating the interpretation of any protection phenotype (Supplementary Figure 2). As expected, hRAP1ΔCT–Venus–TebDB accumulated in foci that co-localized with TRF1 even when TRF2ΔBΔM was expressed (Figure 5A). As indicated by the increased incidence in telomere fusions, co-expressing TebDB fusion proteins with TRF2ΔBΔM caused a further reduction in endogenous TRF2 foci (only 13 out of 107 cells had more than five telomeric TRF2 foci per cell), thereby providing us with a system in which TRF2 is undetectable at most telomeres, whereas the hRAP1 fusion protein localizes prominently to chromosome ends (Figure 5B).
Next, we assayed the incidence of telomere fusions in metaphase spreads of cells co-expressing hRAP1ΔCT–Venus–TebDB and TRF2ΔBΔM. For comparison, telomere fusions were also scored in cells expressing only TRF2ΔBΔM, cells co-expressing TRF2ΔBΔM and hRAP1ΔCT, and cells co-expressing TRF2ΔBΔM, hRAP1ΔCT and Venus–TebDB not fused to each other. The prevalence of telomere fusions observed in metaphase spreads of cells expressing hRAP1ΔCT and TRF2ΔBΔM (Figure 5C) was in sharp contrast with cells co-expressing hRAP1ΔCT–Venus–TebDB and TRF2ΔBΔM (Figure 5D). Scoring of several thousand telomeres revealed that TRF2-independent recruitment of hRAP1 to telomeres caused a 10-fold reduction of end fusions when compared with cells expressing TRF2ΔBΔM alone (P<0.0001; Figure 5E; Supplementary Table I). Importantly, co-expression of hRAP1ΔCT with TRF2ΔBΔM did not provide such protection, as the incidence of telomere fusions was sevenfold higher in these cells (P<0.0001). Furthermore, co-expression of TRF2ΔBΔM with Venus–TebDB or with hRAP1ΔCT and Venus–TebDB not fused to each other provided no end protection, but instead resulted in the highest incidence of fused chromosome ends (Figure 5E). Immunoblotting confirmed that modulation of TRF2ΔBΔM expression was not the mechanism by which hRAP1ΔCT–Venus–TebDB protects telomeres, as the dominant-negative form of TRF2 was expressed at similar levels in all samples (Supplementary Figure 3A). Furthermore, the hRAP1 fusion protein was expressed at levels similar to endogenous hRAP1, confirming that protection is not due to substantial overexpression of hRAP1 (Supplementary Figure 3B).
NHEJ predominantly occurs in the G1 phase of the cell cycle. A substantial shift in the cell cycle profile in favour of S/G2 phase in the presence of the hRAP1 fusion protein could potentially account for apparent end protection in these cells. However, cell cycle analysis revealed very similar profiles for cells transfected with empty vector or Rap1ΔCT–Venus–TebDB and TRF2ΔBΔM (71 versus 74% cells in G1 phase: Supplementary Table II). Samples in which telomere fusions were prevalent (TRF2ΔBΔM alone, Venus–TebDB and TRF2ΔBΔM, hRAP1ΔCT and TRF2ΔBΔM) had higher numbers of cells in G2/M phase, consistent with a mitotic delay caused by telomere fusions. Thus, a shortening of the G1 phase is not responsible for the apparent protection by the hRap1ΔCT–Venus–TebDB protein. Taken together with the inability of Venus-TebDB to protect telomeres from NHEJ, our data strongly suggest that hRAP1 directly mediates protection of telomeres from NHEJ in human cells.
hRAP1 does not inhibit DNA damage signalling at telomeres
Previous studies have shown that NHEJ-dependent telomere fusions depend on DNA damage signalling by ATM and coincide with 53BP1 accumulation (Denchi and de Lange, 2007; Dimitrova et al, 2008). The deletion of either ATM or 53BP1 abrogates the telomere fusion phenotype normally observed in TRF2−/− MEFs, showing the importance of these factors for the joining of deprotected telomeres. Interestingly, TRF2 has been shown to bind to and prevent ATM activation, suggesting that loss of ATM inhibition at telomeres leads to telomere fusions in TRF2−/− MEFs (Karlseder et al, 2004). In our experiments, hRAP1 protected telomeres from NHEJ even in the absence of TRF2, raising the question whether hRAP1 also acts as an inhibitor of damage signalling at telomeres. Alternatively, hRAP1 may regulate a step in the NHEJ pathway, thereby specifically inhibiting NHEJ at telomeres downstream of damage signalling. To distinguish between these possibilities, we assayed the extent of ATM phosphorylation at ser 1981 in cells co-expressing the constructs described above. TRF2ΔBΔM expression invariably resulted in high levels of phosphorylated ATM regardless of the presence or absence of hRAP1 at telomeres (Figure 6A). As cells co-expressing hRAP1ΔCT–Venus–TebDB and TRF2ΔBΔM contained levels of phosphorylated ATM similar to that observed in cells expressing only TRF2ΔBΔM, we conclude that telomeric hRAP1 does not inhibit ATM activation.
Figure 6.
hRAP1 does not inhibit DNA damage signalling at telomeres. (A) ATM activation was analysed in extracts prepared from cells expressing the indicated proteins by immunoblotting with a phosphor-serine 1981-specific anti-ATM antibody. (B) Cells transfected with the indicated constructs were stained with an anti-53BP1 antibody. Telomeres were visualized by the green fluorescence of the Venus-containing fusions proteins. Scale bars represent 5 μm. (C) Quantification of telomere dysfunction-induced foci (TIFs) in cells expressing the indicated proteins. Cells with greater than four or more TIFs per cell were considered TIF positive.
To further assess whether telomeric hRAP1 interferes with damage signalling at TRF2-deficient telomeres, we assayed telomeric 53BP1 localization. Using the previously established threshold for scoring telomere dysfunction-induced foci (TIF)-positive cells (Takai et al, 2003), we found that <6% of cells expressing the vector control were TIF positive (Figure 6B and C). In contrast, 63% of cells expressing TRF2ΔBΔM, and 76% of cells expressing TRF2ΔBΔM and Venus–TebDB were TIF positive (Figure 6C). Importantly, cells co-expressing TRF2ΔBΔM and hRAP1ΔCT–Venus–TebDB also displayed a similar incidence of TIF–positive cells, as 38 out of 56 cells (68%) contained four or more TIFs (Figure 6B and C). Analysis of this data using Fisher's exact test indicated that, although samples transfected with TRF2ΔBΔM were statistically significantly different from cells transfected with empty vector (_P_<0.0001), none of the other differences was statistically significant (_P_>0.2).
Together with the ATM activation, the TIF assay data show that telomeric hRAP1 does not inhibit DNA damage signalling. Instead, these results suggest that hRAP1 protects TRF2-deficient telomeres from engaging in end fusions despite a DNA damage response, either by acting downstream of ATM and 53BP1 or by directly blocking the joining reaction.
Discussion
The ability to target human RAP1 to telomeric DNA independently of TRF2 provided us with a unique opportunity to investigate the roles of TRF2 and hRAP1 in telomere protection. We found that NHEJ of telomeric termini was specifically inhibited upon TRF2-independent recruitment of hRAP1 to telomeric DNA in vitro. Extending these results in vivo, we show that heterologous recruitment of hRAP1 to telomeres is sufficient to avert the uncapping phenotype associated with the expression of dominant-negative TRF2 despite the activation of a DNA damage response. The convergence of biochemical and cell biological data presented here identifies hRAP1 as a critical mediator of telomere protection in human cells.
Current models of NHEJ inhibition at telomeres are largely based on the finding that a telomeric 3′ overhang can invade internal sequences on the same telomere, thereby forming a t-loop that renders the end inaccessible to degradation or fusion (Griffith et al, 1999). As TRF2 can promote t-loop formation in vitro (Stansel et al, 2001), it has been suggested that chromosome fusions caused by the loss of TRF2 from telomeres result ultimately from the dissociation or resolution of t-loops in the absence of TRF2. Although purified recombinant TRF2 is sufficient to induce t-loop formation on telomeric DNA, no biochemical studies have implicated hRAP1 in this process. At least in vitro, t-loops are not required for NHEJ inhibition as 12 telomeric repeats are too short to form a t-loop but are sufficient to protect a DNA terminus from NHEJ (Bae and Baumann, 2007). Interestingly, similarly short but stable telomeres have been observed in vivo as well (Capper et al, 2007; Xu and Blackburn, 2007). It is conceivable that t-loops may protect long telomeres that are capable of forming such structures, whereas very short telomeres are protected by an alternative mechanism involving hRAP1. Further analysis will be required to elucidate the links between hRAP1, t-loops and NHEJ inhibition at telomeres.
The idea that multiple pathways protect telomeres in humans is supported by an emerging literature (Bae and Baumann, 2007; Kim et al, 2008, 2009). Recently, both phosphatase nuclear targeting subunit (PNUTS) and microcephalin (MCPH1) were shown to interact with a domain in TRF2 that is separate from the hRAP1 interaction domain (Kim et al, 2009). In addition, mutation of the PNUTS/MCPH1 binding site in DN-TRF2 reduced its ability to elicit a DNA damage signal at telomeres, suggesting that PNUTS and/or MCPH1 may contribute to telomere protection in another pathway mediated by TRF2. Our study does not address the possibility that TRF1, TIN2, TPP1 and/or POT1 contribute to telomere capping, or that multiple independent pathways exist. However, a key role for hRAP1 is indicated by the observation that telomeric TRF1, TIN2, TPP1 and POT1 are insufficient to prevent widespread telomere fusions, whereas recruitment of hRAP1 has a potent protective effect.
Previous studies have reported chromosome uncapping in response to removal of TRF2 from telomeres by various means, including siRNA-mediated knockdown (Takai et al, 2003; Xu and Blackburn, 2004), expression of dominant-negative forms of TRF2 (van Steensel et al, 1998; Konishi and de Lange, 2008) and TIN2 (Kim et al, 2004) and conditional knockout of TRF2 in mouse embryonic fibroblasts (Celli and de Lange, 2005). As hRAP1 is recruited to telomeres by TRF2, removal of TRF2 from telomeres in these studies would have resulted in the concomitant loss of hRAP1. The assertion that TRF2 and hRAP1 form a functional unit is also supported by the observation that cellular levels of hRAP1 are markedly reduced in murine cells deleted for TRF2 (Celli and de Lange, 2005). We have now shown that hRAP1-dependent protection does not operate through modulation of TRF2 DNA binding, as an hRAP1 fragment incapable of interacting with TRF2 still protects telomeres from NHEJ (Figure 5E). Instead, hRAP1 alone can be sufficient to inhibit NHEJ (Figures 1B, 5B and E), indicating that telomeres are protected from NHEJ at two levels: TRF2 directly inhibits damage signalling, whereas hRAP1 blocks NHEJ downstream of the damage signal.
In light of the findings reported here, it is perhaps surprising that attempts to knock down hRAP1 or generate dominant-negative versions of the protein have not resulted in a telomere fusion phenotype (Li and de Lange, 2003; Xu and Blackburn, 2004; JFS and PB, unpublished data). Although redundant pathways of protection may account for these results, it is also clear that very short telomeres, which can bind little TRF2/hRAP1, are nevertheless capped (Capper et al, 2007; Xu and Blackburn, 2007; Bae and Baumann, 2007). As knockdown experiments cause a reduction, but almost never a complete loss of the target protein, residual hRAP1 may have been sufficient to provide protection in these experiments. The most informative mammalian RAP1 loss-of-function experiment would be a RAP1 knockout. Unfortunately, this experiment has been impeded by the presence of a bidirectional promoter shared by RAP1 and the essential KARS gene (encoding a lysyl-tRNA synthetase), a gene structure conserved among chicken, mouse and human (Tan et al, 2003).
Although hRAP1 has been named on the basis of limited domain and sequence similarity with the budding yeast repressor and activator protein (Rap1), the two proteins have diverged substantially (Li et al, 2000). Unlike hRAP1 that has a single myb domain of unknown function, budding yeast Rap1 has tandem myb-like domains that mediate DNA binding critical for its functions in transcriptional regulation and telomere maintenance (Konig et al, 1996). In Saccharomyces cerevisiae recruitment of Rif2 and Sir4 by the C-terminal domain (CT) of Rap1 is required for NHEJ inhibition at telomeres (Marcand et al, 2008). The CT of hRAP1 mediates homodimerization and interaction with TRF2 (Li et al, 2000). Consistent with divergent modes of inhibition, the CT of hRAP1 was not required for NHEJ inhibition in our experiments and homologues of yeast Rif2 and Sir4 seem to be absent from mammalian genomes. Interestingly, a minor Rap1-dependent, but Rif2- and Sir4-independent NHEJ inhibition pathway has also been noted in yeast (Marcand et al, 2008). This largely uncharacterized pathway is not mediated by the conserved BRCT or CT domains in RAP1, but requires the central region of the protein. It is tempting to speculate that human RAP1 relies predominantly on this mode of NHEJ inhibition.
Further supporting the idea of an evolutionarily conserved mechanism of NHEJ inhibition is the observation that deletion of rap1+ in fission yeast also leads to telomere fusions (Miller et al, 2005). Like in human cells, fission yeast Rap1 is recruited to telomeres by the TRF2-like protein Taz1 (Chikashige and Hiraoka, 2001; Kanoh and Ishikawa, 2001). However, recruitment of fission yeast Rap1 to telomeres in the form of a fusion with the Taz1 DNA-binding domain failed to rescue the chromosome end joining phenotype associated with taz1 deletion in G1-arrested cells (Miller et al, 2005). This may indicate mechanistic differences or simply reflect the possibility that the protective activity of Rap1 was masked in the context of the synthetic fusion protein.
Several DNA repair factors, including the Ku heterodimer, MRE11, RAD50, and PARP1 co-purify with hRAP1 (O'Connor et al, 2004), raising the intriguing possibility that RAP1 contains a domain that directly binds and prevents these proteins from executing DNA repair. Ku and DNA PKcs associate with telomeres (Hsu et al, 1999; d'Adda di Fagagna et al, 2001) and, at least, in vitro hRAP1 does not seem to inhibit the assembly of NHEJ factors at telomeric ends under conditions in which fusions are blocked (NSB, PB; unpublished data). Uncovering the physical and functional interactions between hRAP1 and the NHEJ machinery will now be critical for elucidating the mechanism by which chromosome ends are protected from unsolicited repair events.
Materials and methods
Cloning and purification
Human RAP1 in pGEX-4T was a gift from Z. Songyang. A cDNA encoding TebDB (amino acids 32–227 of Teb1, SpX, SPAC13G7.10) was cloned from an S. pombe cDNA library generously provided by K. Trujillo. TebDB was inserted into pGEX-4T in frame with an N-terminal GST tag and thrombin cleavage site creating pJSCTeb. TebDB was also inserted between the thrombin cleavage site and RAP1 in RAP1–pGEX-4T to generate pJSC3. GST-tagged proteins were expressed in Escherichia coli and bound to glutathione-coupled beads in batch. After elution with glutathione, thrombin (Amersham) cleavage was carried out on GST–TebDB–RAP1-containing eluates. RAP1–TebDB and GST–TebDB were further purified by mono Q ion-exchange chromatography. Protein concentrations were determined by Bradford assay (Bio-Rad).
For mammalian expression, the cDNA for Venus was cloned into pIREShyg2 and TebDB was inserted downstream to create Venus–TebDB (pJFS1). To facilitate nuclear localization of the protein, the DNA sequence coding for the nuclear localization sequence (NLS) PKKKRKVE from SV40 large T antigen was included in the forward primer for TebDB. The hRAP1–Venus–TebDB fusion was generated by inserting cDNAs for hRAP1, TebDB and Venus into pIREShyg2 (pJFS2). hRAP1 was later replaced with hRAP1ΔCT (Addgene plasmid 13252; Li and de Lange, 2003) generating pJFS3. The plasmid pJFS4 (FLAG–TRF2ΔBΔM), containing the cDNA coding for TRF2 amino acids 44–445 with a FLAG epitope tag at the N-terminus, was cloned into pIRESpuro2 using a TRF2 cDNA obtained from Addgene (plasmid 12299; Broccoli et al, 1997). A plasmid containing TRF1 in pBluescript SK+/− (Addgene plasmid 12303; Chong et al, 1995) was used to clone TRF1 as a CT fusion with mCherry into pIREShyg2 (pJFS5).
DNA binding assays
DNA substrates were generated by annealing complementary oligonucleotides. The G-rich strand for the telomeric substrate is 5′-ACGTGGTCAAAGTCTGGAAC(TTAGGG)10-3′, and 5′-ACGTGGTCAAAGTCTGGAAC(TGAGTG)10-3′ for the scrambled substrate. The G-rich DNA oligonucleotides were labelled with [γ-32P]ATP using polynucleotide kinase and annealed with the unlabelled complementary oligonucleotide. The annealed products were purified over G-25 sepharose columns, and used for EMSA reactions at 0.4 nM. Substrates were incubated with recombinant GST–TebDB or RAP1–TebDB in EMSA buffer (50 mM TEA (pH 7.5); 40 mM KCl, 0.5 mM DTT; 100 μg ml−1 BSA) at room temperature for 10 min. DNA and DNA–protein complexes were resolved by electrophoresis on 6% polyacrylamide gels in 0.5 × TBE buffer at 200 V for 5 min followed by 90 min at 90 V. The gels were dried and subjected to PhosphorImager analysis. Apparent dissociation constants _K_d[app] were calculated from electrophoretic mobility shift assays as described above except that the DNA substrate was used at a final concentration of 90 pM.
Immunodepletion/immunoblotting
Immunodepletions and immunoblotting were carried out as described earlier (Celli and de Lange, 2005; Bae and Baumann, 2007). The TRF2 monoclonal antibody 4A794 (cat# 05-521, Millipore), RAP1 polyclonal antibody (cat# A300-306A, Bethyl Labs) and pS1981-ATM antibody (cat# 4526, Cell Signaling Technology) were diluted 1:1000 for use in immunoblotting experiments.
Cell culture and transfection
HeLa S3 cells (ATCC) were grown in medium containing DMEM supplemented with 2 mM GlutaMAX (Invitrogen), non-essential amino acids (Invitrogen) and 10% fetal bovine serum (JRH). GM00558 cells were purchased from the NIGMS Human Genetic Mutant Cell Repository (Camden, NJ) and grown in RPMI medium supplemented with 15% FBS and 2 mM L-glutamine. Cell-free extract was prepared and end joining assays were carried out as described earlier (Bae and Baumann, 2007). For transfection, HeLa cells were seeded at 5 × 105 cells per well in a six-well dish and transfected with 4 μg DNA using Lipofectamine 2000 (Invitrogen). After 4 h, cells were treated with trypsin and re-seeded into two wells of a six-well dish. At 24 h after transfection, antibiotic selection was carried out using medium containing 3.3 μg ml−1 puromycin (Sigma Aldrich) and 250 μg ml−1 hygromycin B (Invitrogen). Dual antibiotic selection was used to ensure that all cells analysed by immunofluorescence, metaphase spread TeloFISH and immunoblotting expressed the relevant proteins, thereby negating any effects due to differences in transfection efficiency.
Immunofluorescence/TIF assay
Cells were seeded onto 22 × 22 mm2 glass coverslips 4 h post-transfection. After 48 h of selection in medium containing puromycin (3.3 μg ml−1) and hygromycin B (250 μg ml−1), cells were washed once with PBS, and incubated with Triton extraction buffer (300 mM sucrose, 20 mM HEPES (pH 7.9), 50 mM NaCl, 3 mM MgCl2 and 0.5% Triton X-100) at 4°C for 2 min. Cells were then washed twice with PBS, and fixed with 4% paraformaldehyde in PBS for 10 min. After fixation, cells were washed with PBS and re-permeabilized with Triton extraction buffer for 10 min. Cells were then washed twice for 5 min with PBS, and blocking was carried out for 45 min in PBS containing 1% BSA. The TRF2 monoclonal antibody 4A794 (Cat# 05-521, Millipore), RAP1 polyclonal antibody (Cat# A300-306A, Bethyl Labs) and 53BP1 polyclonal (Cat# A300-272A) were diluted 1:1000 in PBS with 1% BSA. Coverslips were incubated with primary antibodies for 2 h followed by three 5-min washes in PBS with 1% BSA. Coverslips were then incubated with goat anti-mouse antibodies conjugated with AlexaFluor 594 and goat anti-rabbit antibodies conjugated with AlexaFluor 488 (Invitrogen) diluted 1:1000 in PBS with 1% BSA for 1 h. Coverslips were washed twice in PBS with 1% BSA, stained with DAPI (200 ng ml−1 in PBS) for 5 min, and mounted on slides with Fluoromount G mounting medium. Microscopy was carried out using an AxioPlan microscope with a × 100, 1.4 NA Plan-APOCHROMAT objective (Zeiss) and AxioVision software.
Metaphase spread preparation and telomere FISH
After antibiotic selection for 48 h, cells were treated with colcemid (0.1 μg ml−1) for 4 h and collected by trypsin treatment. After hypotonic swelling in 0.075 M KCl at 37°C for 7 min, cells were pelleted and resuspended/fixed in 3:1 MeOH:CH3COOH. Cells were dropped onto coverslips, washed once with 3:1 MeOH:CH3COOH and heated to 75°C for 1 min. After drying coverslips for 1 h at room temperature, an AlexaFluor 543-labelled locked nucleic acid probe 5′-(CCTAAA)3-3′ was used at 100 nM for telomeric FISH as described (Poon and Lansdorp, 2001; Wang et al, 2004), except that a locking nucleic acid (LNA) probe was substituted for protein–nucleic acid (PNA). Samples were imaged on an AxioPlan microscope using a × 100, 1.4 NA Plan-APOCHROMAT objective (Zeiss) and AxioVision software. Quantification of telomere fusions was carried out on blinded samples to remove experimenter bias. A χ2 test for independence was applied to the incidence of telomere fusions observed by metaphase spread analysis. Expected values were calculated on the basis of the percentage of fused telomeres in cells expressing only TRF2ΔBΔM. Analyses were carried out using GraphPad software.
Supplementary Material
Supplementary figures
Review Process File
Acknowledgments
We thank Titia de Lange and Sunny Songyang for generously sharing plasmids, and the Stowers Institute Microscopy Center and Cytometry Facility for excellent support. We also thank Peter Lansdorp and Martina Gaspari for advice on metaphase spreads and teloFISH, members of the Baumann and Blanchette labs for discussions, and Rachel Helston and Diana Baumann for critical reading of the paper. This study was funded by the Stowers Institute and the Pew Scholars Program in the Biological Sciences sponsored by the Pew Charitable Trusts. JFS is supported by a Madison and Lila Self Graduate Fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bae NS, Baumann P (2007) A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol Cell 26: 323–334 [DOI] [PubMed] [Google Scholar]
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 [DOI] [PubMed] [Google Scholar]
- Blue C, Marcand S, Gilson E (1997) Proteins that bind to double-stranded regions of telomeric DNA. Trends Cell Biol 7: 317–324 [DOI] [PubMed] [Google Scholar]
- Broccoli D, Smogorzewska A, Chong L, de Lange T (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet 17: 231–235 [DOI] [PubMed] [Google Scholar]
- Capper R, Britt-Compton B, Tankimanova M, Rowson J, Letsolo B, Man S, Haughton M, Baird DM (2007) The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev 21: 2495–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli GB, de Lange T (2005) DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol 7: 712–718 [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Hiraoka Y (2001) Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr Biol 11: 1618–1623 [DOI] [PubMed] [Google Scholar]
- Chong L, van Steensel B, Broccoli D, Erdjument-Bromage H, Hanish J, Tempst P, de Lange T (1995) A human telomeric protein. Science 270: 1663–1667 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Hande MP, Tong WM, Roth D, Lansdorp PM, Wang ZQ, Jackson SP (2001) Effects of DNA nonhomologous end-joining factors on telomere length and chromosomal stability in mammalian cells. Curr Biol 11: 1192–1196 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Teo SH, Jackson SP (2004) Functional links between telomeres and proteins of the DNA-damage response. Genes Dev 18: 1781–1799 [DOI] [PubMed] [Google Scholar]
- Denchi EL, de Lange T (2007) Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448: 1068–1071 [DOI] [PubMed] [Google Scholar]
- Dimitrova N, Chen YC, Spector DL, de Lange T (2008) 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456: 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairall L, Chapman L, Moss H, de Lange T, Rhodes D (2001) Structure of the TRFH dimerization domain of the human telomeric proteins TRF1 and TRF2. Mol Cell 8: 351–361 [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97: 503–514 [DOI] [PubMed] [Google Scholar]
- Hanaoka S, Nagadoi A, Nishimura Y (2005) Comparison between TRF2 and TRF1 of their telomeric DNA-bound structures and DNA-binding activities. Protein Sci 14: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock M, Spangler E, Riha K, Puizina J, Shippen DE (2004) Molecular analysis of telomere fusions in Arabidopsis: multiple pathways for chromosome end-joining. EMBO J 23: 2304–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HL, Gilley D, Blackburn EH, Chen DJ (1999) Ku is associated with the telomere in mammals. Proc Natl Acad Sci USA 96: 12454–12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F (2001) spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol 11: 1624–1630 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH, de Lange T (2004) The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol 2: E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee OH, Xin H, Chen LY, Qin J, Chae HK, Lin SY, Safari A, Liu D, Songyang Z (2009) TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat Struct Mol Biol 16: 372–379 [DOI] [PubMed] [Google Scholar]
- Kim SH, Beausejour C, Davalos AR, Kaminker P, Heo SJ, Campisi J (2004) TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem 279: 43799–43804 [DOI] [PubMed] [Google Scholar]
- Kim SH, Davalos AR, Heo SJ, Rodier F, Zou Y, Beausejour C, Kaminker P, Yannone SM, Campisi J (2008) Telomere dysfunction and cell survival: roles for distinct TIN2-containing complexes. J Cell Biol 181: 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig P, Giraldo R, Chapman L, Rhodes D (1996) The crystal structure of the DNA-binding domain of yeast RAP1 in complex with telomeric DNA. Cell 85: 125–136 [DOI] [PubMed] [Google Scholar]
- Konishi A, de Lange T (2008) Cell cycle control of telomere protection and NHEJ revealed by a ts mutation in the DNA-binding domain of TRF2. Genes Dev 22: 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, de Lange T (2003) Rap1 affects the length and heterogeneity of human telomeres. Mol Biol Cell 14: 5060–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T (2000) Identification of human Rap1: implications for telomere evolution. Cell 101: 471–483 [DOI] [PubMed] [Google Scholar]
- Longhese MP (2008) DNA damage response at functional and dysfunctional telomeres. Genes Dev 22: 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand S, Pardo B, Gratias A, Cahun S, Callebaut I (2008) Multiple pathways inhibit NHEJ at telomeres. Genes Dev 22: 1153–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Ferreira MG, Cooper JP (2005) Taz1, Rap1 and Rif1 act both interdependently and independently to maintain telomeres. EMBO J 24: 3128–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Jordan JL, Cross GA, de Lange T, Griffith JD (2001) t-loops at trypanosome telomeres. EMBO J 20: 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti KG, Prescott DM (1999) Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops. Proc Natl Acad Sci USA 96: 14436–14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina T, Woodcock CL (2004) Closed chromatin loops at the ends of chromosomes. J Cell Biol 166: 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MS, Safari A, Liu D, Qin J, Songyang Z (2004) The human Rap1 protein complex and modulation of telomere length. J Biol Chem 279: 28585–28591 [DOI] [PubMed] [Google Scholar]
- Palm W, de Lange T (2008) How shelterin protects mammalian telomeres. Annu Rev Genet 42: 301–334 [DOI] [PubMed] [Google Scholar]
- Pardo B, Marcand S (2005) Rap1 prevents telomere fusions by nonhomologous end joining. EMBO J 24: 3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon SS, Lansdorp PM (2001) Quantitative fluorescence in situ hybridization (Q-FISH). Curr Protoc Cell Biol, Chapter 18, Unit 1814 [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, Karlseder J, Holtgreve-Grez H, Jauch A, de Lange T (2002) DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr Biol 12: 1635–1644 [DOI] [PubMed] [Google Scholar]
- Spink KG, Evans RJ, Chambers A (2000) Sequence-specific binding of Taz1p dimers to fission yeast telomeric DNA. Nucleic Acids Res 28: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansel RM, de Lange T, Griffith JD (2001) T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J 20: 5532–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T (2003) DNA damage foci at dysfunctional telomeres. Curr Biol 13: 1549–1556 [DOI] [PubMed] [Google Scholar]
- Tan M, Wei C, Price CM (2003) The telomeric protein Rap1 is conserved in vertebrates and is expressed from a bidirectional promoter positioned between the Rap1 and KARS genes. Gene 323: 1–10 [DOI] [PubMed] [Google Scholar]
- Tomaska L, Willcox S, Slezakova J, Nosek J, Griffith JD (2004) Taz1 binding to a fission yeast model telomere: formation of t-loops and higher order structures. J Biol Chem 279: 50764–50772 [DOI] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401–413 [DOI] [PubMed] [Google Scholar]
- Vassetzky NS, Gaden F, Brun C, Gasser SM, Gilson E (1999) Taz1p and Teb1p, two telobox proteins in Schizosaccharomyces pombe, recognize different telomere-related DNA sequences. Nucleic Acids Res 27: 4687–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RC, Smogorzewska A, de Lange T (2004) Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119: 355–368 [DOI] [PubMed] [Google Scholar]
- Xu L, Blackburn EH (2004) Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. J Cell Biol 167: 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Blackburn EH (2007) Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol Cell 28: 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
Review Process File