Mesenchymal stem cells produce Wnt isoforms and TGF-β1 that mediate proliferation and procollagen expression by lung fibroblasts (original) (raw)
Abstract
Studies have been carried out previously to determine whether mesenchymal stem cells (MSC) influence the progression of pulmonary fibrosis. Here, we asked whether MSC (derived from mouse bone marrow and human umbilical cord blood) produce factors that mediate lung fibroblast (LF) growth and matrix production. MSC-conditioned media (CM) were found by ELISA to contain significant amounts of PDGF-AA and transforming growth factor-β1 (TGF-β1). Proliferation was increased in a concentration-dependent manner in LF cell lines and primary cells cultured in MSC-CM, but neither anti-PDGF antibodies nor PDGF receptor-specific antibodies affected proliferation, nor did a number of other antibodies to well-known mitogenic factors. However, proliferation was significantly inhibited by the Wnt signaling antagonist, secreted frizzled related protein-1 (sFRP-1). In addition, anti-Wnt1 and anti-Wnt2 antibodies attenuated MSC-CM-induced proliferation, and increased expression of Wnt7b was identified. As would be expected in cells activated by Wnt, nuclear β-catenin was increased. The amount of TGF-β1 in MSC-CM and its biological activity were revealed by activation at acidic pH. The stem cells synthesized and released TGF-β1 that increased α1-procollagen gene expression by LF target cells. Addition of anti-TGF-β to the MSC-CM blocked upregulation of collagen gene expression. These data demonstrate that MSC from mice and humans produce Wnt proteins and TGF-β1 that respectively stimulate LF proliferation and matrix production, two hallmarks of fibroproliferative lung disease. It will be essential to determine whether these factors can play a role in attempts to use MSC for therapeutic approaches.
Keywords: peptide growth factors, pulmonary fibrosis
mesenchymal stem cells (MSC) have been derived from several sources including human umbilical cord blood (CBMSC) and mouse bone marrow (BMSC). These cells have broad potential and have been shown to differentiate into fibroblasts and myofibroblasts as well as bone, adipose, and epithelial cell types (7, 12, 30, 34). Initial genomic studies suggested that CBMSC and BMSC differ in expression of a large number of genes (29, 41). However, it is not understood whether these observed differences would translate to altered responses in models of stem cell therapy. MSC appear to influence the development of inflammation and injury in several model systems (28, 37). In the lung, introduction of MSC has ameliorated bleomycin injury (27, 33, 43) since a BMSC subpopulation expressing IL-1 receptor antagonist (IL-1RN) apparently provides protection from bleomycin-induced inflammation by inhibiting TNF-α and IL-1 production (27). In addition, BMSC were observed in the lungs of asbestos-exposed mice and correlated with decreased fibrosis (16). These data suggest that different MSC subpopulations can significantly modulate the onset of a fibrogenic response. Clearly, there is a great deal to be learned about the mechanisms through which MSC play a role in the development of lung disease and whether the cells could serve as potential therapeutic agents.
One approach to learning key aspects about the biology of MSC is to culture the cells and collect the conditioned medium (CM) to determine whether the cells release factors that control the proliferative and fibrogenic features of pulmonary target cells. We have been interested for some time in the biology of TNF-α, transforming growth factor-β1 (TGF-β1), and the PDGF isoforms that control growth and collagen production by lung fibroblasts (39, 42). Inasmuch as interstitial lung fibroblasts and myofibroblasts are the cells that synthesize collagen during the genesis of fibroproliferative lung disease, we are asking in the work presented here whether MSC express paracrine factors that regulate cell growth and collagen production. The results show that MSC derived from human umbilical cord blood (CB) or from the bone marrow of mice release Wnts 1, 2, and 7b, TGF-β1, and PDGF-AA into the culture medium. The CM induced a fibroblast cell line as well as primary lung fibroblasts to proliferate and express the gene that codes for α1-procollagen (COL1A1) in a concentration-dependent pattern. Our data demonstrate: 1) the Wnt signaling antagonist, secreted frizzled-related protein-1 (sFRP-1), inhibited the proliferative effects of the MSC-CM; 2) Wnt7b expression was measured along with nuclear β-catenin; and 3) anti-TGF-β1 antibody inhibited the induction of collagen gene expression in lung fibroblasts.
METHODS
Culture of Primary MSC
BMSC were provided by the Tulane Center for Gene Therapy. Primary mouse BMSC were isolated from the femurs and tibia of male C57BL/6 mice as previously described (35). Cells were plated, and nonadherent cells were removed by washing with PBS. BMSC were further expanded in BMSC culture medium [Iscove's modified Dulbecco's medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 2 mM l-glutamine (Mediatech, Manassas, VA), 100 U/ml penicillin (Mediatech), 100 μg/ml streptomycin (Mediatech), 0.25 μg/ml amphotericin B (Sigma, St. Louis, MO), 10% FBS (Invitrogen), and 10% horse serum (HyClone, Logan, UT)]. Surface marker expression was determined using flow cytometry. BMSC were negative for CD11b and CD45 and positive for SCA-1, VCAM, and CD29 (5). The BMSC were used up to passage 8.
CBMSC from three different donors were provided by the Adult Stem Cell Core at the University of Vermont. Briefly, human CB was obtained from normal term deliveries, and Ficoll gradient centrifugation was used to isolate CB nucleated cells (38). Adherent cells were expanded in CBMSC culture medium [α-minimum essential medium (αMEM; Invitrogen) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS]. CBMSC were assessed for the expression of MSC surface markers via flow cytometry (38). All experiments were conducted with CBMSC at passages 2-5. On receipt of all MSC, stemness was confirmed by inducing differentiation into osteocytes and adipocytes (data not shown). The CBMSC-CM data presented herein were reproduced independently with cells from each of the three donors.
Fibroblast Culture Conditions
Mouse Swiss 3T3 fibroblasts were purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured in DMEM (Mediatech) supplemented with l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS.
Human lung 16Lu fibroblasts were purchased from ATCC and cultured in MEM (Mediatech) supplemented with l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS, nonessential amino acids (Mediatech), and sodium bicarbonate. Cells were kept subconfluent, and media was changed every 3–4 days. 3T3 and 16Lu fibroblasts were used up to passage 15.
Primary normal human lung fibroblasts (NHLF) were purchased from Lonza (Walkersville, MD) and cultured according to manufacturer's instructions in fibroblast basal medium (FBM) supplemented with hFGF-B, insulin, gentamicin, amphotericin B, and 2% FBS (media and supplements from Lonza). Fibroblasts were isolated from an 11-yr-old female and a 31-yr-old male. Experiments were conducted at passages 2-5. Data are representative of both donors and are not significantly different between the two.
Culturing Fibroblasts in MSC-CM
Subconfluent BMSC (Fig. 1_A_) and CBMSC (Fig. 1_B_) were trypsinized and plated in T-150 flasks at a concentration of 8 × 105 cells per flask. Cells were cultured in 20 ml of serum-free (SF) media for 48 h. Concentrated CM was filter-sterilized and stored at −20°C.
Fig. 1.
Comparison of mesenchymal stem cells (MSC) derived from human umbilical cord blood (CBMSC) and mouse bone marrow (BMSC). BMSC (A) and CBMSC (B) were maintained in subconfluent monolayers. Images were obtained with a phase-contrast microscope at a magnification of ×100.
For all experiments, CM was thawed and concentrated with Centricon Plus-20 centrifugal concentration tubes (5,000 NMWL; Millipore, Bedford, MA) and resuspended at a fivefold concentration in SF DMEM, SF MEM, or SF FBM. The fivefold concentration corresponds to 2 × 105 MSC per milliliter. CM was serially diluted fivefold. Acid activation of TGF-β in CM was achieved by acidifying the concentrated CM with 1 N HCl followed by incubation at room temperature for 10 min. The CM was then neutralized with 1.2 N NaOH/0.5 M HEPES before resuspending fivefold in SF medium.
For the proliferation studies, fibroblasts were plated in 96-well plates at 1 × 103 cells/well in a 96-well plate for bromodeoxyuridine (BrdU) analysis or 2 × 104 cells/well in 12-well plates for cell counts. Cells were quiesced in SF media for 24 h before experimentation. Fibroblasts were cultured in serially diluted MSC-CM for 48 or 96 h. After 48 h, BrdU was added at a final concentration of 10 μM for 6 h. Proliferation was assessed using a cell proliferation ELISA commercial kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Cells were enumerated after 4 days with a hemocytometer. Viability was determined using trypan blue exclusion dye.
For the collagen studies, fibroblasts were plated at 2 × 104 cells/well in 12-well plates and quiesced in SF media for 48 h. Cells were treated with serially diluted MSC-CM for 48 h. Collagen expression was determined by real-time RT-PCR analysis of COL1A1 fibroblast mRNA expression.
Fibroblast Coculture Experiments
Target NHLF were plated at 1 × 104 cells/well on the bottom surface of six-well Transwell plates (0.3-μm pore size) and were cultured in serum-containing FBM for 24 h. Subsequently, MSC or NHLF cells were plated in the top well at 4 × 104 cells/well in serum-containing αMEM (CBMSC), IMDM (BMSC), or FBM (NHLF). Control wells contained αMEM only in the top wells. After 24 h, media in the top wells was replaced with SF αMEM (CBMSC) or SF IMDM (BMSC), and media in the bottom wells was replaced with SF FBM. After 72 h, media was aspirated from all wells, and the top wells were discarded. The target NHLF cells were trypsinized, resuspended in trypan blue, and counted using a hemocytometer.
Quantitative Real-Time RT-PCR
Total RNA was isolated and purified using an RNeasy Mini Kit (Qiagen). Two hundred nanograms of RNA were reverse-transcribed in 20-μl volumes using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. One microliter of cDNA was amplified in 20-μl reactions containing 200 nM COL1A1, TGF-β1, or 36B4 primers in iQ SYBR Green Supermix (Bio-Rad). PCR was performed for 35 cycles at 95°C for 15 s and 60°C for 45 s using a MyiQ Real-Time Detection System (Bio-Rad). All samples were run in duplicate. PCR sequences were: COL1A1, forward, 5′-CTGGTAGCCGTGGTTTCCCT-3′, reverse, 5′-CCAGGGCTTCCAGTCAGACC-3′ (166-bp PCR product); 36B4, forward, 5′-TCTGGAGAAACTGCTGCCTCATA-3′, reverse, 5′-GCCAGCAACATGTCCCTGATC-3′ (90-bp PCR product); TGF-β1, forward, 5′-GGACACCAACTATTGCTTCAGCTCC-3′, reverse, 5′-AGGCTCCAAATGTAGGGGCAGGGCC-3′ (155-bp PCR product). Standard curves showed that PCR efficiency was 98–100% for the assays. Negative controls, such as cDNA reactions without RT or RNA, and PCR mixtures lacking cDNA were included to detect possible contamination. Melt curve analysis was conducted to confirm reaction specificity. Samples were quantitated by the relative standard curve method using standard curves made from serial dilutions of COL1A1, 36B4, and TGF-β1 plasmid standards. Expression of COL1A1 and TGF-β1 were normalized to that of 36B4 for each sample.
PCR Array
Two micrograms of total RNA from quiescent NHLF cells and CBMSC was converted to first-strand cDNA with a RT2 First Strand Kit (SABiosciences, Frederick, MD). Relative expression of an array of genes related to the human Wnt signaling pathway was determined using the NHLF and CBMSC cDNAs as templates in real-time PCR RT2 Profiler arrays (SABiosciences). A total of four arrays were analyzed, two for NHLF and two for CBMSC.
Measurement of Cytokine Concentration in the CM
The concentration of TNF-α, TGF-β1, PDGF-BB, and PDGF-AA in MSC-CM cultured in SF media for 48 h was quantified via ELISA (human and mouse TGF-β1, TNF-α, and PDGF-BB DuoSet and PDGF-AA Quantikine ELISA kits; R&D Systems, Minneapolis, MN). To quantify biologically active TGF-β1, the media was acid-activated according to the manufacturer's instructions and compared with untreated media.
Antibodies
Recombinant, human Dickkopf-1 (Dkk-1), sFRP-1, TGF-β1, PDGF, and recombinant, murine Dkk-1 and sFRP-2 were purchased from R&D Systems. Anti-mouse TGF-β, anti-mouse PDGF receptor-α (PDGFR-α), anti-mouse PDGFR-β, anti-human FGF-2, anti-human FGF-4, anti-human FGF-9, anti-human VEGF, and anti-human Wnt7b were all purchased from R&D Systems. Anti-Wnt1 (A-20), anti-Wnt2 (H-20), and anti-Wnt3 (N-15) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies and recombinant proteins were reconstituted according to the manufacturer's instructions.
Measurement of β-Catenin
NHLF were plated in triplicate at 1 × 105 cells/well in 12-well plates, quiesced in SF media for 48 h, and cultured in fivefold concentrated CBMSC- or BMSC-CM or 33 mM LiCl in SF media for 5 h as a positive control (9). Nuclear and cytoplasmic fractions were isolated according to the manufacturer's instructions (NE-PER Nuclear and Cytoplasmic Extraction Reagents; Pierce Biotechnology, Rockford, IL). Total protein concentrations were measured using the Bradford assay. Total β-catenin was quantified via ELISA according to the manufacturer's instructions (R&D Systems).
Statistics
ANOVA was performed for all statistical analyses using a Tukey-Kramer _t_-test to perform multiple comparisons between all treatment groups. All values are expressed as means ± SE where n = 3 or 4. A P ≤ 0.05 was considered statistically significant.
RESULTS
MSC Produce TGF-β1 and PDGF-AA
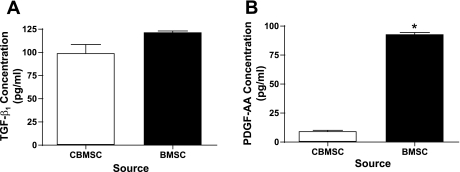
Previously, we presented evidence both in vivo and in vitro that the peptides TNF-α (19), TGF-β1 (20), and PDGF-AA and PDGF-BB (21) play a role in the development of fibroproliferative lung disease. Here, we measured the production of these cytokines in medium conditioned by MSC. To determine the concentration of TNF-α, TGF-β1, PDGF-AA, and PDGF-BB in the supernatant of BMSC and CBMSC, MSC were plated at 80% confluence and cultured in SF media for 48 h. The CM was harvested and measured by ELISA. Gene expression of TGF-β1 was 2.5-fold higher in quiescent CBMSC than in quiescent NHLF as measured by RT-PCR (data not shown). Neither BMSC nor CBMSC produced TGF-β1 that could be detected before acid activation (data not shown). However, following acidification, CM from both MSC types contained similar concentrations of TGF-β1 (∼120 pg/ml; Fig. 2_A_). In contrast, PDGF-AA concentrations were significantly greater in BMSC-CM than CBMSC-CM. BMSC produced ∼90 pg/ml PDGF-AA, and CBMSC produced 10 pg/ml (Fig. 2_B_). PDGF-BB and TNF-α could not be detected by ELISA in the CM from either of the MSC populations (data not shown).
Fig. 2.
Analysis of transforming growth factor-β1 (TGF-β1) and PDGF-AA in MSC-conditioned media (CM). BMSC and CBMSC were plated at 4 × 104 cells per milliliter and quiesced in serum-free (SF) media for 48 h. TGF-β1 (A) and PDGF-AA (B) concentrations were measured in the cell culture supernatants by ELISA. Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from CBMSC, P < 0.05.
MSC-CM Induces Proliferation in Quiescent Lung Fibroblasts
Monolayer cultures.
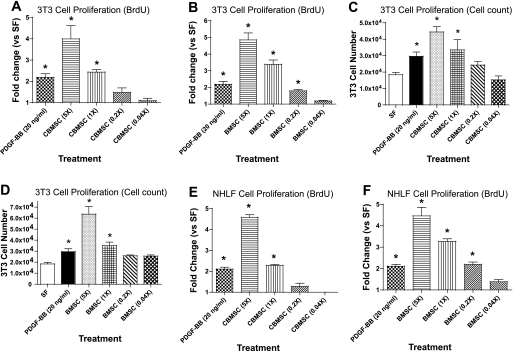
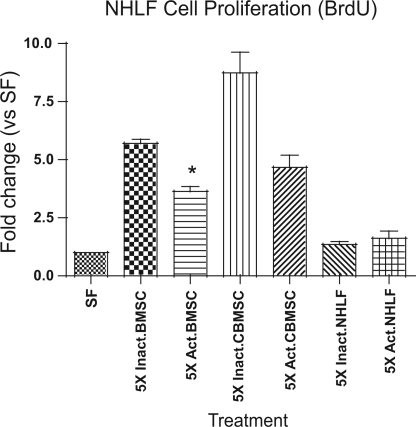
Two primary hallmarks of pulmonary fibrosis are the proliferation of fibroblasts and the production of collagen. To determine whether the cytokines measured in the experiments reported above affect fibroblast proliferation, MSC-CM was concentrated, serially diluted, and applied to quiescent Swiss 3T3 fibroblasts and to NHLF for 48–96 h. Proliferation was assessed by BrdU incorporation and cell counting. CM from both BMSC and CBMSC induced proliferation at similar levels. Recombinant human PDGF-BB was included as the positive control for growth. Fivefold concentrated and unconcentrated CBMSC-CM increased BrdU incorporation 4- and 2-fold, respectively (Fig. 3_A_). Additional concentrations did not induce significant changes in BrdU incorporation. Serial dilutions of BMSC-CM produced similar proliferative effects (Fig. 3_B_). Swiss 3T3 fibroblasts were enumerated via trypan blue exclusion after 4 days of treatment with CBMSC- and BMSC-CM to confirm the BrdU incorporation data, and these counts showed that increased BrdU incorporation correlates with an increased number of cells (Fig. 3, C and D). To confirm that MSC-CM induces proliferation in primary fibroblasts as well as immortalized fibroblasts, primary cells from NHLF were purchased from a commercial source and treated with MSC-CM as in the previous experiments. CBMSC-CM concentrated 5-fold induced an ∼4.5-fold increase in proliferation of NHLF (Fig. 3_E_). In addition, NHLF proliferated at a similar rate as 3T3 fibroblasts following treatment with BMSC-CM (Fig. 3_F_). Together, these data suggest that CM from CBMSC and BMSC contain mitogenic factor(s) capable of inducing fibroblast proliferation at levels similar to 20 ng/ml recombinant human PDGF-BB. Acid activation of 5-fold concentrated BMSC-CM resulted in a significant decrease in NHLF proliferation compared with untreated BMSC-CM (Fig. 4).
Fig. 3.
MSC-CM increases proliferation in quiescent lung fibroblasts. CM from MSC plated at 4 × 104 cells per milliliter and cultured in SF media was concentrated 5-fold and serially diluted. Quiescent Swiss 3T3 fibroblasts were cultured in serially diluted CBMSC- (A) or BMSC-CM (B) for 48 h. PDGF-BB (20 ng/ml) was included as a positive control for proliferation in all experiments. Proliferation was assessed by bromodeoxyuridine (BrdU) incorporation that was measured by ELISA. For cell counts, Swiss 3T3 fibroblasts were cultured for 4 days in serially diluted CBMSC- (C) or BMSC-CM (D). Cell numbers were enumerated in trypan blue. BrdU incorporation was confirmed in quiescent normal human lung fibroblasts (NHLF) cultured for 48 h in CBMSC- (E) or BMSC-CM (F). Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from SF, P < 0.05.
Fig. 4.
Lung fibroblast proliferation is attenuated by acid treatment of MSC-CM. Quiescent NHLF were incubated with acid-treated (Act.) or untreated (Inact.) MSC- and NHLF-CM for 48 h. BrdU incorporation was measured by ELISA and showed that activated TGF-β reduced the proliferation induced by CM. Data represent the means ± SE from quadruplicate wells for each treatment group. *Significantly different from Inactivated BMSC, P < 0.05.
Cocultures.
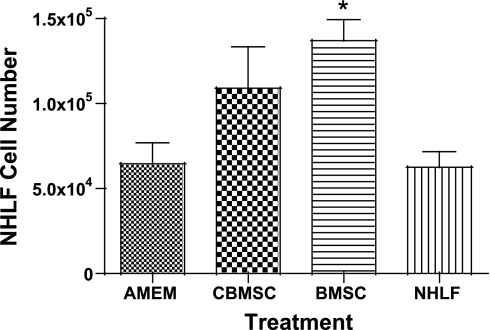
NHLF were grown in coculture with NHLF, BMSC, or CBMSC so that the cells were in close proximity but were separated by a membrane (0.3-μm pore size). NHLF proliferation was significantly increased when cocultured with BMSC (Fig. 5) indicating that the BMSC are releasing a diffusible proliferative factor into the media.
Fig. 5.
BMSC increase proliferation of NHLF cells when grown in coculture. Target NHLF were plated at 1 × 104 cells/well on the bottom surface of Transwell plates (0.3-μm pore size) and cultured in serum-containing fibroblast basal medium (FBM) for 24 h. Subsequently, MSC or NHLF cells were plated at in the top well at 4 × 104 cells/well in serum-containing α-minimum essential medium (AMEM; CBMSC), Iscove's modified Dulbecco's medium (IMDM; BMSC), or FBM (NHLF). Control wells contained AMEM only in the top wells. After 24 h, media in the top wells was replaced with SF AMEM (CBMSC) or SF IMDM (BMSC), and media in the bottom wells was replaced with SF FBM. After 72 h, target NHLF cells were enumerated in trypan blue. It is clear that the MSC in the top well significantly increased target cell numbers in the lower well by means of a diffusible factor. Data represent the means ± SE from triplicate wells for each treatment group. *Significantly different from AMEM and from NHLF, P < 0.05.
sFRP-1 Inhibits the Proliferative Effect of MSC-CM
To determine the cytokine(s) responsible for regulating fibroblast proliferation, several blocking antibodies specific for known MSC mitogenic cytokines were added to the CM. The data in Fig. 1_B_ show that MSC produced measurable levels of PDGF-AA, which is known to induce fibroblast proliferation (11). Anti-PDGFR-α and anti-PDGFR-β were added to the NHLF before treatment with MSC-CM. Growth rates of the NHLF treated with either BMSC- or CBMSC-CM were not significantly affected by the inhibition of the PDGFR (Fig. 6). In addition, a number of other proliferative cytokines such as FGF-2, -4, and -9 and VEGF reportedly are synthesized by BMSC and CBMSC (23, 34). Attempts were made to selectively block these factors by inhibitory antibodies, but each of these failed to suppress the mitogenic effects of the CM (data not shown).
Fig. 6.
Fibroblast proliferation by the CM is not influenced by antibodies to PDGF receptors (PDGFR). The role of PDGF-AA was assessed in the proliferative response. Quiescent NHLF were incubated with anti-PDGFR-α (40 μg/ml) and anti-PDGFR-β (40 μg/ml) 1 h before the addition of SF PDGF-BB (20 ng/ml), BMSC-CM, or CBMSC-CM. BrdU incorporation was measured by ELISA. Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from PDGF-BB, P < 0.05. #Significantly different from SF, P < 0.05.
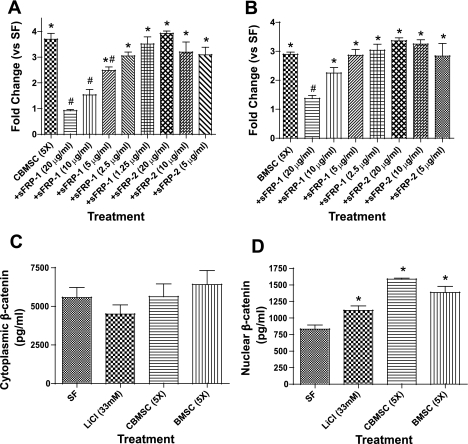
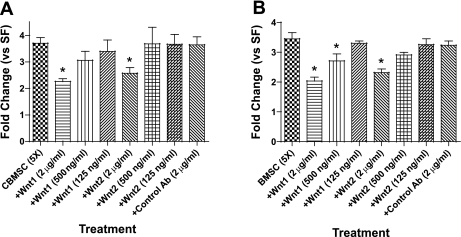
Wnt proteins have been shown to be potent fibroblast mitogens (9, 44), and recent studies have demonstrated that MSC secrete several Wnt proteins (1, 8). To determine whether Wnt plays a role in the induction of proliferation by MSC-CM, several concentrations of recombinant human sFRP-1 and murine sFRP-2 were added to the CM before treating the NHLF. sFRP-1 inhibited NHLF proliferation induced by CBMSC- and BMSC-CM in a concentration-dependent manner (Fig. 7, A and B). However, sFRP-2 did not affect NHLF proliferation at any of the concentrations tested (Fig. 7, A and B). Stimulation of the Wnt signaling pathway has been shown to increase β-catenin concentrations (9). Nuclear and cytoplasmic β-catenin concentrations were measured via ELISA in NHLF following 5-h culture in CBMSC-CM, BMSC-CM, or LiCl (33 mM) as a positive control. Cytoplasmic β-catenin concentrations were not significantly changed in response to either CBMSC or BMSC (Fig. 7_C_), but both CBMSC- and BMSC-CM increased nuclear β-catenin concentrations similar to levels stimulated by LiCl (Fig. 7_D_).
Fig. 7.
MSC-CM induces fibroblast proliferation via a Wnt/β-catenin signaling pathway. The inhibitory effect of secreted frizzled related protein-1 (sFRP-1) and sFRP-2 on NHLF proliferation following CBMSC or BMSC was assessed with a BrdU ELISA. sFRP-1 and sFRP-2 were added in various concentrations to 5-fold concentrated CBMSC- (A) or BMSC-CM (B) for 1 h at 37°C before NHLF treatment. BrdU incorporation was measured via ELISA after 48 h. Cytoplasmic (C) and nuclear (D) β-catenin concentrations were quantified via ELISA in NHLF following 4-h treatment with SF media, 5-fold concentrated CBMSC- or BMSC-CM, or LiCl (33 mM) as a positive control. Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from SF, P < 0.05. #Significantly different from MSC-CM, P < 0.05.
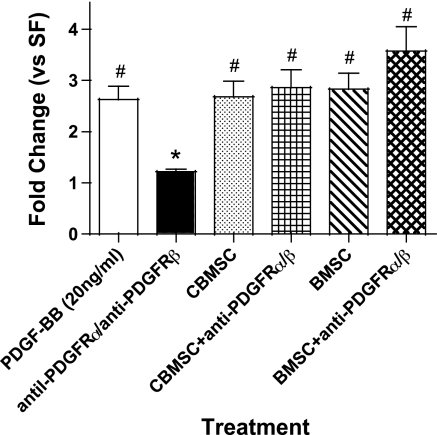
Anti-Wnt1 and 2 Inhibit Fibroblast Proliferation, and Wnt7b Expression is Identified by PCR
MSC have been shown to produce a number of canonical Wnts such as Wnt1, 2, and 7b (1, 23). Anti-Wnt1 and 2 antibodies were added to CBMSC- and BMSC-CM before fibroblast treatment (Fig. 8, A and B). A nonspecific isotype control was added to the CM. The data indicate that both antibodies significantly attenuated fibroblast proliferation in a concentration-dependent fashion (Fig. 8, A and B). Together, these data suggest that MSC-CM contain a sufficient concentration of Wnt proteins to induce lung fibroblast proliferation. Expression of Wnt7b was determined by PCR array to be ∼50-fold higher in quiescent CBMSC than in quiescent NHLF; however, no difference in Wnt1 or Wnt2 expression was detected (data not shown).
Fig. 8.
Anti-Wnt1 and Wnt2 inhibit fibroblast proliferation. Anti-Wnt1, anti-Wnt2, and an isotype control were added to 5-fold concentrated CBMSC- (A) or BMSC-CM (B) for 1 h at 37°C before NHLF treatment. BrdU incorporation was measured via ELISA after 48 h. Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from MSC-CM, P < 0.05. Ab, antibody.
MSC-CM Induces Collagen Gene Expression in Lung Fibroblasts
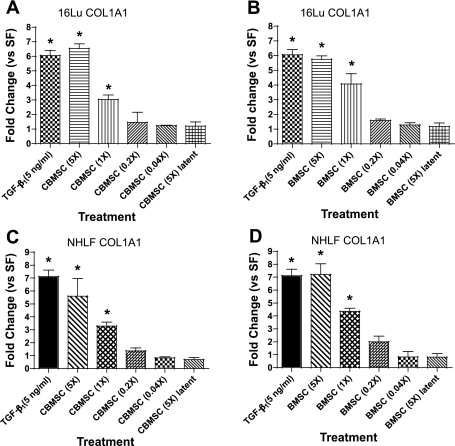
To determine the effect of the TGF-β1 produced by the MSC on collagen gene expression in fibroblasts, CM was concentrated, serially diluted, and applied to quiescent 16Lu and NHLF for 48 h. Gene expression was determined by analyzing COL1A1 levels measured by RT-PCR. When the TGF-β1 in the CM from BMSC or CBMSC remained latent, there was no effect on COL1A1 levels (Fig. 9, A and B). However, acid activation of the CM to remove the latency-associated peptide (24, 31) from TGF-β1 resulted in a significant dose-dependent increase in COL1A1 levels (Fig. 9, A and B). Both CBMSC- and BMSC-CM increased COL1A1 in 16Lu fibroblasts 5- to 7-fold when concentrated 5-fold (Fig. 9, A and B, respectively). In addition, unconcentrated CM from either source increased collagen gene expression approximately 3- to 4-fold. As conducted in the previous set of experiments, primary human fibroblasts were utilized to confirm the results seen with 16Lu fibroblasts. Both CBMSC- and BMSC-activated CM increased procollagen gene expression(s) in NHLF to similar levels as those found in the 16Lu cell line (Fig. 9, C and D).
Fig. 9.
Activated MSC-CM induces expression of the procollagen gene in fibroblast target cells. CM from quiescent MSC plated at 4 × 104 cells per milliliter was concentrated 5-fold. Quiescent 16Lu fibroblasts were cultured in serially diluted acid activated or latent CBMSC- (A) or BMSC-CM (B) for 48 h. TGF-β1 (5 ng/ml) was included as a positive control for collagen gene upregulation. α1-Procollagen (COL1A1) upregulation was determined by quantitative real-time RT-PCR. The collagen gene was also assessed in NHLF treated with activated CBMSC- (C) or BMSC-CM (D) for 48 h. Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from SF, P < 0.05.
Antibody Inhibition of TGF-β1 Blocks the Induction of COL1A1 in Lung Fibroblasts
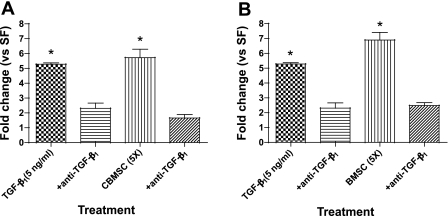
The previous data show that activating TGF-β1 in the CM increases COL1A1 gene expression. To demonstrate that it was the TGF-β1 in the CM that induced collagen gene expression, anti-TGF-β was added to the CM before fibroblast treatment. The data indicate that specific blocking of TGF-β1 abrogates the enhanced collagen expression in fibroblasts treated with MSC-CM (Fig. 10, A and B). Together, these data suggest that the CM is able to induce matrix production in fibroblasts via a TGF-β1-dependent pathway.
Fig. 10.
Anti-TGF-β inhibits collagen gene upregulation in fibroblasts treated with activated MSC-CM. Anti-TGF-β (20 μg/ml) was added to CBMSC- (A) or BMSC-CM (B) for 1 h at 37°C before 16Lu treatment. COL1A1 was measured by RT-PCR after 48 h. Data represent the means ± SE from triplicate wells for each treatment group from 3 separate experiments. *Significantly different from SF, P < 0.05.
DISCUSSION
The objective of our studies reported here was to determine whether a small set of well-characterized peptides are synthesized and released by human and mouse MSC and then to determine whether the factors mediate the growth and matrix production of lung fibroblasts as target cells. To determine the degree of biological variability that is likely to be present in both the stem cells and fibroblast targets, we compared CBMSC from 3 individual donors and BMSC from mice. The fibroblasts were derived from human and murine lungs. Our data demonstrate that CM from MSC derived from these two different sources clearly produced PDGF-AA, but apparently at a concentration insufficient to cause proliferation. On the other hand, latent TGF-β1 that could be readily activated was released at concentrations sufficient to induce collagen gene expression in lung fibroblasts. In addition, we present evidence that CBMSC- and BMSC-CM induce lung fibroblast proliferation via stimulation of the Wnt-β-catenin pathway. Human and mouse signaling proteins and their receptors are highly homologous as determined by amino acid sequence alignments; Wnts 1, 2, and 7b proteins are at least 96% identical, TGF-β1 proteins are 90% identical, and TGF-β and frizzled receptors are 95 and 93% identical, respectively.
Previous reports have demonstrated inhibitory effects of MSC on the growth of target cells such as T lymphocytes and cardiac fibroblasts (13, 18, 26). Conversely, MSC have been shown to induce proliferation and migration in keratinocytes and endothelial cells (3, 32). To our knowledge, the results reported here represent the first demonstration of the mitogenic potential of MSC-CM on lung fibroblast target cells in vitro. The earlier studies indicated above reported suppression of cardiac fibroblast proliferation and collagen production (18, 26), but these differ technically from our studies in several ways. Finally, on this topic, Li and colleagues (18) reported antiproliferative and antifibrotic effects when BMSC-CM was combined with a profibrotic protein such as angiotensin II. Thus conditions for collecting CM appear to vary greatly among these reported studies, and clearly future studies are required to characterize the role of culture conditions on the concentration of paracrine factors in media conditioned by MSC.
In our initial search for mitogenic cytokines, we found that both BMSC- and CBMSC-CM contain PDGF-AA and that BMSC produce ninefold more PDGF-AA than CBMSC. PDGF-AA is a well-established mitogen for mesenchymal cells, including lung fibroblasts, and is upregulated during fibrogenesis in animal models (21) and human lungs (25). However, we found that inhibiting the PDGF α- and β-receptors on lung fibroblasts did not reduce the ability of MSC-CM to enhance proliferation (Fig. 6) and antibodies to the AA and BB isoforms had no effect on cell growth (data not shown). The BB isoform was not detected, and our data suggested that either the concentrations of PDGF-AA in the CM were not sufficient to affect lung fibroblast growth or the AA isoform was not biologically active. Apparently, there is another, more potent proliferative factor in the media that is diffusible to the target cells in our coculture system (Fig. 5).
The Wnt family of proteins regulates a number of cell functions including proliferation, morphology, and migration. Several reports have observed an upregulation of Wnt-related proteins in fibrotic lungs (14). A recent study observed upregulation of Wnt1-inducible signaling proteins 1 and 7b in the lungs of bleomycin-exposed mice (17). Another study found that the Wnt downstream effector, β-catenin, was increased in the nucleus of spindle-shaped mesenchymal cells located in fibroblast foci in the lungs of 16 out of 20 patients diagnosed with idiopathic pulmonary fibrosis (IPF) or usual interstitial pulmonary fibrosis (4). Both BMSC and CBMSC express several Wnt genes such as Wnt1, 2, 4, 5a, 5b, 11, and 16 (1, 23). Our data demonstrate that the Wnt antagonist sFRP-1 significantly inhibits the mitogenic effect of both CBMSC- and BMSC-CM (Fig. 5). sFRP binds directly to Wnts and is hypothesized to inhibit the activation of frizzled/LRP receptors by the Wnt ligands (2). It is currently unknown how the different members of the sFRP family affect the proliferative capacity of the various Wnt proteins (2). In accordance with finding a Wnt ligand playing a role in fibroblast proliferation, we showed there was a clear upregulation of nuclear β-catenin in cells cultured in the MSC-CM (Fig. 7_D_). This suggests that the proliferative response is mediated through the canonical pathway as reported previously for Wnt proteins synthesized by MSC, i.e., Wnts 1, 2, and 7b signal through the canonical β-catenin pathway (36). Detection of increased expression of the Wnt7b gene in a PCR array suggests that this Wnt may contribute to the fibroblast proliferative response. Antibodies to other canonical Wnt proteins, i.e., Wnt1 and Wnt2, significantly attenuated fibroblast proliferation (Fig. 8, A and B), however, increased expression of these genes was not detected by the PCR array. Wnts 3 and 5 did not block cell proliferation in our system. The conservation of the Wnt pathway among many different cell populations (22) suggests that Wnt synthesized by MSC could affect other cell types in a similar manner. For example, studies have demonstrated that Wnt1 induces proliferation in endothelial cells and fibroblasts (10, 44). Future studies will determine whether Wnt synthesis by MSC and the biological activity of the Wnt-β-catenin pathway are altered as the stem cells differentiate and are exposed to other lung cell types in vitro and in vivo.
Expression of several cytokine genes by MSC has been demonstrated (3, 29, 41); however, to our knowledge, this is the first study to quantify the concentration of TGF-β1 and PDGF-AA produced by CBMSC. Our data confirm the findings of previous studies demonstrating TGF-β1 gene expression in MSC (3, 34). Interestingly, the concentration of TGF-β1 we report here in the CM from BMSC and CBMSC is similar to the levels produced by lung fibroblasts (39). In addition, all of the measurable TGF-β1 synthesized by MSC is present in the latent form as reported previously in lung fibroblasts (39). One of the many functions of TGF-β1 is to act as a potent stimulator of matrix production (40) and as an antagonist of cell proliferation (31). It has been well-established that upregulation of TGF-β1 in the lung is a hallmark of IPF (40), and TNF-α has been shown to regulate TGF-β1 expression in lung fibroblasts by activating the ERK-specific MAPK pathway (39). Our repeated attempts to identify TNF-α in the MSC-CM were unsuccessful, and this is consistent with other studies (15), suggesting that when the MSC synthesize TGF-β1, expression of this peptide is not influenced by TNF-α in an autocrine or paracrine fashion. Whether TNF-α from other lung or circulating cells would influence the expression of TGF-β1 by MSC is unknown at this time but could be an important parameter in an inflammatory process. As would be expected, activated TGF-β1 in the CM did block target cell proliferation (Fig. 4).
Activation of latent TGF-β1 is necessary for the ligand to bind to specific cognate receptors and upregulate matrix production in fibroblasts. Several studies have demonstrated that fibrotic lungs contain a number of factors that contribute to activating latent TGF-β1 (6, 24, 31). Consistent with this knowledge, we found that MSC-CM did not enhance collagen gene expression unless the TGF-β therein was activated at low pH. Activation of TGF-β1 in the CB and BMSC-CM upregulated collagen gene expression to levels similar to the TGF-β1-positive controls (Fig. 9). These data suggest that the TGF-β1 in the MSC-CM could act as a profibrotic paracrine factor, but this observation does not necessarily follow what has been seen in vivo. For example, studies demonstrated that amelioration of fibrosis followed bone marrow transplantation in mice exposed to asbestos, and these animals had decreased TGF-β1 in the bronchoalveolar lavage fluid (16). Ortiz and colleagues (27) found that a unique subpopulation of BMSC produce IL-1RN, which blocked TNF-α and IL-1 in bleomycin-treated lungs. These data suggest that MSC could inhibit the accumulation of TGF-β1 by inhibiting the production of TNF-α in alveolar macrophages (27). On the other hand, Xu and coworkers (43) demonstrated that BMSC expressing CXCR4+ contribute to the progression of fibrotic lesions. It is currently unknown whether the CBMSC and BMSC we used produce IL-1RN or are positive for CXCR4. Further investigations will determine how culture conditions as well as differentiation of MSC affects the production of TGF-β1 and its biological properties. At this juncture, our studies suggest that the TGF-β1 produced by MSC could induce matrix production following activation as discussed above.
In summary, our studies show that the MSC derived from mouse BM and human CB produce biologically active Wnts 1 and 2 as well as TGF-β1 (Figs. 8 and 10). Synthesis of these factors was not found to vary significantly between donors or species. Wnt ligand increased accumulation of nuclear β-catenin and induced cell proliferation. The TGF-β1 induced different populations of fibroblast target cells to upregulate the gene that codes for COL1A1. These two activities, fibroblast proliferation and extracellular matrix production, are two key features of lung fibrogenesis. Whether the MSC will influence lung cells in vivo as they have been shown to do here in vitro remains unclear. Certainly, little is known in regard to how exogenous treatment with cytokines or placing the stem cells in coculture conditions with different lung subpopulations would affect production of TGF-β1, Wnts, or other potent mediators. In addition, it will be critical to determine how differentiation into respiratory phenotypes such as myofibroblasts and epithelial cells affects synthesis of these fibroproliferative factors.
GRANTS
This work was supported by National Institutes of Health Research Grants ES-06766 and HL-60532.
REFERENCES
- 1.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25: 1384–1392, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci 121: 737–746, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3: e1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA 98: 7841–7845, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93: 1159–1170, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 21: 514–520, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 22: 849–860, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, Nusse R, Burrus LW. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn 235: 681–690, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin AM, Kitajewski J, D'Amore PA. Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors 25: 25–32, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ingram JL, Rice AB, Geisenhoffer K, Madtes DK, Bonner JC. IL-13 and IL-1beta promote lung fibroblast growth through coordinated up-regulation of PDGF-AA and PDGF-Ralpha. FASEB J 18: 1132–1134, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41–49, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol 179: 2824–2831, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627–2634, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kogler G, Radke TF, Lefort A, Sensken S, Fischer J, Sorg RV, Wernet P. Cytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cells. Exp Hematol 33: 573–583, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Levis J, Loi R, Butnor KJ, Vacek P, Steele C, Mossman BT, Weiss DJ. Decreased asbestos-induced lung inflammation and fibrosis after radiation and bone marrow transplant. Am J Respir Cell Mol Biol 38: 16–25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CC, Yang JY, Huang X, Banerjee SK, Blackburn MR, Baluk P, McDonald DM, Blackwell TS, Nagabhushanam V, Peters W, Voehringer D, Erle DJ. Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med 177: 376–387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep 36: 725–731, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Liu JY, Brass DM, Hoyle GW, Brody AR. TNF-alpha receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestos fibers. Am J Pathol 153: 1839–1847, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JY, Brody AR. Increased TGF-beta1 in the lungs of asbestos-exposed rats and mice: reduced expression in TNF-alpha receptor knockout mice. J Environ Pathol Toxicol Oncol 20: 97–108, 2001 [PubMed] [Google Scholar]
- 21.Liu JY, Morris GF, Lei WH, Hart CE, Lasky JA, Brody AR. Rapid activation of PDGF-A and -B expression at sites of lung injury in asbestos-exposed rats. Am J Respir Cell Mol Biol 17: 129–140, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Markov V, Kusumi K, Tadesse MG, William DA, Hall DM, Lounev V, Carlton A, Leonard J, Cohen RI, Rappaport EF, Saitta B. Identification of cord blood-derived mesenchymal stem/stromal cell populations with distinct growth kinetics, differentiation potentials, and gene expression profiles. Stem Cells Dev 16: 53–73, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Nagaoka I, Trapnell BC, Crystal RG. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest 85: 2023–2027, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett 581: 3961–3966, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100: 8407–8411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panepucci RA, Siufi JL, Silva WA, Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 22: 1263–1278, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA 95: 1142–1147, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab Invest 84: 1013–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 25: 1761–1768, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33: 145–152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 17: 199–206, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA 99: 4397–4402, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 8: 1349–1358, 1997 [PubMed] [Google Scholar]
- 37.Spees JL, Pociask DA, Sullivan DE, Whitney MJ, Lasky JA, Prockop DJ, Brody AR. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med 176: 385–394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med 177: 701–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan DE, Ferris M, Pociask D, Brody AR. Tumor necrosis factor-alpha induces transforming growth factor-beta1 expression in lung fibroblasts through the extracellular signal-regulated kinase pathway. Am J Respir Cell Mol Biol 32: 342–349, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Villanueva AG, Farber HW, Rounds S, Goldstein RH. Stimulation of fibroblast collagen and total protein formation by an endothelial cell-derived factor. Circ Res 69: 134–141, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33: 1402–1416, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Warshamana GS, Corti M, Brody AR. TNF-alpha, PDGF, and TGF-beta(1) expression by primary mouse bronchiolar-alveolar epithelial and mesenchymal cells: tnf-alpha induces TGF-beta(1). Exp Mol Pathol 71: 13–33, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol 37: 291–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young CS, Kitamura M, Hardy S, Kitajewski J. Wnt-1 induces growth, cytosolic beta-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol 18: 2474–2485, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]