Defective CD8 T Cell Memory Following Acute Infection Without CD4 T Cell Help (original) (raw)
. Author manuscript; available in PMC: 2009 Nov 17.
Published in final edited form as: Science. 2003 Apr 11;300(5617):339–342. doi: 10.1126/science.1083317
Abstract
The CD8+ cytotoxic T cell response to pathogens is thought to be CD4+ helper T cell independent because infectious agents provide their own inflammatory signals. Mice that lack CD4+ T cells mount a primary CD8 response to Listeria monocytogenes equal to that of wild-type mice and rapidly clear the infection. However, protective memory to a challenge is gradually lost in the former animals. Memory CD8+ T cells from normal mice can respond rapidly, but memory CD8+ T cells that are generated without CD4 help are defective in their ability to respond to secondary encounters with antigen. The results highlight a previously un-described role for CD4 help in promoting protective CD8 memory development.
Dual recognition of antigen, the requirement for two sites to be recognized as foreign, provides a safeguard against autoimmunity. A strong antibody response by B cells depends on the concurrent activation of CD4+ helper T cells. Similarly, the CD8+ T cell response to noninflammatory immunogens, such as antigen-loaded, transformed, or allogeneic cells, also depends on antigen recognition by CD4+ T cells. In contrast, the CD8 response to acute infections with live virus or intracellular bacteria has been classified as CD4 helper independent. This dichotomy has been neatly explained by the idea that antigen-presenting cell (APC) activation is pivotal to the development of strong primary effector CD8+ T cell responses leading also to long-lasting protective memory. Thus, in cases of immunization with antigen from a noninflammatory source, CD4+ T cells are required to activate APCs through CD40 signaling, “licensing” the APC to stimulate a full-blown CD8 response (1–3). By contrast, infectious agents such as the intracellular Gram-positive pathogen Listeria monocytogenes, which carry a plethora of immunostimulatory signals (such as cell wall components, flagellin, and CpG DNA) (4, 5), activate APCs directly, thereby bypassing the need for CD4 help (6).
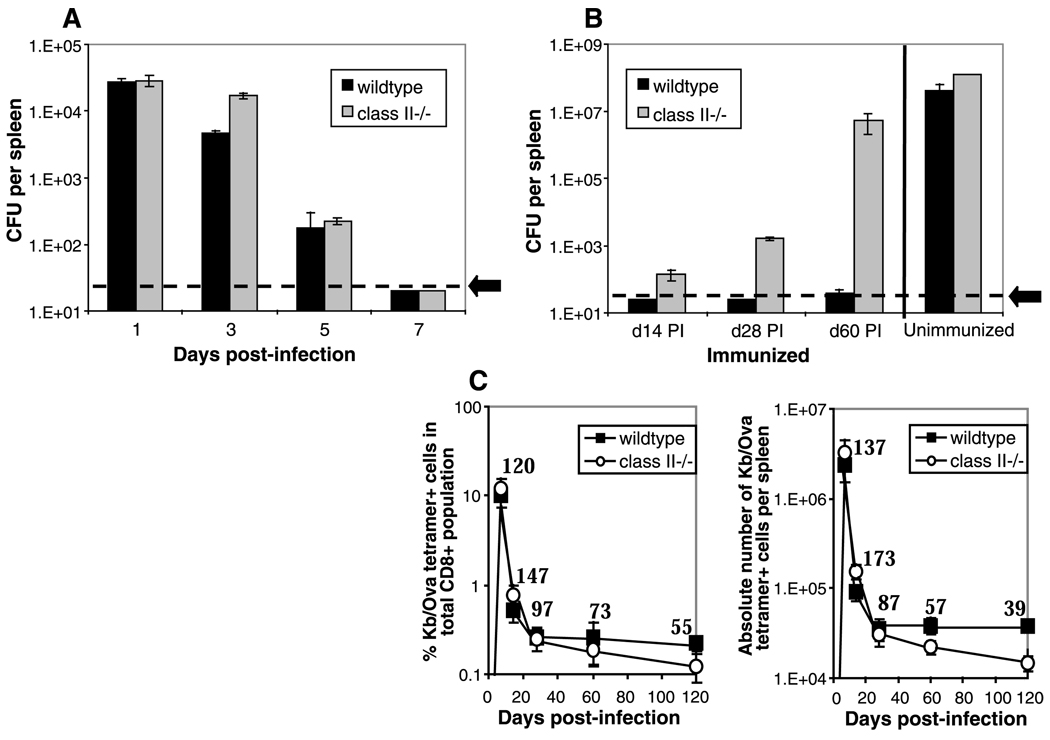
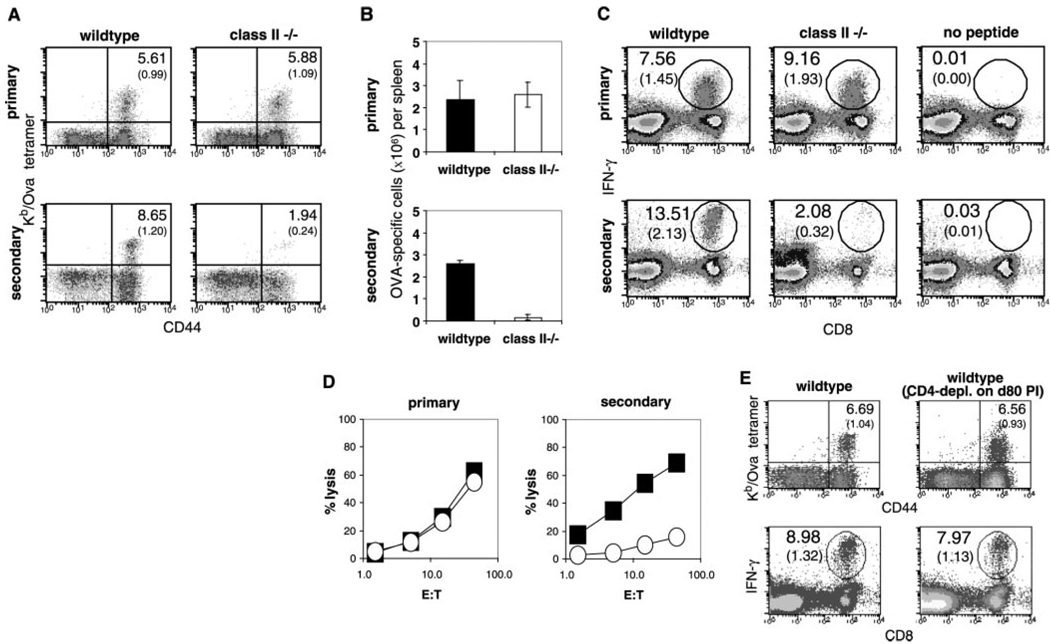
We studied the kinetics of bacterial clearance, the expansion and contraction of antigen-specific CD8+ cells, and the acquisition of protective immunity in intact mice and in mice lacking CD4 T cell function. We used tetramers of class I molecules and the ovalbumin peptide to monitor the CD8+ T cell response to a recombinant strain of Listeria that makes chicken ovalbumin, rL-mOva (7). Consistent with previous work (8, 9), both strains cleared the infection within 7 days (Fig. 1A) and developed equally robust primary CD8 responses to the Ova determinant expressed by the recombinant Listeria (Fig. 1C). Unexpectedly, the ability to resist a second challenge dose of Listeria gradually waned in the class II−/− mice (Fig. 1B). This decline was not due to a loss of memory T cells, because tetramer and intracellular cytokine staining after peptide restimulation revealed that CD8+ memory cell numbers were maintained at close to wild-type levels over a 60-day period after primary immunization. At later time points, the numbers of memory T cells declined to less than half those of wild type (Fig. 1C). At the peak of the primary response, the CD8 response and function appeared normal in both sets of mice. The class II−/− animals generated high numbers of Kb/Ova tetramer-positive staining cells, which were able to produce high levels of interferon-γ (IFN-γ) during in vitro stimulation and were able to lyse peptide-coated target cells (Fig. 2, A to D). After secondary challenge with rLmOva, however, there was a marked deficiency in the response of class II−/− mice, as measured by tetramer-positive staining, the number of IFN-γ-positive cells, or levels of cytotoxic activity (Fig. 2). Quantitation of the Ova-specific CD8 T cells showed that the wild-type and mutant mice contained roughly equal numbers of memory cells at day 60 before the second immunization. However, the wild-type numbers increased about 80-fold in the 3 days after secondary immunization, whereas the numbers of Ova-specific CD8+ cells in class II−/− mice increased less than 10-fold (Fig. 2B). In a separate experiment, even at 5 days after secondary immunization, Ova-specific CD8 cells from class II−/− mice expanded to only a third of the number in wild-type mice. This result revealed that, although naïve CD8+ T cells could respond very well to Listeria infection in the absence of CD4+ T cell help, memory CD8+ T cells in the same animals responded poorly. To determine whether wild-type memory CD8 cells become more helper dependent than their naïve counterparts, we depleted _Listeria_-primed B6 mice of CD4+ cells by injection of an opsonizing CD4 monoclonal antibody 80 days after priming, immediately before secondary immunization. Depleting the CD4 T cells at this late stage after priming had no deleterious effect on the ability of memory CD8 cells in wild-type mice to respond to secondary antigen challenge (Fig. 2E).
Fig. 1.
Mice deficientin CD4+ T cells show a progressive loss in protective immunity. (A) Wild-type C57BL/6 and major histocompatibility complex (MHC) class II–deficient (class II−/−) mice were injected intravenously with 2 × 103 rLmOva and, on the indicated number of days postinfection (PI), the number of live bacteria per spleen was determined (7). (B) Previously immunized mice were challenged intravenously with 105 rLmOva at14, 28, and 60 days PI. Protective immunity was measured by determining the number of colony-forming units (CFU) in spleen 3 days after challenge. An unimmunized set of mice was also given 105 rLmOva as a control. The values shown represent the mean CFU ± SE. The limit of detection in all experiments was 20 CFU per spleen (designated by the dotted line and arrow). (C) Mice were injected intravenously with 2 × 103 rLmOva; 7, 14, 28, 60, and 120 days after immunization, memory CD8+ T cell numbers were measured. The percentage of Kb/Ova tetramer-positive cells in the total CD8+ T cell population (left) and the absolute number of Kb/Ova tetramer-positive cells in the spleen (right) are plotted. Numbers on the graphs represent the percentage of memory CD8+ T cells from class II−/− compared with wild-type mice. The data shown are representative of two independent studies, with two to three mice per group at each time point.
Fig. 2.
Equivalent primary CD8+ T cell response, but weak secondary response, in MHC class II– deficient mice compared with wild-type mice. Naïve and 60-day PI mice were given 2 × 103 or 105 rLmOva, respectively. Primary CD8+ T cell responses were measured 7 days PI by Kb/Ova-specific tetramer staining, intracellular IFN-γ staining, and 51Cr-release cytotoxicity assays (7). To examine secondary CD8+ T cell responses in previously immunized mice, we performed similar assays 3 days after challenge. (A and C) Kb/Ova tetramer and intracellular IFN-γ staining were done to determine the percentage of antigen-specific cells in the CD8+ T cell population (top numbers), as well as in the whole splenocyte pool (bottom numbers in parentheses). (A) CD44+, tetramer+ cells in the CD8+ T cell population. (C) CD8+, IFN-γ–producing cells in total splenocytes. (B) Total numbers of Kb/Ova-specific cells in spleens were determined. (D) Four-hour 51Cr-release assays were performed to measure specific killing of OVA peptide–loaded EL-4 cells by wild-type (filled squares) and MHC class II−/− (open circles) splenocytes taken directly ex vivo. (E) At80 days PI, wild-type mice were given phosphate-buffered saline (PBS) or CD4-depleting antibody, GK1.5, before challenge with bacteria. The data shown are representative of two independent studies, with two mice per group at each time point.
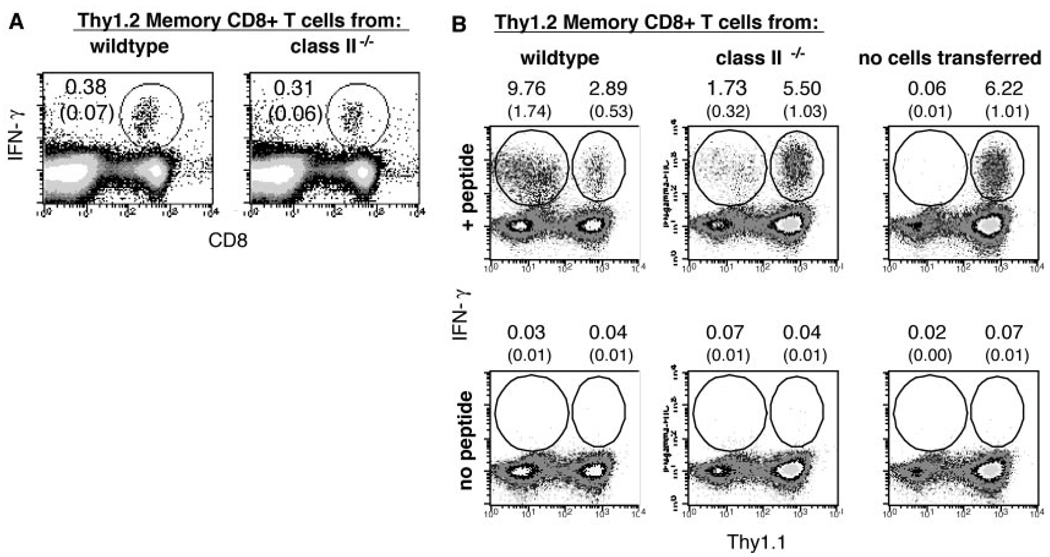
To more rigorously compare the functional properties of memory CD8+ T cells generated in wild-type versus class II−/− mice, we tested the ability of these cells to mount recall responses after adoptive transfer into wild-type hosts. Because the host mice carried an allelic Thy1.1 marker that distinguished their T cells from transferred T cells, the primary response of the naïve host T cells and the secondary response of the transferred memory cells could be simultaneously monitored. Spleen cells from B6 and class II−/− mice that had been primed 80 days previously with rLmOva were depleted of CD4+ cells, and a sample was stained with tetramers and for IFN-γ–producing cells to estimate the frequency of Ova-specific memory cells (Fig. 3A). Equal numbers (104) of wild-type or class II−/− memory CD8+ cells were then adoptively transferred into naïve B6 (Thy1.1×Thy1.2) F1 recipients and challenged with rLmOva. Seven days after immunization, the Ova-specific CD8 response of the mice was assessed by tetramer staining and peptide-stimulated IFN-γ production (Fig. 3B). Naïve B6 (Thy1.1 × Thy1.2) mice that received no adoptively transferred cells showed a typical primary anti-Ova CD8 response. Mice that received 104 wild-type memory cells showed a diminished host response and a large secondary response by the transferred memory cells. In contrast, transferred Ova-specific memory cells derived from the class II−/− mice mounted a much weaker recall response. The slower response of the class II−/− memory cells allowed the naïve (host) T cell response to occur as if there were no competition (Fig. 3B). This provides strong evidence that memory cells generated in the absence of CD4 T cell help during initial priming differ qualitatively from memory cells generated in the presence of normal CD4+ T cell help, resulting in impaired ability to respond to secondary challenge, even if CD4+ T cells are subsequently supplied. Not only were memory CD8 T cells from class II−/− mice less vigorous than wild-type counterparts, they also compared poorly with naïve CD8 T cells (Fig. 3B). It is likely that of 104 injected memory cells, only 10% engraft or “take” in the host, where they compete with an estimated 100 to 200 endogenous, naïve, Ova-specific CD8 T cells (10).
Fig. 3.
Memory CD8+ T cells from MHC class II−/− mice show reduced ability to proliferate after transfer into wild-type mice. CD8+ T cells (Thy1.2) from wild-type and class II−/− mice 80 days after priming were enriched with magnetic anti-CD4 bead depletion (7) and 104 Ova-specific cells adoptively transferred into separate wild-type F1 (C57BL/6 × B6.PL) (Thy1.1 × Thy1.2) hosts. The next day, all mice were injected intravenously with 2 × 103 rLmOva, and CD8+ T cell responses were assayed 7 days later. As a control (to monitor the expansion levels of endogenous CD8+ T cells), F1 hostmice that had not received memory cells were similarly infected. (A) Before adoptive transfer, CD4-depleted cell populations were stained for CD8 and intracellular IFN-γ. The percentages of Ova-specific cells within the CD8+ population (top numbers) and total pool (bottom numbers in parentheses) are shown. (B) On day 7 after infection, intracellular IFN-γ staining of splenocytes was performed. All plots show IFN-γ–producing transferred (Thy1.1−) and host (Thy1.1+) cells within the total cell population. The percentages of antigen-specific cells within the splenic CD8+ (top numbers) and total cell (bottom numbers in parentheses) population are shown.
Other reports have shown the decline of CD8+ T cell memory in mice that lack CD4+ T cells (11–19). In these cases, however, the immunogen was a viral infection. Two problems may confound the issue: First, the infection may become chronic in CD4-deficient animals such that T cells would be continually exposed to persisting antigen. In such situations, it is well established that chronically stimulated CD8 cells require CD4 cells for survival (15–21). Second, the deficient antiviral antibody response in CD4-deficient mice may play a role in the loss of protection. Conversely, antibody plays no role in _Listeria_immunity, making this less of a problem in the system used in our study (22–24).
A recent study led to the suggestion that CD40L-CD40 interaction between CD4 and CD8 cells responding to a cellular form of antigen [male cells expressing histocompatibility-Y (HY) antigen] is essential for the generation of robust CD8 memory cells (25, 26). CD8 cells initially responded well to antigen in the absence of CD4 help but made a “lethargic” response to a secondary immunization. This finding suggests that injections of activating CD40-specific antibody to replace the requirement for CD4 help during CD8 responses (1, 2) may operate at the level of the APC, the CD8+ cell, or both. It will be important to assess the relevance of direct or indirect CD40 interactions between T cells in the CD8 response to immunogens such as Listeria. How might lethargic CD8 memory cells generated in the absence of help be different from normal memory cells? Are they the same clones that have failed to undergo a complete program of differentiation, or are they a different subset of the polyclonal repertoire resulting from a failure to select for the fittest clones to become memory cells? The study of the anti-HY response used monoclonal CD8 T cells, and we can conclude in this case that the generation of lethargic memory CD8 cells is due to a failure to differentiate properly (25, 26), although both possibilities may apply during a polyclonal, endogenous CD8 response.
Our results imply that vaccination schemes that target only CD8 immunity, even though they may stimulate strong, early responses, will fail to provide long-term protection. Defining all of the signals required to produce long-lived, robust memory cells and understanding how they are maintained will aid vaccine design.
Supplementary Material
Online Materials and Methods
Footnotes
References and Notes
- 1.Bennett SR, et al. Nature. 1998;393:478. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 2.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. Nature. 1998;393:480. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 3.Ridge JP, Di Rosa F, Matzinger P. Nature. 1998;393:474. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R. Annu. Rev. Immunol. 2002;20:197. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S. Cell. 2002;111:927. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 6.Rahemtulla A, et al. Nature. 1991;353:180. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 7.Materials and methods are available as supporting material on Science Online.
- 8.Lauvau G, et al. Science. 2001;294:1735. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 9.Ladel CH, Flesch IE, Arnoldi J, Kaufmann SH. J. Immunol. 1994;153:3116. [PubMed] [Google Scholar]
- 10.Blattman JN, et al. J. Exp. Med. 2002;195:657. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. J. Virol. 1996;70:1072. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. J. Virol. 2002;76:12388. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Andreansky S, Diaz G, Hogg T, Doherty PC. J. Immunol. 2002;168:3477. doi: 10.4049/jimmunol.168.7.3477. [DOI] [PubMed] [Google Scholar]
- 14.Riberdy JM, Christensen JP, Branum K, Doherty PC. J. Virol. 2000;74:9762. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalams SA, Walker BD. J. Exp. Med. 1998;188:2199. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battegay M, et al. J. Virol. 1994;68:4700. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matloubian M, Concepcion RJ, Ahmed R. J. Virol. 1994;68:8056. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. J. Exp. Med. 1996;184:8056. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen EM, et al. Nature. 2003;421:852. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 20.Zajac AJ, et al. J. Exp. Med. 1998;188:2205. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zajac AJ, Murali-Krishna K, Blattman JN, Ahmed R. Curr. Opin. Immunol. 1998;10:444. doi: 10.1016/s0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, et al. J. Immunol. 2003;170:1443. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 23.Edelson BT, Cossart P, Unanue ER. J. Immunol. 1999;163:4087. [PubMed] [Google Scholar]
- 24.Edelson BT, Unanue ER. Curr. Opin. Immunol. 2000;12:425. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 25.Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. Eur. J. Immunol. 2002;32:2199. doi: 10.1002/1521-4141(200208)32:8<2199::AID-IMMU2199>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois C, Rocha B, Tanchot C. Science. 2002;297:2060. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 27.We thank B. Dere for assistance. This work was supported by NIH grant AI 19335 and the Howard Hughes Medical Institute.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Materials and Methods