Computer support for determining drug dose: systematic review and meta-analysis (original) (raw)
Abstract
Objective
To review the effectiveness of computer support for determining optimum drug dose.
Design
Systematic review of comparative studies where computers gave advice to clinicians on the most appropriate drug dose. Search methods used were standard for the Cochrane Collaboration on Effective Professional Practice.
Subjects
Comparative studies conducted worldwide and published between 1966 and 1996.
Main outcome measures
For qualitative review, relative percentage differences were calculated to compare effects of computer support in different settings. For quantitative data, effect sizes were calculated and combined in meta-analyses.
Results
Eighteen studies met the inclusion criteria. The drugs studied were theophylline, warfarin, heparin, aminoglycosides, nitroprusside, lignocaine, oxytocin, fentanyl, and midazolam. The computer programs used individualised pharmacokinetic models to calculate the most appropriate dose. Meta-analysis of data from 671 patients showed higher blood concentrations of drug with computer support (effect size 0.69, 95% confidence interval 0.36 to 1.02) and reduced time to achieve therapeutic control (0.44, 0.17 to 0.71). The total dose of drug used was unchanged, and there were fewer unwanted effects of treatment. Five of six studies measuring outcomes of care showed benefit from computer assistance.
Conclusions
This review suggests that using computers to determine the correct dose of certain drugs in acute hospital settings is beneficial. Computers may give doctors the confidence to use higher doses when necessary, adjusting the drug dose more accurately to individual patients. Further research is necessary to evaluate the benefits in general use.
Key messages
- This systematic review of studies examining computer support for determining optimum drug dose showed benefits from computer use
- Computer support led to patients having increased blood concentrations of drug, reduced time to achieve therapeutic benefits, and fewer unwanted effects of treatment
- Computer support helps doctors to tailor drug doses more closely to the needs of individual patients
- All the studies took place in hospitals, and further research is needed to determine the risks and benefits of widespread use of computer support, particularly in general practice, where most prescribing takes place
Introduction
Maintaining therapeutic drug concentrations is a complex task requiring knowledge of medicine and pharmacokinetics, a good rapport with the patients, and some skill in calculating dose. Harm can be caused by miscalculating doses because many drugs have a narrow “window” in which therapeutic benefits can be obtained at a low risk of unwanted effects.
Monitoring drug treatment to optimise effects and minimise dangers can be time consuming and requires meticulous attention to detail. Doctors sometimes make errors of judgment because their capacity to process information is exceeded,1 and their computational skills may be inadequate to perform calculations about drug dose.2 For example, 82 of 150 hospital doctors were unable to calculate how many milligrams of lignocaine were in a 10 ml ampoule of 1% solution.3
Computers, however, are very good at gathering information and performing repetitive calculations. Several computer systems have been designed to help doctors to determine the optimum dose of drugs. We assessed the benefits of these systems to establish whether they should be used more widely.
Methods
Inclusion criteria
We identified all comparative studies in which computers were used to help determine the most appropriate drug dose. The criteria for entry into the review were standard for reviews undertaken by the Cochrane Collaboration on Effective Professional Practice and include methodological and quality criteria for rigorous design of experimental and quasi-experimental studies.4
Methodological criteria were
- Studies using any objective measure of patient outcome or provider behaviour, randomised or quasi-randomised by patient, doctor, practice, or provider of health care
- Interrupted time series with a clearly defined intervention and at least three time points before and three after the intervention
- Non-randomised studies controlled at a second site with data before and after the intervention and appropriate choice of control site.
We included all studies using a reliable, objective, predetermined measure of the process or outcome of health care. This includes studies comparing computer aided decisions either to unassisted decisions or to decisions made using aids such as nomograms, as well as studies in which the computer directly administered the drug to patients (such as with a computer controlled pump). We excluded studies in which the computer simply suggested giving or withholding a drug. The criteria were applied independently by two researchers and any disagreements were resolved by group discussion.
Search strategy
Relevant studies were located from the specialised register of studies of the Cochrane Collaboration on Effective Professional Practice.5 This register is updated by electronic searches and hand searches of relevant journals. We also located references through bibliographies of related topics and contact with experts and pharmaceutical companies. We made specific searches of Medline and Embase from 1966 to June 1996 to identify relevant references. The search terms were “computer assisted decision making” or (prescr* and comput*) and (“randomised controlled trial” or “random allocation” or “double blind method”). Search strategies were modifications of those designed to give a high yield of randomised controlled clinical trials.6 In addition, we hand searched issues of Therapeutic Drug Monitoring published from January 1993 to July 1996.
Outcomes
Outcome measures, determined in advance, were
- Proportion of patients in which drug dose is changed because of computer advice
- Proportion of patients with unwanted effects of treatment
- Proportion of patients with blood concentrations of drug or a physiological measurement within the desired range
- Differences in blood concentrations of drug or physiological measurements attributable to computer support
- Time to achieve therapeutic control
- Proportion of patients with improved outcome attributable to computer advice.
To these we subsequently added changes in the cost of treatment attributable to computer support.
Data extraction
Two researchers reviewed each study independently and, using a standard form, extracted data on methodology, outcomes, and quality criteria.4 We recorded the unit of allocation and analysis, concealment of allocation, blinding, statistical power, follow up of patients and professionals, baseline measurements, and protection of the control group from contamination by the intervention. We calculated the mean difference in outcome with computer support, as a percentage of the mean value without support for all outcomes fitting our criteria for inclusion. These relative percentage differences were used in the narrative part of the review.
Quantitative analysis
Studies with comparable outcomes were divided into groups for meta-analysis according to outcome measure. Of the six predefined outcome measures, only four provided suitable data for meta-analysis: dose of drug; blood concentrations of drug; time to reach therapeutic concentration or effect; and difference between patients’ drug concentration and target concentration. Four separate meta-analyses were performed.
We estimated effect sizes as standardised weighted mean differences for each outcome in each study where the relevant data were available. The effect size is a statistical measure of the impact of the intervention that is independent of the units used to measure an outcome. This measure quantifies the effect of an intervention in units of standard deviation and allows comparison of studies of the same intervention that measured different outcomes.
We used a random effects model to combine the effect sizes to give an overall effect for each subgroup of studies. This model was chosen because the outcomes we combined were for studies on different drugs and different diseases. The random effects model allows quantitative combination of outcomes but does not assume that all interventions have the same underlying effect. If the outcome was measured at different times in the same study, we selected the value nearest the midpoint of the intervention period. When there were related outcomes from the same study we used the mean of the effect sizes.7 In this way only a single effect size for each study was pooled. We performed calculations using the RevMan software provided by the Cochrane Collaboration.
Results
Characteristics of included studies
We identified 23 relevant studies, of which 16 randomised controlled clinical trials8–23 and one non-randomised controlled clinical trial24 met the inclusion criteria. All used reliable outcome measures with adequate blinding of assessment. No interrupted time series or studies controlled at a second site were identified. Sixteen studies used patients as the unit of allocation; one allocated medical firms to intervention and control.9 Three studies did not use the same unit for allocation and analysis.9,12,16 Only two studies reported a power calculation.11,15 Six studies reported adequate concealment of allocation (for example, random numbers in opaque envelopes),8–13 12 followed up more than 80% of patients,8,12,13,15–20,22,24,25 12 reported similar baseline measures between intervention and control groups,8–14,16,19,20,23,24 nine recorded that patient consent had been obtained,8–10,15,18–20,22,23 and eight reported gaining approval from an ethics committee.8,10,12,18–20,22,24 In one study the reviewers thought that there was little room for improvement because the performance of the health professional was adequate without the intervention.23
Most studies were randomised by patient, so the same health professional might have given treatment to study and control groups. If the same person treated both groups it is possible that the effect of computer advice might have carried over into the control group. Such studies would tend to underestimate the effect of computer support. Two studies of continuous infusion anaesthesia, although randomised by patient, had a sufficiently rigorous study design to ensure that this contamination was unlikely to occur.19,24 In these studies a computer controlled pump delivered the drug directly to the patient, and the anaesthetist administered additional anaesthetic agents without knowing the amount of drug given by the computer. In the study randomised by medical firm all firms worked at the same hospital, so the computer advice might have influenced treatment of the control group. The only studies judged to be free of contamination were the two studies of continuous infusion anaesthesia.
Types of computer support systems used
Most of the computer systems used a mathematical model of the pharmacokinetics of the drug in question to predict the required dose (table). These models represent the compartments in the body in which the drug is distributed. Rate constants are used to describe the movements of the drug between different compartments. The models ranged from a simple, one compartment model for theophylline14 to a more complex, three compartment model for fentanyl.24 The starting values for the rate constants were estimated from population data but could then be adjusted as data accumulated from an individual patient. The systems allowed the operator to specify a target blood concentration of drug, which the computer then attempted to achieve. When the effect of the drug was more important than its blood concentration, pharmacodynamic parameters based on population data were added to the model.22
Effects of computer support on outcome
The table shows the effects of computer support on the process and outcome of care.
Drug doses used
—Eleven studies examined change in the drug dose when computer support was used,8,9,11,13,14,17–19,21,23,24 and seven found significant changes.8,13,14,17,19,21,24 Studies on theophylline showed increases in initial dose14,21 and in maintenance dose.13,14 Studies on intravenous anaesthesia showed a reduction in total dose of fentanyl used24 and a reduction in initial dose and maintenance dose of midazolam.19
Drug concentrations within desired range
—Of the seven studies that measured changes in drug concentrations in the body two found significant increases in the proportion of patients with drug concentrations in the therapeutic range with computer support.9–11,13,14,17,21
Physiological control
—Eight studies measured changes in control of a physiological parameter with computer support,8,15,16,18,20,22–24 of which six showed significant benefit.8,15,18,20,22,24 Computer support for anticoagulant control resulted in significant reductions in the time taken to achieve the desired prothrombin time22 and activated partial thromboplastin time.15 In postoperative control of blood pressure with sodium nitroprusside, a computer assisted pump was more effective at keeping blood pressure in the target range than a manually controlled infusion. Babies delivered to women treated with computer controlled oxytocin had a lower lactate concentration in the umbilical cord blood.8
Unwanted effects of drug treatment
—Six studies measured the unwanted effects of drugs,11,12,14,15,22,24 and four found significant reductions associated with computer support.14,15,22,24 Fewer patients treated with theophylline reached toxic drug concentrations when computer advice was used.14 In studies on anticoagulation both the number of patients given too much anticoagulant22 and the total number of adverse events15 were reduced in the intervention groups. The number of hypotensive episodes during cardiac surgery was reduced when fentanyl and midazolam were given via a computer controlled pump.24
Cost of drug treatment
—Only two studies reported economic data, and both looked at computer support for aminoglycoside dose.9,11 In one study the mean direct cost of treatment with computer support was 7102comparedwith7102 compared with 7102comparedwith13 758 in controls (P<0.02), with a benefit to cost ratio of 75.11 The other calculated a cost avoidance (the money potentially saved by the intervention) of $1311 for each patient treated, with a benefit to cost ratio of 4.1.9 These cost savings resulted largely from reduced hospital stays, which was confirmed in one study,14 although another suggested an increased time spent in hospital.10 Another study showed that computer support lengthened the interval between outpatient visits.12
Outcome of medical care
—Six studies directly measured outcomes of care, of which five showed benefits. Three showed significant benefits in clinical improvement scores for asthma,21 treating infection,9 and pain relief after surgery.20 Fewer caesarean sections were required when oxytocin was given by computer controlled pump to augment labour.9 With computer support for hospital treatment of acute asthma, fewer patients subsequently needed convalescent care,13 and there were fewer deaths.14 One study on anticoagulation showed an increase in the number of embolisms, but it may be that the computer system used in this study was set to achieve too low a prothrombin time.12
Overall effect
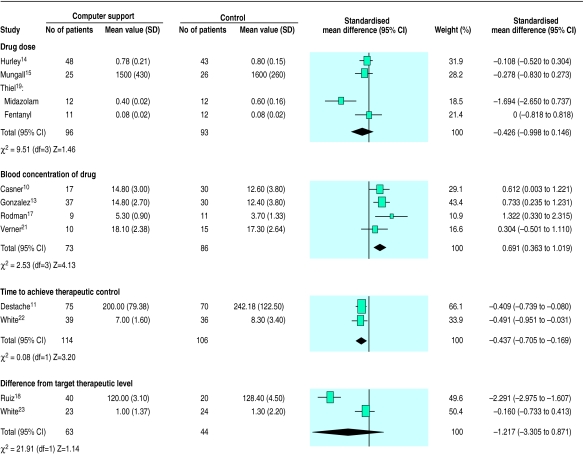
—Eleven studies provided outcomes for quantitative synthesis,10,11,13–15,17–19,21–23 and the figure shows the individual results and meta-analysis for each of the four outcome measures. Patients treated with computer support had higher blood concentrations of the drug (effect size 0.69, 95% confidence interval 0.36 to 1.02) and took less time to reach therapeutic concentrations (−0.44, −0.71 to 0.17). However, computer support had no significant effect on the total amount of drug used (−0.43, −1.00 to 0.15) nor on the difference between the level of a physiological parameter achieved and the target level (−1.22, −3.31 to 0.87). Although the clinical settings of the trials varied widely, only in the case of difference from target level was there evidence of statistical heterogeneity.
Discussion
This review suggests that substantial benefit results from computer support for determining the dose of certain drugs in acute hospital settings. In the studies we identified, unaided doctors were often excessively cautious in estimating drug dose. This caution presumably resulted from an unwillingness to expose patients to adverse effects of drug treatment. Unaided doctors used lower initial doses and maintenance doses than when computer support was available.14,21 Lower doses lead to lower blood concentrations and often to suboptimal therapeutic effects. Although doses with computer support tended to be higher than those used by unaided doctors, no studies reported an increase in unwanted effects due to overdose. This suggests that the computers helped doctors to tailor drug doses more accurately to individual patients. Higher initial doses with computer support gave more rapid therapeutic control,20,22 bringing benefits for patients and reducing the time that they spent in hospital.9,14,22 Unaided doctors tended to exercise caution in checking blood concentrations, which resulted in more blood tests15 and hospital visits.12
The most successful systems were those in which the computer administered drugs directly to patients under medical supervision. Manually controlled infusions resulted in higher doses of anaesthetic agents compared with computer controlled infusions.19,24 This may result from the doctors’ reluctance to expose patients to the risk of unnecessary pain. However, patients treated with computer support experienced less pain,20 suggesting that computer support could help doctors to adjust drug doses more accurately for individual patients.
Methodological issues
Potentially, the most important factor that may undermine our analysis is publication bias—studies with positive results are more likely to be published than those with negative results.26 Our search was exhaustive, and it seems unlikely that large numbers of patients have been randomised to trials that we have not identified. Orwin’s file drawer method suggests that there would need to be 24 unpublished studies showing no benefit from computer support to alter our results significantly.27
Another review in a similar area did not use statistical methods to estimate the overall effects from computer support,28 preferring to present a “vote count” of statistically significant studies. This method risks concluding falsely that an important effect is absent because it assumes that a trial with no significant effect is a negative study.29 The method also does not make full use of available data. Our overview represents an advance from this approach, using established and robust statistical methods to estimate an overall treatment effect.29,30
Our findings need to be read with caution since we identified relatively few studies on a limited range of drugs and our quantitative analysis was based on results derived from only 671 patients. Computer assisted determination of drug dose is a potentially hazardous intervention: it is surprising that the risks and benefits have not been evaluated in large randomised controlled trials. The quality of the studies could be improved: most did not report a power calculation, and sample sizes were often small. A common bias in many of the studies was that the same clinicians treated patients allocated to intervention and control, so the effect of the intervention would tend to spill over into the control group. Contamination of the control group in this manner would tend to make it more difficult to show a benefit from computer support.
More large scale studies are needed to confirm our conclusions, and research is needed to examine the effects of computer support in general use and to develop and implement appropriate systems. Existing studies suggest that computer support for drug dosage will be cost effective, but economic evaluation should be an integral part of any future study. Economic benefits seen in one therapeutic area may not transfer to another clinical situation.
Implications for computer support for drug dose
The scope of computer support systems could be widened to include other, commonly prescribed drugs with a narrow therapeutic window, such as anticonvulsants and lithium. A computer might use the same basic pharmacokinetic model for several different drugs. It is possible that the model could be extended to predict the likelihood and severity of some interactions—for example, those caused by competition for protein binding sites.
A barrier to adopting computer support may be the lack of access to suitable computers and electronic medical records. Computerised records are used in some hospital departments (such as intensive care), but hospitals often rely on paper to record outpatient treatment. In contrast, most general practitioners routinely use electronic records for prescribing31 and have easy access to suitable hardware that could run programs to help them to determine the most appropriate dose of commonly used drugs. General practice computers often already store necessary data such as blood concentrations of drugs, body mass index, and indicators of hepatic and renal function. It may be that the benefits seen in secondary care could be realised on a large scale if programs giving support for drug dose were integrated with the software routinely used in general practice.
Table.
Effects of computer support on the process and outcome of care (ordered by clinical problem)
| Study | Intervention v control | Relative percentage differences between effects of intervention and control (mean intervention value v control value) | P value of difference |
|---|---|---|---|
| Treating acute asthma with theophylline | |||
| Casner 1993, USA10 | Advice based on linear one compartment model (n=17) v usual care (n=18) | Blood concentration of drug increased 19% (15 v 12.6 μg/ml) | >0.05 |
| Time for infusion increased 28.1% (4.1 v 3.2 days) | >0.05 | ||
| Hospital stay increased 29.5% (11.4 v 8.8 days) | >0.05 | ||
| Gonzalez 1989, USA13 | Advice based on bayesian one compartment pharmacokinetic model (n=37) v population based guidelines (n=30) | Initial dose increased 10.5% (4.2 v 3.8 mg/kg) | 0.5 |
| Maintenance dose increased 50% (0.6 v 0.4 mg/kg/hour) | 0.0001 | ||
| Blood concentration of drug increased 19.7% (14.6 v 12.2 mg/l) | <0.002 | ||
| Percentage of patients in therapeutic range at 4 hours increased 28.1% (14.6% v 11.4%) | NA | ||
| Percentage of patients discharged home increased 10.6% (52% v 47%) | >0.05 | ||
| Hurley 1986, Australia14 | Estimate of theophylline clearance based on one compartment linear pharmacokinetic model (n=48) v usual care based on theophylline blood concentrations (n=43) | Initial dose increased 10% (250 v 227 mg) | <0.01 |
| Maintenance dose increased 133% (831 v 698 mg/day) | 0.0023 | ||
| Serum concentration of drug reduced 10.1% (16.1 v 17.9 μg/ml) | >0.05 | ||
| Time for infusion increased 1.4% (22.4 v 22.1 hours) | <0.05 | ||
| Percentage of patients with toxic drug concentrations reduced 50% (18.9% v 37.8%) | 0.04 | ||
| Hospital stay reduced 28% (6.3 v 8.7 days) | 0.027 | ||
| No of deaths reduced (0 v 2) | >0.05 | ||
| Verner 1992, Israel21 | Dose advice based on individualised pharmacokinetic model (n=10) v usual care (n=15) | Initial dose increased 162% (437 v 167 mg) | NA |
| Blood concentration of drug increased 6% (13.8 v 13.0 μg/ml) | <0.05 | ||
| Percentage of time within therapeutic range increased 51% (77% v 51%) | >0.05 | ||
| Clinical improvement score increased 6% (5.3 v 5.0) | >0.05 | ||
| Hospital stay unchanged (4 days) | NA | ||
| Anaesthesia for cardiac surgery with fentanyl | |||
| Alvis 1985, USA24 | Computer controlled pump using three compartment open model (n=10) v manual administration (n=10) | Intervention 1 (computer maintained stable serum drug concentration): | |
| Total dose reduced 38% (19.6 v 27.1 μg/kg) | <0.05 | ||
| No of hypotensive episodes reduced 20% (4 v 5) | >0.05 | ||
| Need for additional anaesthetics increased 14% (8 v 7 events) | >0.05 | ||
| Intervention 2 (computer allowed manual adjustment of serum concentration): | |||
| Total drug dose reduced 16.3% (19.6 v 22.8 μg/kg) | >0.05 | ||
| No of hypotensive episodes reduced 60% (2 v 5) | <0.05 | ||
| Need for additional anaesthetics reduced 43% (4 v 7 events) | <0.05 | ||
| Anaesthesia for cardiac surgery with fentanyl and midazolam | |||
| Theil 1993, USA19 | Computer controlled pump using pharmacokinetic model to achieve target serum concentration (n=12) v infusion controlled by doctor (n=12) | Midazolam: | |
| Initial dose reduced 43% (0.08 v 0.14 μg/kg) | <0.05 | ||
| Maintenance dose reduced 33% (0.4 v 0.6 μg/kg) | <0.05 | ||
| Plasma concentration reduced 47.9% (50 v 96 ng/ml) | <0.05 | ||
| Fentanyl: | |||
| Initial dose increased 25.1% (6.52 v 5.21 μg/kg) | >0.05 | ||
| Maintenance dose unchanged (0.08 μg/kg) | NA | ||
| Total dose increased 14.3% (34.61 v 30.27 μg/kg) | >0.05 | ||
| No of additional drug interventions reduced 8.6% (64 v 70) | >0.05 | ||
| Anticoagulation with heparin | |||
| Mungall 1994, USA15 | Starting doses generated by bayesian model v doctors using nomogram (total n=51) | Drug dose increased 8.4% (1290 v 1190 units) | >0.05 |
| Percentage of patients with APTT ratio >1.5 in first day increased 21% (94% v 78%) | 0.009 | ||
| No of blood tests reduced 18% (2.3 v 2.8 tests/person/day) | <0.05 | ||
| No of adverse events reduced (0 v 24) | <0.05 | ||
| Anticoagulation with warfarin | |||
| Fihn 1994, USA12 | Follow up times suggested by mathematical model (n=301) v usual care (n=319) | Scheduled follow up interval increased 25% (4.4 v 3.5 weeks) | <0.05 |
| Actual follow up interval increased 7% (4.4 v 4.1 weeks) | <0.05 | ||
| No of bleeds reduced 19% (5.4 v 6.7 events/100 patient years) | >0.05 | ||
| No of embolisms increased 71% (2.4 v 1.4 events/100 patient years) | NA | ||
| Poller 1993, UK16 | Computer advice on dose (intervention 1 n=57, intervention 2 n=53, intervention 3 n=12) v usual care (n=64) | Proportion of tests in therapeutic range increased 12%, 5%, and 11% respectively (intervention 1 57%, intervention 2 53%, intervention 3 56%, control 50%) | >0.05 for all interventions |
| White 1987, USA22 | Initial dose suggested by bayesian pharmacokinetic and pharmacodynamic model (n=39) v usual care (n=36) | No of patients with blood concentration above therapeutic level reduced 70% (5% v 17%) | 0.11 |
| Time to reach stable dose reduced 39% (5.4 v 6.7 days) | 0.002 | ||
| Time that blood concentration remained at therapeutic level increased 16% (58 v 42 days) | 0.001 | ||
| Hospital stay reduced by 35% (13 v 20 days) | 0.01 | ||
| Percentage of patients with bleeding complications reduced (0 v 8.3%) | 0.05 | ||
| White 1991, USA23 | Maintenance dose suggested by bayesian pharmacokinetic model (n=24) v usual care (n=25) | Percentage of patients requiring change in dose increased 5% (20% v 15%) | >0.05 |
| Percentage of patients with prothrombin time in target range reduced 14% (43% v 50%) | >0.05 | ||
| Time to next visit increased 6.9% (18.7 v 17.5 days) | >0.05 | ||
| Control of ventricular arrhythmia with lignocaine | |||
| Rodman 1984, USA17 | Initial dose suggested by individualised linear two compartment pharmacokinetic model (n=9) v usual care (n=11) | Dose increased 76.2% (3.7 v 2.1 μg/ml) | <0.01 |
| Infusion rate in first hour increased 96% (83 v 43 mg/kg/min) | <0.01 | ||
| Percentage of patients with blood concentration in therapeutic range increased 70% (89% v 64%) | 0.44 | ||
| Induction of labour with oxytocin | |||
| Willcourt 1994, USA8 | Computer controlled pump with “closed loop” feedback on uterine activity (n=114) v nurse controlled continuous infusion (n=196) | Dose reduced 641% (1.1 v 8.15 mU/min) but computer controlled pump delivered pulsatile oxytocin | 0.006 |
| Duration of infusion reduced 12% (366 v 417 min) | 0.35 | ||
| Cord lactate concentration reduced 11% (3.35 v 3.75 mmol/l) | 0.032 | ||
| Percentage of patients needing caesarean section reduced 15% (11.5% v 13.5%) | 0.037 | ||
| Treating infection with aminoglycosides | |||
| Burton 1991, USA9 | Advice based on bayesian pharmacokinetic model (n=72) v usual care (n=75) | Initial dose increased 3.5% (238 v 230 mg/day) | >0.05 |
| No of patients with peak concentration in therapeutic range increased 37.5% (82.9% v 60.3%) | >0.05 | ||
| No of patients with trough concentration in therapeutic range increased 7.0% (91.3 v 85.3) | >0.05 | ||
| Hospital stay reduced 26% (13 v 17.6 days) | 0.013 | ||
| Percentage of patients with clinical response increased 9.0% (88.2% v 80.9%) | >0.05 | ||
| Destache 1990, USA11 | Patients whose doctors accepted advice based on one compartment bayesian pharmacokinetic model (n=75) v those of doctors who did not (n=70) | No of dose changes increased 78.1% (0.64 v 1.14) | <0.005 |
| Percentage of patients with adequate trough concentrations increased 127% (25% v 11%) | <0.05 | ||
| Duration of treatment increased 0.8% (109.81 v 108.95 hours) | >0.05 | ||
| Time taken to reduce elevated temperature reduced 45% (50 v 92 hours) | <0.05 | ||
| Percentage of patients with nephrotoxicity reduced 44% (8.0% v 14.4%) | >0.05 | ||
| No of drug tests increased 6.8% (3.28 v 3.07) | NA | ||
| Hospital stay reduced 27.4% (13.4 v 18.5 days) | >0.05 | ||
| Postoperative analgesia with fentanyl | |||
| Van den Nieuwenhuyzen 1995, Holland20 | Computer controlled pump using pharmacokinetic model to achieve target drug serum concentration (n=9) v patient controlled morphine pump (n=10) | Time taken to onset of analgesia reduced 60% (20 v 50 min) | <0.05 |
| Time with pain score >3 units reduced 43% (12% v 21%) | <0.05 | ||
| No of demands for additional analgesia reduced 38% (21 v 43) | <0.05 | ||
| Postoperative control of blood pressure with nitroprusside | |||
| Ruiz 1993, Spain18 | Fuzzy logic controlled pump with arterial pressure sensor (n=40) v usual care (n=20) | Infusion rate at one hour increased 36.6% (5.6 v 4.1 μg/kg/min) | >0.05 |
| Time that blood pressure in target range increased 23% (80% v 65%) | <0.001 | ||
| Difference in mean arterial pressure reduced 57% (6.3 v 14.7 mm Hg) | <0.001 |
Figure.
Quantitative effects of computer support for determining drug dose on outcomes of care (standardised weighted mean differences (95% confidence intervals) for computer support versus control)
Acknowledgments
A parallel version of this review will appear in the Cochrane Library. We thank referees in the Cochrane Collaboration on Effective Professional Practice review group for helpful comments on the protocol for this review. Since this review was conducted, the Cochrane Collaboration on Effective Professional Practice has changed its name to the Cochrane Effective Practice and Organisation of Care Group (EPOC).
Footnotes
Funding: NHS research and development programme evaluating methods to promote the implementation of research findings. RW is supported by a Royal College of General Practitioners/BUPA training fellowship. SD was supported by the New Zealand Health Research Council.
Competing interest: None reported.
References
- 1.McDonald CJ. Protocol-based computer reminders, the quality of care and the non-perfectibility of man. N Engl J Med. 1976;295:1351–1355. doi: 10.1056/NEJM197612092952405. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin L. Calculating drug doses. BMJ. 1995;310:1154. doi: 10.1136/bmj.310.6988.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolfe S, Harper NJN. Ability of hospital doctors to calculate drug doses. BMJ. 1995;310:1173–1174. doi: 10.1136/bmj.310.6988.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochrane Collaboration on Effective Professional Practice. The Cochrane Collaboration on Effective Professional Practice data collection checklist. York: Department of Health Sciences and Clinical Evaluation, University of York; 1996. [Google Scholar]
- 5.Bero L, Grilli R, Grimshaw J, Oxman A Cochrane Collaboration, editors. The Cochrane Library. Issue 3. Oxford: Update Software; 1997. The Cochrane Collaboration on Effective Professional Practice (CCEPP) module of the Cochrane database of systematic reviews [updated 03 June 1997] [Google Scholar]
- 6.Dickerson K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. In: Chalmers I, Altman D, editors. Systematic reviews. London: BMJ Publishing Group; 1995. pp. 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal R, Rubin D. Meta analytic procedures for combining studies with multiple effect sizes. Psychol Bull. 1986;99:400–406. [Google Scholar]
- 8.Willcourt RJ, Pager D, Wendel J, Hale RW. Induction of labor with pulsatile oxytocin by a computer-controlled pump. Am J Obstet Gynecol. 1994;170:603–608. doi: 10.1016/s0002-9378(94)70236-5. [DOI] [PubMed] [Google Scholar]
- 9.Burton ME, Ash CL, Hill DP, Jr, Handy T, Shepherd MD, Vasko MR. A controlled trial of the cost benefit of computerized bayesian aminoglycoside administration. Clin Pharmacol Ther. 1991;49:685–694. doi: 10.1038/clpt.1991.86. [DOI] [PubMed] [Google Scholar]
- 10.Casner PR, Reilly R, Ho H. A randomized controlled trial of computerized pharmacokinetic theophylline dosing versus empiric physician dosing. Clin Pharmacol Ther. 1993;53:684–690. doi: 10.1038/clpt.1993.90. [DOI] [PubMed] [Google Scholar]
- 11.Destache CJ, Meyer SK, Rowley KM. Does accepting pharmacokinetic recommendations impact hospitalization? A cost-benefit analysis. Ther Drug Monit. 1990;12:427–433. doi: 10.1097/00007691-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Fihn SD, McDonell MB, Vermes D, Henikoff JG, Martin DC, Callahan CM, et al. A computerized intervention to improve timing of outpatient follow-up: a multicenter randomized trial in patients treated with warfarin. National Consortium of Anticoagulation Clinics. J Gen Intern Med. 1994;9:131–139. doi: 10.1007/BF02600026. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez ER, Vanderheyden BA, Ornato JP, Comstock TG. Computer-assisted optimization of aminophylline therapy in the emergency department. Am J Emerg Med. 1989;7:395–401. doi: 10.1016/0735-6757(89)90046-6. [DOI] [PubMed] [Google Scholar]
- 14.Hurley SF, Dziukas LJ, McNeil JJ, Brignell MJ. A randomized controlled clinical trial of pharmacokinetic theophylline dosing. Am Rev Respir Dis. 1986;134:1219–1224. doi: 10.1164/arrd.1986.134.5.1219. [DOI] [PubMed] [Google Scholar]
- 15.Mungall DR, Anbe D, Forrester PL, Luoma T, Genovese R, Mahan J, et al. A prospective randomized comparison of the accuracy of computer-assisted versus GUSTO nomogram-directed heparin therapy. Clin Pharmacol Ther. 1994;55:591–596. doi: 10.1038/clpt.1994.73. [DOI] [PubMed] [Google Scholar]
- 16.Poller L, Wright D, Rowlands M. Prospective comparative study of computer programs used for management of warfarin. J Clin Pathol. 1993;46:299–303. doi: 10.1136/jcp.46.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodman JH, Jelliffe RW, Kolb E, Tuey DB, de Guzman MF, Wagers PW, et al. Clinical studies with computer-assisted initial lignocaine therapy. Arch Intern Med. 1984;144:703–709. [PubMed] [Google Scholar]
- 18.Ruiz R, Borches D, Gonzalez A, Corral J. A new sodium-nitroprusside-infusion controller for the regulation of arterial blood pressure. Biomed Instrum Technol. 1993;27:244–251. [PubMed] [Google Scholar]
- 19.Theil DR, Stanley TED, White WD, Goodman DK, Glass PS, Bai SA, et al. Midazolam and fentanyl continuous infusion anesthesia for cardiac surgery: a comparison of computer-assisted versus manual infusion systems. J Cardiothorac Vasc Anesth. 1993;7:300–306. doi: 10.1016/1053-0770(93)90009-a. [DOI] [PubMed] [Google Scholar]
- 20.Van den Nieuwenhuyzen MC, Engbers FH, Burm AG, Vletter AA, van Kleef JW, Bovill JG. Computer-controlled infusion of alfentanil versus patient-controlled administration of morphine for postoperative analgesia: a double-blind randomized trial. Anesth Analg. 1995;81:671–679. doi: 10.1097/00000539-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Verner D, Seligmann H, Platt S, Dany S, Almog S, Zulty L, et al. Computer assisted design of a theophylline dosing regimen in acute bronchospasm: serum concentrations and clinical outcome. Eur J Clin Pharmacol. 1992;43:29–33. doi: 10.1007/BF02280750. [DOI] [PubMed] [Google Scholar]
- 22.White RH, Hong R, Venook AP, Daschbach MM, Murray W, Mungall DR, et al. Initiation of warfarin therapy: comparison of physician dosing with computer-assisted dosing. J Gen Intern Med. 1987;2:141–148. doi: 10.1007/BF02596140. [DOI] [PubMed] [Google Scholar]
- 23.White RH, Mungall D. Outpatient management of warfarin therapy: comparison of computer-predicted dosage adjustment to skilled professional care. Ther Drug Monit. 1991;13:46–50. [PubMed] [Google Scholar]
- 24.Alvis JM, Reves JG, Govier AV, Menkhaus PG, Henling CE, Spain JA, et al. Computer-assisted continuous infusions of fentanyl during cardiac anesthesia: comparison with a manual method. Anesthesiology. 1985;63:41–49. doi: 10.1097/00000542-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 25.White KS, Lindsay A, Pryor TA, Brown WF, Walsh K. Application of a computerized medical decision-making process to the problem of digoxin intoxication. J Am Coll Cardiol. 1984;4:571–576. doi: 10.1016/s0735-1097(84)80104-7. [DOI] [PubMed] [Google Scholar]
- 26.Freemantle N, Haines A, Mason J, Eccles M. CONSORT—an important step towards evidence based health care. Ann Intern Med. 1997;126:81–83. doi: 10.7326/0003-4819-126-1-199701010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Wolf F. Meta-analysis: quantitative methods for research synthesis. Newbury Park: Sage; 1986. [Google Scholar]
- 28.Johnston ME, Langton KB, Haynes B, Mathieux A. Effects of a computer-based clinical decision support system on clinician performance and patient outcome. Ann Intern Med. 1994;120:135–142. doi: 10.7326/0003-4819-120-2-199401150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Hedges L, Olkin I. Statistical methods for meta-analysis. London: Academic Press; 1985. [Google Scholar]
- 30.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Computerisation in GP practices 1993 survey. London: Department of Health (Management Executive); 1993. Social Surveys (Gallup Poll) [Google Scholar]