Chronic stress selectively reduces hippocampal volume in rats: a longitudinal MRI study (original) (raw)
. Author manuscript; available in PMC: 2009 Nov 25.
Abstract
The notion of uncontrollable stress causing reduced hippocampal size remains controversial in the posttraumatic stress disorder literature because human studies cannot discern the causality of effect. Here, we addressed this issue by employing structural magnetic resonance imaging in rats to measure the hippocampus and other brain regions before and after stress. Chronic restraint stress produced approximately 3% reduction in hippocampal volume, which was not observed in control rats. This decrease was not signficantly correlated with baseline hippocampal volume or body weight. Total forebrain volume and the sizes of the other brain regions and adrenal glands were all unaffected by stress. This longitudinal, within-subjects design study provides direct evidence that the hippocampus is differentially vulnerable and sensitive to chronic stress.
Keywords: stress, posttraumatic stress disorder, hippocampus, brain imaging, Hypothalamic-pituitary-adrenal axis, prefrontal cortex
Stress is a biologically significant and pervasive factor that negatively impacts memory and hippocampal function in humans and laboratory animals. A number of stress-induced physiological changes have been observed in the hippocampus including alterations in synaptic plasticity, neuronal morphology, neurotoxicity, and neurogenesis in adults [1,2]. Reports of dendritic shrinkage and heightened susceptibility to cell death in the hippocampus associated with severe stress [3,4] are particularly interesting in light of results from recent human imaging studies. Magnetic resonance imaging (MRI) studies have reported that patients diagnosed with posttraumatic stress disorder (PTSD) resulting from traumatic experiences such as military combat, sexual assault, or child abuse have a smaller hippocampus (up to 8% reduction) than comparably matched control subjects [5]. However, because hippocampal volume was only assessed after traumatic events and/or clinical diagnosis it is not possible to determine whether the traumatic experience caused the volume reduction, or if individuals with a smaller hippocampus are more prone to develop PTSD [6]. For example, one MRI study measured the hippocampus volume in 37 individuals within a week of experiencing traumatic events and once more 6 months later (post-stress longitudinal), and found no reliable reduction in those (27% of subjects) later diagnosed with PTSD [7]. However, it is possible that hippocampal morphological/structural changes occurred rapidly within one week or emerge slowly beyond 6 months post-stress period. Also, because of the variability among individuals (e.g., hereditary, lifestyle, and medication history), the finding of smaller hippocampi in PTSD patients is often disputed [8]. To circumvent these potential confounds, the present study employed a longitudinal, within-subjects design and compared hippocampal volume in homogenous inbred laboratory rats before and after a 21 day chronic stress procedure previously confirmed to produce morphological changes in the hippocampus [4].
MATERIALS AND METHODS
Subjects
Twenty adult male Long-Evans rats (300–325 g, Harlan, Madison, WI) were individually housed with ad libitum access to food and water, and maintained on a12-hr light-dark cycle (lights on at 7AM). They were used to measure brain and adrenal volume changes before and after stress procedures. All procedures were performed in strict compliance with Institutional Animal Care & Use Committee (IACUC) guidelines of the University of Wisconsin - Milwaukee.
Procedure
After 7 days of acclimation and handling, 20 rats were randomly assigned to either a control (n=10) or stress (n=10) condition and then transported to the Functional Imaging Research Center at the Medical College of Wisconsin (MCW) for baseline volume measurement. One hour after arrival at the imaging center, rats were anesthetized with Isoflurane (~3%). To minimize motion artifacts, the animal’s head was anchored within the RF coil using a bite bar and ear bars. During scanning body temperature, heart rate, respiratory rate, and blood oxygen saturation level were monitored. Scans were performed on a Bruker 9.4 Tesla animal magnet (AVANCE, Billerica, MA) using a Rapid Acquisition with Relaxation Enhancement (RARE) and FLASH (Fast Low Angle Shot) sequence with a 72 mm quadrature coil (Bruker, Billerica, MA). Two T2-weighted images were acquired to measure hippocampal volumes (spatial resolution: .068 × .068 × .75 mm) and whole brain volume (spatial resolution: .1 × .1 × 1 mm), and one T1-weighted image was acquired to measure adrenal volume (spatial resolution: .195 × .195 × .5 mm).
Stress procedures began one day after the baseline imaging. Body weight and food consumption were measured daily immediately before handling and restraint procedures to assess the effectiveness of the chronic restraint stress procedure. Rats in the stress group were immobilized in a plastic bag (DecapiCone, Braintree Scientific Inc., MA) for 6-hr/day for 21 consecutive days (starting at 10AM). During restraint, control animals stayed in their home cage but food and water were not accessible for the same period to match access with the stress group. After the restraint procedure, stressed rats were placed back in their homecage. One day after the 21 days of stress procedures, all rats were scanned again and then euthanized.
MRI parameters
High resolution hippocampal images were acquired with the following parameters: TR = 4000 ms; TE = 37 ms; number of average = 6; number of slices = 10; slice thickness = .75 mm; field of view = 35 × 35 mm; matrix = 512 × 384 interpolated to 512 × 512; final spatial resolution = .068 × .068 × .75 mm. The first coronal slice started from the anterior commissure (from bregma, about −.2 to −.5 mm), and an additional 9 continuous slices were acquired (from bregma, about −7.7 to −8.0 mm). The parameters for whole brain images were as follows: TR = 4000 ms; TE = 37 ms; number of average = 2; number of slice = 29; slice thickness = 1 mm; field of view = 25.6 × 25.6 mm; matrix = 256 × 256; spatial resolution = .1 × .1 × 1 mm. In addition, the kidney area was scanned to measure the adrenal gland with respiration gated acquisition to prevent motion artifacts: TR = 58 ms; TE = 1.5 ms; number of average = 16; number of slice = 10; slice thickness = .5 mm; field of view = 50 × 50 mm; matrix = 192 × 256 interpolated to 256 × 256; spatial resolution = .195 × .195 × .5 mm.
Volume calculation and statistical analyses
MRI images were reconstructed using the AFNI software suite (http://afni.nimh.nih.gov) originally developed at MCW. In order to objectively outline brain regions and adrenal gland, the image was low-pass filtered (2 pixels) and outlines of the target structures were initially detected using the “trace contour” function in Corel PHOTO-PAINT (Corel Corp., Fremont, CA). The traced contour was overlaid on the original 2D images and manually modified to closely match the target structures. Outlines of the hippocampus, anterior cingulate cortex and retrosplenial granular b cortex were defined according to the previous study of [9] using the rat brain. The hippocampal volume was calculated with 7 slices from the first slice corresponding approximately −2.12 mm from bregma and the last seventh slice corresponding approximately −6.62 mm from bregma. The upper boundaries of the dorsal hippocampus were easily drawn by automatic tracing due to high contrast difference between the hippocampus and the corpus callosum. The ventral hippocampal boundaries were partially guided by automatic tracing and followed by manual tracing. The forebrain volume was computed from 13 slices of whole brain images. The most anterior slice corresponded to a level of approximately 4.5 mm from bregma and the most posterior slice corresponds to a level of approximately −7.5 mm from bregma. The outlines of the brain were drawn using trace contour function and then non-brain areas, such as arteries, pituitary gland and trigeminal nerves, were manually removed. The volume of the anterior cingulate cortex was calculated from 3 whole brain slices corresponding to 1.5 mm to −1.5 mm from bregma, and the volume of the retrosplenial granular b cortex was calculated from 4 whole brain slices corresponding to −1.5 mm to −5.5 mm from bregma. Adrenal gland was verified on 6 to 8 consecutive images showing kidneys, and automatically outlined and manually corrected. All the tracings were drawn and confirmend by two observers who were ‘blind’ to group assignments. After drawing, the size of the target structure was calculated using ImageJ software (National Institute of Health, USA). The total volume of each structure was computed by the summing size of the target areas multiplied by slice thickness. The percent change of hippocampal volume was calculated from the following equation:
%change=Posttreatmentvolume−BaselinevolumeBaselinevolume×100.
Statistical analyses were carried out using SPSS 11 by analysis of variance (ANOVA) with repeated measures, t-test and Pearson correlation.
RESULTS
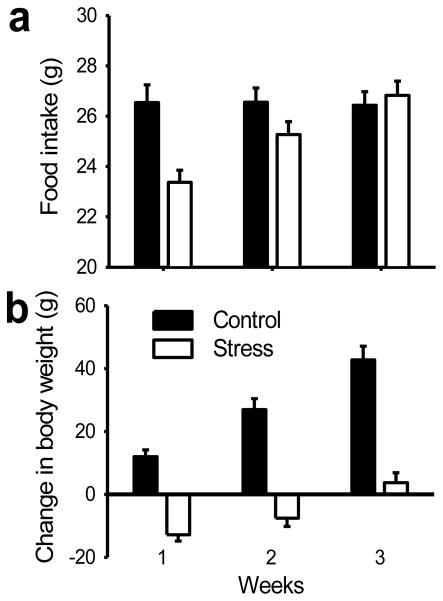
Chronic restraint stress effectively produced a decrease in body weight gain and food intake (Fig 1). Average daily food intake was stable in controls but stressed rats dramatically reduced consumption on the first week (_t_18 = 3.725, p < .01, independent _t_-test), eventually returning to control levels (Fig. 1a). Fig. 1a shows significant increases in food intake in both groups across weeks (_F_2, 36 = 19.043, p < .001), which is accounted for by the increase in food consumption in stressed rats (_F_2, 36 = 21.435, p < .001). The change in body weight (Fig. 1b) was calculated by subtracting the one-week average body weight from the baseline weight prior to stress procedures. The weight gains were also significantly different between groups (_F_1, 18 = 66.815, p < .001). While control animals gained weight normally during the study, stressed rats lost considerable weight during the first week, and gradually recovered back to pre-stress levels by the third week of the study (Fig. 1b). Overall, stressed rats displayed slower weight gain, but controls showed a continuous increase _(F_2, 36 = 11.542, p < .001, an interaction of the week × group).
Figure 1.
Stress effects on 1-week average food intake and body weight gain. (A) Stressed rats showed a decrease in food intake compared to controls. (B) Changes in body weight. Stressed rats displayed decrease of weight, but controls showed continuous increases of weight. All the data are displayed as mean ± s.e.m.
Even though stress resulted in reduced body weight relative to controls, we did not detect a change in total brain volume or adrenal size calculated from MRI images when comparing averages between groups or pre/post stress changes within subjects (Table 1). Specifically, the initial forebrain volumes were not significantly different between groups (_F_1, 18 = 1.211, p > .05), and they did not significantly change after stress in both groups (_F_1, 18 < 1). Furthermore, the adrenal gland was examined because it is a terminal structure of the Hypothalamic-pituitary-adrenal axis that releases stress hormones. Both groups appeared to show increases of adrenal volumes over time although this was not statistically significant (_F_1, 12 = 1.127, _p_ > .05). There was no significant volume difference between groups (_F_1, 12 = 2.829, p > .05).
Table 1.
Region of interest volumes (mm3) for stress and control rats
| Control (n=10) | Stress (n=10) | |||
|---|---|---|---|---|
| Structure | Baseline | Post-stress | Baseline | Post-stress |
| Hippocampus | 93.54 (1.35) | 94.16 (1.29) | 97.20 (1.78) | 94.19 (1.84) |
| Forebrain | 1326.14 (14.29) | 1325.05 (9.52) | 1313.5 (14.55) | 1300.16 (14.63) |
| anterior cingulate cortex* | 12.00 (.43) | 11.97 (.38) | 13.57 (.25) | 13.43 (.44) |
| retrosplenial granular b cortex* | 7.35 (.17) | 7.34 (.25) | 8.11 (.24) | 8.36 (.31) |
| adrenal gland** | 58.89 (4.88) | 62.37 (4.74) | 48.22 (4.95) | 52.25 (5.30) |
Similarly, a series of brain structures chosen as regions of interest based on prior work (e.g., anterior cingulate cortex and retrosplenial granular b cortex; Table 1) failed to show significant alterations in volume. Although a recent human study found decreased anterior cingulate cortex volume in PTSD patients [10], the present stress procedure did not produce any significant volume loss in rats (_F_1, 17 < 1). The retrosplenial granular b cortex (an efferent target of the hippocampus) also did not change in response to chronic stress (_F_1, 17 < 1).
In contrast, hippocampal size did change in the predicted direction as a function of chronic stress. Figure 2a includes representative T2-weighted images showing the hippocampus. Animals in the stress group tended to show reduced hippocampal volume when comparing data collected at the terminal scan to pre-stress baselines (Fig. 2b). On average, rats exposed to chronic restraint stress showed a reduction in hippocampal volume of over 3% (Fig. 2c; _t_9 = 2.511, p < .05, one sample _t_-test to zero baseline). In contrast, control animals did not show a difference in hippocampal volume from pre-stress to post-stress (_p_ > .61, Fig. 2c). There were no reliable correlations between stress-induced reduction in hippocampal volume with either weight loss during the first week of stress (Pearson’s r = .130, p > .05) or terminal body weight (r = −.391, p > .05). Thus, the stress-induced reduction in hippocampal volume cannot be accounted for by group or individual differences in body weight.
Figure 2.
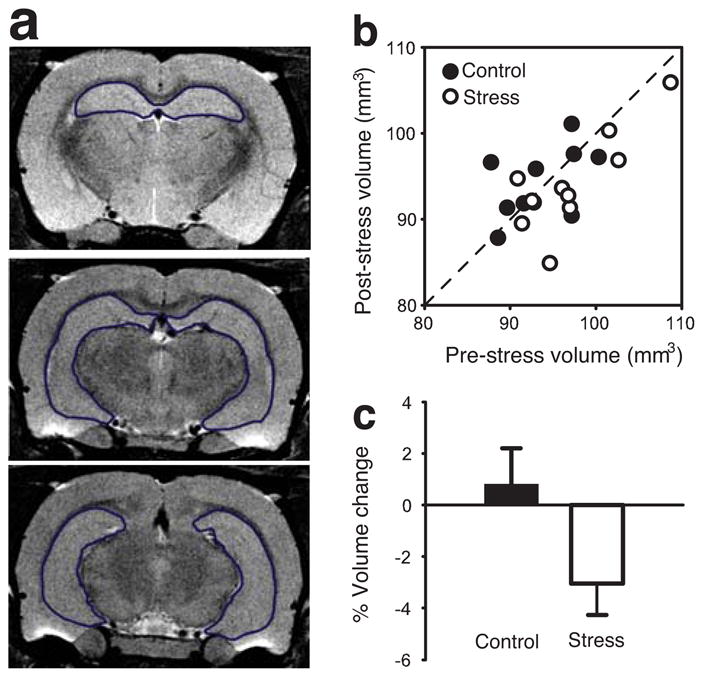
Hippocamapal volume changes following restraint stress. (A) Three representative coronal sections from the MRI brain images. (B) Scatter plot of showing pre- and post-stress hippocampal volume in individual rats. (C) Average percent change of hippocampal volume.
We then examined whether there was a relationship between the variation in hippocampus size at baseline and the variation in hippocampus diminution after stress. The lack of reliable correlation between the two variables (r = −.234, p > .05) indicates that the baseline hippocampus size is not a good predictor of the magnitude of stress effects on the hippocampus. Although the baseline body weight was slightly correlated with the baseline hippocampal volume (r = −.437, p > .054), weight on the final day was not related to post-stress hippocampal volume (r = −.359, p > .05). In addition, changes of adrenal volume were not correlated with changes in total body weight (r = −.094, p > .05).
DISCUSSION
Our results indicate that the hippocampus is particularly vulnerable to stress. Specifically, rats that experienced 6-hours of restraint daily for 21 consecutive days showed a significant reduction in hippocampal volume from the pre-stress baseline level. This reduction was relatively specific to the hippocampus, as anterior cingulate cortex, retrosplenial granular b cortex and adrenal glands did not undergo measurable changes from pre-stress to post-stress conditions. In addition, our initial pilot study has confirmed that chronic restraint procedure increased anxiety measured by elevated plus maze test.
Although several studies have reported decreased hippocampal size in PTSD patients [5] and loss of neurons in the hippocampus associated with chronic stress in rodents (e.g., [11,12]), the present animal structural MRI findings, to our knowledge, provide the first direct evidence that the chronic stress paradigm that is known to produce morphological changes in the hippocampus [4] can reduce the overall size of the hippocampus. Here we report an approximately 3% decrease in hippocampal volume following stress. A previous pilot study using MRI measurement of hippocampal volume in tree shrews (3 animal in each group) showed approximately 5~10% reductions following psychosocial stress or cortisol treatment compared to the pre-stress volume [13]. In humans, Bremner et al. [14] found a 19% reduction of the hippocampus in abused women with PTSD compared to normal control women. Lindauer et al. [15] reported about 10% hippocampal reduction in police officers and firefighters with PTSD. A longitudinal pilot study in children also found reduction of the hippocampal volume between the period shortly after experiencing trauma and measurements made 12 or 18 months later [16]. Although some studies did not find any reliable reductions in the hippocampus from PTSD patients [17], at least one study [6] has compared hippocampal volumes in identical twins—one twin diagnosed with combat-related PTSD and the other co-twin healthy—and found relatively small hippocampi in both twin members, indicating that prior factors (and not PTSD per se) likely contribute to a smaller hippocampus. Our observation of 3% hippocampal reduction, which is smaller than other studies, might be attributed to the habituation to repeated restraint procedures. Girotti et al. [18] have shown that repeated stress reduces cFOS expression in the brain as well as hypothalamic-pituitary-adrenal axis response protecting organisms from excessive corticosterone.
While the chronic restraint procedures used in the present study cannot be easily equated to human PTSD, our approach does control for many of the variables (e.g., genetic and environmental factors) that make interpretation of the patient studies difficult. Moreover, unlike human studies that cannot discern the causality of PTSD effects and unlike prior invasive animal studies that cannot compare neurobiological measurement to a pre-stress baseline within subjects, the use of non-invasive structural MRI allowed us to effectively compare the hippocampus and other structures in the same animals in a longitudinal manner. Here, our various parametric volume measures reveal that initial body weight and other brain volumes (including the hippocampus) did not affect stress induced hippocampal reductions. Regardless of whether the chronic stress procedure employed here produces a valid animal model of PTSD, the present results clearly support the view that stress can alter the size of the hippocampus.
Although the present approach cannot directly address the underlying cellular mechanisms by which stress reduces the size of the hippocampus, our finding is consistent with earlier reports of apotosis, dendritic atrophy and decrease of neurogenesis associated with stress [1],[19]. For example, chronic restraint stress shrinks the adult rat hippocampal dentate gyrus volume by suppressing neurogenesis of granule cells [11]. Additionally, dendritic atrophy and loss of synapses have been observed in CA1, CA3 and dentate gyrus fields in chronically stressed and corticosterone-treated rats [20]. Cellular changes (i.e., smaller neuronal soma size, loss of glial cells) have also been found in the postmortem hippocampus of subjects with major depressive disorders [21]. Changes in hippocampal volume with the selective serotonin reuptake inhibitor (SSRI) antidepressant paroxetine in humans with PTSD are also consistent with plasticity of the hippocampus [22]. Thus, it will be interesting to investigate whether SSRI treatments that up-regulate hippocampal neurogenesis [23] will reverse stress-induced reduction in hippocampal volume. Regarding this issue, Bessa et al. [24] have shown that antidepressants produces dendritic remodeling rather than increases of neurogenesis in the hippocampus. Further, treatment of tianeptine prevented stress induced hippocampal volume reduction in tree shrews [25].
Conclusion
Chronic restraint procedure produced significant reduction of the hippocampal volume compared to pre-stress baseline. This hippocamal specific reduction does not appear to be affected by other confounding factors such as initial body weight or brain volumes.
Acknowledgments
This study was supported by the UWM Research Growth Initiative program, NIH Grant R01MH060668 (F.J.H.), and NIH Grant R01MH64457 (J.J.K.).
References
- 1.McEwen BS, Sapolsky RM. Stress and cognitive function. Current opinion in neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature reviews. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 3.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, Southwick SM, Charney DS. The neurobiology of posttraumatic stress disorder: An integration of animal and human research. In: Saigh P, Bremer JD, editors. Posttraumatic Stress Disorder: A Comprehensive Text. New York: Allyn & Bacon; 1999. [Google Scholar]
- 6.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. The American journal of psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annual review of psychology. 2003;54:229–252. doi: 10.1146/annurev.psych.54.101601.145112. [DOI] [PubMed] [Google Scholar]
- 9.Wolf OT, Dyakin V, Vadasz C, de Leon MJ, McEwen BS, Bulloch K. Volumetric measurement of the hippocampus, the anterior cingulate cortex, and the retrosplenial granular cortex of the rat using structural MRI. Brain Research Protocols. 2002;10:41–46. doi: 10.1016/s1385-299x(02)00181-2. [DOI] [PubMed] [Google Scholar]
- 10.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 11.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. The European journal of neuroscience. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 12.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 13.Ohl F, Michaelis T, Vollmann-Honsdorf GK, Kirschbaum C, Fuchs E. Effect of chronic psychosocial stress and long-term cortisol treatment on hippocampus-mediated memory and hippocampal volume: a pilot-study in tree shrews. Psychoneuroendocrinology. 2000;25:357–363. doi: 10.1016/s0306-4530(99)00062-1. [DOI] [PubMed] [Google Scholar]
- 14.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. The American journal of psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 15.Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biological psychiatry. 2004;56:356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 17.Pederson CL, Maurer SH, Kaminski PL, Zander KA, Peters CM, Stokes-Crowe LA, et al. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. Journal of traumatic stress. 2004;17:37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- 18.Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain research. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biological psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biological psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 24.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. Molecular psychiatry. 2008. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. [DOI] [PubMed] [Google Scholar]
- 25.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]