Induction of the CXC Chemokine Interferon-γ-Inducible Protein (IP)-10 Regulates the Reparative Response Following Myocardial Infarction (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 6.
Abstract
Rationale
Interferon-γ-inducible protein (IP)-10/CXCL10, an angiostatic and antifibrotic chemokine with an important role in T cell trafficking, is markedly induced in myocardial infarcts, and may regulate the reparative response.
Objective
To study the role of IP-10 in cardiac repair and remodeling.
Methods and Results
We studied cardiac repair in IP-10 null and WT mice undergoing reperfused infarction protocols and examined the effects of IP-10 on cardiac fibroblast function. IP-10 deficient and WT animals had comparable acute infarct size. However, IP-10 absence resulted in a hypercellular early reparative response and delayed contraction of the scar. Infarcted IP-10 −/− hearts exhibited accentuated early dilation, followed by rapid wall thinning during infarct maturation associated with systolic dysfunction. Although IP-10 null and WT mice had comparable cytokine expression, IP-10 absence was associated with marked alterations in the cellular content of the infarct. IP-10 −/− infarcts had more intense infiltration with CD45+ leukocytes, Mac-2+ macrophages and α-smooth muscle actin (α-SMA)+ myofibroblasts than WT infarcts but exhibited reduced recruitment of the subpopulations of leukocytes, T lymphocytes and α-SMA+ cells that expressed CXCR3, the IP-10 receptor. IP-10 did not modulate cardiac fibroblast proliferation and apoptosis, but significantly inhibited basic Fibroblast Growth Factor-induced fibroblast migration. In addition, IP-10 enhanced growth factor-mediated wound contraction in fibroblast-populated collagen lattices.
Conclusions
Endogenous IP-10 is an essential inhibitory signal that regulates the cellular composition of the healing infarct and promotes wound contraction, attenuating adverse remodeling. IP-10-mediated actions may be due, at least in part, to direct effects on fibroblast migration and function.
Keywords: infarction, remodeling, chemokines, fibrosis, wound healing
INTRODUCTION
Myocardial infarct healing is dependent on activation of an inflammatory cascade, followed by granulation tissue formation and deposition of collagen-based matrix1,2. Rapid induction of inflammatory chemokines, cytokines and adhesion molecules in the infarcted myocardium results in chemotactic recruitment of leukocytes. Neutrophils and activated macrophages clear the wound from dead cells and debris while secreting mediators that promote fibroblast growth and angiogenesis. The intense, but transient, inflammatory response is followed by repression of cytokine synthesis and activation of fibrogenic and angiogenic pathways. Activated myofibroblasts produce extracellular matrix proteins, while neovessels provide oxygen and nutrients necessary for the metabolically active wound. As the infarct matures, fibroblasts and vascular cells undergo apoptosis, and a hypocellular collagenous scar is formed. Optimal repair of the infarcted myocardium is dependent on timely induction and suppression of inflammatory pathways and on endogenous mechanisms that ensure containment of the fibrotic response within the area of the infarct. Disturbances in the mechanisms involved in regulation of the reparative process result in formation of a defective scar with altered mechanical properties, leading to increased adverse remodeling.
The chemokines are inflammatory mediators with an essential role in leukocyte trafficking. Several members of the chemokine family are markedly and consistently induced in healing infarcts, modulating the post-infarction inflammatory response through recruitment of leukocyte subpopulations3,4,5,6,7. The CC chemokine monocyte chemoattractant protein (MCP)-1/CCL2 plays a crucial role in chemotaxis and activation of mononuclear cells in the infarcted myocardium5. CXC chemokines containing the ELR motif are potent neutrophil chemoattractants and are critically involved in infiltration of the infarcted heart with granulocytes6. However, recent evidence suggests that certain chemokines may modulate inflammatory processes through actions beyond their leukocyte chemotactic properties8. The ELR-negative CXC chemokine CXCL10/interferon-γ-inducible protein (IP)-10 is involved in trafficking of effector T-cells9, but also modulates fibroblast10 and endothelial cell phenotype11, exerting angiostatic12 and anti-fibrotic effects13. We have previously demonstrated that IP-10 synthesis is markedly induced in reperfused canine14 and murine15 myocardial infarcts and suggested that its upregulation in the infarcted heart may be an essential regulatory mechanism in infarct healing. Fibrogenic and angiogenic growth factors, such as basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-β, and vascular endothelial growth factor (VEGF), are rapidly induced in the infarcted heart; their expression extends in the infarct border zone16 creating an environment that could promote expansion of the fibrotic response into the non-infarcted myocardium. We hypothesized that through its antifibrotic and angiostatic effects, IP-10 may prevent excessive infiltration of the infarcted heart with fibroblasts and may limit uncontrolled angiogenesis, serving as a “stop signal” that limits expansion of the fibrotic reaction. To test this hypothesis we studied cardiac injury, repair, and post-infarction remodeling in IP-10 null mice. Our findings suggest that IP-10 plays an essential role in the reparative response following myocardial infarction by modulating the composition of the cellular infiltrate and by enhancing contraction of the healing scar. IP-10 exerts direct actions on growth factor-stimulated fibroblasts reducing their migratory capacity and enhancing their ability to induce contraction of collagen lattices.
METHODS
1. Murine ischemia/reperfusion protocols
All animal studies were approved by the animal protocol review committee at Baylor College of Medicine. IP-10 −/− mice and wildtype (WT) C57/BL/6 controls were used for myocardial infarction experiments using an established closed-chest model of coronary occlusion/reperfusion5.
2. Infarct size determination
To assess the size of acute infarcts the Evans blue-triphenyltetrazolium chloride (TTC) staining method was used.
3. Immunohistochemistry and quantitative histology
For histopathological analysis murine hearts were fixed in zinc-formalin (Z-fix; Anatech, Battle Creek, MI), and embedded in paraffin. Immunohistochemical staining was performed using established protocols17.
4. TUNEL staining and immunofluorescence
Identification of apoptotic myofibroblasts in myocardial infarction was performed using the fluorescent In situ Cell Death Detection Kit (Roche) combined with α-smooth muscle actin (SMA) immunofluorescence.
5. Preparation of single cell suspensions from myocardial infarction
Single cell suspensions were obtained from infarcted WT (n=6) and −/− (n=6) hearts after 72h of reperfusion.
6. Flow cytometry
Flow cytometric identification of the cells was performed through simultaneous labeling with two of the following antibodies: FITC labeled anti-CD3 (clone BD Biosciences 145-2C11); PE-labeled anti-CXCR3 (R&D Systems); PE-Cy5 labeled anti-CD45 (BD Biosciences, clone 30-F11). For intracellular staining, cells were fixed and permeabilized; subsequently, cells prelabeled with surface markers were incubated with anti-α-SMA.
7. Perfusion fixation and assessment of ventricular volumes
For assessment of post-infarction remodeling, infarcted hearts after 7 and 28 days days of reperfusion were used for perfusion-fixation as previously described5. Left Ventricular End-Diastolic Volume (LVEDV) and scar size were assessed using established methods. Anterior wall (AW), posterior wall (PW) and mid-septal wall (S) thickness were assessed at the mid-papillary level.
8. Echocardiography
Short axis M-mode echocardiography was performed prior to instrumentation and after 7, 14 and 28 days of reperfusion (WT: n=10, IP-10 −/−: n=12) using a 25 MHz probe (Vevo 770; Visualsonics. Toronto ON). Left Ventricular End-Diastolic Diameter (LVEDD) and Left Ventricular End-systolic Dimension (LVESD) were measured as indicators of cardiac remodeling and Left Ventricular Fractional Shortening (LVFS) was calculated as an indicator of systolic function.
9. RNA extraction and Ribonuclease protection assay (RPA)
RNA was extracted from sham and infarcted hearts. Ribonuclease protection assays were performed (RiboQuant; Pharmingen) as previously described17.
10. Cardiac fibroblast isolation and transwell migration assay
Mouse cardiac fibroblasts were isolated by enzymatic digestion with a collagenase buffer. Cardiac fibroblast migration was studied using a colorimetric transwell system.
11. Assessment of contraction in collagen lattices populated with cardiac fibroblasts
Cardiac fibroblasts at passage 3 were used to examine the effects of IP-10 on growth factor-induced collagen lattice contraction.
12. Cell proliferation assay
Proliferation of cardiac fibroblasts was assessed using a colorimetric BrdU Cell Proliferation ELISA kit (Roche Applied Biosciences, Indianapolis, IN).
13. Assessment of cardiac fibroblast apoptosis
Apoptosis in isolated cardiac fibroblasts was assessed using the Cell Death Detection ELISAPLUS (Roche Applied Biosciences, Indianapolis, IN).
14. Statistical analysis
Statistical analysis was performed using ANOVA followed by t-test corrected for multiple comparisons (Student-Newman-Keuls). Paired t-test was used to compare echocardiographic endpoints before and after infarction. Data were expressed as mean±SEM. Statistical significance was set at 0.05.
RESULTS
1. IP-10 induction in the infarcted myocardium is associated with recruitment of CXCR3+ cells
RPA demonstrated marked IP-10 mRNA upregulation in the infarcted myocardium after 6h of reperfusion (Fig. 1A). Although IP-10 levels were also elevated in the non-infarcted segment, the difference did not reach statistical significance. In order to examine whether IP-10 upregulation is associated with infiltration of the infarcted myocardium with cells expressing the IP-10 receptor, CXCR3, we performed flow cytometry on single cell suspensions harvested from control and infarcted hearts (1h ischemia/72h reperfusion). Myocardial infarction resulted in a marked increase in the absolute number of nucleated cells isolated from the heart (Table 1). The infarcted myocardium contained a significantly higher number of CD45+ leukocytes, CD3+ T lymphocytes and α-SMAc+ cells than the control heart (Table 1). Myocardial infarction resulted in intense infiltration with CXCR3+ cells (Figure 1B, Table 2) leading to a marked increase in the number of CXCR3+/CD45+ leukocytes and CXCR3+/CD3+ T lymphocytes (Table 2, Figure 1C–D). In addition, infarcts contained abundant CXCR3+ cells that did not express hematopoietic cell markers. Almost 20% of the CXCR3+ cells in the infarcted heart expressed α-SMA (vs. less than 5% in control hearts) reflecting the accumulation of myofibroblasts expressing the IP-10 receptor (Table 2, Figure 1E).
Figure 1.
A: IP-10 mRNA levels markedly increased in infarcted segments (I) after 6h of reperfusion (**p<0.01 vs. sham). IP-10 expression in non-infarcted segments (NI) was higher than in sham hearts; however, the difference did no reach statistical significance. B–E: Infiltration of the infarcted heart with cells expressing the IP-10 receptor, CXCR3. Flow cytometry of cell suspensions from the infarcted heart after 72h of reperfusion demonstrated marked increases in the number of CXCR3-positive cells. Representative histograms are shown comparing a control mouse heart (black area under the curve) and an infarcted heart (white area). The x-axis represents the intensity of the CXCR3-PE signal, whereas the y-axis shows the number of positive cells. Infarcted hearts had significantly higher number of nucleated DRAQ5+/CXCR3+ cells (B). Marked influx of CD45+ leukocytes (C), CD3+ T cells (D) and α-SMA+ myofibroblasts (E) expressing CXCR3 was noted in the infarcted heart. Quantitative analysis of the findings is shown in Table 1. F–I: IP-10 null and WT mice had comparable acute infarct size.. F: TTC/Evans Blue staining was used to assess the necrotic region, the viable myocardium and the area at risk (AAR). The AAR (G), the size of the acute infarct (H) and the ratio infarct size:AAR (I) were comparable between IP-10 null and WT mice.
Table 1.
Flow cytometric identification of the cellular infiltrate in WT and IP-10 −/− infarcts.
| Control | Infarction(1h ischemia/72h reperfusion) | |||
|---|---|---|---|---|
| WT | IP-10 −/− | WT | IP-10 −/− | |
| Nucleated cells (N/w) | 388.3±66.3 | 346.5±45.5 | 9091.6±526.2** | 15245.4±2210.8# |
| CD45+ (N/w) | 61.7±8.4 | 72.8±9.4 | 5936.0±231.1** | 7041.2±234.9## |
| CD3+/CD45+ (N/w) | 0.4±0.1 | 0.2±0.1 | 755.3±37.3** | 835.7±63.65pNS |
| CD3+ (% of CD45+) | 0.9±0.1 | 0.8±0.1 | 12.3±0.4** | 10.5±0.5# |
| α-SMA+/DRAQ5+ (N/w) | 9.8±1.6 | 10.3±2.1 | 1279±199.7** | 2482.1±396.2# |
| α-SMA+ (% of nucleated cells) | 2.5±0.1 | 2.4±0.5 | 10.5±1.4** | 13.7±0.8## |
Table 2.
Recruitment of CXCR3+ cells in the infarcted heart is dependent on IP-10
| Control | Infarction(1h ischemia/72h reperfusion) | |||
|---|---|---|---|---|
| WT | IP-10 −/− | WT | IP-10 −/− | |
| CXCR3+/DRAQ5+ (N/w) | 49.1±9.7 | 54.3±8.5 | 1790.3±106.5** | 639.0±94.5## |
| CXCR3+/CD45+ (N/w) | 16.0±2.5 | 13.4±3.5 | 1118.4±88.6** | 433.9±57.3## |
| CXCR3+ (% of CD45+) | 18.45±0.9 | 17.8±2.1 | 16.7±1.1 | 5.2±0.6## |
| CXCR3+/CD3+ (N/w) | 5.7±1.0 | 5.4±1.3 | 607.1±62.9** | 202.3±29.0## |
| CD3+ (% of CD45+) | 0.8±0.04 | 0.7±0.1 | 12.3±0.4** | 10.5±0.5## |
| CXCR3/α-SMA+ (N/w) | 16.4±3.1 | 15.2±3.4 | 1218.9±80.5** | 328.8±36.7## |
| CXCR3/α-SMA+ (% of nucleated cells) | 0.9±0.1 | 1.1±0.2 | 5.3±0.8** | 1.38±0.2## |
2. IP-10 null and WT mice exhibit comparable acute infarct size
In the absence of injury, IP-10 null and WT mice had comparable structural and functional characteristics (Online supplement). Following reperfused infarction, IP-10 null mice and WT animals had comparable mortality rates (WT: 15.6 % vs. IP-10 −/−: 17.4%; pNS). Infarct size and the ratio of the infarct size to the area at risk (infarct:AAR) were comparable between WT and IP-10 null animals after 24h of reperfusion (Figure 1G–J) suggesting that IP-10 absence did not affect susceptibility of cardiomyocytes to ischemic injury.
3. IP-10 absence is associated with enhanced adverse remodeling and early expansion of the scar
Both echocardiographic and quantitative morphometric analysis demonstrated that IP-10 absence is associated with accentuated early remodeling. After 7 days of reperfusion LVEDV assessed through morphometry (Figure 2B) and echocardiographically-derived LVEDD (Online Table I) were significantly higher in infarcted IP-10 null hearts when compared with WT animals. Although there was a trend towards a lower LVFS in IP-10 null hearts, the difference between IP-10 −/− and WT animals did not reach statistical significance at this timepoint. Seven days after reperfused infarction IP-10 null mice exhibited significantly larger scars in comparison to WT animals (Figure 2C). Because acute infarct size was comparable between groups (Figure 1), the increased scar area suggested expansion, or impaired contraction, of the fibrotic region. Serial echocardiographic imaging demonstrated that, although both IP-10 null and WT hearts continued to dilate, and cardiac dimensions remained higher in IP-10 null animals after 28 days of reperfusion, the difference was no longer statistically significant (Online Table I). However, after 28 days of reperfusion IP-10 null hearts had significantly lower LVFS, indicating accentuated systolic dysfunction (Online Table I). Development of late systolic impairment in IP-10 null hearts was associated with progressive thinning of the left ventricular walls (Figure 2D). Thus, in comparison to WT, IP-10 −/− hearts had markedly increased dilative remodeling over the first week following infarction associated with a more extensive fibrotic area. As the scar matured, the differences in chamber dilation between WT and IP-10 null hearts were attenuated; however, IP-10 −/− mice developed rapid wall thinning and accentuated systolic dysfunction (Figure 2D, Online Table I).
Figure 2.
IP-10 absence results in early expansion of the fibrotic region and increased adverse remodeling followed by late wall thinning and development of systolic dysfunction. Chamber dimensions (A) and LVEDV (B) significantly increased following infarction in both IP-10 null and WT animals indicating adverse remodeling (**p<0.01 vs. sham). Both morphometric (A, B) and echocardiographic studies (E, Online Table I) demonstrated that IP-10 null mice had enhanced remodeling and expansion of the scar after 7 days of reperfusion in comparison to WT animals. B: Morphometrically-derived LVEDV was significantly higher in IP-10 null mice after 7 days of reperfusion (#p<0.05 vs. WT). Although LVEDV remained higher in IP-10 null hearts after 28 days of reperfusion, the difference between WT and −/− animals was not statistically significant. C: IP-10 −/− animals had increased scar size after 7 days of reperfusion (*p<0.05 vs. WT). Scar size was comparable between groups after 28 days of reperfusion. D. After 7 days of reperfusion, thickness of the remodeling septum (S) and posterior wall (PW) was higher in IP-10 null mice (*p<0.05, **p<0.01 vs. WT). However, after 28 days, the rapid scar contraction (C) in IP-10 null mice was associated with thinning of the infarcted anterior wall (AW) and the remodeling septum (S) (#p<0.05, ##p<0.01 vs. 7d IP-10 −/−). E: Representative echocardiographic images of the infarcted WT and IP-10 −/− hearts after 7 and 28 days of reperfusion. Quantitative analysis of the echocardiographic findings is shown in Online Table I.
4. Effects of IP-10 loss on the post-infarction inflammatory response
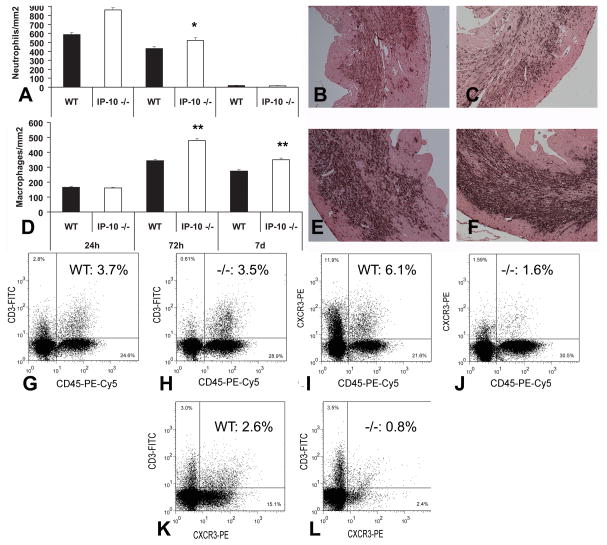
IP-10 deficiency was associated with alterations in the composition of the inflammatory infiltrate in the infarcted heart. IP-10 null mice had increased infiltration with neutrophils after 24h and 72h of reperfusion (Figure 3A–C) and enhanced macrophage density in the infarcted myocardium after 3–7 days (Figure 3D–F). The increased recruitment of hematopoietic cells in IP-10 null infarcts was confirmed through flow cytometry of cell suspensions harvested from the infarcted myocardium. After 72h of reperfusion, the number of nucleated cells and the number of CD45+ leukocytes were significantly higher in infarcted IP-10 −/− hearts when compared with WT infarcts (Table 1); however, the number of CD3+ T-lymphocytes was comparable between groups (Figure 3G–H). IP-10 deficiency resulted in impaired recruitment of CXCR3+ cells in the healing infarct (Table 2). The number of CXCR3+/CD45+ leukocytes and CXCR3+/CD3+ T cells was markedly reduced in IP-10 null infarcts (Table 2, Figure 3I–L). Despite the significant alterations in cellular composition of the inflammatory infiltrate, IP-10 absence had relatively subtle effects on expression of inflammatory cytokines and chemokines (Online Figure I/Table II).
Figure 3.
A–C: IP-10 null mice exhibited increased neutrophil infiltration in the infarcted myocardium after 24–72h of reperfusion (A). Representative images show neutrophil immunohistochemistry in WT (B) and IP-10 null (C) infarcts. D–F: Macrophage density was increased in the infarcted myocardium after 72h-7 days of reperfusion (D). Representative images show Mac-2 staining in WT (E) and IP-10 null (F) infarcts (*p<0.05, **p<0.01 vs. WT). Counterstained with eosin. G–L: Lymphocyte infiltration was assessed using flow cytometry of cell suspensions harvested from the infarcted heart. Representative experiments are presented; quantitative analysis is shown in Tables 1 and 2. IP-10 null infarcts had an increased number of CD45+ hematopoietic cells; however, the number of CD3+ T lymphocytes was comparable between WT (G) and IP-10 null (H) infarcts. The percentage of CXCR3+/CD45+ hematopoietic cells (I–J) and CXCR3+/CD3+ lymphocytes (K–L) was significantly lower in IP-10 null infarcts indicating the critical role of IP-10 in recruitment of CXCR3+ cells.
5. IP-10 null mice exhibit an accentuated early fibrotic response
IP-10 deficiency was associated with an accentuated early fibrotic response. Infarct myofibroblasts were identified as spindle-shaped α-SMA+ cells localized outside the vascular media (Figure 4A–B). Myofibroblast density was significantly increased in IP-10 null infarcts after 72 h of reperfusion (Figure 4C). In order to examine whether increased myofibroblast accumulation in IP-10 −/− infarcts was due to enhanced cellular proliferation, we performed dual immunohistochemistry combining α-SMA labeling with staining for ki-67, an indicator of cellular proliferation (Figure 4A–B). The number of ki-67+ cells and ki-67+/α-SMA+ myofibroblasts was comparable between groups suggesting that IP-10 deficiency does not affect cellular proliferation (Figure 4D–E). Flow cytometry of cell suspensions from the infarcted heart confirmed the immunohistochemical findings demonstrating a significantly higher number of α-SMA+ cells in IP-10 null infarcts (Table 1, Figure 4F–G). Despite the increased infiltration of IP-10 null infarcts with myofibroblasts, recruitment of the CXCR3+ subset of α-SMA-expressing cells was markedly reduced in the absence of IP-10 (Table 2, Figure 4H–I). Accumulation of myofibroblasts resulted in enhanced deposition of collagen in the infarct and in the neighboring border zone of IP-10 null hearts after 7 days of reperfusion (Figure 4J–L). Enhanced fibrotic remodeling of the infarcted heart in IP-10 null animals was not associated with increased expression of TGF-β isoforms (Figure 4M–O4), FGF-1, and FGF-2 (Online Supplement). In addition, assessment of Matrix Metalloproteinases (MMP) and Tissue Inhibitor of Metalloproteinases (TIMP) mRNA expression showed only subtle differences between IP-10 null and WT hearts (Online Table III).
Figure 4.
IP-10 deficient mice have accentuated early fibrosis following infarction. A–B: Dual immunohistochemistry combining α-SMA staining (red) to identify myofibroblasts and ki-67 staining (black) to label proliferating cells in WT (A) and IP-10 null (B) infarcts (72h reperfusion). Numerous proliferating myofibroblasts were noted (B- inset). C: Peak myofibroblast density was significantly higher in IP-10 null infarcts (*p<0.05 vs. WT). D–E: However, proliferating cell density (D) and the number of proliferating α-SMA-positive myofibroblasts (E) were comparable between groups. F–I: Flow cytometry on cell suspensions from infarcted hearts also showed a significantly higher number of α-SMA+ cells in IP-10 null infarcts (Table 1). Representative experiments identifying α-SMA+ nucleated cells in WT (F) and IP-10 −/− (G) infarcts are shown. Although IP-10 absence resulted in increased myofibroblast infiltration, recruitment of the CXCR3+ subset of α-SMA+ cells was reduced (Table 2). Representative experiments from WT (H) and IP-10 −/− (I) infarcts are demonstrated J–L: Sirius red staining identified the collagen network in WT (J) and IP-10 −/− (K) infarcts. L: IP-10 −/− mice exhibited significantly higher collagen content in the infarct and the peri-infarct zone neighboring the scar (**p<0.01 vs. WT). M–O: Enhanced fibrosis in IP-10 null infarcts was not associated with increased mRNA expression of TGF-β isoforms.
In order to examine whether increased myofibroblast density in the absence of IP-10 is due to reduced apoptosis of infarct myofibroblasts we performed dual fluorescence combining TUNEL staining, to identify apoptotic cells, and α-SMA immunofluorescence (Figure 5A–B). After 7 days of reperfusion the density of apoptotic nuclei was significantly higher in IP-10 null infarcts, when compared with WT scars (Figure 5C). Although only a small number of TUNEL+/α-SMA+ myofibroblasts was detected in the infarcted hearts (perhaps reflecting the rapid clearance of these cells from the infarct, or the loss of α-SMA expression as these cells undergo apoptosis), the density of apoptotic myofibroblasts was significantly higher in IP-10 null infarcts (Figure 5D).
Figure 5.
A–D: Apoptosis in the infarcted heart. Dual fluorescence combines TUNEL staining (green) to identify apoptotic nuclei and α-SMA immunofluorescence (red) to label myofibroblasts. Representative sections from WT (A) and IP-10 −/− (B) infarcts are shown. Myofibroblasts were identified as spindle-shaped α-SMA-immunoreactive cells (arrows) located outside the vascular media (arrowhead). Counterstained with DAPI (blue). IP-10 null infarcts had increased density of apoptotic cells (C) and apoptotic myofibroblasts (D) (*p<0.05 vs. WT) after 7 days of reperfusion. The findings reflected the increased density of granulation tissue cells during the proliferative phase of healing in IP-10 −/− infarcts and their apoptotic clearance during scar maturation. E–G: CD31 staining was used to assess microvascular density in the infarct, the neighboring subepicardial and subendocardial zone (Nei) and the remote remodeling myocardium (Rem) in WT (E) and IP-10 null (F) hearts. G: Vascular density was significantly lower in IP-10 null infarcts (**p<0.01, *p<0.05 vs. WT). H–I: IP-10 stimulation did not induce proliferation in serum-starved cardiac fibroblasts and did not modulate the effects of serum (1%–5%) or bFGF (I) on fibroblast proliferation (**p<0.01 vs. control). J: In addition, IP-10 had no effects on fibroblast apoptosis. Stimulation with cycloheximide (CHX) was used as a positive control (**p<0.01 vs. serum-stimulated cells).
6. IP-10 deficiency is associated with decreased microvascular density in the infarct and peri-infarct area
Because IP-10 has potent angiostatic properties18, we examined whether expansion of fibrosis in IP-10 null infarcts is associated with uncontrolled angiogenesis (Figure 5E–G). Surprisingly, IP-10 −/− mice showed significantly decreased microvascular density in the infarct and peri-infarct area in comparison with WT animals (Figure 5E–G).
7. IP-10 does not modulate cardiac fibroblast proliferation
In order to dissect the mechanisms responsible for the antifibrotic actions of IP-10, we examined whether recombinant mouse IP-10 modulates proliferative activity of isolated mouse cardiac fibroblasts. Incubation with 1–5% Fetal Calf Serum (FCS) markedly enhanced cardiac fibroblast proliferation (Figure 5H). IP-10 (10–250ng/ml) had no effect on the proliferative activity of serum-stimulated cardiac fibroblasts (Figure 5H). Stimulation of serum-starved cells with bFGF, induced dose-dependent fibroblast proliferation (Figure 5I). Co-incubation with IP-10 did not affect bFGF-mediated proliferative activity (Figure 5I).
8. IP-10 does not induce cardiac fibroblast apoptosis
Because fibroblast apoptosis plays an important role in regulation of myofibroblast density in the infarct, we examined the effects of IP-10 on cardiac fibroblast apoptosis. Using a cell death detection ELISA, we found that incubation with IP-10 (10–100ng/ml) did not induce cardiac fibroblast apoptosis (Figure 5J). Cycloheximide was used as a positive control and markedly increased cardiac fibroblast apoptosis.
9. IP-10 attenuates bFGF-mediated fibroblast migration
Because migration of fibroblasts in the infarcted area plays an important role in the development of peri-infarct fibrosis we examined the effects of IP-10 in modulating cardiac fibroblast migratory activity. Using a transwell migration assay we demonstrated that bFGF induces cardiac fibroblast migration. Pre-incubation with IP-10 one hour prior to bFGF stimulation prevented growth-factor mediated cardiac fibroblast migration (Figure 6A–D).
Figure 6.
A–D: IP-10 inhibits bFGF-mediated fibroblast migration. A–C: Using a transwell assay we compared migration of serum-starved cardiac fibroblasts (A: control, B: bFGF stimulation, C: bFGF+IP-10). D: bFGF induced cardiac fibroblast migration (**p<0.01 vs. control), whereas IP-10 inhibited bFGF-induced migratory activity (#p<0.05 vs. bFGF). E–M: IP-10 enhanced growth factor-mediated wound contraction in fibroblast-populated collagen lattices. Fibroblast populated collagen lattices were stimulated with serum and the growth factors TGF-β1 and bFGF in the presence or absence of IP-10. E: control, F: 1%FCS, G: TGF-β1, H: bFGF, I: IP-10(100 ng/ml), J: FCS (1%)+ IP-10(100 ng/ml), K: TGF-β1+IP-10(100 ng/ml), L: bFGF+IP-10(100 ng/ml). M: Stimulation with serum, TGF-β1, or bFGF induced marked contraction of the collagen gel. IP-10 had no effects on serum-starved cells, but enhanced wound contraction in growth factor- or serum-stimulated fibroblast populated lattices (**p<0.01 vs. Control; #p<0.01 vs. growth factor stimulation without IP-10).
10. IP-10 enhances growth factor-mediated wound contraction in fibroblast-populated collagen lattices
Infarct healing is associated with contraction of the wound. Because IP-10 null animals have increased scar size in comparison with WT infarcts, we examined whether IP-10 modulates contraction of fibroblast-populated collagen lattices. Fibroblast stimulation with 1% serum, TGF-β1 (50 ng/ml), or bFGF (50 ng/ml) induced collagen lattice contraction. IP-10 had no effects in the absence of serum or growth factors. However, incubation with IP-10 (100 ng/ml) significantly enhanced the effects of serum, TGF-β1 and bFGF on collagen lattice contraction (Figure 6E–M)
DISCUSSION
Our study suggests that the CXC chemokine IP-10 is an essential molecular signal in regulation of the reparative response following myocardial infarction. In the absence of IP-10, formation of a hypercellular wound with impaired contractile properties is associated with accelerated early dilative remodeling. At a later stage, during the maturation phase of healing, clearance of the abundant macrophages and myofibroblasts recruited in IP-10 null infarcts, results in rapid wall thinning and leads to the development of systolic dysfunction (Figure 2). The deleterious effects of IP-10 deficiency are not due to increased susceptibility of cardiomyocytes to ischemic injury because acute infarct size was comparable between WT and IP-10 null mice (Figure 1). The mechanisms responsible for the effects of IP-10 gene disruption on the remodeling infarcted heart appear to involve alterations in recruitment of inflammatory cells and changes in functional activation of myofibroblasts that ultimately may result in perturbation of the mechanical properties of the wound.
A growing body of evidence suggests that IP-10 is involved in the pathogenesis of several chronic inflammatory conditions, including multiple sclerosis19, sarcoidosis, atherosclerosis20,21, heart, lung and small bowel transplant rejection22. The proinflammatory effects of IP-10 appear to be mediated predominantly through recruitment of effector T cells9 in sites of inflammation. IP-10 gene disruption in hypercholesterolemic ApoE −/− mice results in a 2-fold reduction in atherosclerotic lesion formation, associated with increased density of regulatory T cells and enhanced expression of the inhibitory cytokines interleukin (IL)-10 and TGF-β21. We found that IP-10 absence resulted in significant alterations in the composition of the inflammatory infiltrate in the infarcted heart (Figure 3). Histology and flow cytometry demonstrated that IP-10 null infarcts contain higher numbers of CD45+ leukocytes and Mac2+ macrophages than WT infarcts. In addition, IP-10 absence markedly reduced recruitment of leukocyte subsets expressing its main receptor, CXCR3. However, in contrast to the important role of IP-10-induced alterations in modulating inflammatory mediator synthesis in models of chronic inflammation, IP-10 absence did not affect the balance between pro-inflammatory and anti-inflammatory cytokines in acute myocardial infarction. Infarcted IP-10 null and WT hearts showed comparable expression of the inflammatory cytokines tumor necrosis factor (TNF)-α, IL-1β and IL-6 and of the inhibitory cytokines TGF-β and IL-10 at all timepoints examined (Online Table II). Thus, the role of the altered inflammatory cell content in mediating the protective actions of endogenous IP-10 in post-infarction cardiac remodeling is unclear.
Investigations in models of fibrosis demonstrated that IP-10/CXCR3 interactions play an important regulatory role in the pathogenesis of fibroproliferative processes. Both IP-10 null and CXCR3 −/− mice exhibit increased fibrosis in a model of bleomycin-induced lung injury13,23, whereas CXCR3 deficiency was associated with formation of hypercellular and weakened wounds in a model of full-thickness cutaneous wounding24. On the other hand, transgenic mice overexpressing IP-10 in keratinocytes had delayed and disorganized repair of cutaneous wounds associated with impaired blood vessel formation25. Both quantitative histology and flow cytometry demonstrated increased accumulation of myofibroblasts in the infarcted heart (Figure 4). Enhanced fibrosis in IP-10 null infarcts was not due to altered synthesis of TGF-β isoforms.
What is the mechanism responsible for enhanced myofibroblast accumulation in IP-10 null infarcts? IP-10 may regulate myofibroblast infiltration by inhibiting proliferation and migration of cardiac fibroblasts, or by enhancing fibroblast apoptosis. Our in vivo studies showed comparable density of proliferating myofibroblasts in IP-10 null and WT infarcts. In vitro experiments demonstrated that IP-10 does not inhibit growth factor-mediated cardiac fibroblast proliferation (Figure 5) and does not exert pro-apoptotic actions, but markedly attenuates fibroblast migration (Figure 6). Similar IP-10-mediated inhibitory effects on fibroblast migration were observed in mouse pulmonary fibroblasts13. In addition, Shiraha and co-workers demonstrated that IP-10-induced inhibition of motility in epidermal growth factor (EGF)-stimulated human dermal fibroblasts was not due to a disruption of EGF Receptor signaling at the ligand or receptor level, but was associated with inhibition of calpain activation10.
Although enhanced inflammatory cell and myofibroblast infiltration may alter the reparative response following myocardial infarction, these alterations do not explain the increased adverse remodeling observed in infarcted IP-10 null hearts. Our experiments identified a novel mechanism that may be responsible for the effects of IP-10 in cardiac remodeling. Using a fibroblast-populated collagen lattice model we found that IP-10 enhanced the ability of growth factor-stimulated murine cardiac fibroblasts to induce collagen contraction (Figure 6E–M). Thus, in addition to inhibition of the migratory potential of cardiac fibroblasts, IP-10 appears to increase their wound-contracting properties. IP-10-mediated reduction of the volume of the healing wound may alter the mechanical properties of the scar playing a role in the pathogenesis of dilative remodeling. The cellular basis for the effects of IP-10 on contraction of the collagen matrix is unknown. Wound contraction is a complex process that involves cell contraction, cell tractional forces, and cell elongation ultimately resulting in elimination of water from between the collagen fibers27. Previous studies demonstrated that IP-10 stimulation did not affect basal cell morphometry, but prevented EGF-mediated dermal fibroblast compaction resulting in an elongated cell morphology10; this mechanism may be in part responsible for enhanced collagen lattice contraction. Modulation of integrin-mediated interactions between transdifferentiated myofibroblasts and the matrix may also be implicated.
We have identified a chemokine-mediated pathway that plays an essential role in infarct healing, through mechanisms that may involve alterations in recruitment of inflammatory cells, and modulation of fibroblast phenotype and function. Our study highlights the importance of endogenous IP-10, as a key orchestrator of the fibrotic reparative response following myocardial infarction. Defects in the pathways involved in regulation of infarct healing may be associated with impaired wound contraction and perturbation of the mechanical properties of the infarcted heart leading to accentuated remodeling in patients with myocardial infarction.
Supplementary Material
supp1
Acknowledgments
SOURCES OF FUNDING: Supported by NIH R01 HL-76246, R01 HL-85440, the Alkek Foundation, the Medallion Foundation (N.G.F.) and NIH R01 CA-69212 (A.D.L.).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
MCP-1
monocyte chemoattractant protein
IP-10
interferon-γ-inducible protein-10
FGF
fibroblast growth factor
TGF
transforming growth factor
VEGF
vascular endothelial growth factor
WT
wildtype
TTC
triphenyltetrazolium
α-SMA
α-smooth muscle actin
LVEDV
left ventricular end-diastolic volume
AW
anterior wall
PW
posterior wall
S
septal
LVEDD
left ventricular end-diastolic diameter
LVESD
left ventricular end-systolic diameter
LVFS
left ventricular fractional shortening
RPA
ribonuclease protection assay
MMP
matrix metallproteinases
TIMP
tissue inhibitor of metalloproteinases
FCS
fetal calf serum
ApoE
apolipoprotein E
IL
interleukin
TNF
tumor necrosis factor
EGF
epidermal growth factor
Footnotes
Subject codes: [115] Remodeling, [130] Animal models of human disease, [147] Growth factors/cytokines, [151] Ischemic biology - basic studies, [4] Acute myocardial infarction.
DISCLOSURES: none
References
- 1.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. [PubMed] [Google Scholar]
- 4.Liehn EA, Merx MW, Postea O, Becher S, Djalali-Talab Y, Shagdarsuren E, Kelm M, Zernecke A, Weber C. Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. J Cell Mol Med. 2008;12:496–506. doi: 10.1111/j.1582-4934.2007.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 6.Tarzami ST, Miao W, Mani K, Lopez L, Factor SM, Berman JW, Kitsis RN. Opposing effects mediated by the chemokine receptor CXCR2 on myocardial ischemia-reperfusion injury: recruitment of potentially damaging neutrophils and direct myocardial protection. Circulation. 2003;108:2387–2392. doi: 10.1161/01.CIR.0000093192.72099.9A. [DOI] [PubMed] [Google Scholar]
- 7.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 10.Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–254. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 13.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–1430. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 15.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 17.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 22.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, Newsome J, Hebda PA, Wells A. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171:484–495. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians. 1998;110:183–196. [PubMed] [Google Scholar]
- 26.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16:472–479. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supp1