ROLE OF LIPIDS AND ACTIN IN THE FORMATION OF CLATHRIN-COATED PITS (original) (raw)
. Author manuscript; available in PMC: 2009 Nov 30.
Published in final edited form as: Exp Cell Res. 2006 Sep 30;312(20):4036–4048. doi: 10.1016/j.yexcr.2006.09.025
Abstract
Assembly of clathrin coated pits and their maturation into coated vesicles requires coordinated interactions between specific lipids and several structural and regulatory proteins. In the presence of primary alcohols, phospholipase D generates phosphatidylalcohols instead of PA, reducing stimulation of phosphatidyl inositol 5-kinase (PI5K) and hence decreasing formation of phosphoinositide-4,5-biphosphate (PIP2). Using live-cell imaging, we have shown that acute treatment of cells with 1-butanol or other small primary alcohols induces rapid disassembly of coated pits at the plasma membrane and blocks appearance of new ones. Addition of exogenous PIP2 reverses this effect. Coated pits and vesicles reappear synchronously upon removal of 1-butanol; we have used this synchrony to assess the role of actin in coated vesicle assembly. Prolonged inhibition of actin polymerization by latrunculin A or cytochalasin D reduced by ~50% the frequency of coated pit formation without affecting maturation into coated vesicles. As in control cells, removal of 1-butanol in the continued presence of an actin depolymerizer led to synchronous appearance of new pits, which matured normally. Thus, remodeling of the actin cytoskeleton is not essential for clathrin coated vesicle assembly but may indirectly affect the nucleation of clathrin coated pits.
Keywords: Endocytosis, clathrin, 1-butanol, live-cell imaging, actin, phosphoinositides
INTRODUCTION
The growth, invagination, pinching and uncoating of clathrin coate pits are governed by protein-protein and protein-lipid interactions (reviewed in [1, 2]). Phosphoinositide 4,5 bi-phosphate (PIP2) has been proposed to play a central role in coated pit mediated endocytosis [1, 2]. The clathrin adaptor AP-2 binds to PIP2 [3] and this interaction is in part responsible for recruitment of AP-2 to the plasma membrane [4]. Phosphatidylinositol 4-phosphate 5-kinase (PIP5K), the enzyme that generates PIP2, interacts with AP-2 through a direct contact with μ2-adaptin, one of its subunits [5]. Depletion of PIP5KIα by RNAi treatment in Hela cells decreased the cellular level of PIP2 and reduced the clathrin-dependent endocytosis of transferrin, whereas overexpression of PIP5KIα had the opposite effect, while also increasing the number of clathrin coated pits at the plasma membrane [6].
Another way to stimulate the activity of PIP5K is by exposing it to phosphatidic acid (PA), the product of the transphosphatidylation reaction catalyzed by phospholipase D (PLD) [7]. Normal activity of PLD is required for clathrin-mediated internalization of the angiotensin- and μ-opioid receptors and for degradation of epidermal growth factor (EGF) receptor [8–10]. In the presence of primary alcohols, such as 1-butanol, PLD generates phosphatidylalcohols instead of PA [11], leading to a lower activation of PIP5K and a concomitant reduction in the amount of PIP2 generated by PIP5K. Treatment with 1-butanol was used to show the dependence on PIP2 of AP-2 recruitment to lysosomal membranes in vitro [12] and also to dissect aspects of vesicular traffic between the endoplasmic reticulum and the Golgi apparatus [12–15].
Here we show that treatment of cells with 1-butanol, as well as other primary alcohols, blocks the endocytosis of transferrin and the formation of coated pits and vesicles. We observe rapid disappearance of coated pits and a complete block in the appearance of new ones. The latter effect of 1-butanol on coated pit formation is completely reversed if cells are incubated with liposomes enriched with PIP2, consistent with the essential role of PIP2 in coated pit assembly. We also observed formation of new coated pits just seconds after removal of the primary alcohols. The coated pits mature to coated vesicles with kinetic parameters similar to those measured in untreated cells. The appearance of new pits is almost synchronous, forming during the first ~3 min with a much higher frequency than usual, before returning to normal values.
We have used this synchronization to dissect the role of actin in coated pit assembly. In yeast cells the actin and clathrin-based machineries are interlinked in sustaining normal endocytosis [16]. In mammalian cells, however, the role of actin in the clathrin-based endocytic pathway is less well defined. Actin is recruited to assembling coated vesicles in mammalian cells [17–19] and it has therefore been suggested that it also critical for coated pit formation [19] and membrane scission [17, 18]. Pharmacological interference (Latrunculin A or B or Cytochalasin D) with actin assembly prevents to variable degrees (0 and 80% inhibition) the uptake of transferrin [6, 20–24]. We have interfered pharmacologically with actin assembly in the presence or absence of 1-butanol. We find that inhibiting the actin polymerization has no detectable effects on the growth and maturation of an individual coated pit as it transforms into a fully formed coated vesicle, nor does it have detectable effects on the final uncoating step. In contrast, there is a progressive decrease in the frequency of new coated pit formation, an increase in the number of large clathrin/AP-2 clusters, and a coalescence of active zones, the plasma membrane regions available for pit assembly [25]. As in control cells, 1-butanol exposure to cells pre-treated with Latrunculin B led to the rapid loss of coated pits and vesicles, which reappeared promptly when 1-butanol was removed. We suggest that the actin cytoskeleton helps create diffusion barriers or confinement zones at the plasma membrane of mammalian cells that favor the buildup of phosphoinositides and protein factors required for efficient formation of coated pits.
MATERIALS AND METHODS
Cell Culture and transfection
BSC-1, COS-7 and HeLa cells were grown as previously described [25]. BSC-1 and HeLa cell lines stably expressing EGFP-fusion chimeras of AP-2 or LCa or galactosyltransferase (GalT) were described previously [25, 26]. We have generated cell lines stably expressing EGFP-fusion chimeras of AP-1, AP-3, Arf1, Caveolin-1, β-COP, or ε-COP by transfection with the respective plasmids (see below) followed by selection and maintenance with complete medium supplemented with geneticin (G418, 0.5–0.7 mg/ml). All other constructs were transiently expressed. Cells were prepared for fluorescence microscopy experiments as previously described [25]. The following chemicals and fluorescent probes were employed: LatA, LatB, CytoD (Sigma), HEPES (VWR), geneticin (Invitrogen, Carlsbad, CA), Alexa Fluor 594 human transferrin (Molecular Probes), Phalloidin (Molecular Probes).
Plasmids
Rat clathrin light chain A1, dynamin2, sigma-1, sigma-2, sigma-3a (tetrameric clathrin adaptors small chains), Arf1, rab22, Caveolin1 were fused to fluorescent proteins (EGFP, Tomato) using C1 or N1 terminal fusion vectors (BD Biosciences Clontech). All transfections were carried out using FuGENE 6 (Roche Diagnostics, Indianapolis, IN).
Preparation of cells for imaging
The preparation of cells for imaging was as previously described [25]. For t-butanol and 1-butanol applications the imaging medium was replaced with imaging medium containing indicated % v/v of compound. Washouts were performed by replacing back the imaging medium.
Image acquisition
Confocal microscopy image acquisition was carried out with the same instrumentation as in [25] Exposure times varied between 500 and 1000 milliseconds.
TIRF/EPI experiments were performed using an inverted microscope (Axiovert 200M; Carl Zeiss, Thornwood, NY) equipped with a Zeiss TIRF module and a 100X 1,45NA Zeiss TIRF lens. EPI fluorescence illumination was provided through Lambda DG-4 illumination unit (Sutter Instruments, Novato, CA). A 470 nm solid state laser (Crystal lasers, Reno, NV) was the source of illumination in TIR. The measured penetration depth of the TIR illumination was 90 nm. Endocytosis of the coated pits was quantified using normalized EPI and TIRF fluorescence profiles: fluorescence records were normalized to their maximum intensity, the area in between the EPI and TIRF fluorescence traces after the peak in the EPI signal was measured as an indication of endocytosis.
Image processing and analysis of dynamics of AP-2 spots
Particle identification was performed following criteria described previously [25]. All particle tracks were individually validated. Calculation of several descriptors (lifetime, maximal intensity distributions, etc) was performed using an image analysis application developed using Matlab 7 (Mathworks, Natick, MA).
RESULTS
1-butanol blocks transferrin uptake by preventing the formation of clathrin coated pits and vesicles
Transferrin binds to its receptor, which is internalized by a clathrin-dependent pathway. A brief (5 min) incubation of Hela cells with 1–2% 1-butanol prevented the uptake of fluorescently labeled Alexa-594 transferrin in a dose-dependent manner (Fig. 1C–D). This endocytic block was significantly less pronounced in cells treated with t-butanol (Fig. 1 E–F). The effect of 1-butanol is completely reversible, as shown by the full recovery of transferrin uptake monitored in cells following washout of the alcohol (Fig. 1G–H). Analogous results were obtained with other primary alcohols such as 1-propanol and ethanol (data not shown). We then used live cell imaging [25] to follow the effect of 1-butanol on the formation of coated pits and vesicles [25]. BSC1 cells display a characteristic punctate pattern at the plasma membrane, corresponding to coated pits and vesicles labeled here with EGFP- LCa (for clathrin) or σ2-EGFP (for AP-2) (Fig. 2A, untreated [25]. These punctate patterns are lost upon incubation with 2% 1-butanol (Fig. 2A, 1-butanol and supplementary Movies 1–2); a similar effect was observed for other coated pit and coated vesicle components such as dynamin (Fig. 2B) or epsin and auxilin-1 (data not shown). Treatment with other primary alcohols such as 1-propanol or ethanol led to a similar disappearance of coated pits and vesicles (Fig. 2C). On the contrary, treatment with the secondary and tertiary alcohols 2-propanol and t-butanol did not affect coated pits labeled with AP-2 (Fig. 2D).
Fig. 1. 1-butanol blocks Tf uptake.
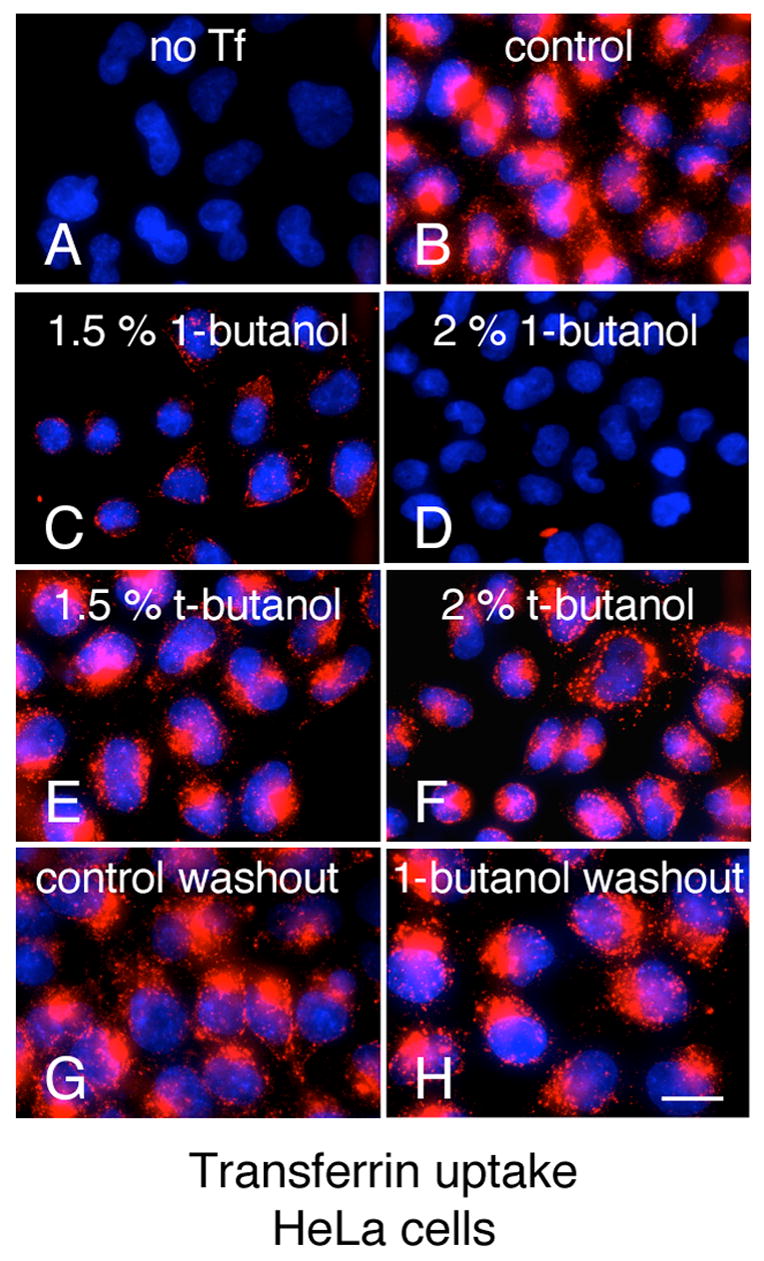
(A–F) Hela cells were pre incubated for 5 min at 37° C with PBS/Glucose/BSA (PBS-GB) (A,B), PBS-GB with 1,5% v/v 1-butanol (C), PBS-GB with 2% v/v 1-butanol (D), PBS-GB with 1,5% v/v t-butanol (E), and PBS-GB with 2% v/v t-butanol (F); followed by incubation with 10 μg/ml Alexa594-Tf for 2 min at RT (B–F) and 5 min at 37°C in the respective media (A–F); cells were subsequently transferred to 4°C and acid washed with (150mM NaCl, 1mM MgCl2, 0.125mM CaCl2, 0.1M Glycine pH 2.5) (G–H) Hela cells were pre-incubated with media alone (G) or with 2 % v/v 1-butanol (H) for 5 min at 37°C, followed by an additional 5 min in media without 1-butanol and Tf uptake and acid wash were carried out similarly as in A–F. Bar, 20 μm.
Fig. 2. 1-butanol and other primary alcohols disassemble clathrin coated pits.
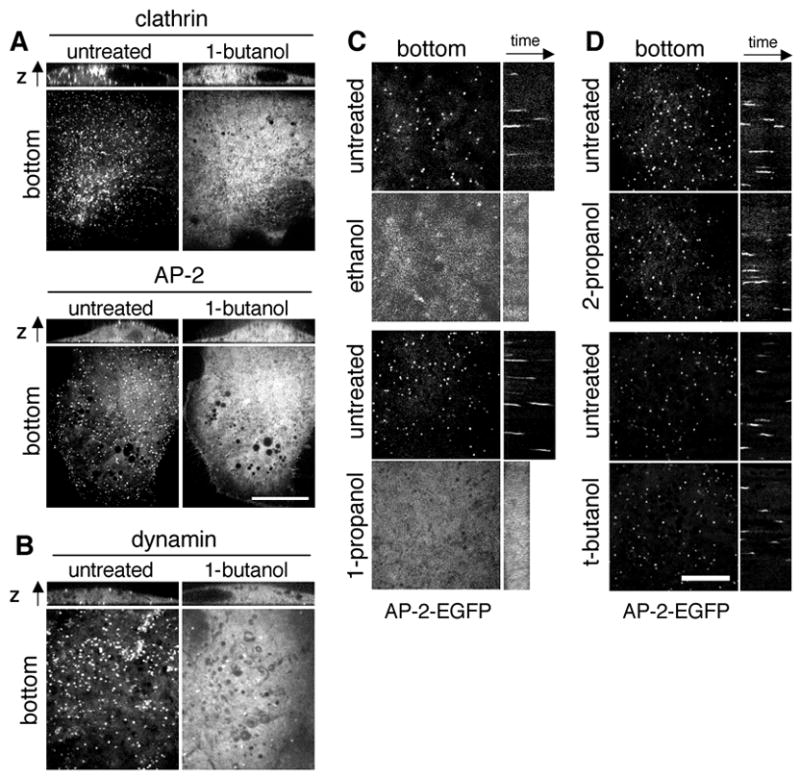
(A) Confocal section of BSC-1 cells stably expressing EGFP-LCa (BSC-1-EGFP-LCa), σ2-EGFP (BSC-1-σ2-EGFP), imaged before and during (~ 3min) treatment with 2% v/v 1-butanol. The fluorescence along the z-axis is shown on top of each confocal section. Bar, 20 μm. (B) Confocal section of COS cells transiently expressing dynamin2-EGFP. Cells were imaged in the same way as in panel A. (C) Treatment with the primary alcohols ethanol and 1-propanol disassembles coated pits. Confocal section of BSC-1-σ2-EGFP imaged before and during (~3min) treatment with 2.5% v/v ethanol (top) or 2% v/v 1-propanol (bottom). Kymographs are shown on the right of each confocal section. (D) The secondary alcohol 2-propanol and tertiary alcohol t-butanol disassemble coated pits and vesicles. Confocal section of BSC-1-σ2-EGFP imaged before, during (~ 3min) treatment with 2.5% v/v 2-propanol (top) or t-butanol (bottom). Kymographs are shown on the right of each confocal section. Bar, 10 μm.
The disruptive effect of 1-butanol on the formation of coated pits and vesicles is very fast, leading to the complete loss of coated structures within 5–7 sec after alcohol addition. A representative kymograph for this experiment, performed with BSC1 cells simultaneously expressing σ2-EGFP and Tomato-LCa, shows the abrupt loss of both signals within seconds of exposing the cell to 2% 1-butanol (Fig. 3A and B). Not only is appearance of new pits prevented, but also maturation of pits exposed to 1-butanol during the growth phase stops, due to their rapid dissolution (Fig. 3A). We used alternate imaging by total internal reflection fluorescence (TIRF) and wide-field epifluorescence (EPI) illumination to follow the fate of dissolving pits labeled with σ2-EGFP (Fig. 3 C–F). Loss of the TIRF signal before loss of its corresponding EPI signal reflects the normal movement of the AP-2 signal as the mature coated vesicle moves towards the cell interior and out of the evanescent field, just before uncoating (Fig. 3 C and E, untreated). In contrast, both TIRF and EPI signals disappeared together in dissolving pits of cells treated with 1-butanol (Fig. 3 D and F, 1-butanol), indicating that dissolution occurred at the plasma membrane and did not involve invagination. Similar results were obtained in cells expressing EGFP-LCa (not shown). The disruptive effect of 1-butanol on coated pits requires presence of a membrane beneath the clathrin coat, as shown by absence of coat dissolution in cells first bathed with hypertonic medium to induce the formation of clathrin micro-cages lacking enclosed vesicles [27] and then treated with 1-butanol (Fig. 3 G, EGFP-LCa, sucrose).
Fig. 3. 1-butanol leads to the simultaneous disassembly of AP-2 and clathrin without coated vesicle internalization.
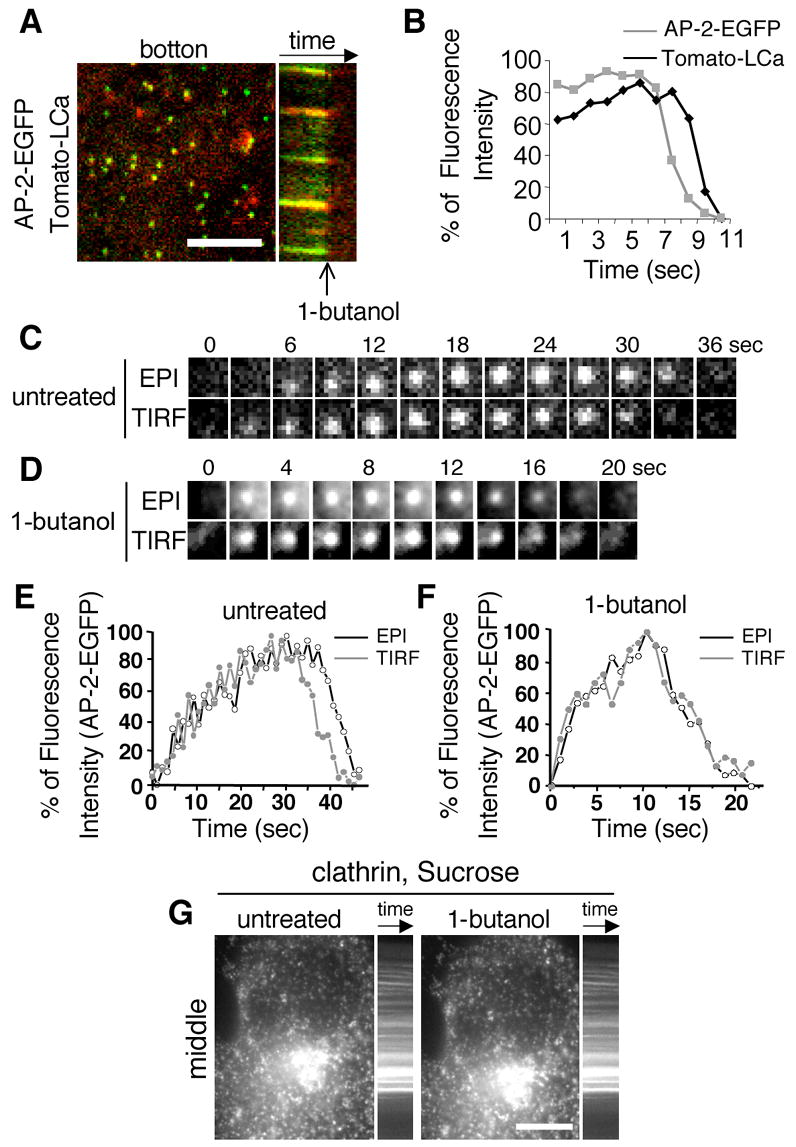
(A) Kymograph of the disassembly of the coated pits of a BSC-1 cell co-expressing σ2-EGFP (green) and Tomato-LCa (red) upon incubation with 2% 1-butanol. Bar 10 μm. (B) Alignment of the relative fluorescent intensity of clathrin (black line) and AP-2 (grey line) of the disassembling pits depicted in 2B. (C) Time-lapse series acquired under alternating wide field/epi-fluorescence and TIRF illumination of a typical AP-2 coated pit of an untreated cell. (D) Time-lapse acquisition under alternating wide field/epi-fluorescence and TIRF illumination of a typical AP-2 coated pit treated with 2% 1-butanol. (E) Graphic depiction of the florescent intensity profile of a typical AP-2 coated of an untreated cell; note the reduction in the TIRF signal intensity (black) prior to the ensuing reduction in the epifluorescence signal (grey). (F) Graphic depiction of the florescent intensity profile of a typical AP-2 coated (such as the one depicted in (C)) treated with 2% 1-butanol; note the concomitant reduction in epifluorescence (grey) and TIRF (black) signal intensities. (G) Confocal section of BSC1 cells stably expressing EGFP-LCa upon hypertonic treatment (0.45 M sucrose, which leads to the formation of clathrin microcages [27]). Cells were imaged before and after (~3min) treatment with 2% v/v 1-butanol. A kymograph of the locked pits is shown. Bars, 10 μm.
The acute effect of the 1-butanol treatment also resulted in loss of the punctate pattern characteristic of the clathrin adaptors AP-1 and AP-3 specific for the secretory pathway (Supplementary Fig. 1). In contrast, it had no detectable effect on the localization of proteins unlinked to clathrin coats such as caveolin-1-EGFP, β-COP-EGFP, ε-COP-EGFP and Rab22-EGFP (Supplementary Fig. 1). 1-butanol also had no significant effects on the localization of Arf1-EGFP, on the organization of the Golgi apparatus as monitored with GalT-EGFP, or on chimeras of EGFP fused to protein domains that are targeted to membranes by their binding preferences to phosphoinositide 3 phosphate (3x-FYVE-GFP; [28]) or phosphoinositide 3,4 biphosphate and phosphoinositide 3,4,5 triphosphate (PLC-γPH Domain-GFP (Supplementary Fig. 1;[29]). Thus, the inhibition of transferrin uptake induced by 1-butanol treatment is readily explained by the disappearance of endocytic clathrin coated pits and vesicles.
Coated pits and coated vesicles reform upon washout of 1-butanol
The inhibition of transferrin uptake produced by 1-butanol was reversed upon removal of the alcohol (Fig. 1 H), and this effect correlated with the reformation of clathrin coated pits and vesicles (Fig. 4). A burst of newly formed coated pits appeared within seconds of removing the 1-butanol from the medium, and all of these new pits matured to form coated vesicles. BSC-1 cells stably expressing EGFP-LCa or σ2-EGFP were first treated with 2% v/v 1-butanol for 1 min to remove all coated pits and vesicles. The medium bathing the cells was then exchanged with one lacking 1-butanol, and the cells were imaged for up to 5 min. Within 1 min after initiation of the washout period we observed a transient ~3 fold increase in the surface density of newly formed coated pits, which corresponded to a similar increase in their formation rate (Fig. 4 A–B and supplementary Movies 3–4); 3–5 min after the washout period began, the surface density, and hence the formation rate returned to the same value observed in untreated cells (Fig. 4 B). All newly formed coated pits matured to endocytic coated vesicles, regardless of when the coat started to assemble, with characteristics similar to those observed in untreated cells (Fig. 4 C–D and supplementary Movie 5). Coated vesicles formed 1 min after initiation of the washout period had shorter lifetimes and where correspondingly smaller (as determined by the maximum fluorescence intensity achieved just prior to the uncoating step [25]) than those formed in the absence of the alcohol (supplementary Fig. 2). The lifetimes and sizes of the coated vesicles formed subsequently were indistinguishable from those of untreated cells. We found similar disassembly and reformation of coated pits upon treatment with 1-butanol and after its washout, respectively, in U373mg astrocytes and in Hela cells stably expressing EGFP-LCa (not shown) or σ2-EGFP (Fig. 4 E and F; and supplementary Movie 6). The washout of the other primary alcohols ethanol and 1-propanol also led to synchronous reformation of coated pits and vesicles (Supplementary Fig. 3).
Fig. 4. Washout of 1-butanol results in a transient burst of coated pit nucleations.
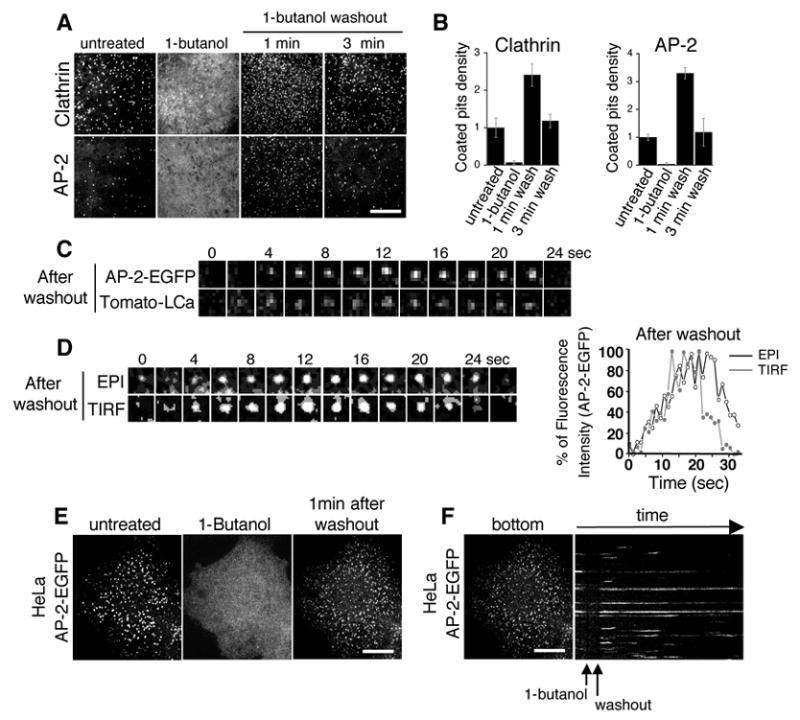
(A) BSC1-EGFP-LCa and BSC1-σ2-EGFP were incubated with 2% 1-butanol for 5 min. 1-butanol was washed out and cells were imaged after 1 and 3 minutes. Bar, 10 μm. (B) Graphic depiction of the relative coated pit density (normalized to the untreated level; from 3 independent experiments) under the experimental conditions described in (A). (C) Confocal time lapse acquisition of a typical coat formed after the 1-butanol washout in a cell co-expressing σ2-EGFP (green) and Tomato-LCa (red), note the concomitant recruitment, signal intensification and disappearance of AP-2 and clathrin. (D) Left: EPI-TIRF time lapse acquisition of a typical σ2-GFP coat formed after the 1-butanol washout. Right: Graphic depiction of the relative fluorescent intensities of a typical coated pit depicted immediately after 1-butanol washout under TIRF (grey) and EPI (black) illuminations, note the internalization, ie. a reduction in TIRF signal prior to the reduction in epifluorescence signal intensity. (E) HeLa cells stably expressing σ2-EGFP were incubated with 2% butanol for 5 min. Subsequently 1-butanol was washed out and cells were imaged after 1 and 3 minutes. Bar, 10 μm. Note the disassembly of all AP-2 containing structures and the increase in density and decrease in average intensity of AP-2 coated pits upon 1-butanol washout. (F) Kymograph representation of the cell depicted in (A), note the return of signal to preexisting structures as well as the new nucleations in previously unoccupied locations. Bar, 10 μm.
Liposomes containing phosphoinositide 4,5 bisphosphate allow the formation of coated pits and vesicles in cells treated with 1-butanol
If the loss of clathrin coated pit/vesicle assembly is a specific consequence of PIP2 depletion induced by the 1-butanol treatment, then replenishing cells with exogenous PIP2 in the presence of 1-butanol should rescue coated pit formation and endocytosis. We tested this hypothesis by treating BSC-1 cells stably expressing σ2-EGFP with 2% v/v 1-butanol for 1 minute to produce a complete loss of coated pits and vesicles. Subsequent imaging of the cells in the presence of the alcohol but after incubation with liposomes (50 μg/ml final concentration) containing a mixture of 5% PIP2, 45% phosphatidyl-ethanolamine (PE) and 50% phosphatidyl-choline (PC) showed a complete recovery of the formation of coated pits and vesicles, beginning as early as one min after exposure to the liposomes (Fig. 5 A and B and supplementary Movie 7). Coated vesicles that formed under this condition had an average lifetime (35 +/− 11 sec, Fig. 5 C), very similar to that found in the absence of the alcohol (supplementary Fig. 2). The control experiment, done by adding liposomes containing equimolar amounts of (PE) and (PC) but lacking PIP2 showed no recovery of coated pit and coated vesicle formation (Fig. 5 A and B, control liposomes). We conclude from these observations that the PIP2 delivered by the liposomes was sufficient to correct the biosynthetic defect of PIP2 elicited by 1-butanol, and that PIP2 is essential for clathrin coated pit and coated vesicle formation.
Fig. 5. Liposomes containing phosphoinositide 4,5 bisphosphate allow the formation of coated pits and vesicles in cells treated with 1-butanol.
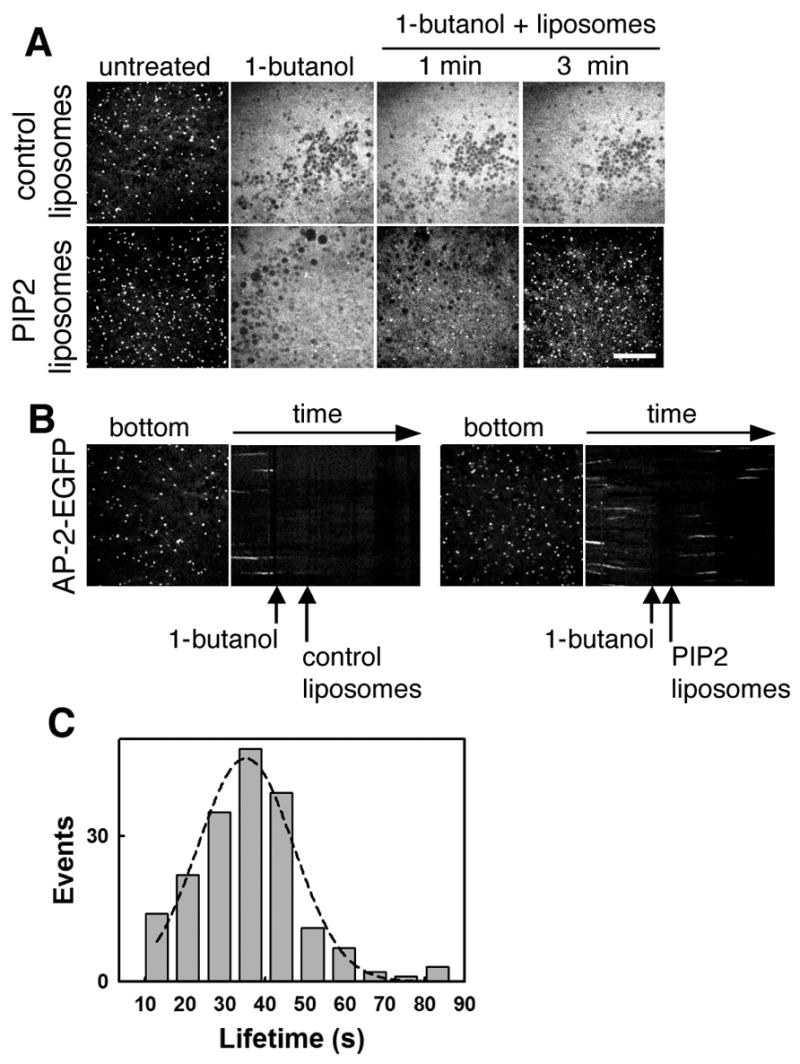
(A) Recovery of coated pit formation in the presence of PIP2 containing liposomes. BSC-1-σ2-EGFP cells were treated with 2% v/v 1-butanol for 1 minute. Cells were then incubated with liposomes (50 μg/ml final concentration) containing either equal amounts of PE and PC (upper panel) or 5% PIP2 in addition to 45% PE and 50% PC (lower panel) in the presence of 2% (v/v) 1-butanol; and imaged for an additional 3 minutes. Bar, 10 μm. (B) Kymograph representations of the cell depicted in (A). Note the return of new nucleations and coated vesicles formation in cells treated with PIP2 containing liposomes (right) but not with control liposomes (left). (C) Clathrin coated pits formed in the course of PIP2 addition on cells treated with 2% v/v 1-butanol have normal lifetime. The histogram plot displays the lifetimes of AP-2 spots calculated from the time-lapse depicted in A and B. The data derives from n=182 pits.
Inhibition of actin polymerization leads to formation of AP-2 clusters and a reduction in coated pits and vesicles
After verifying that 1-butanol treatment (up to 20 min) does not affect actin polymerization (Supplementary Fig. 4), we used the synchronization of coated pit formation after removal of 1-butanol as a tool to investigate further the role of actin polymerization in the endocytic event. Actin polymerization was inhibited by sequestering actin monomers with Latrunculin B (LatB) (Fig. 6) or Latrunculin A (LatA) (supplementary Fig. 5, left panels), or by capping the growing end of actin fibers with Cytochalasin D (CytoD) (supplementary Fig. 5 right panels). Incubation of BSC1 cells with LatB (2.5 μg/ml) resulted in a progressive accumulation of AP-2 structures that appeared larger than diffraction limit (highlighted in the bottom region of Fig. 6 A, LatB), indicating that they may correspond to clusters of coated pits or coated vesicles unresolved from each other, as previously suggested [19]. About 60% of the membrane-bound AP-2 had accumulated in these structures (Fig. 6 A and B) after 15 min of incubation with LatB (a time at which no more actin stress fibers could be detected, Supplementary Fig. 3, LatB). The AP-2 content of the majority of these structures was 3-4 times higher than the amount contained in the largest coated vesicles formed during normal conditions (Fig. 6 B). At this point, we also observed ~50% reduction in the formation rate of new coated pits (Fig. 6 C). The growth characteristics of the remaining pits and vesicles as defined by their maximum size and lifetime remained unchanged, however, even when the cells were exposed to LatB for 25 min. (Fig. 6 D, supplementary Movie 8). We observed a similar accumulation of relatively static coat structures paralleled by the progressive decrease of coated pits and vesicles, which nonetheless exhibited normal dynamics, in cells extensively treated with LatA or Cytocholasin D (supplementary Fig. 5).
Fig. 6. Actin depolymerization with LatB does not affect the disassembly of coated pits by 1-butanol, their reappearance upon 1-butanol washout or the dynamic parameters of individual pits.
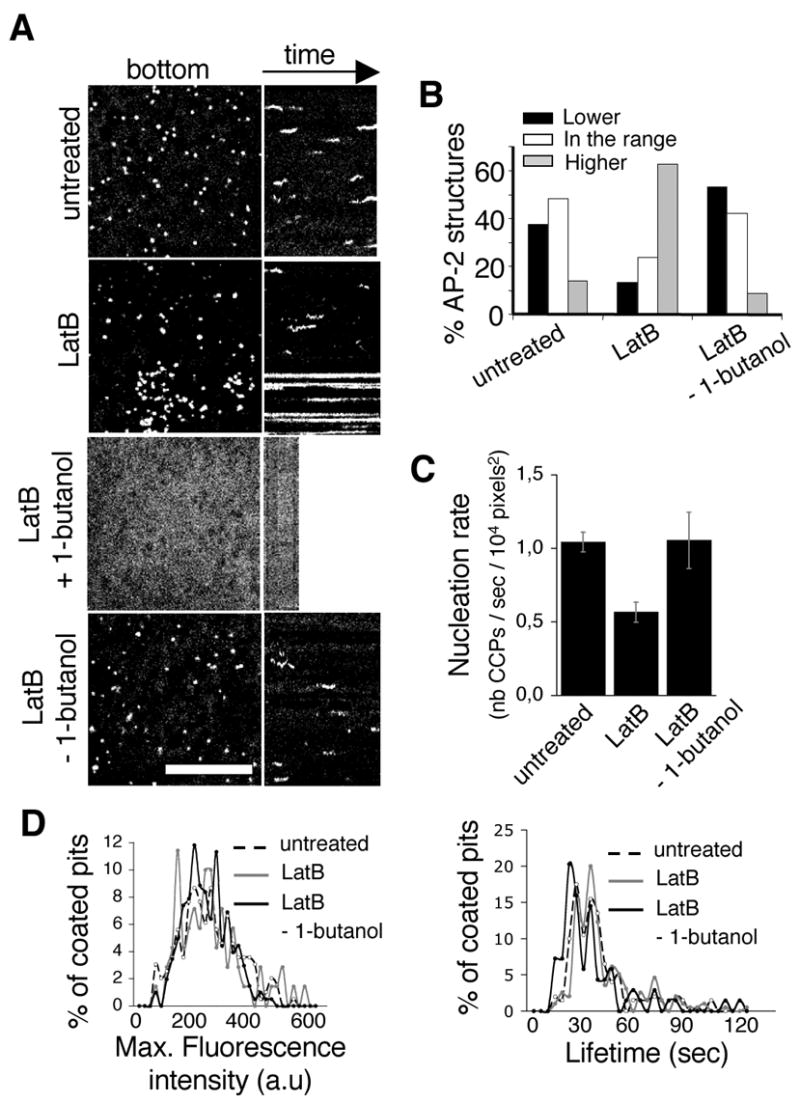
(A) Kymograph of BSC1-σ2-EGFP cell prior to incubation with LatB (“untreated”); 15 min after the addition of 2.5 μg/ml LatB (“LatB”); during the incubation with 2% 1-butanol +2.5 μg/ml LatB (“LatB + 1-butanol”); and during the washout of 2% 1-butanol in the presence of 2.5 μg/ml LatB (“LatB – 1-butanol”). Note the static, high-intensity objects in the LatB treated cell as well as the absence of AP-2 pits in the beginning of the 1-butanol washout and their dynamic nature thereafter. Bar, 10 μm. (B) Graphic representation of the profile of intensity of AP-2 structures at any given frame (snapshots) in “untreated” cells (n=112), “LatB” cells (n=236) and “LatB – 1-butanol” cells (n=136). Pits were divided in 3 classes: under the range of the maximum intensity of dynamic pits (‘Lower’, black columns), in the range of the maximum intensity of dynamic pits (white columns), and over the range of the maximum intensity of dynamic pits (‘Higher’, grey columns). Note the similar profiles of “untreated” and “LatB – 1-butanol” cells in contrast to the accumulation of large structures in the LatB treated cells. (C) Nucleation rate of dynamic pits (number of clathrin coated pits/sec/104 pixels2) in untreated (left, n=196), “LatB” (n=72), or “LatB – 1-butanol” cells (n=203). (D) Distribution of the maximum intensities and lifetimes of dynamic pits in cells: untreated (dashed line, n=196), “LatB” (grey, n=72) and “LatB – 1-butanol” (black, n=203). Note the similarity of the profiles of the parameters of dynamic pits in all three conditions.
Depolymerization of the actin cytoskeleton affects coated motility and alters the spatial distribution of coated pit formation
As reported by Gaidarov et al. [30], depolymerization of actin had a marked effect on the movement of coated pits. In control cells, coated pits, tracked in their growing phase (initial 20s), displayed an average immediate velocity (Euclidian displacement of the centroid divided by time) of 0.032 +/− 0.011 μm/s (Fig. 7 A). The displacement of the coated pits was linearly correlated with the square root of time, as is characteristic of a two-dimensional random walk (Fig. 7 B and supplementary Fig. 6B and D); the overall displacement of ~150 nm was well within the endocytic permissive zones previously observed in BSC1 cells [25]. Inhibition of actin polymerization by LatB (Fig. 7 A), LatA or CytoD treatment (supplementary Fig. 6 A and C) led to increases in both the average immediate velocity of coated pits (to ~ 0.087 +/− 0.017 μm/s) and their average displacement, which became ~ 500 nm (Fig. 7 B and supplementary Fig. 6 B and D), but this motions were correlated within neighborhoods, as if due to some concerted displacement of the plasma membrane. Furthermore, depolymerization of the cytoskeleton led to an increase in the clustering of sites of formation of coated pits. In the absence of polymerized actin there is an increase in the probability of formation of an additional coated pit at a given distance from any coated pit (as measured by the Ripley L function calculated with PASSAGE software, [31]), over an increased radius (Fig. 7 C) These observations are consistent with the dissolution of local barriers in the context of the maintenance of the overall ratio of areas in which coated pits can or cannot form.
Fig. 7. Actin depolymerization abrogates the upregulation in nucleation resulting from removal of 1-butanol.
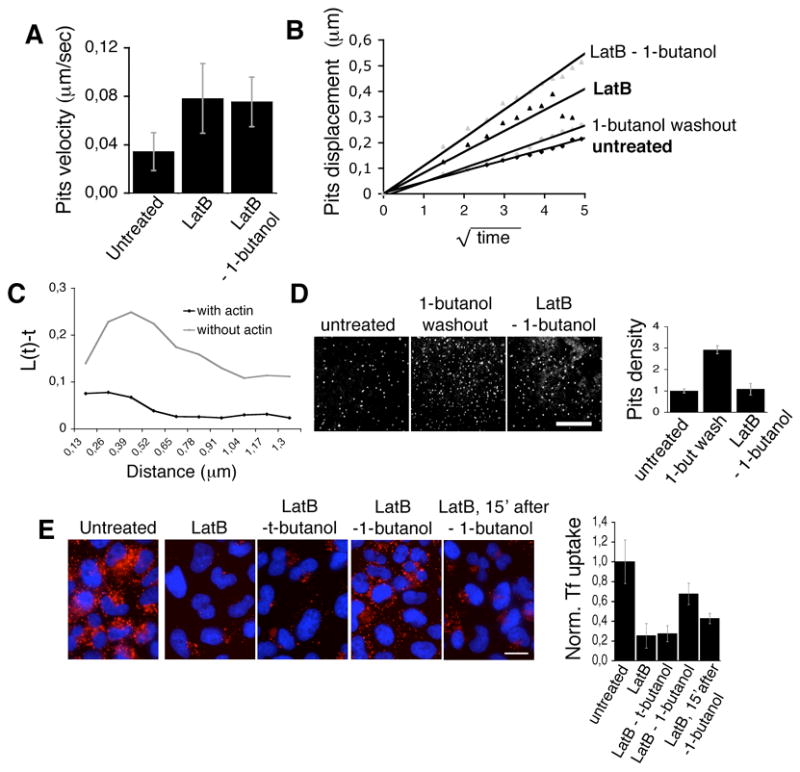
(A) Lateral displacement of dynamic coated pits (μm/sec) in untreated (left, n=55), “LatB” (middle, n=52) and “LatB – 1-butanol” (right, n=56). Note the similar lateral displacement of the pits upon depolymerized actin irresepective of the 1-butanol treatment. (B) Displacement of dynamic coated pits in untreated (black diamonds), 1-butanol washout” cells (gray diamonds), “LatB” cells (black triangles) and “LatB – 1-butanol” cells (gray triangles). (E) Ripleys L function of the clustering tendencies of the points of origin of clathrin coated pits in cells in which the actin cytoskeleton was intact (lower curve) or in which the actin cytoskeleton was depolymerized (upper curve); given the fact that the 1-butanol washout treatment had no effect on the clustering tendencies (data not shown), results from cells in washout or untreated conditions were compiled together. (D) Confocal image of BSC1-σ2-EGFP cells, untreated, during the burst of nucleation following 1-butanol washout or “ LatB – 1-butanol” cells. (Bar, 10 μm) and graphic depiction of the relative coated pit density (normalized to the untreated level; from 3 independent experiments). (E) Transferrin uptake in Hela cells following LatB and butanol treatments. Hela cells were pre-incubated at 37° C for 15 min with imaging medium (untreated, left) or medium containing LatB (2.5 μg/ml). Cell samples were then incubated for 2 min with the resepective combinations of LatB and alcohols. The alcohol was washed out in the presence of LatB for 2 or 15 minutes, after which cells were incubated with 10 μg/ml Alexa594-Tf for 2 min at RT and 5 min at 37°C in PBS/Glucose/BSA (PBS-GB; untreated), or PBS-GB + LatB. Cells were subsequently transferred to 4°C, acid washed and fixed. Bar, 20 μm. (Right) Bar plot illustrates the quantitation of Tf uptake in the conditions depicted, normalized to the level of untreated cells.
Normal coated pits and vesicles reform in the absence of polymerized actin upon 1-butanol washout
The variable effects of actin disassembly on coated pit and coated vesicle formation have made it difficult to reach a simple interpretation linking the actin and clathrin systems. Therefore, we took advantage of the significantly more rapid effects of 1-butanol on coated pit and coated vesicle dynamics as a way to facilitate the analysis. As in control cells, we found that 1-butanol led to rapid and complete disappearance of AP-2 structures in cells pre-incubated with LatB (Fig. 6 A). When the 1-butanol was removed from cells in the continued presence of the actin polymerization inhibitory compounds new coated pits and vesicles promptly appeared (Fig. 6 A). The dynamics of these assembling pits and vesicles were indistinguishable from the corresponding values determined in control cells that had never been treated with 1-butanol or with actin-depolymerizing agents (Fig. 6 D and supplementary Movie 9); the surface density of the newly formed coated pits did not show the transient ~3 fold increase observed in cells treated only with 1-butanol (Fig. 7 D). The large patches of AP-2, which normally appear 10–20 minutes in the presence of actin depolymerizing drugs (see Fig. 6 A, LatB), were completely absent during the first minutes after the onset of the 1-butanol washout, despite the absence of polymerized actin; eventually these structures reappeared (supplementary Movie 9). The transient recovery of coated pit and coated vesicle formation just described corresponded to reactivation of a functional clathrin-based endocytic pathway, as determined by following the receptor-mediated uptake of transferrin (Fig. 7 E). The uptake of transferrin, which was partially inhibited in the presence of LatB, recovered to 60–80% of control values during the first minutes of the 1-butanol washout period, followed by a sharp reduction 15 min later if LatB remained in the medium. A similar experiment using t-butanol did not activate the uptake of transferrin after washing the alcohol (Fig. 7 E), as expected for a compound that has no effect on coated pit and vesicle formation.
DISCUSSION
We have addressed here the role of PIP2 and actin on coated pit and coated vesicle formation, using as an experimental tool the rapid and acute effect of primary alcohols on assembly and disassembly of endocytic clathrin structures. The primary alcohols, 1-butanol, 1-propanol and ethanol, all had the same three effects on the formation of clathrin coats at the plasma membrane: (1) they induced rapid disassembly of existing coated pits; (2) they inhibited formation of new ones; (3) they removal caused a transient increase in the number of new coated pits and vesicles that formed immediately after washout. The first two effects are probably caused by specific interference with the activity of PLD and to a lesser extent by more general inhibitory effects related to alcohols in general. The third effect, significant up-regulation of the formation of new pits, probably comes from reversing the inhibition of PLD activity. Exposure of PLD to primary alcohols results in the production of phosphatidyl alcohol instead of PA, thus creating an imbalance in the production of PIP2 mediated by PI5K, itself activated by PA. We propose that the resulting acute reduction in PIP2 (or of other phosphoinositides whose biosynthesis is linked to PIP2) not only impairs the nucleation and recruitment of coat components required to initiate and sustain coated pit assembly but also promotes dissolution of coat components from pits before they complete assembly into mature coated vesicles. In support of a direct role of PIP2 in coat formation we show that in the continued presence of 1-butanol, addition of PIP2 containing liposomes leads to uptake of PIP2 and rapid recovery of normal coated structures.
A number of proteins that regulate coat formation are known to interact with phosphoinositides. The best characterized are the tetrameric AP-2 and the monomeric CALM clathrin adaptors, present in all endocytic coated pits and vesicles. Their interactions with PIP2 are mediated by lipid binding sites located at the N-terminus of α-adaptin and of CALM, respectively; mutations in these lipid binding sites reduce their targeting to clathrin coated pits and vesicles. A decrease in PIP2 levels could therefore reduce the ability to recruit AP-2 or CALM (or some of other protein) to sites of coat assembly. Dissolution of coats, through inhibition of PLD by 1-butanol, probably reflects a release of AP-2 from the membrane together with clathrin triskelions bound to them; it is unlikely that such dissolution occurs by an activation of the standard uncoating process, however. Standard uncoating sustained by coated vesicles requires Hsc70 and its co-factor auxilin, a process marked by a burst in the recruitment of auxilin to fully assembled coated vesicles just prior to their uncoating [32, 33]; in contrast, auxilin is not recruited to disappearing coated pits in cells treated with 1-butanol (not shown).
Knockdown by RNAi of PLD2 inhibits transferrin recycling from endosomes back to the cell surface, but knockdown of PLD1 or PLD2 does not prevent transferrin internalization [34]. It is hard to correlate these observations directly with the data presented here, as the RNAi experiments were performed in a much longer time frame (days) than ours (minutes). It is possible, for example, that cells exposed to a long term perturbation in their PLD activity respond by upregulating other enzymes to compensate for the defect; indeed such compensation has been observed upon depletion by RNAi of different PIP5K isoforms [6].
The onset of the reversal in the inhibition of coated pit assembly is rapid and starts 5–7 sec after removal of the primary alcohols; the frequency of new coated pits appearing during the first minutes of the reversal is always higher than in cells untreated cells. This transient increase can be explained by an overshoot in the amount of available PIP2 at the onset of the wash period. The temporary increase of PIP2 could be generated, if for example, PI4P, the substrate of PIP5K, accumulated during the 1-butanol treatment because of lack of consumption by inactive PIP5K; upon removal of 1-butanol, PIP5K is stimulated again by freshly synthesized PA, leading to a temporary increase in the amount of PIP2. This model has been proposed as an explanation for the overshoot in actin dynamics observed in Dictyostelium upon removal of 1-butanol [35].
Previous studies focused on understanding the role of actin in coated pit and coated vesicle formation have linked actin dynamics with clathrin function [6, 16–19, 23, 24, 30, 36–38]. Highlight of these studies include: (1) transient capture of actin particularly at late stages in the formation of a coated pit/vesicle Yarar, 2005 #45;Merrifield, 2002 #19;Merrifield, 2005 #20}; (2) co-localization of actin binding proteins such as cortactin, Hip1R and N-WASP [39] with clathrin structures but only partial inhibition of endocytosis during RNAi knockdown of these proteins [36, 37, 40]; (3) variable inhibition of transferrin uptake and clathrin dynamics in cells treated with compounds that interfere with actin dynamics [18, 19, 23, 24, 36].
We have confirmed that treatment of cells with agents that prevent actin dynamics hinder assembly of clathrin coated pits and vesicles and endocytosis of transferrin. We find that the inhibitory effects are progressive: they become more apparent with longer exposure to the actin-polymerization inhibiters. If actin polymerization is required for coated vesicle formation, we expected that cells first treated for an extensive period with LatB, in order to achieve a severe block in coated pit assembly, followed by 1-butanol, to eliminate the remaining pits, would not form new coated pits and vesicles upon removal of the alcohol. Instead, we observed exactly the opposite: recovery of transferrin uptake, accompanying the reappearance of new coated pits, which matured to form coated vesicles following normal assembly dynamics. The period of efficient coated pit formation lasted several minutes, until the cells eventually reverted to the regime with decreased frequency of new pits and reappearance of clathrin/AP-2 clusters, as observed under conditions of extensive actin depolymerization. These results lead us to conclude that actin does not have an essential role in any of the events required to form a coated vesicle, including nucleation, assembly of the coat, budding of the underlying membrane to form the mature coated vesicle, and uncoating. Nonetheless, sustained coat assembly fails in the absence of a functional actin cytoskeleton. Relaxation of barriers by extensive actin depolymerization creates a new situation with large patches of membrane accessible to coat assembly, but where the local lipid concentration is lower than the required threshold to ensure efficient nucleation and capture of coat components. This adjustment would result in a decrease in the frequency of coated pits, and those that form would start at localized regions with sufficient local accumulation of lipids to sustain assembly as detected by the increase in pit clustering. Relaxation of the actin barriers has the additional effect of reducing the frequency of new coats appearing during the early stages of alcohol washout, as expected if the same amounts of lipids, but at a lower concentration, are recovered in a larger unconstrained region.
It is thought that the actin cytoskeleton helps define regions of constrained lateral diffusion for proteins and lipids at the plasma membrane by imposing actin-based barriers [41]. Presence of such corrals might impose a diffusion barrier to phosphoinositides generated locally, thus favoring an increase in their local concentration that might facilitate recruitment of lipid-binding coat components.
In BSC1 cells, the regions where assembly of coated pits occurs can be modeled by permissive zones of 200 to 400 nm in diameter, surrounded by rings of 100 nm in width that are inactive [25]. Similar active regions are observed in many types of cells in culture, including Hela, COS, U373mg astrocytes [25, 33], as well as in primary adipocytes [42]. Our modeling is consistent with electron microscopic observations that show the presence of endocytic zones, which contain pits, intercalated with regions rich in cytoskeleton and devoid of coated pits [24]. Santini and colleagues showed that G-protein coupled receptor activation generated a localized increase within, “coated pit zones”, in the number of coated pits, a phenomenon that was dependent on the presence of a functional actin cytoskeleton [43]. Relaxation of barriers by extensive actin depolymerization creates a new situation with large patches of membrane accessible to coat assembly, but where the local lipid concentration is lower that a required threshold to ensure efficient nucleation and capture of coat components. This adjustment would result in a decrease in the frequency of coated pits, and those that form would start at localized regions with sufficient local accumulation of lipids to sustain assembly as detected by the increase in pit clustering.
Supplementary Material
Supplementary Fig. 1
Confocal section of BSC1 cells stably expressing σ1-EGFP, σ3-EGFP, Caveolin1-EGFP, GalT-EGFP, Arf1-EGFP, β -COP, ε -COP or Rab22-EGFP or BSC1 cells transiently expressing PLC-γ– PH Domain-EGFP or 3x-FYVE-EGFP. Cells were imaged before and after (∼ 3min) treatment with 2% v/v 1-butanol. The fluorescence along the z-axis is shown on top of each confocal section. Note that treatment with 2% v/v 1-butanol affects only the localization of AP-1 and AP-3. Bars, 10 μm.
Supplementary Fig. 2
Clathrin coated pits formed in the course of the 1-butanol washout have normal dynamic parameters of size and lifetime. The graphic distribution compares maximum fluorescence intensities (determined just prior to the uncoating of coated vesicles) and lifetimes of AP-2 spots calculated from time-series acquired before incubation with 1-butanol, or 1 min or 5 min after 1-butanol washout. BSC-1 cells stably expressing σ2-EGFP were incubated with 2% 1-butanol for 5 min. Subsequently 1-butanol was washed out and cells were imaged after 1 and 5 minutes. The data derives from 5 cells (n=932, 1974 and 707 pits for before 1-butanol application, and 1 min and 5 min after washout respectively).
Supplementary Fig. 3
Ethanol and 1-propanol (but not 2-propanol) disassemble coated pits and the removal of these primary alcohols results in a transient burst of coated pit nucleations. Confocal section of BSC-1-σ2-EGFP imaged before, during (∼ 3min) treatment with 2.5% v/v ethanol (A), 2% v/v 1-propanol (B) or 2-propanol (C) and after washout. Kymographs are shown on the right of each confocal section**.** Note the effect of the primary alcohols ethanol and 1-propanol but not of the secondary alcohol 2-propanol. Bar, 10 μm.
Supplementary Fig. 4
1-butanol treatment and washout do not affect actin polymerization. (A) Phalloidin staining of BSC1 cells fixed after incubation with media alone, 2% v/v 1-butanol or during the washout. (B) Phalloidin staining of in “LatB treated”, “LatB + 1-butanol” and “LatB – 1-butanol” cells. (C) Phalloidin staining of in “LatA treated”, “LatA + 1-butanol” and “LatA – 1-butanol” cells. (D) Phalloidin staining of in “CytoD treated”, “CytoD + 1-butanol” and “CytoD – 1-butanol” cells. Note the intact polymerized actin network in both conditions.
Supplementary Fig. 5
Actin depolymerization with Latrunculin A or Cytochalasin D does not affect the dissolution of coated pits by 1-butanol nor the nucleation resulting from its washout. (A) Kymograph depictions of a BSC1-σ2-EGFP cell prior to incubation with LatA (untreated), 15 min after the addition of 1 μg/ml LatA (“LatA”) during the incubation with 2% 1-butanol in the presence of 1 μg/ml LatA (“LatA + 1-butanol”) and during the washout of 2% 1-butanol in the presence of 1 μg/ml LatA (“LatA – 1-butanol”). Note the static, high-intensity objects in the Latrunculin A treated cell as well as the absence of AP-2 pits in the beginning of the washout and their dynamic nature thereafter. Bar, 10 μm. (B) Graphic representation of the profile of intensity of AP-2 structures at any given frame (snapshots) in “untreated” cells (n=212), “LatA treated” cells (n=211) and “LatA – 1-butanol” cells (n=196). Pits were divided in 3 classes: under the range of the maximum intensity of dynamic pits ('Lower', black columns), in the range of the maximum intensity of dynamic pits (white columns), and over the range of the maximum intensity of dynamic pits ('Higher', grey columns). Note the similar profiles of “untreated” and “LatA – 1-butanol” cells in contrast to the accumulation of large structures in the LatA treated cells. (C) Distribution of the maximum intensities and lifetimes of dynamic pits in untreated (dashed line, n=99), “LatA treated” (grey, n=52) and “LatA – 1-butanol” cells (black, n=47). (D) Kymograph depictions of a BSC1-σ2-EGFP cell prior to incubation with CytoD (untreated), 15 min after the addition of 25 μg/ml CytoD (“CytoD”) during the incubation with 2% 1-butanol in the presence of 25 μg/ml CytoD (“CytoD + 1-butanol”) and during the washout of 2% 1-butanol in the presence of 25 μg/ml CytoD (“CytoD – 1-butanol”). Note the static, high-intensity objects in the CytoD treated cell as well as the absence of AP-2 pits in the beginning of the washout and their dynamic nature thereafter. Bar, 10 μm. (E) Graphic representation of the profile of intensity of AP-2 pits at any given frame (snapshots) in untreated cells (n=224 pits), “CytoD” cells (n=114 pits) and “CytoD – 1-butanol” cells (n=155 pits). Pits were classified as in (B) (F) Distribution of the maximum intensities and lifetimes of dynamic pits in untreated (dashed line, n=165), “CytoD” cells (grey, n=134) and “CytoD – 1-butanol” cells (black, n=236).
Supplementary Fig. 6
(A) Lateral displacement of dynamic pits (μm/sec) in untreated (left, n=72), “LatA treated” (middle n=79) and “LatA – 1-butanol” cells (right, n=70). Note the similar lateral displacement of the pits during 1-butanol washout in the presence of LatA. (B) Displacement of dynamic coated pits in untreated (black diamonds), “LatA treated” cells (black triangles) and “LatA – 1-butanol” cells (grey triangles). (C) Lateral displacement of dynamic pits (μm/sec) in untreated (left, n=65), “CytoD” cells (middle n=77) and “CytoD – 1-butanol” cells (right, n=73). Note the similar lateral displacement of the pits during 1-butanol washout in the presence of CytoD. (D) Displacement of dynamic coated pits in untreated (black diamonds), “CytoD treated” cells (black triangles) and “CytoD – 1-butanol” cells (grey circles). (E) Confocal image of BSC1-σ2-EGFP cells, untreated, during the burst of nucleation following 1-butanol washout or “ LatA – 1-butanol” or “CytoD – 1-butanol” cells. (Bar, 10 μm) and graphic depiction of the relative coated pit density (normalized to the untreated level; from 3 independent experiments). (F) Transferrin uptake is partially inhibited in cells incubated with LatA or CytoD but returned to normal levels in the first minutes following the washout of 1-butanol but not t-butanol. Hela cells were pre-incubated at 37° C for 15 min with imaging medium (untreated, left) or medium containing LatA (1 μg/ml) or CytoD (25μg/ml). Cell samples were then incubated for 2 min with the respective combinations of the actin depolymerizing drugs and alcohols. The alcohol was washed out in the presence of LatA or CytoD for 2 or 15 minutes, after which cells were incubated with 10 μg/ml Alexa594-Tf for 2 min at RT and 5 min at 37°C in PBS/Glucose/BSA (PBS-GB; untreated), or PBS-GB + LatB. Cells were subsequently transferred to 4°C, acid washed and fixed. Bar, 20 μm. (G) Bar plot illustrates the quantitation of Tf uptake in the conditions depicted in (A), normalized to the level of untreated cells.
Supplementary Fig. 7
Model; Normal condition (top). At the plasma membrane, phosphatidylcholine (PC) is transphosphatidylated to phosphatidic acid (PA) by phospholipase D2 (PLD2). PA stimulates the lipid kinase PIP5K which phosphorylates PI(4)P, leading to the localized production of PIP2. A localized concentration of PIP2 and coat components, enabled by the organizing presence of the actin cytoskeleton initiates the cascade of molecular interactions which culminate in coated pit nucleation. Primary alcohols, like 1-butanol, compete with water in the transphosphatidylation reaction of PC and lead to the production of phosphatidylbutanol (P-But) in place of PA. Subsequently, in the absence of PA, PI5K is not stimulated, resulting in an abrupt drop in the levels of PIP2. In the absence of PIP2, the cell is unable to support the nucleation/maintenance of coated pits. While this is our current working model, the formal demonstration of each of the proposed steps will be the carried out in future work.
Supplementary Movie 1
Effect of 1-butanol on the dynamic behavior of coated pits and vesicles labeled with EGFP-LCa. Time-lapse series (70 frames, ∼ 2,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing EGFP-LCa using the spinning disk confocal microscope, 2% v/v 1-butanol was added at frame 25. Note that upon addition of 1-butanol the punctuate pattern, characteristic of the membrane distribution of coated pits, was radically altered to a diffuse distribution of clathrin.
Supplementary Movie 2
Effect of 1-butanol on the dynamic behavior of coated pits and vesicles labeled with σ2-EGFP Time-lapse series (50 frames, ∼ 3,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope, 2% v/v 1-butanol was added at frame 15. Note that upon addition of 1-butanol the punctuate pattern, characteristic of the membrane distribution of coated pits, was radically altered to a uniform distribution of AP-2.
Supplementary Movie 3
Synchronized appearance of coated pits labeled with EGFP–LCa upon removal of 1-butanol. Time-lapse series (150 frames, ∼ 2,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing EGFP-LCa using the spinning disk confocal microscope (continuation of Supplementary Movie 1). The washout of the 2% v/v 1-butanol was performed at frame 18. Note the synchronized burst of coated pit nucleations upon the washout of 1-butanol.
Supplementary Movie 4
Synchronized appearance of coated pits labeled with σ2-EGFP upon removal of 1-butanol. Time-lapse series (80 frames, ∼ 3,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope (continuation of Supplementary Movie 2). The washout of 2% v/v 1-butanol was performed at frame 5. Note the synchronized burst of coated pit nucleations upon the washout of 1-butanol.
Supplementary Movie 5
Synchronized appearance of coated pits labeled with σ2-EGFP and Tomato-LCa upon removal of 1-butanol. Time-lapse series (110 frames, ∼ 2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing σ2-EGFP and transiently expressing Tomato-LCa using the spinning disk confocal microscope. The washout of the 2% v/v 1-butanol was performed at frame 25. Note the synchronized burst of coated pit nucleations containing AP-2 and clathrin upon the washout of 1-butanol.
Supplementary Movie 6
Effect of 1-butanol application and washout on the dynamic behavior of coated pits and vesicles labeled with σ2-EGFP in HeLa cells. Time-lapse series (120 frames, ∼ 2,2 s intervals acquired at 37°C) obtained from a HeLa cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope. 2% v/v 1-butanol was added at frame 2 and washed out at frame 25 without stop of the capture. Note that the objects that are larger than the diffraction limit as well as coated pits were dispersed upon application of 2% v/v 1-butanol and that upon washout of 1-butanol, in addition to a return of AP-2 and clathrin to the same large structures, coated pits formed de-novo at new sites on the remaining membrane areas.
Supplementary Movie 7
Addition of liposomes containing PIP2 in the presence of 1-butanol rescues the formation of clathrin coated pits and vesicles. Time-lapse series (225 frames acquired at 37°C, ∼ 2 s intervals) obtained from BSC-1 cells stably expressing σ2-EGFP. Cells were treated with 2% v/v 1-butanol for 1 minute (added at frame 30) to produce a complete loss of coated pits and vesicles. Liposomes (50 μg/ml final concentration) containing a mixture of 5% PIP2, 45% phosphatidyl-ethanolamine (PE) and 50% phosphatidylcholine (PC) were then added (frame 65) to the medium still containing 1-butanol. This addition resulted in a complete recovery of the formation of coated pits and vesicles, beginning as early as 1 min after exposure to the liposomes.
Supplementary Movie 8
Effect of Latrunculin B on the dynamic behavior of coated pits and vesicles labeled with σ2-EGFP. Time-lapse series (120 frames, ∼ 2,2 s intervals acquired at 37°C) was initiated 15 min after addition of 2.5 μg/ml LatB and was obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope. Note the accumulation of large static structures but also coated pits with similar dynamic behavior to those of untreated cells.
Supplementary Movie 9
Effect of 1-butanol, in presence of Latrunculin B, on the dynamic behavior of coated pits and vesicles with σ2-EGFP. Time-lapse series (80 frames, ∼2.2 s intervals acquired at 37°C) was initiated 25 min after addition of 2.5 μg/ml LatB was obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope. 2 % v/v 1-butanol were added at frame 5 and washed out at frame 40. Note that both the larger structures induced by Latrunculin as well as the remaining coated pits were dispersed by incubation with 1-butanol applied in the presence of Latrunculin, followed by the resumption of new coated pit nucleations after removal of 1-butanol.
Acknowledgments
We thank Dr. Tal Pupko for helping with the clustering analysis. Supported by NIH GM 075252 and GM62566 (T.K); EMBO Long Term Fellowship (E.B.); American Heart Foundation (S.S.); Dorot Fellowships and Fulbright Foundation (M.E.). We acknowledge the generous support of the Perkin Fund to purchase part of the imaging equipment used here for live cell imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans. 2005;33:1285–9. doi: 10.1042/BST0331285. [DOI] [PubMed] [Google Scholar]
- 2.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 3.Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–7. [PubMed] [Google Scholar]
- 4.Gaidarov I, Keen JH. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146:755–64. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci U S A. 2006;103:11934–9. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padron D, Wang YJ, Yamamoto M, Yin H, Roth MG. Phosphatidylinositol phosphate 5-kinase Ibeta recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J Cell Biol. 2003;162:693–701. doi: 10.1083/jcb.200302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones DR, Sanjuan MA, Merida I. Type Ialpha phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett. 2000;476:160–5. doi: 10.1016/s0014-5793(00)01702-6. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Xu L, Foster DA. Role for phospholipase D in receptor-mediated endocytosis. Mol Cell Biol. 2001;21:595–602. doi: 10.1128/MCB.21.2.595-602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch T, Brandenburg LO, Liang Y, Schulz S, Beyer A, Schroder H, Hollt V. Phospholipase D2 modulates agonist-induced mu-opioid receptor desensitization and resensitization. J Neurochem. 2004;88:680–8. doi: 10.1046/j.1471-4159.2003.02189.x. [DOI] [PubMed] [Google Scholar]
- 10.Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell. 2004;15:1024–30. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris AJ, Frohman MA, Engebrecht J. Measurement of phospholipase D activity. Anal Biochem. 1997;252:1–9. doi: 10.1006/abio.1997.2299. [DOI] [PubMed] [Google Scholar]
- 12.Arneson LS, Kunz J, Anderson RA, Traub LM. Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J Biol Chem. 1999;274:17794–805. doi: 10.1074/jbc.274.25.17794. [DOI] [PubMed] [Google Scholar]
- 13.Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathre P, Shome K, Blumental-Perry A, Bielli A, Haney CJ, Alber S, Watkins SC, Romero G, Aridor M. Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. Embo J. 2003;22:4059–69. doi: 10.1093/emboj/cdg390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 17.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–8. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 18.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–75. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyman T, Shmuel M, Altschuler Y. Actin is required for endocytosis at the apical surface of Madin-Darby canine kidney cells where ARF6 and clathrin regulate the actin cytoskeleton. Mol Biol Cell. 2006;17:427–37. doi: 10.1091/mbc.E05-05-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–5. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 22.Moskowitz HS, Heuser J, McGraw TE, Ryan TA. Targeted chemical disruption of clathrin function in living cells. Mol Biol Cell. 2003;14:4437–47. doi: 10.1091/mbc.E03-04-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskowitz HS, Yokoyama CT, Ryan TA. Highly cooperative control of endocytosis by clathrin. Mol Biol Cell. 2005;16:1769–76. doi: 10.1091/mbc.E04-08-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–71. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Massol RH, Larsen JE, Fujinaga Y, Lencer WI, Kirchhausen T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol Biol Cell. 2004;15:3631–41. doi: 10.1091/mbc.E04-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenmark H, Aasland R, Driscoll PC. The phosphatidylinositol 3-phosphate-binding FYVE finger. FEBS Lett. 2002;513:77–84. doi: 10.1016/s0014-5793(01)03308-7. [DOI] [PubMed] [Google Scholar]
- 29.Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- 30.Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg MS. PASSAGE. 2001. Pattern Analysis, Spatial Statistics, and Geographic Exegesis. Version 1.1.2.3. Tempe, AZ. [Google Scholar]
- 32.Lee DW, Wu X, Eisenberg E, Greene LE. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci. 2006;119:3502–12. doi: 10.1242/jcs.03092. [DOI] [PubMed] [Google Scholar]
- 33.Massol RH, Boll W, Griffin AM, Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A. 2006;103:10265–70. doi: 10.1073/pnas.0603369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padron D, Tall RD, Roth MG. PLD2 Is Required for Efficient Endocytic Recycling of Transferrin Receptors. Mol Biol Cell. 2005 doi: 10.1091/mbc.E05-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zouwail S, Pettitt TR, Dove SK, Chibalina MV, Powner DJ, Haynes L, Wakelam MJ, Insall RH. Phospholipase D activity is essential for actin localization and actin-based motility in Dictyostelium. Biochem J. 2005;389:207–14. doi: 10.1042/BJ20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–17. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- 37.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–70. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schafer DA. Coupling actin dynamics and membrane dynamics during endocytosis. Curr Opin Cell Biol. 2002;14:76–81. doi: 10.1016/s0955-0674(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 39.Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–15. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- 40.Engqvist-Goldstein AE, Zhang CX, Carreno S, Barroso C, Heuser JE, Drubin DG. RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol Biol Cell. 2004;15:1666–79. doi: 10.1091/mbc.E03-09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–81. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellve KD, Leonard D, Standley C, Lifshitz LM, Tuft RA, Hayakawa A, Corvera S, Fogarty KE. Plasma membrane domains specialized for clathrin-mediated endocytosis in primary cells. J Biol Chem. 2006;281:16139–46. doi: 10.1074/jbc.M511370200. [DOI] [PubMed] [Google Scholar]
- 43.Santini F, Gaidarov I, Keen JH. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J Cell Biol. 2002;156:665–76. doi: 10.1083/jcb.200110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1
Confocal section of BSC1 cells stably expressing σ1-EGFP, σ3-EGFP, Caveolin1-EGFP, GalT-EGFP, Arf1-EGFP, β -COP, ε -COP or Rab22-EGFP or BSC1 cells transiently expressing PLC-γ– PH Domain-EGFP or 3x-FYVE-EGFP. Cells were imaged before and after (∼ 3min) treatment with 2% v/v 1-butanol. The fluorescence along the z-axis is shown on top of each confocal section. Note that treatment with 2% v/v 1-butanol affects only the localization of AP-1 and AP-3. Bars, 10 μm.
Supplementary Fig. 2
Clathrin coated pits formed in the course of the 1-butanol washout have normal dynamic parameters of size and lifetime. The graphic distribution compares maximum fluorescence intensities (determined just prior to the uncoating of coated vesicles) and lifetimes of AP-2 spots calculated from time-series acquired before incubation with 1-butanol, or 1 min or 5 min after 1-butanol washout. BSC-1 cells stably expressing σ2-EGFP were incubated with 2% 1-butanol for 5 min. Subsequently 1-butanol was washed out and cells were imaged after 1 and 5 minutes. The data derives from 5 cells (n=932, 1974 and 707 pits for before 1-butanol application, and 1 min and 5 min after washout respectively).
Supplementary Fig. 3
Ethanol and 1-propanol (but not 2-propanol) disassemble coated pits and the removal of these primary alcohols results in a transient burst of coated pit nucleations. Confocal section of BSC-1-σ2-EGFP imaged before, during (∼ 3min) treatment with 2.5% v/v ethanol (A), 2% v/v 1-propanol (B) or 2-propanol (C) and after washout. Kymographs are shown on the right of each confocal section**.** Note the effect of the primary alcohols ethanol and 1-propanol but not of the secondary alcohol 2-propanol. Bar, 10 μm.
Supplementary Fig. 4
1-butanol treatment and washout do not affect actin polymerization. (A) Phalloidin staining of BSC1 cells fixed after incubation with media alone, 2% v/v 1-butanol or during the washout. (B) Phalloidin staining of in “LatB treated”, “LatB + 1-butanol” and “LatB – 1-butanol” cells. (C) Phalloidin staining of in “LatA treated”, “LatA + 1-butanol” and “LatA – 1-butanol” cells. (D) Phalloidin staining of in “CytoD treated”, “CytoD + 1-butanol” and “CytoD – 1-butanol” cells. Note the intact polymerized actin network in both conditions.
Supplementary Fig. 5
Actin depolymerization with Latrunculin A or Cytochalasin D does not affect the dissolution of coated pits by 1-butanol nor the nucleation resulting from its washout. (A) Kymograph depictions of a BSC1-σ2-EGFP cell prior to incubation with LatA (untreated), 15 min after the addition of 1 μg/ml LatA (“LatA”) during the incubation with 2% 1-butanol in the presence of 1 μg/ml LatA (“LatA + 1-butanol”) and during the washout of 2% 1-butanol in the presence of 1 μg/ml LatA (“LatA – 1-butanol”). Note the static, high-intensity objects in the Latrunculin A treated cell as well as the absence of AP-2 pits in the beginning of the washout and their dynamic nature thereafter. Bar, 10 μm. (B) Graphic representation of the profile of intensity of AP-2 structures at any given frame (snapshots) in “untreated” cells (n=212), “LatA treated” cells (n=211) and “LatA – 1-butanol” cells (n=196). Pits were divided in 3 classes: under the range of the maximum intensity of dynamic pits ('Lower', black columns), in the range of the maximum intensity of dynamic pits (white columns), and over the range of the maximum intensity of dynamic pits ('Higher', grey columns). Note the similar profiles of “untreated” and “LatA – 1-butanol” cells in contrast to the accumulation of large structures in the LatA treated cells. (C) Distribution of the maximum intensities and lifetimes of dynamic pits in untreated (dashed line, n=99), “LatA treated” (grey, n=52) and “LatA – 1-butanol” cells (black, n=47). (D) Kymograph depictions of a BSC1-σ2-EGFP cell prior to incubation with CytoD (untreated), 15 min after the addition of 25 μg/ml CytoD (“CytoD”) during the incubation with 2% 1-butanol in the presence of 25 μg/ml CytoD (“CytoD + 1-butanol”) and during the washout of 2% 1-butanol in the presence of 25 μg/ml CytoD (“CytoD – 1-butanol”). Note the static, high-intensity objects in the CytoD treated cell as well as the absence of AP-2 pits in the beginning of the washout and their dynamic nature thereafter. Bar, 10 μm. (E) Graphic representation of the profile of intensity of AP-2 pits at any given frame (snapshots) in untreated cells (n=224 pits), “CytoD” cells (n=114 pits) and “CytoD – 1-butanol” cells (n=155 pits). Pits were classified as in (B) (F) Distribution of the maximum intensities and lifetimes of dynamic pits in untreated (dashed line, n=165), “CytoD” cells (grey, n=134) and “CytoD – 1-butanol” cells (black, n=236).
Supplementary Fig. 6
(A) Lateral displacement of dynamic pits (μm/sec) in untreated (left, n=72), “LatA treated” (middle n=79) and “LatA – 1-butanol” cells (right, n=70). Note the similar lateral displacement of the pits during 1-butanol washout in the presence of LatA. (B) Displacement of dynamic coated pits in untreated (black diamonds), “LatA treated” cells (black triangles) and “LatA – 1-butanol” cells (grey triangles). (C) Lateral displacement of dynamic pits (μm/sec) in untreated (left, n=65), “CytoD” cells (middle n=77) and “CytoD – 1-butanol” cells (right, n=73). Note the similar lateral displacement of the pits during 1-butanol washout in the presence of CytoD. (D) Displacement of dynamic coated pits in untreated (black diamonds), “CytoD treated” cells (black triangles) and “CytoD – 1-butanol” cells (grey circles). (E) Confocal image of BSC1-σ2-EGFP cells, untreated, during the burst of nucleation following 1-butanol washout or “ LatA – 1-butanol” or “CytoD – 1-butanol” cells. (Bar, 10 μm) and graphic depiction of the relative coated pit density (normalized to the untreated level; from 3 independent experiments). (F) Transferrin uptake is partially inhibited in cells incubated with LatA or CytoD but returned to normal levels in the first minutes following the washout of 1-butanol but not t-butanol. Hela cells were pre-incubated at 37° C for 15 min with imaging medium (untreated, left) or medium containing LatA (1 μg/ml) or CytoD (25μg/ml). Cell samples were then incubated for 2 min with the respective combinations of the actin depolymerizing drugs and alcohols. The alcohol was washed out in the presence of LatA or CytoD for 2 or 15 minutes, after which cells were incubated with 10 μg/ml Alexa594-Tf for 2 min at RT and 5 min at 37°C in PBS/Glucose/BSA (PBS-GB; untreated), or PBS-GB + LatB. Cells were subsequently transferred to 4°C, acid washed and fixed. Bar, 20 μm. (G) Bar plot illustrates the quantitation of Tf uptake in the conditions depicted in (A), normalized to the level of untreated cells.
Supplementary Fig. 7
Model; Normal condition (top). At the plasma membrane, phosphatidylcholine (PC) is transphosphatidylated to phosphatidic acid (PA) by phospholipase D2 (PLD2). PA stimulates the lipid kinase PIP5K which phosphorylates PI(4)P, leading to the localized production of PIP2. A localized concentration of PIP2 and coat components, enabled by the organizing presence of the actin cytoskeleton initiates the cascade of molecular interactions which culminate in coated pit nucleation. Primary alcohols, like 1-butanol, compete with water in the transphosphatidylation reaction of PC and lead to the production of phosphatidylbutanol (P-But) in place of PA. Subsequently, in the absence of PA, PI5K is not stimulated, resulting in an abrupt drop in the levels of PIP2. In the absence of PIP2, the cell is unable to support the nucleation/maintenance of coated pits. While this is our current working model, the formal demonstration of each of the proposed steps will be the carried out in future work.
Supplementary Movie 1
Effect of 1-butanol on the dynamic behavior of coated pits and vesicles labeled with EGFP-LCa. Time-lapse series (70 frames, ∼ 2,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing EGFP-LCa using the spinning disk confocal microscope, 2% v/v 1-butanol was added at frame 25. Note that upon addition of 1-butanol the punctuate pattern, characteristic of the membrane distribution of coated pits, was radically altered to a diffuse distribution of clathrin.
Supplementary Movie 2
Effect of 1-butanol on the dynamic behavior of coated pits and vesicles labeled with σ2-EGFP Time-lapse series (50 frames, ∼ 3,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope, 2% v/v 1-butanol was added at frame 15. Note that upon addition of 1-butanol the punctuate pattern, characteristic of the membrane distribution of coated pits, was radically altered to a uniform distribution of AP-2.
Supplementary Movie 3
Synchronized appearance of coated pits labeled with EGFP–LCa upon removal of 1-butanol. Time-lapse series (150 frames, ∼ 2,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing EGFP-LCa using the spinning disk confocal microscope (continuation of Supplementary Movie 1). The washout of the 2% v/v 1-butanol was performed at frame 18. Note the synchronized burst of coated pit nucleations upon the washout of 1-butanol.
Supplementary Movie 4
Synchronized appearance of coated pits labeled with σ2-EGFP upon removal of 1-butanol. Time-lapse series (80 frames, ∼ 3,2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope (continuation of Supplementary Movie 2). The washout of 2% v/v 1-butanol was performed at frame 5. Note the synchronized burst of coated pit nucleations upon the washout of 1-butanol.
Supplementary Movie 5
Synchronized appearance of coated pits labeled with σ2-EGFP and Tomato-LCa upon removal of 1-butanol. Time-lapse series (110 frames, ∼ 2 s intervals acquired at 37°C) obtained from a BSC1 cell constitutively expressing σ2-EGFP and transiently expressing Tomato-LCa using the spinning disk confocal microscope. The washout of the 2% v/v 1-butanol was performed at frame 25. Note the synchronized burst of coated pit nucleations containing AP-2 and clathrin upon the washout of 1-butanol.
Supplementary Movie 6
Effect of 1-butanol application and washout on the dynamic behavior of coated pits and vesicles labeled with σ2-EGFP in HeLa cells. Time-lapse series (120 frames, ∼ 2,2 s intervals acquired at 37°C) obtained from a HeLa cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope. 2% v/v 1-butanol was added at frame 2 and washed out at frame 25 without stop of the capture. Note that the objects that are larger than the diffraction limit as well as coated pits were dispersed upon application of 2% v/v 1-butanol and that upon washout of 1-butanol, in addition to a return of AP-2 and clathrin to the same large structures, coated pits formed de-novo at new sites on the remaining membrane areas.
Supplementary Movie 7
Addition of liposomes containing PIP2 in the presence of 1-butanol rescues the formation of clathrin coated pits and vesicles. Time-lapse series (225 frames acquired at 37°C, ∼ 2 s intervals) obtained from BSC-1 cells stably expressing σ2-EGFP. Cells were treated with 2% v/v 1-butanol for 1 minute (added at frame 30) to produce a complete loss of coated pits and vesicles. Liposomes (50 μg/ml final concentration) containing a mixture of 5% PIP2, 45% phosphatidyl-ethanolamine (PE) and 50% phosphatidylcholine (PC) were then added (frame 65) to the medium still containing 1-butanol. This addition resulted in a complete recovery of the formation of coated pits and vesicles, beginning as early as 1 min after exposure to the liposomes.
Supplementary Movie 8
Effect of Latrunculin B on the dynamic behavior of coated pits and vesicles labeled with σ2-EGFP. Time-lapse series (120 frames, ∼ 2,2 s intervals acquired at 37°C) was initiated 15 min after addition of 2.5 μg/ml LatB and was obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope. Note the accumulation of large static structures but also coated pits with similar dynamic behavior to those of untreated cells.
Supplementary Movie 9
Effect of 1-butanol, in presence of Latrunculin B, on the dynamic behavior of coated pits and vesicles with σ2-EGFP. Time-lapse series (80 frames, ∼2.2 s intervals acquired at 37°C) was initiated 25 min after addition of 2.5 μg/ml LatB was obtained from a BSC1 cell constitutively expressing σ2-EGFP using the spinning disk confocal microscope. 2 % v/v 1-butanol were added at frame 5 and washed out at frame 40. Note that both the larger structures induced by Latrunculin as well as the remaining coated pits were dispersed by incubation with 1-butanol applied in the presence of Latrunculin, followed by the resumption of new coated pit nucleations after removal of 1-butanol.