IL-10 promotes neuronal survival following spinal cord injury (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 1.
Abstract
We have previously reported that the anti-inflammatory cytokine IL-10 induces a number of signaling cascades through the IL-10 receptor in spinal cord neurons in vitro to activate NF-κB transcription Bcl-2 and Bcl-xL and that after exposure to glutamate IL-10 blocks cytochrome c release and caspase cleavage. In the current study we used a herpes simplex virus (HSV)-based vector to express IL-10 in spinal cord in vivo. Injection of the vector 30 minutes after lateral hemisection injury resulted in increased neuronal survival in the anterior quadrant of the spinal cord and improved motor function up to 6 weeks after injury, that correlated with translocation of p50 and p65 NF-κB to the nucleus and increased expression of Bcl-2 and Bcl-xL in anterior quadrant neurons. Inhibition of cytochrome c release and caspase 3 cleavage was seen in homogenates of injured spinal cord treated by the IL-10 vector. Taken together with in vitro studies that demonstrate direct neuroprotective effects of IL-10 acting through the neuronal IL-10 receptor, these results suggest that IL-10 may provides direct neuroprotective effects in spinal cord injury separate from and in addition to the known anti-inflammatory effects, and point to the possibility that IL-10 delivery by gene transfer may be a useful adjunctive therapy for spinal cord injury.
Keywords: spinal cord injury, cytokine, interleukin-10, gene therapy, herpes simplex virus, apoptosis
Introduction
IL-10, a prototypical anti-inflammatory cytokine, suppresses cellular immunity and inhibits the synthesis and release of pro-inflammatory mediators such as TNFα, IL1-β, IL-6, IL-8 and IL-12 (Howard et al., 1992; Moore et al., 2001). In the nervous system IL-10 has been shown to improve outcomes in models of stroke (Dietrich et al., 1999; Grilli et al., 2000), experimental autoimmune encephalomyelitis (Sloane et al., 2009), traumatic or excitotoxic spinal cord injury (Abraham et al., 2004; Brewer et al., 1999; Jackson et al., 2005). The beneficial effects of IL-10 in vivo have been ascribed to IL-10-mediated down-regulation of the secondary inflammatory response and cytokine expression profile that results from tissue injury (Bethea et al., 1999; Plunkett et al., 2001). More recently, in addition to the anti-inflammatory effects it has been demonstrated that IL-10 exerts direct effects on neurons. Expression of IL-10 receptor mRNA and protein has been found in spinal cord neurons in vitro where IL-10 activates signaling pathways involved in neuronal survival and growth (Zhou et al in press). In the setting of cellular stress caused by glutamate toxicity or serum deprivation, IL-10 also provides an anti-apoptotic effect in retinal ganglion cell line (Boyd et al., 2003), cerebellar granule cells (Bachis et al., 2001) and spinal cord neurons (Zhou et al in press).
Here we report that delivery of IL-10 by HSV-vector to the spinal cord after traumatic injury prevented the development of an apoptotic cascade characterized by enhanced release of cytochrome c from mitochondria and cleavage of caspase 3. Increased Bcl-2 and Bcl-xL protein expression in anterior horn correlates temporally with the increased p50 and p65 NF-κB seen in nuclei of the motor neuron pool of the anterior horn. Treatment with IL-10 expressing vector results in significant neuronal survival and improved motor behavior outcomes. We show that motor neurons express the IL-10 receptor and propose that the neuroprotective effects of IL-10 after tissue injury may be in part mediated by direct interactions through the neuronal receptor.
Methods
Vectors
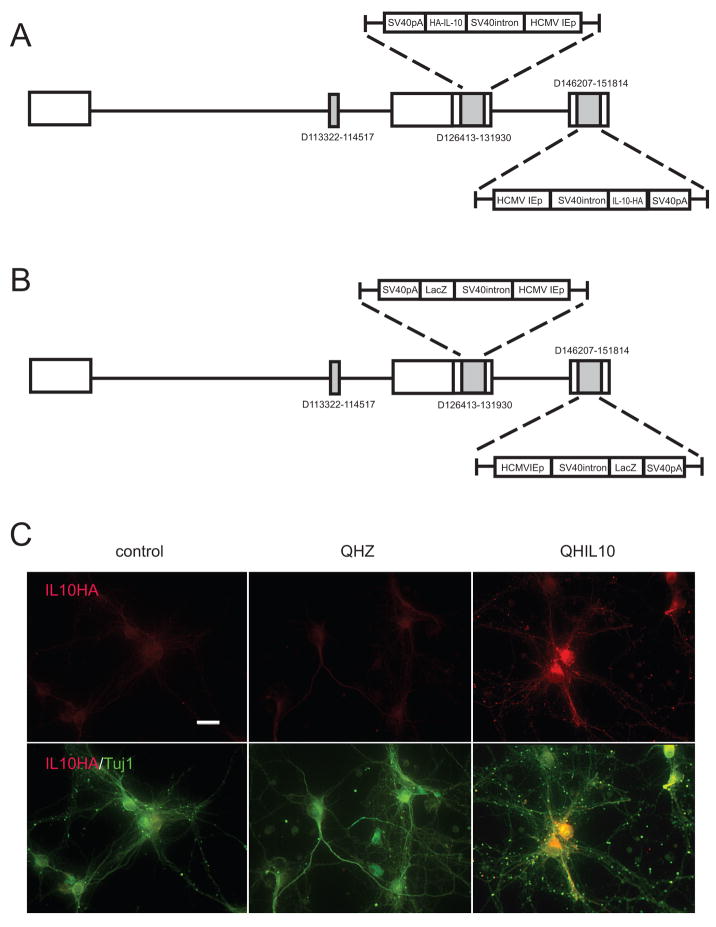
Two vector constructs were employed in this study. QHIL10 contains the full-length rat IL10 gene tagged with hemagglutinin (HA) under the control of the human cytomegalovirus immediate-early promoter (HCMV IEp). Control vector QHLacZ is identical to QHIL10, but contains the _lac_Z gene product in place of IL10-HA. The construction of vectors has been described previously (Zhou et al., 2008).
Cell Culture
Spinal cord from 17-day-old rat embryos were treated with 0.25% trypsin-EDTA (Gibco-BRL, Carlsbad, CA) for 30 minutes at 37°C, dissociated and plated on poly-D-lysine-coated coverslips at 1.5 × 106 cells per well in a 6-well plate with 2ml of defined Neurobasal Media containing B27, Glutamax I, Albumax I, and penicillin/streptomycin according to manufacturer’s recommendation (Invitrogen, Carlsbad, CA). After 10 days in culture, the cells were transfected with either QHIL10 or QHLacZ at a multiplicity of infection (MOI) of 1 for 2 hours. Fresh medium was replaced and collected 48 hours later for determination of IL10 by enzyme-linked immunosorbent assay (ELISA). Spinal cord neurons were also examined for expression of IL10 protein by immunocytochemistry and Western blot.
ELISA
The amount of IL10 released from the transduced spinal cord neuron was determined using a commercial IL10 ELISA kit (R&D Systems, Minneapolis, MN).
Experimental Animals
Female Sprague–Dawley rats, 200–250g, underwent a laminectomy at T11–12 vertebral bodies and a lateral hemisection of the spinal cord performed at the T13 level as described (Dong et al., 2003; King et al., 2006). Thirty minutes after hemisection 2 μl containing 1.2 × 107 pfu of vector was injected into 2 sites on the side of the injury just above and below the hemisection. There were three groups in each experiment: 1) sham (skin-muscle incision only); 2) lateral hemisection treated with control vector QHZ; and 3) lateral hemisection treated with experimental vector QHIL10.
Behavioral study
Recovery of hindlimb function was assessed using the Basso-Beattie-Bresnahan (BBB) motor rating scale (Basso et al., 1995). The animals were placed in an open field and rated on a 21–point scale for use of the hindlimb ipsilateral to the lesion. The measures were performed prior to surgery and at weekly intervals thereafter. Test sessions were 4 min in duration, and scores were obtained from 1 or 2 blinded observers.
Semiquantitative RT-PCR analysis
Total RNA was isolated from cells via TRIzol (Invitrogen). cDNA prepared from mRNA isolated from spinal cord neurons or rat spinal cord was amplified using following primer sets: β-actin-forward (5′-CAG TTC GCC ATG GAT GAC GAT ATC-3′) and β-actin-reverse (5′-CAC GCT CGG TCA GGA TCT TCA TG-3′) for β-actin, IL-10-HA-forward (5′-ATG CTT GGC TCA GCA CTG CTA TGT TGC-3′) and IL-10-HA-reverse (5′-GCG TAG TCG GGC ACG TCG TAG-3′) for IL-10-HA. All reactions involved initial denaturation at 94°C for 5 min followed by 28 cycles (94°C for 30 sec, 68°C for 3 min and 1 cycle 68°C for 8 min using a GeneAmp PCR 2700 (Applied Biosystems, Foster City, CA). Each animal experiment represents the results of samples from 5 different animals. Data presented as mean ± SEM.
Western blot
Cultured spinal cord neurons, spinal cord tissue were homogenized in buffer containing 50 mM Tris, 10 mM NaCl, 1% NP40, 0.02% sodium azide, and protease inhibitor cocktail (Sigma Aldrich) at pH 7.4. Aliquots containing 20 μg of protein were dissolved in Laemmli buffer and boiled at 95°C for 5 min, and the proteins separated by 12% Tris-Glycine SDS-PAGE (Bio-Rad Laboratories, Hercules, CA), transferred to nitrocellulose membranes, blocked, and then incubated with primary antibodies at 4°C. Primary antibodies included an antibody against anti-caspase 3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-cytochrome c (Santa Cruz Biotechnology), anti-Bcl-2 (Santa Cruz Biotechnology), anti-HA (Sigma, St. Louis, MO), or anti-β-actin (Sigma), then incubated with HRP-conjugated secondary antibody, followed by enhanced chemiluminescence detection (Amersham Biosciences, Arlington Heights, IL). Chemiluminescence detection values were used to quantitate the Western blot results. A ratio of each band of interest to the appropriate internal control was obtained and the statistical significance of the difference between control and experimental groups determined. Each animal experiment represents the results of samples from 5 different animals. Data presented as mean ± SEM.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde, washed with 0.2% Triton X-100 in phosphate buffered saline (PBS), and blocked with 5% normal goat serum in PBS. Primary antibodies were anti-Tuj1 (Covance Research Products, Berkeley, CA) and anti-HA (Sigma). The secondary antibodies were fluorescently tagged with Alexa Fluor (Molecular Probes). Animals were perfused 48 hrs after injury with 4% paraformaldehyde in phosphate buffer. The spinal cord was cryoprotected in 30% sucrose and 35 μm cryosections labeled with anti-Bcl-2, anti-Bcl-xL, anti-p65, anti-p50, anti IL-10R (all Santa Cruz Biotechnology) or anti-NeuN (Chemicon) followed by Alexa Fluor 488 or 594 tagged secondary antibodies (Molecular Probes).
Quantitative Image Analysis
Images were acquired at room temperature with a Nikon E1000 microscope with air objectives (40×, NA 0.95; 20×, NA 0.75) and a digital camera (Cool Snap ES, Photometrics). The original images were acquired using Metamorph software. Digital images were manipulated and arranged using Photoshop 7.0 (Adobe). Serial 30 μm cryostat sections were obtained and the number of NeuN-labeled neurons within the ventrolateral quadrant and the number of NF-κB and Bcl-2 and Bcl-xL, positive motor neurons determined by counting labeled cells in one session every approximately 750 μm by an experimenter blinded to the animal treatment group.
Data Analysis
The statistical significance of the difference between vector-treated and control animals was determined by one-way analysis of variance with post hoc comparisons when appropriate. Parametric statistics, using the general linear model for repeated measures, were applied for the behavior studies and used to identify significant effects of treatment over time using SPSS 12.0 for Windows (SPSS, Chicago, IL). Data are expressed as mean ± standard error of mean (SEM), with P < 0.05 considered significant.
Results
Characterization of IL-10 expressing HSV vector in spinal cord
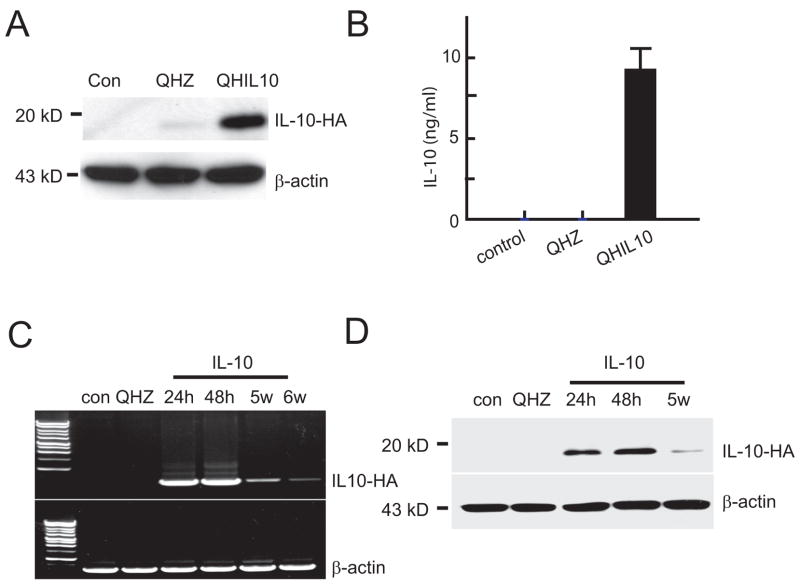
We used vector-mediated gene transfer to express IL-10 in vivo in order to explore the potential role of IL-10 on neuroprotection in a model of spinal cord injury in view of the effects that we have reported in spinal cord neurons in vitro. For this purpose, we utilized a nonreplicating HSV-based vector expressing IL-10 (vector QHIL10, Fig 1A). Primary neurons in vitro transduced with QHIL10 at a multiplicity of infection of 1 resulted in robust expression of IL-10 in the transduced cells (Fig 1C). IL-10 could be detected by Western blot of cell lysate (Fig 2A) and release of IL-10 from the infected cells into the medium was detected by ELISA (Fig 2B). Injection of 2 μl of vector in to spinal cord resulted in the expression of IL-10 mRNA detected by RT-PCR that was highest in samples taken at 24–48 hrs after inoculation of the vector, but could be detected up to 6 weeks injection of the vector (Fig 2C). The production of transgene-mediated IL-10 protein in injected spinal cord was confirmed by Western blot using an antibody directed against the HA epitope (Fig 2D).
Figure 1.
HSV vector QHIL10 expresses IL-10 in spinal cord neurons in vitro. Vector constructs QHIL10 (A) and QHZ (B). (C) E17 spinal cord neurons (10 DIV) 48 hr after infection with QHIL10 at MOI 1 express transgene-mediated IL-10 recognized by its HA tag (anti-βIII tubulin green; anti HA red); scale bar = 10 μm.
Figure 2.
(A, B). Western blot of cell lysate (A) and ELISA of culture medium (B) from spinal cord neurons 48 hr after infection. (C, D) IL-10 mRNA determined by RT-PCR (C) and IL-10 protein determined by Western blot (D) of spinal cord after inoculation of vector QHIL10 or vector QHZ into adult thoracic spinal cord in vivo.
IL-10 promotes neuronal survival following spinal cord injury
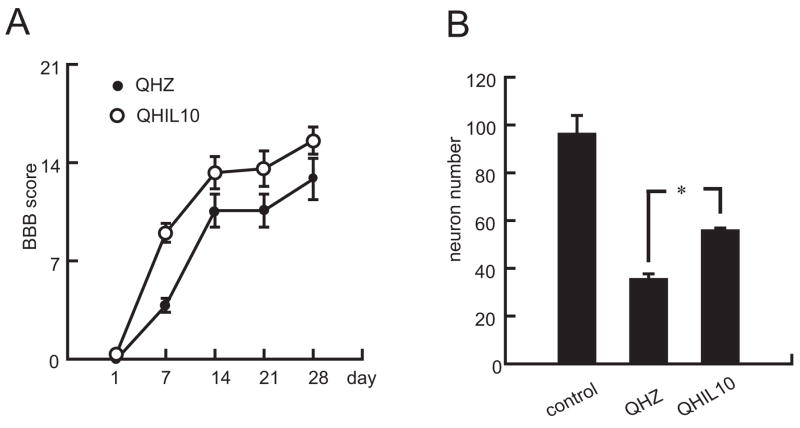
SCI causes significant muscle weakness below the level of the lesion. Initial spontaneous recovery is often seen followed by stable motor deficits. Consequent behavioral manifestations below the level of the injury in rodents are measured by motor disability scores (BBB) (Basso et al., 1995). Animals inoculated with the IL-10 expressing HSV vector above and below the site of injury 30 min after lateral hemisection of the thoracic spinal cord showed a statistically significant improvement in BBB score over the course of 4 wks after trauma compared to animals inoculated with the control vector QHZ (Fig 3A). Inoculation of QHIL10 also increased the number of NeuN-positive neurons in the ventral horn of spinal cord (Fig 3B)
Figure 3.
Vector-produced IL-10 protects neurons in vivo resulting in enhanced recovery following SCI. (A) Inoculation of the IL-10 expressing vector directly into spinal cord 30 minutes after T13 hemisection resulted in improved locomotor function assessed by BBB; P < 0.05 by repeated measures analysis; n = 8 animals per group. (B) The number of NeuN positive neurons in ventral horn of spinal cord around the site of injury 48 hr after spinal cord injury was also increased by injection of QHIL10 compared to animals injected with QHZ.
Activation of the neuroprotective cascade by IL-10 in spinal cord injury
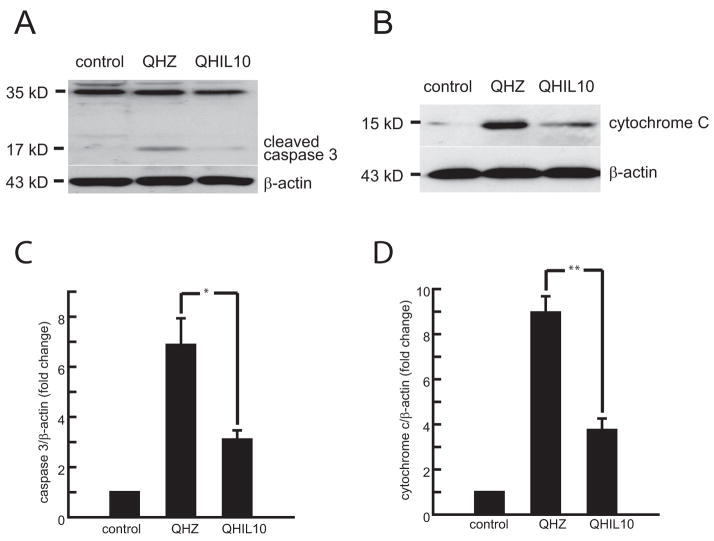
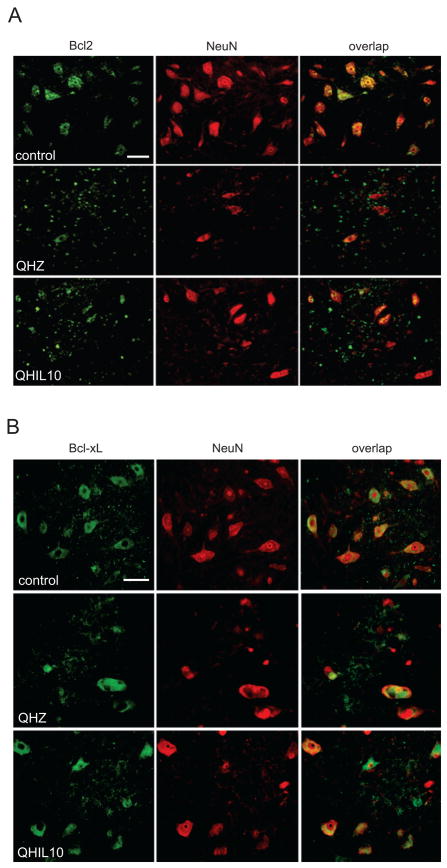
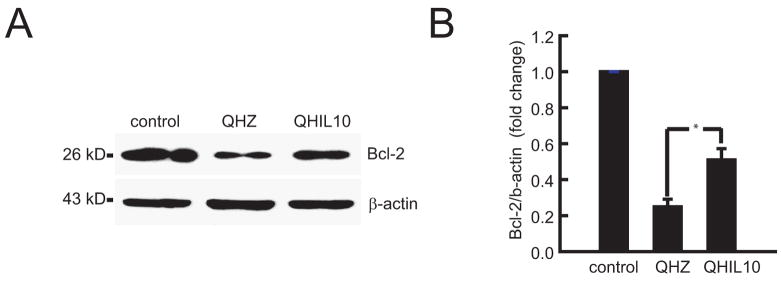
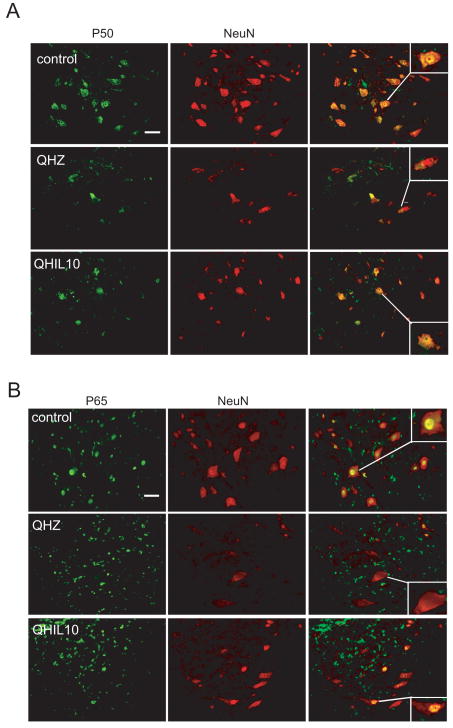
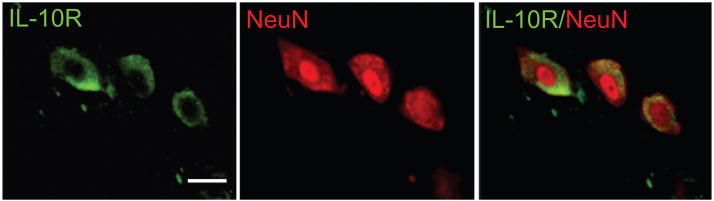
Trauma to the spinal cord results in substantial tissue damage and activation of apoptotic pathways (Citron et al., 2000; Springer et al., 1999). In order to assess if the neuronal survival observed after IL-10 treatment is mediated through the inhibition of cellular apoptosis as described in SCI. We analyzed anterior quadrant of the spinal cord just below the level of the injury in rats treated with QHIL10 or control vector and we found that IL-10 caused a marked reduction in cleaved caspase 3 induced by injury (Fig 4A) and in release of cytochrome c from mitochondria (Fig 4B). These findings were associated with increased expression of Bcl-2 and Bcl-xL in motor neurons of the ventral horn (Fig 5A,B and Fig 6) that correlated with an increase in nuclear p50 and p65 NF-κB in those cells (Fig 7A,B). Because of evidence in vitro that IL 10 can signals through the neuronal IL-10 receptor to provide direct neuroprotective effects (Zhou et al., 2009 in press), we used immunocytochemistry to examine the cellular distribution of IL-10 receptor in the spinal cord. We observed a very significant labeling of IL-10-R in motor neurons of the anterior horn (Fig 8).
Figure 4.
Inoculation of the IL-10 expressing vector reduced the amount of cleaved caspase 3 (A) and cytochrome c (B) in thoracic spinal cord homogenates taken 2.5 mm above and below T13 hemisection, 48 h after injury. Quantification of the bands by chemiluminescence (C,D); n = 5, mean ± SEM; *P<0.05 and **P<0.01.
Figure 5.
Double label immunohistochemistry of Bcl-2 (A) and Bcl-xL (B) (both green) and the neuronal marker NeuN (red) in ventral horn 2 mm below the lesion 48 hr after T13 hemisection. Scale bar = 20 μm.
Figure 6.
Vector-mediated IL-10 expression increases Bcl-2 in ventral horn neurons of spinal cord after SCI. (A) Western blot. (B) quantification of the Western blot; n = 5, mean ± SEM; *P<0.05.
Figure 7.
Vector mediated IL-10 expression prevents translocation of p50 and p65 subunits of NF-κB in VH spinal neurons after SCI. Double label immunohistochemistry of p50 (A) and p65 (B) (both green) with the neuronal marker NeuN (red) in ventral horn 2 mm below the site of injury 48 h after T13 hemisection. Scale bar = 20 μm.
Figure 8.
IL-10 receptor co-localizes with the neuronal marker Neu-N in spinal cord neurons in vivo. Bar =10 μm.
Discussion
As a pluripotent cytokine Il-10 has been shown to have important beneficial effects in the nervous system. In transgenic animals lacking expression of the IL-10 gene the degree of tissue damage following stroke injury or after quisqualate injury to the spinal cord was significantly decreased compared to control mice suggesting a protective effect of IL-10 (Abraham et al., 2004; Grilli et al., 2000). In models of traumatic or excitotoxic injury to the spinal cord delivery of IL-10 induced recovery of motor function, reduced pain and decreased tissue damage (Bethea et al., 1999; Brewer et al., 1999; Jackson et al., 2005; Plunkett et al., 2001). However in other studies systemic administration of IL-10 alone or in combination with methylprednisolone failed to improved motor behavior or improved axonal growth (Takami et al., 2002). The differences in outcomes may relate to the particular injury model, animal species and route or dose of IL-10 administration. The therapeutic effects of IL-10, in these animal models, have been ascribed to its anti-inflammatory properties, decrease astroglia activation and cytokine production. Further interest in IL-10 has been brought by clinical studies showing elevated serum concentration of proinflammatory cytokines TNF-α, IL-6 and IL-1 receptor antagonist and GM1 ganglioside autoantibodies in patients with SCI without infectious or other medical complications (Davies et al., 2007). The potential importance of cytokine dysregulation in primary disease of the nervous system was also suggested by a recent study showing that levels of IL-10 are markedly reduced in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis compared to control subjects (Mitchell et al., 2009).
In spinal cord injury induction of cell death occurs by activation of apoptotic pathways manifested by rapid upregulation of caspase 3 expression (Citron et al., 2000), cytochrome c release from mitochondria caspase 3 clevage and activation downstream of caspase 9 (Springer et al., 1999). Evidence that IL-10 could change the outcome of spinal cord injury by suppressing cell death mechanisms in neurons were suggested by in vitro studies using a retinal ganglion cell line and cerebellar granule neurons (Bachis et al., 2001; Boyd et al., 2003).
In spinal cord neurons in vitro, IL-10 engages Jak–Stat 3, PI3K-AKT and GSK3-β signaling cascades downstream from the IL-10 receptor that lead to enhanced p50/p65 NF-κB mediated transcription of prosurvival peptides Bcl-2 and Bcl-xL blocking the activation of similar apoptotic pathways that follow spinal cord injury (Zhou et al., 2009 in press). In vivo we now report that vector delivery of IL-10 to the spinal cord after injury engages similar p50/p65 NF-κB mediated antiapoptotic pathway in neurons with increased levels of Bcl-2 and Bcl-xL, blocking cytochrome c release and caspase activation blocking caspase while concomitantly preserving motor neuron survival. The limited motor recovery with QHIL10 (BBB score up to 15) reflects the improvement of hip girdle function and knee stabilization, reflecting the preservation of motor neurons in the upper lumbar spinal cord observed in these SCI treated animals in which the IL-10 vector was delivered just above and below T13.
Our studies point to the potential therapeutic implications of IL-10 signaling to neurons, and the utility of local expression of IL-10 by vector-mediated gene transfer to the spinal cord in the setting of spinal cord injury. The observation that after injury local delivery of IL-10 by HSV-mediated gene transfer to the spinal cord promotes neuronal survival and improves behavioral outcomes through cellular mechanisms similar to those activated in vitro suggests that the neuroprotective effects of IL-10 in vivo are in part mediated through the neuronal IL-10 receptor. The combination of anti-inflammatory properties and neuroprotective effects suggest that IL-10 may have added therapeutic potential in the clinical setting.
Acknowledgments
The authors acknowledge the excellent technical assistance of Vikram Thakur Singh in propagation of the vector, and Carrissia Holloway and Jere Christian in animal care. These studies were supported by grants from the Department of Veterans Affairs and the NIH to Drs. Mata and Fink.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham KE, McMillen D, Brewer KL. The effects of endogenous interleukin-10 on gray matter damage and the development of pain behaviors following excitotoxic spinal cord injury in the mouse. Neuroscience. 2004;124:945–952. doi: 10.1016/j.neuroscience.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21:3104–3112. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Boyd ZS, Kriatchko A, Yang J, Agarwal N, Wax MB, Patil RV. Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Invest Ophthalmol Vis Sci. 2003;44:5206–5211. doi: 10.1167/iovs.03-0534. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Bethea JR, Yezierski RP. Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp Neurol. 1999;159:484–493. doi: 10.1006/exnr.1999.7173. [DOI] [PubMed] [Google Scholar]
- Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, Landis ME, Festoff BW. Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol. 2000;166:213–226. doi: 10.1006/exnr.2000.7523. [DOI] [PubMed] [Google Scholar]
- Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. 1999;158:444–450. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci. 2003;23:8682–8691. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Howard M, O’Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336:173–183. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26:4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RM, Freeman WM, Randazzo WT, Stephens HE, Beard JL, Simmons Z, Connor JR. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology. 2009;72:14–19. doi: 10.1212/01.wnl.0000333251.36681.a5. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bethea JR, Wood PM, Kleitman N, Bunge MB. Methylprednisolone and interleukin-10 reduce gray matter damage in the contused Fischer rat thoracic spinal cord but do not improve functional outcome. J Neurotrauma. 2002;19:653–666. doi: 10.1089/089771502753754118. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06263.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]