Small Cajal Body–specific RNAs of Drosophila Function in the Absence of Cajal Bodies (original) (raw)
Abstract
During their biogenesis small nuclear RNAs (snRNAs) undergo multiple covalent modifications that require guide RNAs to direct methylase and pseudouridylase enzymes to the appropriate nucleotides. Because of their localization in the nuclear Cajal body (CB), these guide RNAs are known as small CB-specific RNAs (scaRNAs). Using a fluorescent primer extension technique, we mapped the modified nucleotides in Drosophila U1, U2, U4, and U5 snRNAs. By fluorescent in situ hybridization (FISH) we showed that seven Drosophila scaRNAs are concentrated in easily detectable CBs. We used two assays based on Xenopus oocyte nuclei to demonstrate that three of these Drosophila scaRNAs do, in fact, function as guide RNAs. In flies null for the CB marker protein coilin, CBs are absent and there are no localized FISH signals for the scaRNAs. Nevertheless, biochemical experiments show that scaRNAs are present at normal levels and snRNAs are properly modified. Our experiments demonstrate that several scaRNAs are concentrated as expected in the CBs of wild-type Drosophila, but they function equally well in the nucleoplasm of mutant flies that lack CBs. We propose that the snRNA modification machinery is not limited to CBs, but is dispersed throughout the nucleoplasm of cells in general.
INTRODUCTION
The two major ribosomal RNAs (rRNAs) and the spliceosomal small nuclear RNAs (snRNAs) U1, U2, U4, U5, and U6 contain many posttranscriptionally modified nucleotides, which are crucial for RNA–RNA and RNA–protein interactions, as well as spliceosome function (Yu et al., 1998; Zhao and Yu, 2004, 2007). The most abundant modifications in both rRNAs and snRNAs are 2′-_O_-methylation and pseudouridylation, which are directed by small box C/D and box H/ACA guide RNAs, respectively. Each guide RNA molecule is associated with a set of four core proteins: box C/D RNAs form RNP particles with fibrillarin (the methyltransferase), Nop56, Nop58, and a 15.5-kDa protein, whereas box H/ACA RNAs associate with NAP57/dyskerin (the pseudouridine synthase), GAR1, NHP2, and NOP10 proteins (reviewed in Yu et al., 2004; Matera et al., 2007). Most guide RNAs are concentrated in the nucleolus, where they are involved in posttranscriptional modifications of rRNA. Because of their localization, they are referred to as small nucleolar RNAs (snoRNAs). However, the guide RNAs that mediate modification of the snRNAs are preferentially found in another nuclear organelle, the Cajal body (CB). These guide RNAs are called small CB-specific RNAs (scaRNAs).
The first scaRNA to be studied in detail was U85 scaRNA (Jády and Kiss, 2001; Darzacq et al., 2002). U85 is a remarkable double guide RNA that contains both box C/D and box H/ACA motifs. In a set of detailed experiments it was shown that human U85 scaRNA guides both 2′_-O-_methylation at C45 and pseudouridylation at U46 in U5 snRNA. Modification takes place in the nucleus after import of the U5 snRNA from the cytoplasm (Jády et al., 2003). CB localization of U85 scaRNA was originally demonstrated by fluorescent in situ hybridization (FISH) in mammalian and Drosophila cultured cells (Darzacq et al., 2002; Richard et al., 2003), and subsequently in various tissues of the fly (Liu et al., 2006b, 2009).
CBs are small nuclear bodies found in a wide variety of eukaryotic organisms, including mammals, amphibians, insects, and plants. They are most often recognized by immunostaining for the marker protein coilin (Andrade et al., 1991; Raska et al., 1991) and by their unusually high concentration of splicing snRNPs relative to the rest of the nucleus (Carmo-Fonseca et al., 1991; Matera and Ward, 1993; Spector, 1993). The discovery of scaRNAs added a convenient way to identify CBs by FISH. Because Drosophila coilin was difficult to recognize on the basis of sequence comparison, we originally used U85 scaRNA and other markers to identify the Drosophila CB (Liu et al., 2006b). More recently we identified Drosophila coilin and showed that it colocalizes with U85, snRNAs, fibrillarin, and the survival motor neuron (SMN) protein in CBs in a wide variety of larval and adult cells (Liu et al., 2009). We also generated two coilin-null strains, which lack CBs detectable by probes for any of the standard CB markers. Thus coilin is essential for normal CB organization in Drosophila. Nevertheless, coilin-null flies are homozygous viable and fertile.
These data raise two important questions. Are the usual CB components (other than coilin) present in coilin-null flies, but are simply not concentrated in a CB? And do reactions that normally take place in the CB occur in the absence of a morphologically recognizable CB? Here we examine U85 and six other scaRNAs in both wild-type and coilin-null flies. We show that scaRNAs are present at normal levels in the mutants, although they are not concentrated in a CB. We also found methylation and pseudouridylation of U1, U2, U4, and U5 snRNAs in both wild-type and coilin-null flies. Thus, cytologically detectable CBs are not required for normal function of scaRNAs in Drosophila.
MATERIALS AND METHODS
Flies
Drosophila melanogaster strains were maintained at 21–23°C on a standard cornmeal-based medium. A y w stock was used as control. Two coilin null lines, coil199 and coil203, were generated by site-specific mutation using zinc-finger nucleases (Beumer et al., 2008; Liu et al., 2009). Drosophila S2 cells were used as an additional wild-type control.
Templates for In Vitro Transcription
Using a nucleotide database search similar to that described by Tycowski et al. (2009), we independently identified a guide RNA for 2′_-O-_methylation of Drosophila U4 snRNA at position A65. A standard RACE-PCR strategy was used to determine the 5′ and 3′ ends of the RNA.
Drosophila snRNAs and box C/D guide RNAs were amplified from genomic DNA isolated from y w flies using PCR with gene specific primers. The oligos we used are listed in Supplemental Table S1. Most 5′ oligos contain a T3 promoter to make sense RNA, whereas 3′ oligos contain a T7 promoter to make antisense RNA. PCR fragments were either cloned into pCRII-TOPO (Invitrogen, Carlsbad, CA) or pGEM-T-Easy (Promega, Madison, WI) vectors (U1, U2, U4, and U5 snRNAs; mgU2-25, mgU2-41, mgU4-65, mgU5-38, and putative mgU2-31) or were digested with EcoRI + XbaI and cloned into pUC19 (mgU2-28, mgU5-42, and U85 scaRNA). Otherwise PCR products were purified and used as templates (pug28S-3186 and pug28S-3327). To obtain mgU2-28 lacking the CB-specific localization sequence, referred to as the CAB box, oligos SD104 and SD105 were annealed and extended with Taq DNA polymerase at 55°C for 15 min. The DNA fragment was cloned into pGEM-T-Easy vector, and the plasmid was used as a template for in vitro transcription. To make a 5′ truncated fragment of mgU2-28, oligo SD94 was annealed with a standard T7 oligo and used as a template for transcription.
RNA Extraction
For Northern blot and primer extension analysis total RNA was extracted from adult females, 3rd instar larvae, pupae, and S2 cell cultures using the RiboPure kit (Ambion, Austin, TX). RNA was treated with RNase-free DNase I (Roche, Indianapolis, IN) and checked for traces of DNA by PCR. A qualitative estimate of RNA was made by RT-PCR and by electrophoresis on an agarose gel. After ethanol precipitation the RNA was dissolved in nuclease-free water, and its concentration was measured by spectrophotometry.
Northern Blot Analysis
For Northern analysis of total RNA, 20 μg samples were separated on an 8% denaturing polyacrylamide gel (8 M urea, 1× TBE buffer [89 mM Tris, 89 mM borate, and 2 mM EDTA]) and electrophoretically transferred onto a nylon membrane (GeneScreen from Perkin Elmer, Norwalk, CT, or Zeta-Probe GT from Bio-Rad, Richmond, CA). After UV cross-linking of the RNA, the membranes were stained with methylene blue to estimate the amount of transferred RNA. Hybridization was performed for 2–3 h in Rapid-Hyb Buffer (GE Healthcare, Piscataway, NJ) at 65°C for U85 RNA detection or at 50–55°C for other box C/D RNAs. Single-stranded DNAs complementary to full-length Drosophila scaRNAs were labeled with [α-32P]dCTP in a reverse transcription reaction and were used as probes. Blots were washed briefly in 2× SSC (0.15 M NaCl, 0.015 M Na citrate, pH 7.0) and then in 2× SSC, 0.1% SDS for 20 min, followed by two times in 0.1× SSC, 0.1% SDS for 30 min at the hybridization temperature. Membranes were exposed to Kodak x-ray film (Eastman Kodak, Rochester, NY) for 1–7 d or for 2–7 h in the case of snRNA detection.
Fluorescent Primer Extension Analysis to Map 2′-O-Methylation and Pseudouridylation
For mapping 2′_-O-_methylation and pseudouridylation sites in Drosophila snRNAs we developed a nonradioactive modification of methods based on reverse transcription (Kiss and Jády, 2004). To detect 2′_-O-_methylation, we performed primer extension with a low concentration of deoxynucleotide triphosphate (dNTP); the dNTP concentration varied from 0.004 to 0.01 mM and was optimized for each snRNA. Control reactions were done at a high concentration of dNTPs (0.5 mM). Pseudouridines were mapped after reacting the test RNA with CMC [_N_-cyclohexyl-_N_′-(2-morpholinoethyl) carbodiimide metho-_p_-toluene sulfonate; Sigma-Aldrich, St. Louis, MO] followed by alkali treatment. In vitro–transcribed RNAs, either untreated or treated with CMC, were used as negative controls for modifications. RNA sequencing products were used to determine the exact position of modified nucleotides. Chain termination mixes contained 270 μM dNTPs, where one dNTP was partially replaced with acyNTP (New England Biolabs, Beverly, MA) at a 1:3 (dNTP:acyNTP) ratio. Oligonucleotides terminally labeled with a 6-FAM fluorescent dye were used as primers; 3–6 pmol of 6-FAM-oligo was used per reaction (oligos are listed in Supplemental Table S1). Reaction products were purified by ethanol precipitation, lyophilized, and kept at −20°C until use. Fragments were separated on 50-cm capillary columns in POP4 acrylamide polymer on a capillary electrophoresis instrument (ABI Prism 3100 Genetic Analyzer; Applied Biosystems, Foster City, CA) using techniques and parameters suggested by the manufacturer. The Gene Scan-500 Liz Size Standard (Applied Biosystems) was included in each run to align fragments from different samples. GeneMapper version 3.7 software (Applied Biosystems) was used to screen the data, identify peaks, and precisely determine positions of modified nucleotides.
In Vitro Modification Assays
Capped Drosophila sense-strand RNAs were transcribed in vitro from linearized plasmids using T3 RNA polymerase according to standard procedures. After DNase I treatment and DNase I inactivation, RNAs were purified using MicroSpin G25 columns (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Xenopus oocytes were injected with 9.2 nl (85 fmol) of a guide RNA into the giant nucleus or germinal vesicle (GV), then with 18.4–23 nl (∼0.5 pmol) of Drosophila U2 snRNA into the cytoplasm using a Nanoject microinjection apparatus (Drummond Scientific, Broomall, PA). Both RNAs were synthesized with an m7Gppp cap at the 5′ end. As a control, U2 snRNA alone was injected into oocytes. After overnight incubation in OR2 medium (Wallace et al., 1973) at room temperature, GVs were manually isolated in 5:1 KCl-NaCl isolation medium (Gall et al., 1991). RNA was extracted using the RNAqueous-micro kit (Ambion) and analyzed for 2′_-O-_methylation mapping.
In a second type of assay we used Xenopus GVs isolated in mineral oil. These nuclei retain their physiological activity for hours (Lund and Paine, 1990; Paine et al., 1992; Handwerger et al., 2003; Deryusheva and Gall, 2004). Furthermore, U2 snRNA injected into such nuclei is properly modified (Yu et al., 2001). GVs (n = 150) were collected in a minimal amount of oil in a 0.5-ml tube. Micrococcal nuclease buffer (50 mM Tris-HCl, 5 mM CaCl2, and 0.1 mg/ml BSA, pH 7.9) was added to the GVs under oil to a final volume of 14–15 μl. The tube was then centrifuged at 20,800 × g for 2 min to break down nuclear envelopes and release the GV contents. The tube now contained three fractions: yolk on the bottom, GV extract, and oil with nuclear envelopes at the interface. While still under oil, the GV extract was treated with 1 μl micrococcal nuclease (100 gel units/μl; New England Biolabs) for 30 min at 30–35°C. Micrococcal nuclease was then inactivated by adding EGTA to a final concentration of 10 mM. RNasin, reconstitution buffer (final concentration 20 mM HEPES-NaOH, pH 8, 120 mM KCl, and 5 mM MgCl2) and in vitro–transcribed RNAs were added to the micrococcal nuclease–treated GV extract. RNAs were either 100 ng full-length target snRNA alone or 100 ng target RNA along with a two- to threefold excess of corresponding guide RNA. After overnight incubation at room temperature, RNA was extracted and analyzed.
FISH
Various tissues from Drosophila third instar larvae and adult flies were examined as whole mounts. Flies were dissected in Grace's medium (Grace, 1962) and tissues were fixed for 10 min in 4% paraformaldehyde, 2.5% acetic acid in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4). Acetic acid added to the fixative improves probe penetration and increases the efficiency of hybridization. After washing in PBS, samples were equilibrated for several hours in hybridization mix (50% formamide, 5× SSC, pH 7.0, 10 mM citric acid, 50 μg/ml heparin, 500 μg/ml yeast tRNA, and 0.1% Tween 20). Fluorescent RNA probes labeled with Alexa-488-UTP, Alexa-546-UTP (Invitrogen), or Cy5-UTP (GE Healthcare) were prepared by in vitro transcription from DNA clones, PCR products, or deoxyoligonucleotides as described previously (Liu et al., 2006a,b). Probes were diluted in the hybridization mix to a final concentration of 50–100 ng/ml. Tissues for in situ hybridization were incubated at 42°C overnight. DAPI (4,6-diamidino-2-phenylindole, 1 μg/ml) was added to the hybridization mix as a stain for DNA. For colocalization analysis by two-color FISH, Alexa-488 and Cy5 labels were used to obtain complete separation of the fluorescence signals. Images were taken with a 40× (NA 1.25) or a 63× (NA 1.40) planapochromatic objective on a laser-scanning confocal microscope (Leica SP2 or SP5. Deerfield, IL). Images were taken with the laser intensity and photomultiplier gain adjusted so that pixels in the region of interest were not saturated (“glow-over” display). In most cases contrast and relative intensities of the blue (DAPI), green (Alexa-488), red (Alexa-546), and far red (Cy5) images were adjusted with Photoshop (Adobe Systems, San Jose, CA).
RESULTS
Known and Predicted Guide RNAs for Nucleotide Modifications in Drosophila snRNAs
Human U85 scaRNA is a guide RNA concentrated in the CB, where it directs 2′_-O-_methylation of C45 and pseudouridylation of U46 in human U5 snRNA (Jády and Kiss, 2001). Drosophila U85 scaRNA is concentrated in the CB of the fly (Jády and Kiss, 2001; Liu et al., 2006b, 2009), where it presumably directs the corresponding modifications of Drosophila U5 snRNA at C46 and U47. Two box H/ACA and several box C/D RNAs have been described as putative guide RNAs for modification of Drosophila snRNAs (Yuan et al., 2003; Huang et al., 2005a,b; Tycowski et al., 2009; Table 1). Independently, we identified one of these Drosophila guide RNAs, mgU4-65. Aside from U85, however, the cytological localization of the Drosophila guide RNAs has not been reported. Moreover, their function as guide RNAs to direct specific snRNA modifications remains to be demonstrated.
Table 1.
2′-_O_-methyl and pseudouridine modifications in Drosophila snRNAs
| Internal modifications | Predicted modification guide RNA | ||||
|---|---|---|---|---|---|
| Name used here | GenBank accession no. | Alternative names used | References* | ||
| U1 snRNA | |||||
| U5 | Ψ | ||||
| U6 | Ψ | ||||
| U22 | No | pug28S-3327 | AJ544026, AJ629274, AJ629275, AJ629276 | Dm-586, SnoR586, Ψ28S-3327 | b, c |
| U2 snRNA | |||||
| G25 | 2′-_O_-methyl | mgU2-25 | BK006790 | e | |
| C28 | 2′-_O_-methyl | mgU2-28 | AJ543999 | Dm-700, SnoR700, _Dm_mgU2-28, MeU2-C28 | b, c, d, e |
| A31 | No | mgU2-31 | AJ784390 | MeU2-A31 | f |
| G34 | 2′-_O_-methyl | ||||
| U35 | Ψ | ||||
| U38 | Ψ | ||||
| U40 | Ψ | ||||
| C41 | 2′-_O_-methyl | mgU2-41 | BK006791 | e | |
| U42 | Ψ | ||||
| U44 | Ψ | ||||
| U45 | Ψ | ||||
| U48 | 2′-_O_-methyl | ||||
| U55 | Ψ | ||||
| U4 snRNA | |||||
| U36 | Ψ | ||||
| U59 | No | pug28S-3186 | AJ809574, AJ544025 | Dm-165, SnoR165, Ψ28S-3186 | b, c |
| A65 | 2′-_O_-methyl | mgU4-65 | BK006792 | e, this study | |
| U79 | Ψ | ||||
| U5 snRNA | |||||
| U32 | Ψ | ||||
| G38 | 2′-_O_-methyl | mgU5-38 | BK006793 | e | |
| U42 | 2′-_O_-methyl | mgU5-42 | AJ544002 | Dm-755, SnoR755, MeU5-U42 | b, c, e |
| U44 | Ψ | ||||
| C46 | 2′-_O_-methyl | U85 | AF308282 | MeU5-C46 | a, c |
| U47 | Ψ | ||||
| U54 | Ψ |
Posttranscriptional Nucleotide Modifications in Drosophila snRNAs
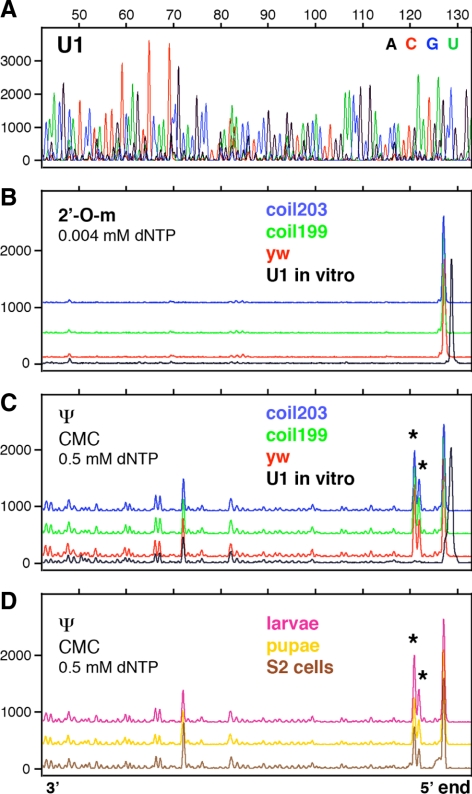
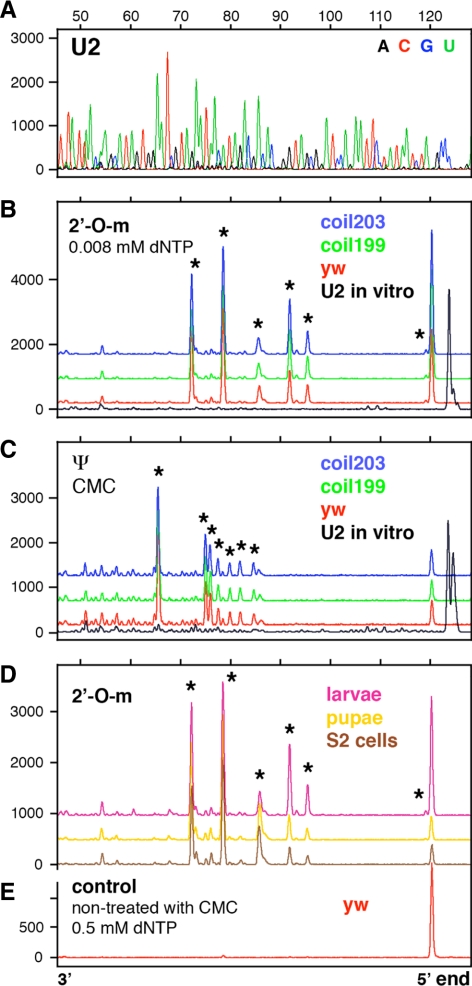
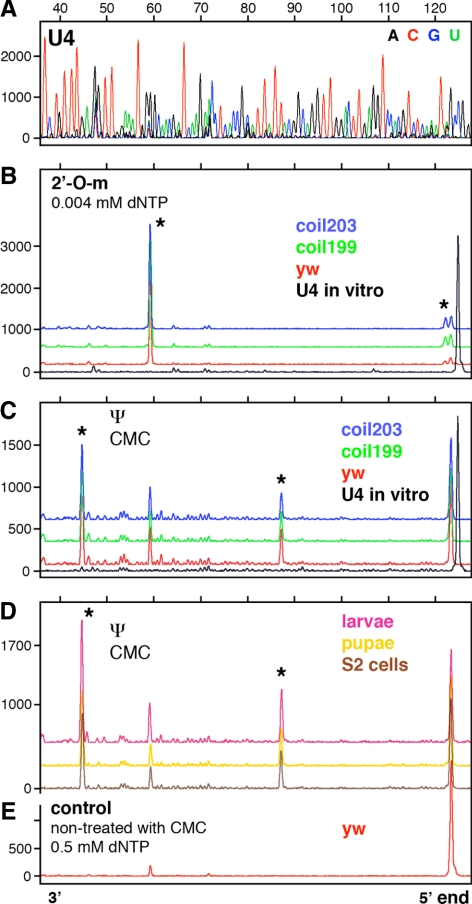
Modifications in Drosophila U5 snRNA have been described in detail, but less is known about modifications in Drosophila U1, U2, and U4 snRNAs (Myslinski et al., 1984; Reddy and Busch, 1988; Szkukalek et al., 1995; Huang et al., 2005b). To obtain a more complete list of modifications, we carried out primer extension reactions with fluorescently labeled oligonucleotides, followed by separation on capillary columns. Our data confirm previously described modifications in Drosophila U1, U2, U4, and U5 snRNA. In addition we identified 11 new modifications in U2 and U4 (Table 1 and Figures 1–3; Supplemental Figure S1). We did not find any stage-specific modifications or differences in modification patterns between samples (Figures 1–3).
Figure 1.
Mapping of 2′_-O-_methyl groups and pseudouridines in Drosophila U1 snRNA by primer extension. Terminations of the primer extension reaction occur one nucleotide downstream of the actual modification and appear as peaks above the baseline. The strong peak at the 5′ end represents the end of the template molecule. The X-axis shows fragment length in nucleotides determined by the migration of size standards. The Y-axis shows peak intensities in arbitrary units (traces are shifted vertically to avoid overlap). Details are given in Materials and Methods. (A) Sequencing reaction run on in vitro–transcribed U1 snRNA allows the position of terminations to be determined. (B) There are no detectable methylation peaks in U1 snRNA derived from adult control flies (y w), two coilin-null mutants (coil199 and coil203), or in vitro–transcribed U1 snRNA. (C) There are two strong pseudouridine peaks near the 5′ end of U1 snRNA from adult y w and mutant flies (stars at Ψ5 and Ψ6), but not in the control in vitro–transcribed U1 snRNA (black). Multiple small peaks in all the traces represent terminations caused by uridine itself. (D) U1 snRNA derived from S2 cells, larvae, and pupae shows the same two pseudouridine peaks as U1 derived from adult flies (stars at Ψ5 and Ψ6). Note the absence of pseudouridylation at position U22 in all samples.
Figure 2.
Mapping of 2′_-O-_methyl groups and pseudouridines in Drosophila U2 snRNA. Conditions as in Figure 1. (A) Sequencing reaction on in vitro–transcribed U2 snRNA. (B) Six 2′_-O-_methyl groups are detectable in U2 snRNA derived from y w and coilin-null mutant flies (stars at Am1, Gm25, Cm28, Gm34, Cm41, and Um48). (C) Seven pseudouridine modifications are detectable in U2 snRNA derived from y w and coilin-null flies (stars at Ψ35, Ψ38, Ψ40, Ψ42, Ψ44, Ψ45, and Ψ55). (D) U2 snRNA derived from S2 cells, larvae, and pupae shows the same six 2′_-O-_methyl peaks as U2 derived from adult flies (stars at Am1, Gm25, Cm28, Gm34, Cm41, and Um48). (E) U2 snRNA from y w flies shows no peaks when treated with high concentration of dNTP during the extension reaction. Note the absence of methylation at A31 in all samples.
Figure 3.
Mapping of 2′_-O-_methyl groups and pseudouridines in Drosophila U4 snRNA. Conditions as in Figure 1. (A) Sequencing reaction on in vitro–transcribed U4 snRNA. (B) Two 2′_-O-_methyl groups are detectable in U4 snRNA derived from y w and coilin-null mutant flies (stars at Am1 and Am65). (C) Two pseudouridine modifications are detectable in U4 snRNA derived from y w and coilin-null flies (stars at Ψ36 and Ψ79). The prominent peak at A65 (no star) is due to methylation at this site, which can cause termination of the extension reaction in CMC-treated RNA. (D) U4 snRNA derived from S2 cells, larvae, and pupae shows the same two pseudouridine peaks as U4 derived from adult flies (stars at Ψ36 and Ψ79). (E) U4 snRNA from y w flies shows one tiny peak (Am65) when treated with high concentration of dNTP during the extension reaction. Note the absence of pseudouridine at U59 in all samples.
Significantly, we failed to detect modifications at certain sites for which there are putative guide RNAs (Table 1). Thus, we did not see pseudouridylation of U1 snRNA at U22 (Figure 1, C and D). The putative guide RNA for this modification, pug28S-3327, is quite abundant by Northern blot analysis (Yuan et al., 2003) and concentrates in nucleoli (data not shown). We also failed to detect 2′_-O-_methylation at the A31 position in Drosophila U2 snRNA isolated from several developmental stages (Figure 2, B and D). In this case the theoretical guide RNA was not detected by RT-PCR from total RNA, again isolated from several different stages (data not shown). Finally, we did not see pseudouridylation at U59 in U4 snRNA isolated from different stages (Figure 3, C and D), even though the putative guide RNA, pug28S-3186, is abundant (Yuan et al., 2003).
In Vitro Assays to Test the Predicted Role of Novel Box C/D RNAs in snRNA Modification
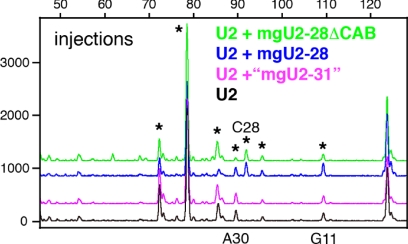
We used Xenopus oocytes and their GVs for two assays of snRNA modification. In the first assay we injected in vitro–transcribed Drosophila snRNAs into the cytoplasm of Xenopus oocytes. We isolated GVs from the injected oocytes after overnight incubation and tested for 2′_-O-_methylation of the injected RNAs by primer extension. By choosing suitable oligonucleotides as primers, we could selectively distinguish the injected Drosophila snRNA from endogenous Xenopus snRNAs. When in vitro–transcribed Drosophila U2, U4, or U5 snRNAs were injected into oocytes, they became methylated, showing that endogenous Xenopus guide RNPs can recognize and direct the modification of in vitro–transcribed Drosophila snRNAs. U4 and U5 were modified in the pattern shared by Xenopus and Drosophila (data not shown). However, when Drosophila U2 snRNA was injected into oocytes, it was methylated in the pattern expected for Xenopus U2 rather than that of Drosophila U2. Thus, 2′_-O-_methylation occurred at two _Xenopus_-specific sites (A30 and G11) and at all sites shared between Xenopus and Drosophila. Significantly, the _Drosophila_-specific methylation at C28 was not detected (Figure 4). The predicted guide RNA for methylation of C28 in Drosophila is mgU2-28. Thus, we could test the ability of Drosophila mgU2-28 to function as a guide RNA by injecting it into the Xenopus oocyte along with unmodified Drosophila U2 snRNA. In this case we injected U2 into the cytoplasm and mgU2-28 directly into the GV. After overnight incubation the injected U2 was tested for modification by primer extension. We detected an extra peak corresponding to 2′_-O-_methylation of C28 (Figure 4). This experiment demonstrates that in vitro–transcribed Drosophila mgU2-28 can be assembled into an efficient modification RNP in the Xenopus GV and act as the guide for its predicted site in Drosophila U2 snRNA. As a negative control we injected a 30-nt fragment of mgU2-28 that contains only the region complementary to the U2 target (underlined) and adjacent sequences (GgUUACCUUGCACUUUGAUCGCUGAGGCAG). This truncated RNA did not support methylation at C28 (data not shown).
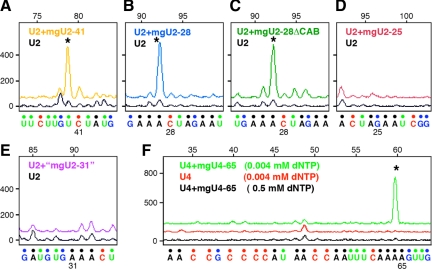
Figure 4.
Modification of Drosophila U2 snRNA injected into Xenopus oocytes. When U2 was injected alone, it became methylated in the pattern expected for Xenopus U2 (black trace); that is, at sites common to the two species (stars at Cm25, Gm34, Cm41, and Um48), at _Xenopus_-specific sites (stars at Gm11 and Am30), but not at a _Drosophila_-specific site (star at Cm28). When U2 was coinjected with the Drosophila guide for C28 (mgU2-28), an extra peak corresponding to methylation at C28 was observed (blue trace). Methylation at C28 was also observed when the CAB box of its guide RNA was mutated (green trace; mgU2-28ΔCAB). Coinjection of U2 and the putative guide “mgU2-31” did not change the modification pattern (purple trace). If this guide RNA were functional, an additional peak at A31 would be present.
Our second assay for snRNA modifications involves a Xenopus GV extract to which we add unmodified Drosophila snRNAs and putative guide RNAs. It has been known for a long time that intact GVs isolated in mineral oil (as opposed to a saline solution) retain physiological activity for at least 16 h, including RNA transcription (Lund and Paine, 1990; Paine et al., 1992) and modification of injected U2 snRNA (Yu et al., 2001). When we work with a GV extract, as opposed to intact GVs, we can remove endogenous Xenopus RNAs with micrococcal nuclease. Thus we can use a depleted Xenopus GV extract to examine the modification of in vitro–transcribed Drosophila snRNAs by in vitro–transcribed guide RNAs. In this way we can directly test whether a putative guide RNA mediates a specific modification. A modification reaction was scored as positive if the peak corresponding to the predicted modification was higher than the control (no guide RNA) when the primer extension reaction was performed at low dNTP concentration, but was similar to the control at high dNTP concentration. The in vitro method was successful with U2 and U4 snRNAs, but for some reason in vitro–transcribed U5 snRNA was unstable when incubated in Xenopus GV extracts. We examined five putative guide RNAs using the in vitro method, three of which gave positive results.
mgU2-28.
Methylation at C28 is Drosophila specific, not being found in vertebrate U2. This modification was carried out by Drosophila mgU2-28 in the in vitro assay (Figure 5B), as it was in the injection experiment described earlier (Figure 4).
Figure 5.
Modification of Drosophila U2 snRNA in RNA-depleted Xenopus GV extract. In each case in vitro–transcribed U2 snRNA was added to the extract along with a putative in vitro–transcribed guide RNA. (A) Addition of guide RNA mgU2-41 resulted in a peak of methylation at C41 (yellow trace, star) that was higher than in the control without the guide (black trace). (B) Guide RNA mgU2-28 produced a strong methylation peak at C28 (blue trace, star). (C) Methylation at C28 was also observed when the CAB box of guide RNA mgU2-28 was mutated (mgU2-28ΔCAB, green trace, star). (D) The putative guide RNA mgU2-25 had no effect on methylation (red trace). (E) The putative guide RNA “mgU2-31” had no effect on methylation (purple trace). (F) Modification of Drosophila U4 snRNA in RNA-depleted Xenopus GV extract. Each trace represents a primer extension reaction in which in vitro–transcribed U4 snRNA was added to the extract. Addition of guide RNA mgU4-65 resulted in a peak of methylation at A65 (green trace, star). No peak was seen in the control without the guide (red trace) or in a reaction with the guide but carried out at high concentration of dNTPs (black trace).
mgU2-41.
Methylation at C41 occurs normally in Drosophila U2 (Figure 2, B and D). The modification was carried out by Drosophila mgU2-41 in the in vitro assay (Figure 5A).
mgU4-65.
Methylation at A65 occurs normally in Drosophila U4 (Figure 3B). The modification was carried out by mgU4-65 in the in vitro assay (Figure 5F).
mgU2-25.
Methylation of Drosophila U2 at G25 was detectable in all RNA samples that we analyzed from several different stages and strains of flies (Figures 2, B and D). Furthermore, the putative guide RNA, mgU2-25 is easily demonstrable by Northern blotting (Tycowski et al., 2009; Figure 6). Nevertheless, we failed to detect this modification when in vitro–transcribed U2 was incubated with in vitro–transcribed mgU2-25 (Figure 5D). It is possible that some posttranscriptional modifications must occur in a defined sequence and that a partially modified substrate might be required for mgU2-25 to function. Alternatively, methylated G25 may fail to arrest reverse transcriptase in the primer extension reaction. We noted that the G25 modification was difficult to detect in Drosophila U2 that had been injected into Xenopus oocytes (Figure 4). Similarly, methylation of G25 (and G19) was not detected in an earlier study in which transiently expressed human U2 was analyzed in simian COS-7 cells (Jády et al., 2003).
Figure 6.
Northern blots of Drosophila scaRNAs. For each scaRNA there are three lanes that contain total RNA from adult y w, coil199, and coil 203 flies, respectively. Both coil199 and coil 203 are coilin-null mutants that have no detectable CBs in their cells; y w flies serve as controls. In each case the abundance of the scaRNA is approximately the same for mutant and control flies. Seven Northern blots were run separately with their own size markers. Images of the autoradiographs were grouped into one figure with a common size scale in nucleotides.
mgU2-31 (MeU2-A31).
FlyBase (http://flybase.org) lists snoRNA:MeU2-A31 as a putative guide RNA for methylation of A31 in U2 snRNA. However, we were not able to detect an endogenous RNA corresponding to this sequence by RT-PCR, nor did we see the A31 modification itself in U2 RNA isolated from flies at different stages (Figure 2, B and D). Thus we were not surprised to find that in vitro–transcribed MeU2-A31 failed to support modification of U2 in our in vitro assays, both by injection and in GV extract (Figures 4 and 5E). Furthermore, the predicted sequence of this box C/D RNA does not have canonical C- and D-box motifs or typical secondary structure. For these reasons we think it is unlikely that the sequence identified as MeU2-A31 is a genuine guide RNA in Drosophila.
Box C/D RNAs That Guide Modifications in snRNAs U2, U4, and U5 Are Concentrated in CBs
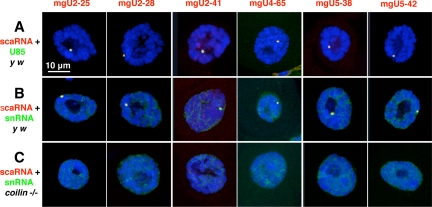
Drosophila box C/D guide RNAs possess a conserved motif called the CAB box, which is assumed to be a CB-specific localization signal (Tycowski et al., 2009). To determine whether these RNAs are all found in the CB of Drosophila, we carried out two-color FISH experiments, using Drosophila U85 scaRNA as a CB marker. In earlier studies we showed that U85 is specifically localized in the CB of various somatic and germline cells (Liu et al., 2006b, 2009). In every case the guide RNAs were colocalized with U85 in the CB. Representative images of Malpighian tubule nuclei are shown in Figure 7A. In addition we carried out two-color FISH experiments using the guide RNAs and their corresponding target snRNAs. Again, we saw colocalization in the CB of Malpighian tubule cells (Figure 7B). Unlike scaRNAs, which can be detected only in the CB, the snRNAs are concentrated in the CB but also occur diffusely throughout the nucleoplasm (Figure S2). We also carried out two-color FISH experiments with similar results on a variety of other tissues, including adult ovary and testis, as well as larval salivary glands, Malpighian tubules, fat bodies, and brains (data not shown).
Figure 7.
Confocal images of Malpighian tubule nuclei after two-color FISH for scaRNAs and their cognate snRNAs. The three images in each column show nuclei hybridized with a probe for the scaRNA listed at the top of the column (red probe). The six nuclei in each row were hybridized with U85 scaRNA (A) or the cognate snRNA for the guide RNA (B and C). For instance, the first nucleus in row A was hybridized with probes for mgU2-25 scaRNA (red) and U85 scaRNA (green), whereas the last nucleus in row B was hybridized with probes for mgU5-42 (red) and U5 snRNA (green). Nuclei in rows A and B are from control y w flies and show an easily detectable CB that is labeled with both probes. Nuclei in row C are from coilin-null flies in which a CB is not detectable. All nuclei are stained with the DNA-specific dye DAPI (blue).
CB Localization Is Not Required for Modification of snRNAs
The high concentration of guide RNAs and their corresponding snRNA substrates in the CB suggests that modification itself normally occurs in the CB. If this is the case, the question arises whether CB localization is required for snRNA modification. To address this issue, we examined snRNA modification in flies mutant for the gene that codes for coilin protein. Coilin is widely used as a marker for the CB and occurs at high concentration in Drosophila CBs. We recently described two coilin mutants (coil199 and coil203) that are homozygous viable and fertile despite being completely null for coilin. These flies not only lack coilin, but they fail to form distinct foci of other CB markers, including fibrillarin, the survival motor neuron (SMN) protein, U2, U4, and U5 snRNA, and U85 scaRNA (Liu et al., 2009; Figure 7C). Distinct foci are also lacking for the Box C/D scaRNAs described here: mgU2-25, mgU2-28, mgU2-41, mgU4-65, mgU5-38, and mgU5-42 (Figure 7C). Despite their failure to concentrate in cytologically detectable CBs, the guide RNAs occur at normal levels in tissues of the coilin null mutants, as shown by Northern blots (Figure 6). Furthermore, snRNAs from the mutants are modified at all of the usual sites (Figures 1, B and C, 2, B and C, and 3, B and C; Supplemental Figure S1). These data show that efficient posttranscriptional modification of snRNAs does not depend on concentration of the snRNAs or their modification guide RNAs in cytologically detectable CBs. Furthermore, snRNA modification in coilin-null flies shows that coilin itself is not involved in the modification process, as previously demonstrated in cells from coilin knockout mice (Jády et al., 2003).
The CAB Box Is Not Required for Modification of an snRNA
Earlier experiments showed that the CAB box of U85 scaRNA is required for appropriate localization to the CB (Richard et al., 2003). Furthermore, localization of human scaRNA ACA57 to the CB was recently shown to require binding of a conserved WD40 protein to the CAB box (Tycowski et al., 2009). These experiments raise the question whether the CAB box and the WD40 protein might also be required for the modification reactions themselves. Our in vitro modification assays allow us to test this question directly. We synthesized a mgU2-28 molecule in which the Drosophila CAB box (CGCCUUAAUG) was replaced with the sequence CGCG. When injected into Xenopus oocytes along with unmodified U2 snRNA, the mutated form of mgU2-28 was fully functional as a guide RNA for methylation of C28 (Figure 4). Similarly, the mutated mgU2-28 was functional in our second assay system, which uses a cell-free extract made from oil-isolated GVs (Figure 5C). Thus, the CAB box is not required for mgU2-28 to function as a guide RNA. It is probable, therefore, that the WD40 protein does not play a direct role in the modification reaction.
DISCUSSION
It has been known for a long time that rRNA and splicing snRNAs undergo 2′_-O-_methylation and pseudouridylation at specific nucleotides. These modifications are controlled by guide RNAs acting in concert with a corresponding methyltransferase (fibrillarin) and pseudouridylase (NAP57/dyskerin; reviewed in Reddy and Busch, 1988; Matera et al., 2007). It is generally believed that modification of rRNA takes place in the nucleolus. This view is consistent with the fact that rRNA is transcribed in the nucleolus, where it is rapidly assembled into ribosomal subunits and permanently exported to the cytoplasm. On the other hand, four of the snRNAs, U1, U2, U4, and U5, are transcribed in the nucleus and exported to the cytoplasm for assembly into snRNP particles, and then they return to the nucleus. Thus the cytological site of their modification is not obvious from the maturation pathway.
That modification of U2 snRNA occurs in the nucleus was shown by injection experiments using Xenopus oocytes (Yu et al., 2001) and confirmed by transient expression of U2 constructs in COS-7 cells (Jády et al., 2003). Specific evidence for involvement of the CB in snRNA modification came from the discovery of U85 scaRNA (Jády and Kiss, 2001) and the demonstration that it and other scaRNAs are localized in the CB of HeLa cells (Darzacq et al., 2002; Jády et al., 2003; Richard et al., 2003). Localization in the CB is mediated by an evolutionarily conserved sequence (UGAG), referred to as the CB-specific localization sequence or CAB box. This sequence was identified in U85 and several other scaRNAs from human, Drosophila, and Arabidopsis (Richard et al., 2003). Recently, several new box C/D scaRNAs have been described from Drosophila. These contain a related but somewhat different CAB box, which was shown to bind a conserved WD40 protein. Furthermore the CB localization of human ACA57 scaRNA was found to depend on its association with the WD40 protein (human WDR79; Tycowski et al., 2009).
The conventional view, therefore, is the following. U1, U2, U4, and U5 are transcribed in the nucleus and quickly exported to the cytoplasm, where they assemble into snRNP particles. They then return to the nucleus, specifically to the CB (Sleeman and Lamond, 1999). In the CB they undergo 2′_-O-_methylation and pseudouridylation, guided by scaRNAs. The scaRNAs themselves are targeted to the CBs by their CAB boxes in association with the WD40 protein.
Elegant experiments from the Kiss laboratory provided strong evidence that snRNA modification takes place in the CB itself. Their key experiments involved targeting short fragments of snRNAs to the CB and to the nucleolus. Fragments of U2 and U5 snRNA were targeted to the CB by inserting them into the coding region of U87 scaRNA and transfecting into mouse cells. Localization of the construct in the CB was demonstrated by FISH, and modification at the expected positions in the fragments was revealed by modification mapping (Jády et al., 2003). On the other hand, when the U2 and U5 fragments were targeted to the nucleolus by insertion into the mouse pW ribosomal minigene, they did not become modified (Ganot et al., 1999; Jády et al., 2003). These experiments demonstrate that the activity responsible for modification of U2 and U5 snRNAs is absent from the nucleolus. They also show clearly that modification occurs when the U2 or U5 fragment is attached to a scaRNA (U87), some of which is detectable in the CB. We would argue that the experiment does not formally rule out the possibility that some of the modification machinery exists at lower concentration in the nucleoplasm, where it could be functional.
We will discuss evidence from three sources that modification of snRNAs can occur in the nucleoplasm: coilin mutants, Xenopus GVs, and scaRNAs in the nucleoplasm.
Coilin Mutants
Our earlier studies showed that almost all cells in wild-type Drosophila have prominent CBs detectable with probes for a variety of standard CB components, including coilin, fibrillarin, SMN, U2 and U5 snRNAs, and U85 scaRNA (Liu et al., 2006b, 2009). In the present study we show concentration of six additional scaRNAs in Drosophila CBs: mgU2-25, mgU2-28, mgU2-41, mgU4-65, mgU5-38, and mgU5-42. By showing colocalization of the snRNAs with their guide RNAs, these observations on wild-type flies fit the hypothesis that snRNA modification itself occurs in the CB.
Questions began to arise when we first obtained two coilin-null mutants, coil199 and coil203. CBs were not detectable in cells of the mutants using antibody or FISH probes for any of the usual CB components (Liu et al., 2009 and Figure 7C). Because the mutant flies were fully viable and fertile, it seemed unlikely that they were deficient in important aspects of U snRNP biogenesis. The results presented here show that they are not: scaRNAs are present at the same level as in wild-type flies (Figure 6), and more importantly, U1, U2, U4, and U5 snRNAs are properly modified in the mutants (Figures 1–3; Supplemental Figure S1). Clearly, therefore, the modification machinery exists and is functional in Drosophila cells that lack CBs by standard morphological and molecular criteria.
Coilin-null mutants are also known for two other organisms, Arabidopsis and the mouse. As in Drosophila, the Arabidopsis coilin mutant (no cajal body-1 or ncb-1) lacks morphologically detectable CBs, yet the plant is perfectly viable (Collier et al., 2006). Biochemical data are not available, but the absence of a gross organismal phenotype suggests that splicing is not seriously affected, as it would be if snRNA modification were impaired (Yu et al., 1998; Zhao and Yu, 2004, 2007). The evidence from the coilin knockout mouse is less clear. These mice exhibit a semilethal phenotype in which some individuals die as embryos, and the surviving adults have significant fertility and fecundity defects (Walker et al., 2009). Cultured cells derived from the knockouts exhibit three types of “residual” bodies, each of which contains a subset of CB components. In principle, therefore, snRNA modification could take place in the residual bodies that contain snRNAs and their guide RNAs (Tucker et al., 2001; Jády et al., 2003).
Xenopus GV
The Xenopus GV provides additional evidence that scaRNAs can function in the nucleoplasm. Shortly after the first description of scaRNAs, a Xenopus H/ACA guide RNA was found that directs pseudouridylation of U2 snRNA at two different sites. This guide RNA, designated pugU2-34/44, was localized in the nucleoplasm of the GV, not in the nucleoli or CBs (Zhao et al., 2002). The corresponding human guide RNA is scaRNA U92 (Darzacq et al., 2002). Our experiments with Xenopus GV extract demonstrate clearly that snRNA modification can take place in the nucleoplasm in the absence of CBs. In this case, GVs are collected from living oocytes under oil and centrifuged to remove the lampbrush chromosomes, nucleoli, CBs, and other nuclear organelles. The resulting extract can be depleted of endogenous snRNAs and scaRNAs by treatment with micrococcal nuclease. The extract supports modification of in vitro–transcribed Drosophila U2 and U4 snRNAs by _Drosophila-_specific guide RNAs. Modification even takes place when the guide RNA mgU2-28 lacks the canonical CB localization sequence (CAB box). These data show conclusively that modification can take place in the nucleoplasm of Xenopus GVs and that the CB localization signal itself is not required.
scaRNAs in the Nucleoplasm?
How can the data from coilin mutants and the Xenopus GV be reconciled with the strong evidence that CBs are involved in snRNA modification? We believe that the answer is reasonably straightforward. We suggest that under normal physiological conditions scaRNAs occur both in CBs and in the nucleoplasm. Because their concentration is high in CBs, they are detectable there by in situ hybridization, whereas their low concentration in the nucleoplasm puts them below the usual level of detection. This hypothesis simply extends to scaRNAs what is well known for other CB components, particularly from FRAP (fluorescence recovery after photobleaching) experiments (Handwerger et al., 2003; Deryusheva and Gall, 2004; Dundr et al., 2004). Even coilin, the canonical CB marker, is widely distributed in the nucleoplasm, although it is concentrated in CBs (Bellini and Gall, 1998; Matera, 1998). We suggest that technical issues lead to the mistaken impression that scaRNAs are limited to CBs and by extension must function there.
This impression is further fostered by the common assertion that CBs are “universal nuclear organelles,” of which we are as guilty as any (Gall, 2003). CBs have been identified in many eukaryotic organisms including mammals, amphibians, insects, angiosperm plants, and probably budding yeast. In the sense of phylogenetic distribution, it is probably safe to speak of CBs as universal nuclear organelles. On the other hand, it is easy to find individual cells or cell types that lack morphologically detectable CBs, and in this important sense CBs are not universal organelles. In the first articles to describe coilin, the authors examined five human and one marsupial cell lines, and five tissue types from the mouse. The number of CBs per nucleus varied from 0 to 6, with up to 20% of some cell types having no detectable body (Andrade et al., 1991; Raska et al., 1991). In a recent study only 15% of human WI-38 cells showed focal staining for coilin (Ghule et al., 2008). CBs are absent from spermatocytes and from germline stem cells in the female germarium of Drosophila (Liu et al., 2009). CBs are absent or are difficult to demonstrate in some adult vertebrate tissues (Young et al., 2000), whereas they are prominent in others, such as nerve cells (Pena et al., 2001). It is reasonable to suppose that snRNA modification and splicing occur in all of these normal cells that lack CBs. Thus, general considerations have never favored the view that specific nuclear functions must invariably take place in cytologically defined CBs.
Concluding Remarks
We suggest that scaRNAs and other CB components normally exist in the nucleoplasm as macromolecular complexes, which are too small to be resolved individually by conventional light microscopy, but appear as background fluorescence after immunostaining or FISH. These complexes can assemble into larger aggregates, which are then resolved as CBs of various sizes (Kaiser et al., 2008). Coilin is essential for the assembly process, as shown by the absence of CBs in coilin-null Drosophila, Arabidopsis, and mouse. However, assembly into cytologically detectable CBs is not required for function, at least not for the scaRNA-dependent modification of splicing snRNAs.
Given the fact that cells can manage without CBs, the question remains: what role do CBs play when they are present? A common suggestion is that CB formation leads to an increase in effective concentration of macromolecules and hence increased activity based on mass action. Another possibility is that CBs might sequester specific components and thereby regulate the rate of reactions in the nucleoplasm. Future studies will need to address the extent to which CBs act as positive or negative regulators of specific biochemical reactions.
Supplementary Material
[Supplemental Materials]
ACKNOWLEDGMENTS
We thank Allison Pinder and Alison Singer for technical help. This work was supported by National Institutes of Health Grant GM-33397 to J.G.G. J.G.G. is American Cancer Society Professor of Developmental Genetics.
Abbreviations used:
CB
Cajal body
GV
germinal vesicle
scaRNA
small Cajal body–specific RNA.
Footnotes
REFERENCES
- Andrade L.E.C., Chan E.K.L., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J. Exp. Med. 1991;173:1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini M., Gall J. G. Coilin can form a complex with the U7 small nuclear ribonucleoprotein. Mol. Biol. Cell. 1998;9:2987–3001. doi: 10.1091/mbc.9.10.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer K. J., Trautman J. K., Bozas A., Liu J.-L., Rutter J., Gall J. G., Carroll D. Efficient gene targeting in Drosophila by direct embryo injection with zinc-finger nucleases. Proc. Natl. Acad. Sci. USA. 2008;105:19821–19826. doi: 10.1073/pnas.0810475105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Tollervey D., Barabino S.M.L., Merdes A., Brunner C., Zamore P. D., Green M. R., Hurt E., Lamond A. I. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 1991;10:195–206. doi: 10.1002/j.1460-2075.1991.tb07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Pendle A., Boudonck K., van Rij T., Dolan L., Shaw P. A distant coilin homologue is required for the formation of Cajal bodies in Arabidopsis. Mol. Biol. Cell. 2006;17:2942–2951. doi: 10.1091/mbc.E05-12-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X., Jády B. E., Verheggen C., Kiss A. M., Bertrand E., Kiss T. Cajal body-specific small nuclear RNAs: a novel class of 2′-O-methylation and pseudouridylation guide RNAs. EMBO J. 2002;21:2746–2756. doi: 10.1093/emboj/21.11.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryusheva S., Gall J. G. Dynamics of coilin in Cajal bodies of the Xenopus germinal vesicle. Proc. Natl. Acad. Sci. USA. 2004;101:4810–4814. doi: 10.1073/pnas.0401106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M., Hebert M. D., Karpova T. S., Stanek D., Xu H., Shpargel K. B., Meier U. T., Neugebauer K. M., Matera A. G., Misteli T. In vivo kinetics of Cajal body components. J. Cell Biol. 2004;164:831–842. doi: 10.1083/jcb.200311121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlyBase. A Database of Drosophila Genes & Genomes. MeU2–A31. FlyBase Annotation ID: CR34705. 2009. [accessed 29 October 2009]. http://flybase.org.
- Gall J. G. The centennial of the Cajal body. Nat. Rev. Mol. Cell Biol. 2003;4:975–980. doi: 10.1038/nrm1262. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Callan H. G., Wu Z., Murphy C. Lampbrush chromosomes. In: Kay B. K., Peng H. B., editors. Xenopus laevis: Practical Uses in Cellular and Molecular Biology. vol. 36. San Diego, CA: Academic Press; 1991. pp. 149–166. [Google Scholar]
- Ganot P., Jády B. E., Bortolin M. L., Darzacq X., Kiss T. Nucleolar factors direct the 2′-O-ribose methylation and pseudouridylation of U6 spliceosomal RNA. Mol. Cell. Biol. 1999;19:6906–6917. doi: 10.1128/mcb.19.10.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghule P. N., Dominski Z., Yang X. C., Marzluff W. F., Becker K. A., Harper J. W., Lian J. B., Stein J. L., van Wijnen A. J., Stein G. S. Staged assembly of histone gene expression machinery at subnuclear foci in the abbreviated cell cycle of human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:16964–16969. doi: 10.1073/pnas.0809273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace T. D. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962;195:788–789. doi: 10.1038/195788a0. [DOI] [PubMed] [Google Scholar]
- Handwerger K. E., Murphy C., Gall J. G. Steady-state dynamics of Cajal body components in the Xenopus germinal vesicle. J. Cell Biol. 2003;160:495–504. doi: 10.1083/jcb.200212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. P., Zhou H., He H. L., Chen C. L., Liang D., Qu L. H. Genome-wide analyses of two families of snoRNA genes from Drosophila melanogaster, demonstrating the extensive utilization of introns for coding of snoRNAs. RNA. 2005a;11:1303–1316. doi: 10.1261/rna.2380905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. P., Zhou H., Qu L. H. Maintaining a conserved methylation in plant and insect U2 snRNA through compensatory mutation by nucleotide insertion. IUBMB Life. 2005b;57:693–699. doi: 10.1080/15216540500306983. [DOI] [PubMed] [Google Scholar]
- Jády B. E., Darzacq X., Tucker K. E., Matera A. G., Bertrand E., Kiss T. Modification of Sm small nuclear RNAs occurs in the nucleoplasmic Cajal body following import from the cytoplasm. EMBO J. 2003;22:1878–1888. doi: 10.1093/emboj/cdg187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády B. E., Kiss T. A small nucleolar guide RNA functions both in 2′-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J. 2001;20:541–551. doi: 10.1093/emboj/20.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser T. E., Intine R. V., Dundr M. De novo formation of a subnuclear body. Science. 2008;322:1713–1717. doi: 10.1126/science.1165216. [DOI] [PubMed] [Google Scholar]
- Kiss T., Jády B. E. Functional characterization of 2′-O-methylation and pseudouridylation guide RNAs. Methods Mol. Biol. 2004;265:393–408. doi: 10.1385/1-59259-775-0:393. [DOI] [PubMed] [Google Scholar]
- Liu J.-L., Wu Z., Nizami Z., Deryusheva S., Rajendra T. K., Gao H., Beumer K. J., Carroll D., Matera A. G., Gall J. G. Coilin is essential for Cajal body organization in Drosophila melanogaster. Mol. Biol. Cell. 2009;20:1661–1670. doi: 10.1091/mbc.E08-05-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. L., Buszczak M., Gall J. G. Nuclear bodies in the Drosophila germinal vesicle. Chromosome Res. 2006a;14:465–475. doi: 10.1007/s10577-006-1062-5. [DOI] [PubMed] [Google Scholar]
- Liu J. L., Murphy C., Buszczak M., Clatterbuck S., Goodman R., Gall J. G. The Drosophila melanogaster Cajal body. J. Cell Biol. 2006b;172:875–884. doi: 10.1083/jcb.200511038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Paine P. L. Nonaqueous isolation of transcriptionally active nuclei from Xenopus oocytes. Methods Enzymol. 1990;181:36–43. doi: 10.1016/0076-6879(90)81110-g. [DOI] [PubMed] [Google Scholar]
- Matera A. G. Of coiled bodies, gems, and salmon. J. Cell Biochem. 1998;70:181–192. [PubMed] [Google Scholar]
- Matera A. G., Terns R. M., Terns M. P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007;8:209–220. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- Matera A. G., Ward D. C. Nucleoplasmic organization of small nuclear ribonucleoproteins in cultured human cells. J. Cell Biol. 1993;121:715–727. doi: 10.1083/jcb.121.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslinski E., Branlant C., Wieben E. D., Pederson T. The small nuclear RNAs of Drosophila. J. Mol. Biol. 1984;180:927–945. doi: 10.1016/0022-2836(84)90264-x. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Johnson M. E., Lau Y. T., Tluczek L. J., Miller D. S. The oocyte nucleus isolated in oil retains in vivo structure and functions. Biotechniques. 1992;13:238–246. [PubMed] [Google Scholar]
- Pena E., Berciano M. T., Fernandez R., Ojeda J. L., Lafarga M. Neuronal body size correlates with the number of nucleoli and Cajal bodies, and with the organization of the splicing machinery in rat trigeminal ganglion neurons. J. Comp. Neurol. 2001;430:250–263. doi: 10.1002/1096-9861(20010205)430:2<250::aid-cne1029>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L.E.C., Ochs R. L., Chan E.K.L., Chang C.-M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp. Cell Res. 1991;195:27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Reddy R., Busch H. Small nuclear RNAs: RNA sequences, structure, and modification. In: Birnstiel M. L., editor. Structure and Function of Major and Minor Small Nuclear Ribonucleoprotein Particles. Berlin: Springer; 1988. pp. 1–37. [Google Scholar]
- Richard P., Darzacq X., Bertrand E., Jády B. E., Verheggen C., Kiss T. A common sequence motif determines the Cajal body-specific localisation of box H/ACA scaRNAs. EMBO J. 2003;22:4283–4293. doi: 10.1093/emboj/cdg394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman J. E., Lamond A. I. Newly assembled snRNPs associate with coiled bodies before speckles, suggesting a nuclear snRNP maturation pathway. Curr. Biol. 1999;9:1065–1074. doi: 10.1016/s0960-9822(99)80475-8. [DOI] [PubMed] [Google Scholar]
- Spector D. L. Nuclear organization of pre-mRNA processing. Curr. Opin. Cell Biol. 1993;5:442–447. doi: 10.1016/0955-0674(93)90009-f. [DOI] [PubMed] [Google Scholar]
- Szkukalek A., Myslinski E., Mougin A., Luhrmann R., Branlant C. Phylogenetic conservation of modified nucleotides in the terminal loop 1 of the spliceosomal U5 snRNA. Biochimie. 1995;77:16–21. doi: 10.1016/0300-9084(96)88099-0. [DOI] [PubMed] [Google Scholar]
- Tucker K. E., Berciano M. T., Jacobs E. Y., LePage D. F., Shpargel K. B., Rossire J. J., Chan E.K.L., Lafarga M., Conlon R. A., Matera A. G. Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product. J. Cell Biol. 2001;154:293–307. doi: 10.1083/jcb.200104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Kukoyi A., Steitz J. A. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol. Cell. 2009;34:47–57. doi: 10.1016/j.molcel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. P., Tian L., Matera A. G. Reduced viability, fertility and fecundity in mice lacking the Cajal body marker protein, coilin. PLoS One. 2009;4:e6171. doi: 10.1371/journal.pone.0006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes: III. Optimum incubation conditions. J. Exp. Zool. 1973;184:321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Young P. J., Le T. T., Man N. T., Burghes A.H.M., Morris G. E. The relationship between SMN, the spinal muscular atrophy protein, and nuclear coiled bodies in differentiated tissues and cultured cells. Exp. Cell Res. 2000;256:365–374. doi: 10.1006/excr.2000.4858. [DOI] [PubMed] [Google Scholar]
- Yu Y.-T., Terns R. M., Terns M. P. Mechanisms and functions of RNA-guided RNA modification. In: Grosjean H., editor. Topics in Current Genetics: Fine-Tuning of RNA Functions by Modification and Editing. vol. 12. Berlin: Springer; 2004. pp. 223–262. [Google Scholar]
- Yu Y. T., Shu M. D., Narayanan A., Terns R. M., Terns M. P., Steitz J. A. Internal modification of U2 small nuclear (sn)RNA occurs in nucleoli of Xenopus oocytes. J. Cell Biol. 2001;152:1279–1288. doi: 10.1083/jcb.152.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. T., Shu M. D., Steitz J. A. Modifications of U2 snRNA are required for snRNP assembly and pre-mRNA splicing. EMBO J. 1998;17:5783–5795. doi: 10.1093/emboj/17.19.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G., Klambt C., Bachellerie J. P., Brosius J., Huttenhofer A. RNomics in Drosophila melanogaster: identification of 66 candidates for novel non-messenger RNAs. Nucleic Acids Res. 2003;31:2495–2507. doi: 10.1093/nar/gkg361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li Z. H., Terns R. M., Terns M. P., Yu Y. T. An H/ACA guide RNA directs U2 pseudouridylation at two different sites in the branchpoint recognition region in Xenopus oocytes. RNA. 2002;8:1515–1525. [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yu Y. T. Pseudouridines in and near the branch site recognition region of U2 snRNA are required for snRNP biogenesis and pre-mRNA splicing in Xenopus oocytes. RNA. 2004;10:681–690. doi: 10.1261/rna.5159504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yu Y. T. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res. 2007;35:550–558. doi: 10.1093/nar/gkl1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Materials]