The Optx2 homeobox gene is expressed in early precursors of the eye and activates retina-specific genes (original) (raw)
Abstract
Vertebrate eye development begins at the gastrula stage, when a region known as the eye field acquires the capacity to generate retina and lens. Optx2, a homeobox gene of the sine oculis-Six family, is selectively expressed in this early eye field and later in the lens placode and optic vesicle. The distal and ventral portion of the optic vesicle are fated to become the retina and optic nerve, whereas the dorsal portion eventually loses its neural characteristics and activates the synthesis of melanin, forming the retinal pigment epithelium. Optx2 expression is turned off in the future pigment epithelium but remains expressed in the proliferating neuroblasts and differentiating cells of the neural retina. When an _Optx2_-expressing plasmid is transfected into embryonic or mature chicken pigment epithelial cells, these cells adopt a neuronal morphology and express markers characteristic of developing neural retina and photoreceptors. One explanation of these results is that Optx2 functions as a determinant of retinal precursors and that it has induced the transdifferentiation of pigment epithelium into retinal neurons and photoreceptors. We also have isolated optix, a Drosophila gene that is the closest insect homologue of Optx2 and Six3. Optix is expressed during early development of the fly head and eye primordia.
Development of the eye begins in a zone of primitive ectoderm at the anterior end of the gastrula stage embryo. This region, known as the eye field, is the first to acquire the potential to generate ocular tissue (1, 2). As morphogenesis proceeds, eye development becomes progressively localized to retinal primordia in the forebrain neural plate and to lens primordia located in the head ectoderm. (3, 4). One gene expressed in these ocular primordia is Pax6, a homeobox gene that encodes a transcription factor required to initiate formation of the lens (5–9). Pax6 has a highly conserved Drosophila homologue, eyeless, that also is required for insect eye development (10). Rx/rax, another homeobox gene belonging to the Pax-aristaless family, also is expressed in the early eye field (11, 12) and is essential for formation of the mouse optic vesicle (12). To better understand the role of regulatory genes in establishing the eye, we have isolated and characterized Optx2, a homeobox gene of the sine oculis family (13, 14) that is expressed in the early eye field and both ocular primordia.
MATERIALS AND METHODS
Isolation of cDNA Clones.
A library of random cDNA amplimers was prepared from embryonic day 7 (E7) chicken retina mRNA (15). Following the method of Lovett (16), 500–800-bp amplimers were hybridized at 60°C with biotinylated probe from the chicken Six3 homeobox, washed at 60°C in 0.1X SSC, selected by streptavidin capture, and cloned. Complete 3′ ends were obtained by screening an E15 chicken retina λgt10 cDNA library from Ruben Adler. 5′ ends were obtained by rapid amplification of cDNA ends-PCR (Marathon kit, CLONTECH). Full length mouse Optx2 clones were isolated from λZapII phage cDNA libraries of retinas from 3-week-old C57BL mice, provided by Donald Zack. Drosophila optix was isolated from mRNA of 18 hr Drosophila embryos (17) by lowered stringency PCR of hexanucleotide-primed cDNA with partially degenerate primers 5′-AAGAAGTTCCC(A/C)CT(G/C)CC(A/C)(A/C)G(G/C)AC(A/C)AT(A/C/T)TGG-3′ and 5′-C(T/G)(A/G)TC(C/G)C(T/G)(T/C)TG(C/G)C(T/G)(C/G)C(T/G)GTTCTTGAACCA(A/G)TT-3′ generated a 203-bp amplimer corresponding to Optx2 amino acids 117 to 181. PCR with primers 5′-ATGTTCCAGCT(G/C)CC(C/G)AC(C/G)CT(C/G)AACTTC(A/T)(C/G)(C/G)CC(C/G)GA(A/G)CA-3′(Optx2 amino acids 1–14), and 5′-GGTGGGATTCGGGTAGGGATCCTGTA-3′ (optix homeobox) gave a 467-bp amplimer clone, which was used for in situ hybridization to Drosophila embryos and polytene chromosomes.
In Situ Hybridization.
Wholemount in situ hybridization and sectioning of staged chicken embryos (18) was done as described earlier (8), with the exception that hybridization and 50% formamide post-RNase washes were both carried out at 65°C. Optx2 probe 1 extended from amino acid 1 to amino acid 184. Probe 2, 276 nt long, extended from amino acid 188 into the 3′-untranslated region. In situ hybridization to cultured cells used the same protocol but with 5-min protease pretreatment. Digoxigenin-labeled riboprobes were made from chicken visinin cDNA provided by Carol Freund, David Valle, and Ruben Adler of Johns Hopkins University. Ruben Adler and Terri Belecky-Adams of Johns Hopkins University provided iodopsin riboprobe. Chicken Chx10 probe was prepared as described (19). Drosophila embryos were fixed, dechorionated (17), and hybridized with a 467 nt optix riboprobe after the chicken protocol (8). The same probe was hybridized to Drosophila polytene chromosomes by standard methods (17) and interpreted with the help of Deborah Andrew of Johns Hopkins University. Ian Duncan of Washington University provided YAC DYEO2–19 (20).
Immunocytochemistry.
Using clarified 5% milk-saline-Tween as a blocker (21), cells fixed as described previously (8), were incubated overnight at 4°C with a 1:3,000 dilution of rabbit anti-chicken visinin antibody generously provided by Naomasa Miki and Che-Hui Kuo of Osaka University (22). Detection was with biotinylated goat anti-rabbit IgG (1 mg/ml) at a 1:200 dilution and rhodamine avidin D (5 mg/ml) at 1:500 dilution. mAb specific for chicken cone opsins (COS-1) was provided by Pál Röhlich of Semmelweis University and used at 1:100 dilution. Cells were overlaid with a 9:1 mixture of glycerol:100 mM Tris pH 8.0, followed by immunofluorescent detection of rhodamine or green fluorescent protein (GFP).
Cell Cultures and Transfection.
Retinal pigment epithelium (RPE) from E7 White Leghorn chickens was isolated (23) and trypsinized to sheets of 50–500 cells. RPE from six E7 embryos was seeded onto two 24-well plates coated by adsorption of 100 μg/ml Matrigel (Collaborative Research). Cells were allowed to attach and spread for 24 hr and then transfected. The plasmid cmv_Optx2_ was constructed by placing the full coding region of mouse Optx2 cDNA under the control of a cytomegalovirus (CMV) promoter in pKW10 (24). Transfecting DNA mixtures contained either CMVZ-BV, a plasmid expressing beta-galactosidase constructed by F. M. Boyce or pEGFP-N1, a CMV-promoter plasmid expressing GFP (CLONTECH) (25). Each well was transfected for 2 hr with a solution containing 2 μl of Lipofect AMINE in 200 μl of OptimMEM medium (Life Sciences, Gaithersburg, MD) and 160 ng of CsCl-purified plasmid DNA. For assay by in situ hybridization, the standard transfection mixture contained 60 ng of CMVZ-BV + 60 ng of cmv_Optx2_. Controls contained 120 ng of CMVZ-BV to provide the same input of DNA and CMV promoter. For fluorescent detection, the standard transfection contained 40 ng of pEGFP-N1 and 120 ng of cmv_Optx2_. Medium was then replaced with 10% fetal bovine serum-supplemented Medium 199. Alternatively, transfected cells were grown in serum-free neuronal culture medium (26), which gave better neuronal morphology. Modified for chicken cells, neuronal culture medium consisted of Medium 199 (Life Sciences) supplemented with insulin (25 μg/ml), transferrin (50 μg/ml), progesterone (20 nM), putrescine (100 μM), sodium selenite (30 nM), basic fibroblast growth factor (bFGF; 5 ng/ml), Matrigel (2 μg/ml), penicillin (100 units/ml), and streptomycin (100 μg/ml). Cultures were grown for 1–3 days and then processed for immunohistochemistry or in situ hybridization.
RESULTS
Optx2 is a Member of the sine oculis Family.
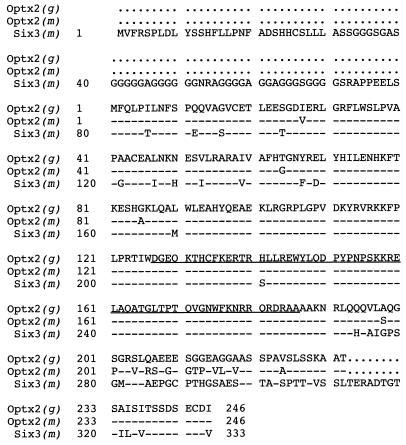
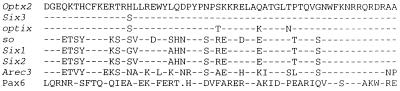
cDNA clones of chicken Optx2 (_opti_c Six gene 2) were originally isolated by hybridization with chicken Six3. Longer clones were assembled into a 1,683 nt contig (Fig. 1), which contains a 741-nt coding region and corresponds to the single 1.75-kb band we observed on Northern blots of E15 chicken embryo retina mRNA. At the N terminus of the predicted 246-aa protein is a 126-aa Six domain. In other members of the sine oculis-Six family, this region binds DNA promoter elements (27) and interacts with protein domains of transcription factors (28). This is followed by a 60-aa DNA-binding homeodomain (Fig. 2) belonging to a highly diverged family of homeobox genes (13). The C-terminal region consists of a moderately conserved 46-aa serine-rich sequence, followed by 14 highly conserved amino acids that show homology with amino acids 290–295 in the C-terminal domain of sine oculis. For the expression studies, it was essential to establish that the amino acid sequence MFQL is the correct amino terminus of Optx2. This was of concern because the Kozak consensus predicts weak initiation of translation (30) and MFQL occupies an internal position in the closely related Six3 polypeptide (29). Comparison of chicken and mouse Optx2 cDNA sequences just upstream of the predicted start site revealed no extended amino acid homology in any reading frame or alternative translation start sites. Possible splice acceptor sites that could connect to an alternative upstream exon (31) also were not found. This start site assignment is supported by sequence comparisons with human OPTX2 clones (unpublished data).
Figure 1.
Comparison of Chicken Optx2 amino acid sequence with Mouse Optx2 and Six3. Top line: chicken Optx2 amino acid sequence. Line indicates homeodomain. Second line: mouse Optx2. Third line: mouse Six3. Dash: identity to chicken Optx2. Dots indicate absence of corresponding amino acid, such as in the N terminus of Six3.
Figure 2.
Comparison of homeodomain sequences. Lines 1–8: Homeodomain amino acid sequence, Optx2, chicken and mouse. Comparison with Six3, mouse (29, 49); optix, Drosophila melanogaster; sine oculis, D. melanogaster (13, 14); Six1, mouse; Six2, mouse (41); Arec3/Six4, mouse (27; 49); and Pax6, mouse (6). Dash indicates identity to Optx2. Dot indicates absence of corresponding amino acid.
Optx2 is Expressed Throughout Chicken Eye Development.
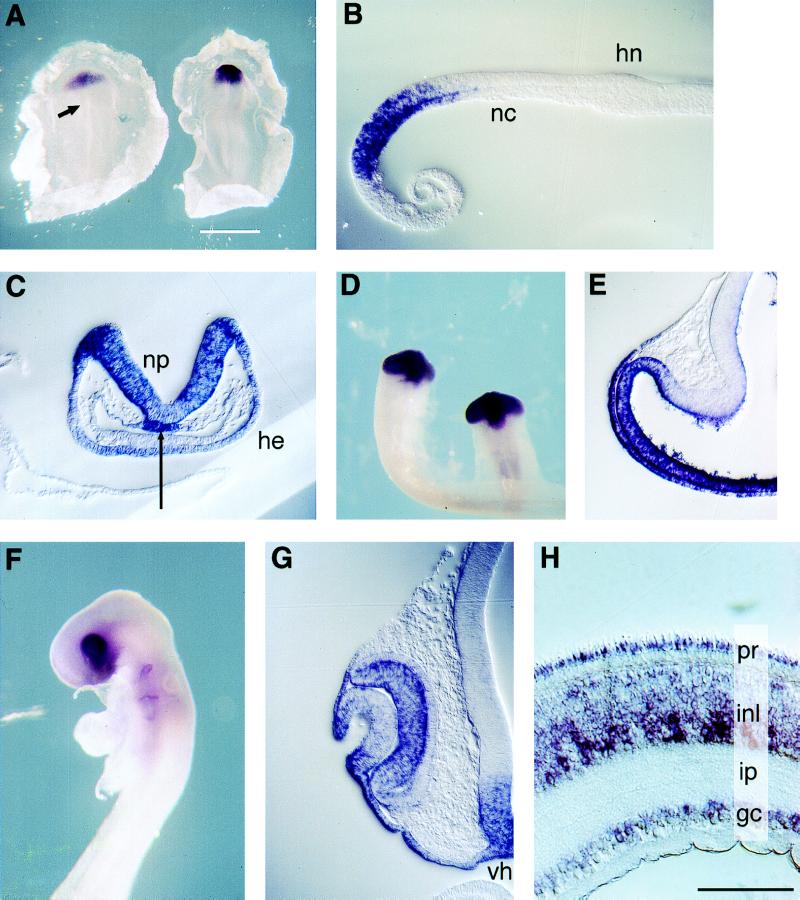
In situ hybridization of chicken embryos (stages 3–17) was carried out with two nonoverlapping antisense Optx2 riboprobes and a sense strand control. Conditions were adjusted to eliminate cross-hybridization between chicken Six3 and Optx2. The earliest expression of Optx2 was found in the prechordal mesoderm of stage 4 gastrulas (not shown). In the stage 5 gastrula, Optx2 expression was observed in prechordal plate mesoderm and in an oval domain of epiblast corresponding to the eye field (Fig. 3A and B). In contrast, stringent hybridization conditions revealed no expression of chicken Six3 in any gastrula stage chicken embryos and only low levels of expression in the early neural plate (data not shown). During the neural plate stages, Optx2 mRNA was detected throughout the presumptive forebrain, in the head ectoderm (Fig. 3 A and C), and in the prechordal plate mesoderm (Fig. 3C, arrow). After neural tube closure, Optx2 mRNA was detected in the optic vesicles and ventral forebrain, with no expression detected at more caudal levels of the body axis (Fig. 3D). Optx2 expression decreased in the dorsal forebrain, but high levels remained in the distal and ventral portions of the optic vesicle and forebrain (Fig. 3E). Within the surface ectoderm, the strongest Optx2 expression was found in the early lens placode, which overlies the optic vesicle (Fig. 3E). By the lens pit stage (stage 16), a decrease in Optx2 expression was observed in the center of the lens placode, but strong signal remained along its edges and in the nearby surface ectoderm, (Fig. 3 F and G) with expression detected in the embryonic day 4 corneal epithelium (not shown). Optx2 mRNA was detected in the undifferentiated neural retina (Fig. 3G) but was nearly absent from the outer layer of the optic cup, a region of neural epithelium that gives rise to the RPE. During later development of the neural retina, in situ hybridization detected Optx2 signal in neuroblasts (not shown) and in each layer of differentiated cells, including the photoreceptors (32) (Fig. 3H).
Figure 3.
Expression of Optx2 during chicken development. Wholemount in situ hybridization of embryos and explants; 10 μm sections. Orientation: dorsal up (B_–_G). Blue/purple signal: Optx2 mRNA. (A Left) Gastrula (st 5), dorsal view, anterior up. Signal in eye field region. Arrow: Hensen’s node. (A Right) Neural plate embryo (st 7). Signal in prospective forebrain and head ectoderm. (B) Stage 5 embryo, sagittal section, anterior left. Signal in prechordal mesoderm and overlying ectoderm of eye field. nc, notochord; hn, Hensen’s node. (C) Neural fold embryo (st 7+) transverse section through forebrain. Signal in neural plate, head ectoderm, and prechordal plate (arrow). (D) Optic vesicle stage (st 11), ventral view. Signal in the ventral forebrain and optic vesicles, with none detected at more posterior levels of the head or body. (E) Optic vesicle, forebrain (st 12+), transverse section, midline on the right. Optx2 signal restricted to distal and ventral optic vesicle, ventral forebrain. Note signal in optic vesicle neurectoderm and overlying surface ectoderm (lens placode). (F) Optic cup stage embryo (st 16). Signal in branchial arch clefts is an artifact. (G) Stage 16, transverse section, eye faces left. Note absence of signal in outer layer of optic cup (RPE). Decreased signal in the central lens placode, in contrast to the future cornea and conjunctiva. Signal in ventral hypothalamus (vh) and overlying ectoderm. (H) E17 chicken neural retina. Cone outer segments visible on upper edge of specimen. Optx2 mRNA is detected in all cell layers, including photoreceptor inner segment. pr, photoreceptors; inl, inner nuclear layer; ip, inner plexiform layer; gc, ganglion cells. (Scale: A, white bar = 1,000 μm; B, C, E, and G white bar = 100 μm; D, white bar = 500 μm; E, white bar = 300 μm; and H, black bar = 50 μm).
Optx2 Induces Phenotypic Changes in RPE Cells.
The persistence of Optx2 expression in the neural retina and its selective inactivation in the RPE could each be essential for the proper development of these tissues. To test whether the ectopic expression of Optx2 in differentiated RPE cells can alter their phenotype, primary explant cultures of RPE from day 7 embryos (stages 31–32) were transfected with the plasmid cmv_Optx2_, in which mouse Optx2 is expressed constitutively from a CMV promoter. After 48 hr, cell cultures transfected with cmv_Optx2_ showed a considerable increase in the number of cells that clearly expressed Chx10 or visinin mRNA, which are markers for the developing neural retina (Table 1). The Chx10 homeobox gene is normally expressed in retinal neuroblasts and differentiated bipolar cells. (19, 33). The visinin gene, which encodes a calcium-binding regulator of visual transduction, is highly retina-specific. It is expressed in all photoreceptors and occasionally in other retinal cell types (22). Neither Chx10 nor visinin is expressed in the RPE, either in vivo or in cell culture. Nearly all of the visinin positive cell bodies were spindle shaped or spherical. Approximately one-half of the Chx10-positive cells had spindle or spherical shapes, whereas the rest had a flat, epithelial appearance.
Table 1.
Induction of retinal markers by Optx2
| Chx10+ | visinin+ | GFP + total | GFP + visinin+ | ||||
|---|---|---|---|---|---|---|---|
| Untransfected | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reporter alone | 0 | 2 | 6 | 1 | 0 | 889 | 0 |
| Reporter + cmv_Optx2_ | 130 | 280 | 398 | 102 | 84 | 807 | 107 |
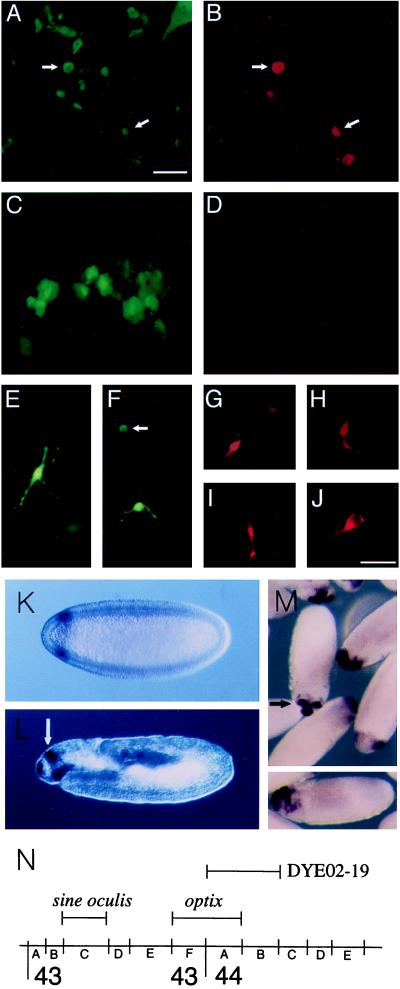
To better examine visinin expression and morphology in individual cells, a GFP reporter plasmid (pEGFP-N1) was cotransfected with cmv_Optx2_. At 72 hr after transfection, approximately one-half of the GFP-expressing cells had rounded cell bodies with one or more long, thin processes (Figs. 4 A, B, and E). Approximately 13% of cells expressing GFP also expressed visinin (Table 1; Fig. 4 A and B). In control cultures transfected with pEGFP-N1 alone, GFP-expressing cells retained their normal epithelial appearance, and none of these cells expressed visinin (Fig. 4 C and D). Although a significant fraction of the transfected cells had multiple, branched processes (Fig. 4 E), these neuron-like cells were generally visinin-negative. Most of the visinin-positive GFP-expressing cells had rounded cell bodies with two narrow processes extending from opposite ends (Fig. 4 B and F, arrow), a morphology suggestive of undifferentiated neuroepithelial cells. A smaller number were tapered at one end (Fig. 4, G and H) and extended a single process from the opposite end (Fig. 4H), a shape characteristic of cultured photoreceptors (32). The frequency of _Optx2_-mediated effects on cell phenotype were dependent on dosage of the Optx2 plasmid and appeared to be rapid. Full morphological changes and visinin expression were observed 48 hr after transfection. Expression of the red cone pigment, iodopsin, was not detected in any of the transfected cells, either with the mAb COS-1 (35) or by in situ hybridization. During normal retinal development, visinin is first expressed shortly after a cell is committed to photoreceptor differentiation, whereas iodopsin appears ≈7 days later (36). These results suggest that the culture conditions of the experiment support only the early stages of photoreceptor differentiation. In experiments assayed by in situ hybridization, rare visinin-expressing cells were found in control cultures transfected with cmv-LacZ alone. This very low level background of visinin or Chx10 expression was observed also in mock-transfected and untransfected cultures (not shown). Lipofectin reagents used in transfection did not appear to cause a substantial increase in this background. In experimental controls transfected with the GFP reporter alone, none of the GFP-expressing RPE cells showed expression of visinin.
Figure 4.
Induction of neural retina markers in RPE; Expression and map position of the Drosophila optix gene. E7 RPE cells were transfected with 120 ng of cmv_Optx2_ and 40 ng of cmvGFP (A, B, and E–J) or with 160 ng of cmvGFP plasmid DNA alone (C and D). After 72 hr, cultures were stained with anti-visinin (rhodamine). Fluorescence detection with rhodamine-red (B, D, and G_–_J) or GFP-green (A, C, E, and F). A and B depict the same set of cells, as do C and D. The cell in E is visinin-negative. In F, the cell indicated by an arrow is also visinin-positive, whereas the other cell is visinin-negative. (G and H) Visinin-positive cells induced in E7 RPE, with the appearance of immature photoreceptors developing in culture. (I and J) Visinin and GFP-positive cells derived from post-hatch RPE. (Scale: bars in A and J = 50 μm for A_–_H and J; I = 100 μm.) Drosophila: In situ hybridization with optix probe was carried out with 0–12 hr Drosophila embryos. (K) Stage 5 (2.5 hr) blastoderm embryo, anterior to left, dorsal up, Nomarski optics. Circumferential band of optix expression, 93–85% egg length. (L) 11–12 (7 hr) embryo, before germ band contraction. Parasagittal optical section, orientation as before. Optix signal in dorsal, bilaterally paired structures (white arrow), the clypeolabrum (anterior end of embryo), and roof of the stomodeum. (M) In center, stage 13 embryo, view of dorso–anterior aspect, showing signal in paired dorsal sites and clypeolabrum. (M Lower) gastrulating embryo. Dorso–anterior expression of optix. (N) Map position of optix at 43F–44A and sine oculis, as determined by hybridization to polytene chromosomes. Bar indicates extent of YAC DYE02–19, which contains optix.
Removal of the retina and the presence of acidic fibroblast growth factor (aFGF or bFGF) can trigger the conversion of embryonic RPE into neural retina, either in vivo or in vitro (37–40). This phenomenon is highly stage-dependent, occurring only in chicken embryos earlier than E5, and requires intact epithelial layers (34, 37). To determine whether our cultures of E7 RPE were susceptible to this effect, RPE cells were grown for 3 days in Medium 199 containing 20 ng/ml aFGF or bFGF and 2% serum. The cultures showed no significant increase in the background level of Chx10 or visinin-expressing cells. To determine whether the inductive effect of Optx2 is equally stage-dependent, we transfected RPE cells of post-hatch day 1 chickens with cmv_Optx2_ and pEGFP-N1. Although DNA transformation efficiency in this mature RPE was much lower than for E7 RPE, several of the cells that gave strong GFP signals exhibited altered morphology and the activation of visinin expression (Fig. 4 I and J). No expression of visinin was observed in mature RPE cells transfected with pEGFP-N1 alone.
Optix Is the Drosophila Orthologue of Optx2 and Six3.
Drosophila sine oculis has two close vertebrate homologues, Six1 and Six2, but neither is expressed during mouse eye development (41). It has therefore been suggested that Six3, a related gene that is expressed selectively in the eye, is the true functional homologue of sine oculis (29). To date, Optx2 and its close relative, Six3, are the only members of the vertebrate Six gene family known to be selectively expressed in the eye. One problem with this suggestion is that the Six1 and Six2 homeodomains show 93% and 95% identity with sine oculis, whereas Six3 shows only 70% amino acid identity. To resolve this issue, we isolated cDNA clones of a Drosophila gene, optix, which has a homeodomain that is 93% identical to Optx2. This gene appears to be the true orthologue of both Six3 and Optx2. In situ hybridization to polytene chromosomes located optix at 43F–44A (Fig. 4N), whereas PCR and Southern hybridization detected _optix_-coding sequences in the yeast artificial chromosome DYE02–19, which extends from 44A1–2 to 44B5–6 (20). This places optix very close to sine oculis, which is located at 43C (13, 14). It may be significant that the mammalian orthologues of sine oculis and optix also are genetically linked. Six2 and Six3 are closely linked on mouse chromosome 17 (29), whereas the SIX1 and OPTX2 genes map very near each other on human chromosome 14 (unpublished data). The expression patterns of Optx2 and optix are also strikingly similar, in that both are expressed during early development of the head. In situ hybridization of Drosophila embryos revealed the first expression of optix mRNA in a band around the head end of the stage 5 blastoderm embryo, at 93% to 85% egg length (Fig. 4K). By the gastrula stage, the site of expression had shifted to the dorsal–anterior region of the embryo (Fig. 4M). At stage 12, expression was found in the clypeolabrum, the stomodaeum, and in ectoderm dorsal to the future supraesophageal ganglion (Fig. 4L, arrow). This dorsal ectoderm contains two bilateral foci of optix expression (Fig. 4M, arrow) that might correspond to the eye-antennal disk primordia (43). Aside from sine oculis, examination of the Fly Base registry has not yet revealed the existence of any Drosophila eye mutants that map near the optix locus.
DISCUSSION
Expression of Optx2 During Early Head and Eye Development.
Optx2 expression is strikingly confined to the developing head, especially precursors of the eye, pituitary, and hypothalamus. In this respect, the expression pattern is similar to that of the closely related Six3 gene, but there are several significant differences. One difference is that Optx2 is expressed in the gastrula, whereas mouse (29) and chicken Six3 (unpublished results) are not. Optx2 is expressed in the primitive ectoderm of the eye field and also in the underlying prechordal plate mesoderm. The prechordal plate mesoderm is a source of inductive signals that divide the eye field into bilateral eye primordia and direct the central eye field ectoderm to establish midline structures such as the pituitary (1, 4, 44). The association of Optx2 expression with eye and pituitary development opens the possibility that mutations in this gene cause defects in both of these structures. In fact, rare human birth defects involving the complete failure of eye development and severe pituitary deficiency are associated with de novo deletions in the 14q22-q23 region of chromosome 14 (45, 46). We have found that human OPTX2 maps to 14q22-q23, suggesting that haploinsufficiency of OPTX2 could be a primary cause of the birth defects in these individuals.
Determination of Retinal Precursors.
Earlier studies have shown that overexpression of Six3 in fish embryos causes small lenses to form in the otic placodes (47). In this paper, we present evidence that ectopic expression of the related Optx2 gene can induce differentiated RPE cells to activate genes characteristic of the neural retina and assume a neuronal or neuroblast morphology. One possible interpretation is that Optx2 expression brings about the transdifferentiation of RPE cells into true precursors of photoreceptors and retinal neurons (48). If Optx2 normally functions as a determinant that establishes the retina, it is possible that its expression reactivates retinal development in differentiated RPE cells. Chicken embryonic RPE cells normally retain the capacity to transdifferentiate into retinal neurons in response to aFGF or bFGF, but this capacity is entirely lost after E4 (37–40). _Optx2_-mediated induction of visinin expression is effective with RPE from E8 or post-hatch chickens, suggesting that direct regulation of gene expression within the cell bypasses the requirement for extracellular signaling molecules and stage-dependent factors affecting competence. An alternative explanation of the results is that we are not observing true transdifferentiation, but an _Optx2-_mediated activation of Chx10 and visinin transcription that does not cause permanent or biologically relevant changes in the basic identity of the cell. Whichever explanation applies, the activation of visinin appears to be relatively specific for Optx2. We have not yet observed the phenomenon with mouse Six3, Pax6, Eya2, or other genes that we have constitutively expressed in the RPE.
The Context of Insect Eye Development.
Although the eyes of insects are structurally very different from those of vertebrates, there is growing evidence that their early development shares a central genetic program that has been extremely conserved during evolution. For example, eyeless, the Drosophila Pax6 homologue, has the remarkable ability to induce eye development at various sites on legs, wings, and antennae when expressed ectopically in imaginal discs of the larva (10). Very recently, it has been shown that transcription factors encoded by sine oculis, eyes absent, and dachshund genes also have a capacity to initiate ectopic eye development. They also interact synergistically (28, 42). On the basis of these studies, it has been suggested that a small network of genes function in a combinatorial manner to initiate insect eye development. The current molecular model is that these eye-specification genes are each required as components of a protein complex that regulates transcription during eye development. In this scheme, sine oculis binds to promoter elements via DNA-binding activities of the Six domain and homeodomain. Eyes-absent protein does not bind DNA and is instead recruited to the regulatory complex by binding to the Six domain of sine oculis (28).
It seems likely that these mechanisms and principles will apply also to the process of vertebrate eye development, but we need to be cautious in assuming that sine oculis is the Drosophila model for the vertebrate _Six-_family genes Six3 (28, 29) and Optx2. It is now clear that another gene, optix, which is specifically expressed in the early head and ocular primordia, is a much closer structural homologue. The separate structural identities of sine oculis and optix orthologues have been faithfully maintained since the divergence of insects and vertebrates, and it seems likely that this reflects the conservation of important functional differences between the two genes. For reasons that remain unclear, only the orthologues of optix appear to have retained a role in vertebrate eye development.
Acknowledgments
The authors are very grateful for key advice and reagents from Terry Belecky-Adams, Ruben Adler, Deborah Andrew, Philip Beachy, Carol Freund, Morton Goldberg, Doris von Kessler, Jeremy Nathans, David Valle, and Donald Zack of Johns Hopkins University; Ales Cvekl, Stanislav Tomarev, and Joram Piatigorsky of the National Institutes of Health, Bethesda, MD; Naomasa Miki and Che-Hui Kuo of Osaka University, Osaka Japan; Pál Röhlich of Semmelweis University, Budapest, Hungary; F. M. Boyce of Harvard University, Boston, MA, and Ian Duncan of Washington University, St. Louis, MO. We thank Ruben Adler for comments on the text. This work has been supported by a Career Development Award from Research to Prevent Blindness, the Knights Templar Eye Foundation and National Institutes of Health Grants EY10729 and EY10813 to O.H.S.
ABBREVIATIONS
RPE
retinal pigment epithelium
GFP
green fluorescent protein
aFGF or bFGF
acidic or basic fibroblast growth factor
CMV
cytomegalovirus
E
embryonic day
st
stage
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AF050131 (Optx2, gallus gallus), AF050130 (Optx2, mus musculus), and AF050132 (optix, Drosophila melanogaster)].
References
- 1.Adelmann H B. J Exp Zool. 1929;54:291–371. [Google Scholar]
- 2.Clarke L F. Physiol Zool. 1936;9:102–128. [Google Scholar]
- 3.Saha M S, Spann C L, Grainger R M. Cell Differ Dev. 1989;28:53–172. doi: 10.1016/0922-3371(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Tierney C, Wen L, Wu J Y, Rao Y. Development (Cambridge, UK) 1997;124:603–615. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaser T, Lane J, Housman D. Science. 1990;250:823–827. doi: 10.1126/science.2173141. [DOI] [PubMed] [Google Scholar]
- 6.Walther C, Gruss P. Development (Cambridge, UK) 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 7.Hill R E, Favor J, Hogan B L M, Ton C, Saunders G F, Hanson I M, Prosser J, Jordan T, Hastie N D, van Heyningen V. Nature (London) 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 8.Li H S, Yang J M, Jacobson R D, Pasko D, Sundin O. Dev Biol. 1994;162:181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- 9.Hogan B L M, Horsburgh G, Cohen J, Hetherington C M, Fisher G, Lyon M F. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- 10.Halder G, Callaerts P, Gehring W J. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa T, Kozak C A, Cepko C L. Proc Natl Acad Sci USA. 1997;94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathers P H, Grinberg A, Mahon K A, Jamrich M. Nature (London) 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 13.Cheyette B N R, Green P J, Martin K, Garren H, Hartenstein V, Zipursky S L. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 14.Serikaku M A, O’Tousa J E. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froussard P. Nucleic Acids Res. 1992;20:2900. doi: 10.1093/nar/20.11.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovett M, Kere J, Hinton M. Proc Natl Acad Sci USA. 1991;88:9628–9632. doi: 10.1073/pnas.88.21.9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardue M L. In: Drosophila, A Practical Approach. Roberts D B, editor. Oxford: IRL Press; 1986. [Google Scholar]
- 18.Hamburger V, Hamilton H. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 19.Belecky-Adams T, Tomarev S, Li H-S, Ploder L, McInnes R, Sundin O, Adler R. Invest Ophthalmol Visual Sci. 1997;38:1293–1303. [PubMed] [Google Scholar]
- 20.Ajioka J W, Smoller D A, Jones R W, Carulli J P, Vellek A E, Garza D, Link A J, Duncan I W, Hartl D L. Chromosoma. 1991;100:495–509. doi: 10.1007/BF00352200. [DOI] [PubMed] [Google Scholar]
- 21.Sundin O H, Eichele G. Development (Cambridge, UK) 1992;114:841–852. doi: 10.1242/dev.114.4.841. [DOI] [PubMed] [Google Scholar]
- 22.Yamagata K, Goto K, Kuo C H, Kondo H, Miki N. Neuron. 1990;4:469–476. doi: 10.1016/0896-6273(90)90059-o. [DOI] [PubMed] [Google Scholar]
- 23.Agata K, Kobayashi H, Itoh Y, Mochii M, Sawada K, Eguchi G. Development (Cambridge, UK) 1993;118:1025–1030. doi: 10.1242/dev.118.4.1025. [DOI] [PubMed] [Google Scholar]
- 24.Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I, Busslinger M. Genes Dev. 1992;6:1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G, Gurtu V, Kain S R. Biochem Biophys Res Commun. 1996;227:707–711. doi: 10.1006/bbrc.1996.1573. [DOI] [PubMed] [Google Scholar]
- 26.Okabe S, Forsberg-Nilsson K, Spiro A C, Segal M, McKay R D G. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K, Ohto H, Ikeda K, Roeder R G. Nucleic Acids Res. 1996;24:303–310. doi: 10.1093/nar/24.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pignoni F, Hu B, Zavitz K, Xiao J, Garrity P A, Zipursky S L. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 29.Oliver G, Mailhos A, Wehr R, Copeland N G, Jenkins N A, Gruss P. Development (Cambridge, UK) 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- 30.Kozak M. Biochimie. 1994;76:815–821. doi: 10.1016/0300-9084(94)90182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green M R. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- 32.Adler R. The Retina, Part 1. San Diego: Academic; 1986. [Google Scholar]
- 33.Liu I S, Chen J D, Ploder L, Vidgen D, van der Kooy D, Kalnins V I, McInnes R R. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 34.Reh T A, Jones M, Pittack C. CIBA Found Symp. 1991;160:192–204. doi: 10.1002/9780470514122.ch10. [DOI] [PubMed] [Google Scholar]
- 35.Cerhati P, Szel A, Röhlich P. Invest Ophthalmol Visual Sci. 1989;30:74–81. [PubMed] [Google Scholar]
- 36.Bruhn S L, Cepko C L. J Neurosci. 1996;16:1430–1439. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coulombre J L, Coulombre A J. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 38.Park C M, Hollenberg M J. Dev Biol. 1991;148:322–333. doi: 10.1016/0012-1606(91)90341-y. [DOI] [PubMed] [Google Scholar]
- 39.Pittack C, Grunwald G B, Reh T A. Development (Cambridge, UK) 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- 40.Guillemot F, Cepko C L. Development (Cambridge, UK) 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- 41.Oliver G, Wehr R, Jenkins N A, Copeland N G, Cheyette B N R, Hartenstein V, Zipursky S L, Gruss P. Development (Cambridge, UK) 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- 42.Chen R, Amoui M, Zhang Z, Mardon G. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 43.Campos-Ortega J A, Hartenstein V. The Embryonic Development of Drosophila Melanogaster. Berlin: Springer; 1996. [Google Scholar]
- 44.Chiang C, Litingtung Y, Lee E, Young K E, Corden J L, Westphal H, Beachy P A. Nature (London) 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 45.Bennett C P, Betts D R, Seller M J. J Med Genet. 1991;28:280–281. doi: 10.1136/jmg.28.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott J, Maltby E L, Reynolds B. J Med Genet. 1993;30:251–252. doi: 10.1136/jmg.30.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Mech Dev. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- 48.Adler R, Hatlee M. Science. 1989;243:391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- 49.Kawakami K, Ohto H, Takizawa T, Saito T. FEBS Lett. 1996;393:259–263. doi: 10.1016/0014-5793(96)00899-x. [DOI] [PubMed] [Google Scholar]