Urate predicts rate of clinical decline in Parkinson disease (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 1.
Abstract
Context
The risk of Parkinson disease (PD) and its rate of progression may decline with increasing blood urate, a major antioxidant.
Objective
To determine whether serum and cerebrospinal fluid (CSF) concentrations of urate predict clinical progression in patients with PD.
Design, Setting, and Participants
800 subjects with early PD enrolled in the DATATOP trial. Pre-treatment urate was measured in serum for 774 subjects and in CSF for 713.
Main Outcome Measures
Treatment-, age- and sex-adjusted hazard ratios (HRs) for clinical disability requiring levodopa therapy, the pre-specified primary endpoint.
Results
The HR of progressing to endpoint decreased with increasing serum urate (HR for 1 standard deviation increase = 0.82; 95% CI = 0.73 to 0.93). In analyses stratified by α-tocopherol treatment (2,000 IU/day), a decrease in the HR for the primary endpoint was seen only among subjects not treated with α-tocopherol (HR = 0.75; 95% CI = 0.62 to 0.89, versus those treated HR = 0.90; 95% CI = 0.75 to 1.08). Results were similar for the rate of change in the United Parkinson Disease Rating Scale (UPDRS). CSF urate was also inversely related to both the primary endpoint (HR for highest versus lowest quintile = 0.65; 95% CI: 0.54 to 0.96) and to the rate of change in UPDRS. As with serum urate, these associations were present only among subjects not treated with α-tocopherol.
Conclusion
Higher serum and CSF urate at baseline were associated with slower rates of clinical decline. The findings strengthen the link between urate and PD and the rationale for considering CNS urate elevation as a potential strategy to slow PD progression.
Introduction
In humans, urate is a major antioxidant as well as the end product of purine metabolism.1,2 Its high concentrations in cerebrospinal fluid (CSF) and blood have been attributed to mutations in the urate oxidase gene occurring late in hominid evolution.3 Oxidative damage is suspected to contribute to the neurodegenerative process in Parkinson disease (PD)4,5 and antioxidants like urate may provide an endogenous defense against the development and progression of PD.
Prospective epidemiological studies have demonstrated that healthy individuals with higher blood urate concentrations are at reduced risk for developing PD.6-8 Similarly, a lower risk of PD has also been reported among individuals consuming diets that increase serum urate,9 and among those with a history of gout.51,52 Recently, we found that higher urate blood concentrations in patients recently diagnosed with PD predict a slower rate of disease progression, assessed by both clinical and neuroimaging measures.10 These studies suggest that urate measured systemically may serve as a robust predictor of the brain neurodegeneration that leads to the initiation and progression of PD.
The studies also raise the possibility that CNS urate directly protects against the neuronal degeneration underlying clinical deterioration in PD. CSF may reflect more closely the microenvironment of degenerating neurons than does blood.11 Accordingly, we utilized the clinical database from a completed multi-center randomized, placebo-controlled trial (DATATOP, or Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism)12,13 to test the hypothesis that higher urate concentrations in both CSF and blood specimens from PD patients predict a slower rate of clinical disease progression.
Methods
Study design
The DATATOP study was a two-year double-blind, randomized trial originally designed to test the hypothesis that long-term treatment of early PD with the monoamine oxidase type-B inhibitor deprenyl (selegiline) and/or the antioxidant α-tocopherol would extend the time until the emergence of disability requiring therapy with levodopa.12 The 800 participants were enrolled between September 1987 and November 1988 at 28 sites across the United States and Canada.
Study population
Subjects enrolled in the study had typical and early PD (Hoehn and Yahr stage I and II) of less than 5 years duration, and were excluded if use of symptomatic PD medication, severe tremor, serious dementia (Mini-Mental Status Exam score ≤ 22), or depression (Hamilton Depression Scale score of ≥16). Subjects were reviewed and examined by neurologists who were PD specialists. After baseline evaluation, study participants were randomized according to a 2×2 factorial design to one of four treatment assignments: deprenyl (10 mg/d) and α-tocopherol placebo, α-tocopherol (2000 IU/d) and deprenyl placebo, active deprenyl and α-tocopherol, or double placebo.14
Serum and CSF urate and covariates
Urate was measured in serum samples collected at the baseline visit prior to treatment assignment.. Serum was shipped without freezing to a central commercial clinical laboratory (SciCor, Indianapolis, IN) for immediate enzymatic assay of urate concentrations, which were available for 774 of the 800 enrolled subjects. Values maintained in a digitized database were not analyzed with respect to disease progression outcome measures until their retrieval in 2006 specifically for this purpose.
CSF was collected at baseline after overnight bedrest from 730 subjects (i.e., 91% of enrollees, with technical difficulties in performing lumbar punctures precluding the collection of the others)15 and at the end of the study in 486 subjects. Specimens were rapidly frozen for storage at −70 °C after first splitting all CSF collection tubes into aliquots with or without metabisulfite preservative added.15 Baseline and final CSF urate concentrations were measured in 1991 by high-performance liquid chromatography with electrochemical detection (HPLC-ECD) from collection tubes containing the 18th to 20th ml of lumbar CSF flow in two selected subsets totaling 290 subjects who had provided both baseline and final CSF collections.16 The values of CSF urate at baseline correlated well with those at the end of treatment or follow-up in both subsets (Spearman coefficient = 0.7; p<0.0001), a result that supports the reproducibility of the assay as well as relatively stable within-person CSF urate concentrations. For the present analyses, in 2008 we obtained CSF aliquots from the same collection tubes (containing no metabisulfite preservative) and repeated the measurement of urate concentrations by HPLC-ECD. For these assays, 50 μM α-methyldopa served as an internal standard. Baseline CSF urate could be determined in 713 participants (i.e., 98% of those from whom baseline CSF was obtained and stored). Although mean CSF urate values were lower than those measured in 1991, a good correlation was found between original urate concentrations and those measured in 2008 among the 277 individuals in both sets (Spearman coefficient = 0.72; p<0.0001). Furthermore, baseline serum urate concentrations were correlated more strongly with baseline CSF urate measured in 2008 (r=0.73) than in 1991 (r=0.58). These results provide evidence of the stability of urate in these samples, and of the accuracy of CSF urate measurements.
Clinical evaluation and Outcomes
Following the baseline visit and initiation of study drugs, subjects were scheduled for visits every three months until 24 months had elapsed.14 At each visit the site investigator evaluated the subject for disability sufficient to require dopaminergic therapy, the primary endpoint for the study, and for the secondary response variables, including the Unified Parkinson Disease Rating Scale (UPDRS; sum of the motor, cognitive, and activity of daily living subscales).14 Because the UPDRS is modified by the dopaminergic treatment instituted at endpoint, the annualized rate of change in UPDRS was determined based on change from baseline to endpoint (or final visit if endpoint was not reached) for each subject, and was calculated as: ([total UPDRS at the last assessment before initiation of dopaminergic treatment - total UPDRS at baseline] / number of days between the two assessments) × (365 days/year). The vital status and dates of death of participants in DATATOP were collected in 2001 to 2002, as previously described.17 The shortest time elapsed between enrollment and vital status update was 13 years. Information was available for 768 subjects with baseline serum urate measurement.
Statistical analysis
In the original trial, the hazard ratio (HR) for the primary endpoint was 0.50 (95% CI: 0.41 to 0.62) among patients assigned to deprenyl, and 0.91 (0.74 to 1.12) among patients assigned to α-tocopherol.14 Accordingly, all the analyses were adjusted for assignment to deprenyl versus placebo.
Serum urate
Cox proportional hazards models were used to estimate the hazard ratios (HRs) of reaching the endpoint according to quintiles of baseline serum urate, adjusting for sex, age (in 5-year groups), and treatment assignment (deprenyl versus placebo). Initial analyses were conducted using quintiles based on the combined urate distribution in men and women. However, because this categorization as expected resulted in a markedly skewed distribution within sex, we also conducted analyses based on sex-specific quintiles. Tests for trend were conducted by including serum urate as a continuous variable in the proportional hazard models. Potential confounding was assessed by adjusting the regression analyses for body mass index (BMI), and use of antihypertensive drugs or non-steroidal anti-inflammatory drugs (use versus no-use). With the exception of BMI, these adjustments did not affect the results. Therefore, only the treatment-, age- and sex-adjusted, or treatment-, age-, sex- and BMI-adjusted results are presented. Possible interactions were explored by including, in the proportional hazard model, the cross-product of serum urate (continuous variable) with age (continuous in years), sex, or deprenyl and α-tocopherol treatments. None of the interaction terms was significant, and only results that do not include these terms are reported. The results of these exploratory analyses, however, suggested a possible interaction between α-tocopherol treatment and serum urate. Because both α-tocopherol and urate have antioxidant properties, this interaction has some biological plausibility. This interaction was examined further by estimating the HRs for the primary outcome in groups of subjects classified according to both their serum urate and treatment group.
The relation between serum urate and rate of change in UPDRS was assessed by linear regression, using both common quintiles of serum urate and sex-specific quintiles, as outlined above. The association between serum urate and time from study enrollment until death was investigated using Cox proportional hazard models adjusted for treatment, age, sex, and smoking history (pack-years), with or without further adjustment for cardiac morbidity at baseline.
CSF urate
Analyses were conducted as for serum urate.
Results
Serum urate
Serum urate at baseline was available for 97% (510 men and 264 women) of the 800 subjects enrolled in the trial. Selected characteristics of these subjects are shown in Table 1. As expected, serum urate concentrations were correlated positively with male sex, BMI, use of thiazide diuretics, and hypertension. (Table 1.) Use of calcium channel blockers was reported by only 17 patients and showed no relationship to serum urate concentration.
Table 1.
DATATOP - Baseline characteristics of study participants according to quintiles of baseline serum urate
| Quintile of baseline serum urate | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | |
| Serum urate (mg/dL) | < 3.91 | 3.91 –4.60 | 4.61 –5.20 | 5.21 –6.20 | ≥ 6.21 | 5.1 |
| Subjects (n) | 162 | 140 | 149 | 165 | 158 | 774 |
| Female % | 75.9 | 40.7 | 24.2 | 17.0 | 12.7 | 34.1 |
| Age (median) | 62 | 63 | 61 | 63 | 63 | 62 |
| BMI (mean) | 23.7 | 26.2 | 26.1 | 27.1 | 28.4 | 26.3 |
| Current smokers % | 9.3 | 5.7 | 12.1 | 10.3 | 6.3 | 8.8 |
| Hypertension (% usingantihypertensive drugs) | 6.8 | 7.1 | 7.4 | 8.5 | 15.7 | 9.2 |
| Thiazides (% using) | 2.5 | 2.9 | 3.4 | 4.2 | 6.9 | 4.0 |
| NSAIDs (% using) | 22.2 | 22.1 | 20.1 | 27.3 | 17.6 | 21.9 |
| Cardiac comorbidity(%) | 17.9 | 25.0 | 24.8 | 27.9 | 36.5 | 26.5 |
Overall, 369 (48%) of these participants progressed to disability sufficient to require levodopa therapy during follow-up. The HR of reaching this primary endpoint declined with increasing concentrations of serum urate, and was 36 % lower among subjects in the top quintile as compared to those in the bottom quintile of serum urate (HR = 0.64; 95% CI: 0.44 to 0.94; p for trend = 0.002) (Table 2). This association was stronger in men than in women, although a test for interaction of urate with sex was not significant (Table 2). Further, in both sexes, the HR for reaching the primary endpoint decreased with increasing BMI (p for trend = 0.05 in men, = 0.02 in women). After adjustment for BMI, the association between serum urate and the primary clinical endpoint was partially attenuated; the HR for a 1-SD increase in serum urate was 0.85 in men (p = 0.04), and 1.01 in women (p = 0.94).
Table 2.
DATATOP Hazard ratios (HR)† for reaching the endpoint according to common quintiles of baseline serum urate, or corresponding to 1 standard deviation (SD = 1.4 mg/dL) increase in serum urate
| Serumuratequintile | ALL (n=774) | Men (n=510) | Women (n=264) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Serumuratemg/dL | HR (95% CI) | pvalue | n | HR (95% CI) | pvalue | n | HR (95% CI) | pvalue | |
| 1 | <3.91 | 1.00 (Ref) | - | 39 | 1.00 (Ref) | - | 123 | 1.00 (Ref) | - |
| 2 | 3.91–4.60 | 0.88 (0.62 – 1.25) | 0.47 | 83 | 0.88 (0.52 – 1.49) | 0.63 | 57 | 0.90 (0.53 – 1.51) | 0.68 |
| 3 | 4.61–5.20 | 1.04 (0.73 – 1.47) | 0.83 | 113 | 1.17 (0.71 – 1.91) | 0.54 | 36 | 0.86 (0.45 – 1.62) | 0.63 |
| 4 | 5.21–6.20 | 0.80 (0.55 – 1.15) | 0.23 | 137 | 0.78 (0.48 – 1.29) | 0.33 | 28 | 1.34 (0.68 – 2.64) | 0.40 |
| 5 | ≥6.21 | 0.64 (0.44 – 0.94) | 0.02 | 138 | 0.67 (0.41 – 1.11) | 0.12 | 20 | 0.58 (0.23 – 1.50) | 0.26 |
| Serum urate, 1 SD | 0.82 (0.73 – 0.93) | 0.002 | 0.81 (0.70 – 0.94) | 0.005 | 0.89 (0.71 – 1.12) | 0.32 |
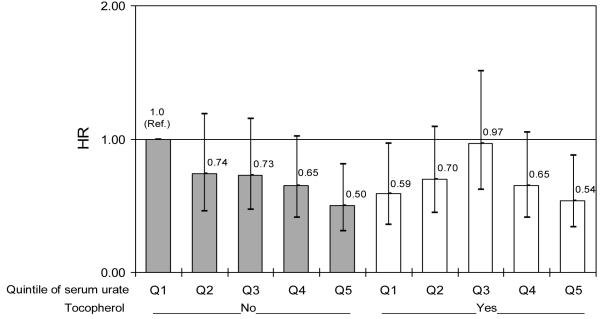
When subjects were classified simultaneously according to serum urate concentration and α-tocopherol treatment, a decreasing HR for reaching the primary endpoint with increasing quintiles of serum urate was observed among untreated subjects (figure 1.A) (HR = 0.75; 95% CI = 0.62 to 0.89, p = 0.001), but not among those treated (HR = 0.90; 95% CI = 0.75 to 1.08, p = 0.24). Conversely, randomization to α-tocopherol treatment appeared to lower the HR of reaching the primary endpoint among subjects in the lowest quintile of serum urate (HR: 0.59; 95% CI: 0.36 to 0.97), but not among those with higher serum urate (figure 1.A). Further analyses were conducted within sex. In men, the HR for a 1-SD increase in serum urate was 0.74 (95% CI: 0.59 to 0.92; p = 0.008) among subjects not on α-tocopherol, and 0.88 (95% CI: 0.71 to 1.08; p =0.21) among subjects on α-tocopherol. In women, the corresponding HRs were 0.73 (0.52 to 1.02; p = 0.06) for subjects not on α-tocopherol, and 1.04 (0.69 to 1.59; p = 0.84) for subjects on α-tocopherol. For neither men nor women was the interaction between α-tocopherol and serum urate significant (p for interaction = 0.55 in men, and p = 0.06 in women). No significant interaction was found between serum urate concentration and deprenyl treatment; a decreasing HR with increasing serum urate was found in the placebo-placebo and in the deprenyl-placebo groups, but not in the placebo-tocopherol or deprenyl-tocopherol group (results in supplementary Table 1s).
Figure 1.A. Hazard ratio (HR) for reaching endpoint according to assignment to α-tocopherol (vitamin E) and quintile of baseline serum urate.
(referenced to placebo-treated subjects in the lowest quintile). Bars indicate 95% confidence intervals for HRs, adjusted for age, gender, and treatment group (deprenyl or placebo)
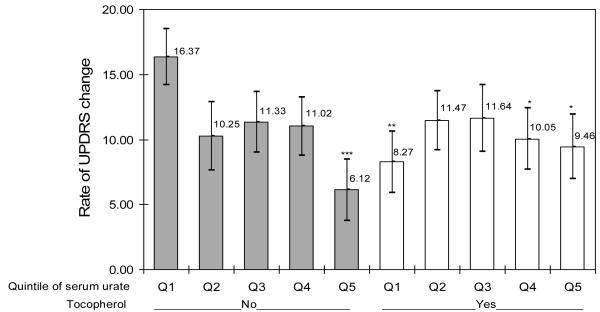
The change in UPDRS between baseline and either the time of primary endpoint or the end of follow-up was available for 760 of the 774 subjects with baseline serum urate. Overall, the rate of UPDRS change declined with increasing serum urate (p, for trend = 0.03). As observed above for the primary endpoint, results were more robust in men though there was no statistically significant interaction with sex. Among men, the adjusted rate of UPDRS change declined from 14.8 points per year for subjects in the lowest quintile of serum urate to 8.9 for those in the highest quintile (p for trend = 0.03); comparable results among women were 11.0 and 8.2 (p for trend = 0.4). The relation between serum urate and the rate of UPDRS change was modified by α-tocopherol treatment (p for interaction = 0.009) (figure 2.A). Among subjects not assigned to α-tocopherol, the rate of UPDRS change was 9.8 points lower in the highest serum urate quintile than in the lowest quintile (p=0.003), whereas no difference was observed for subjects assigned to α-tocopherol (0.5 points higher in highest as compared with lowest quintile, p = 0.89). No significant interaction was found between serum urate and deprenyl treatment (supplementary Table 2s).
Figure 2.A. Annualized rate of UPDRS change according to assignment to α-tocopherol (vitamin E) and quintile of baseline serum urate.
Bars indicate standard errors of the mean, Adjusted for age, gender, and treatment group (deprenyl or placebo)
Significantly different from lowest quintile of urate placebo-treated group: *p<0.05; **p<0.01; ***p<0.001
Two hundred and eleven (41.3%) men and 81 (31%) women were identified as having died after 13 years of follow-up. In men and women combined, after adjustment for deprenyl treatment, age, sex, pack years of smoking, and cardiovascular comorbidity at baseline, serum urate was not significantly associated with mortality. In men, however, the relation between serum urate and mortality was a U-shaped curve, with the lowest mortality in the fourth quintile of urate values (Table 3). In women, a suggestion of increased mortality at any urate concentration above those in the lowest quintile was not substantiated statistically. No significant interactions between serum urate and α-tocopherol were found in analyses on mortality.
Table 3.
DATATOP Hazard ratios (HR) † for death from any cause according to common quintiles of baseline serum urate
| Serum uratequintile | All (n = 768) | Men (n = 504) | Women (n = 264) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | pvalue | HR (95% CI) | pvalue | HR (95% CI) | pvalue | |
| 1 | 1.00 (Ref) | - | 1.00 (Ref) | - | 1.00 (Ref) | - |
| 2 | 1.17 (0.77 – 1.78) | 0.47 | 0.66 (0.38 – 1.15) | 0.14 | 1.68 (0.90 – 3.11) | 0.10 |
| 3 | 1.20 (0.78 – 1.83) | 0.41 | 0.66 (0.38 – 1.12) | 0.12 | 1.30 (0.64 – 2.66) | 0.47 |
| 4 | 1.11 (0.72 – 1.72) | 0.62 | 0.60 (0.35 – 1.02) | 0.06 | 1.88 (0.89 – 3.97) | 0.10 |
| 5 | 1.48 (0.96 – 2.27) | 0.06 | 0.89 (0.53 – 1.50) | 0.67 | 1.96 (0.89 – 4.33) | 0.10 |
| Multivariate HR (95% CI) | Multivariate HR (95% CI) | Multivariate HR (95% CI) | ||||
|---|---|---|---|---|---|---|
| 1 | 1.00 (Ref) | - | 1.00 (Ref) | - | 1.00 (Ref) | - |
| 2 | 1.12 (0.73 – 1.71) | 0.61 | 0.63 (0.36 – 1.10) | 0.10 | 1.66 (0.90 – 3.08) | 0.11 |
| 3 | 1.14 (0.74 – 1.74) | 0.56 | 0.63 (0.37 – 1.08) | 0.09 | 1.29 (0.63 – 2.64) | 0.49 |
| 4 | 1.05 (0.68 – 1.62) | 0.83 | 0.56 (0.33 – 0.95) | 0.03 | 1.85 (0.87 – 3.93) | 0.11 |
| 5 | 1.38 (0.89 – 2.12) | 0.15 | 0.83 (0.49 – 1.39) | 0.47 | 1.89 (0.83 – 4.30) | 0.13 |
CSF urate
Mean urate concentrations in CSF collected at baseline were higher in men (0.42 mg/dL) than in women (0.28 mg/dL) and were substantially lower than in serum, as expected.18 Despite the lower concentrations of CSF urate, a strong correlation was found between CSF and serum urate (r=0.73; p<0.0001).
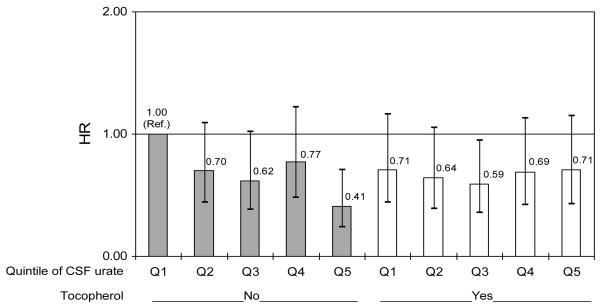
The primary clinical endpoint of disability was reached by 342 (48%) of the 713 subjects for whom CSF urate was available. Overall, the HR of reaching the endpoint of disability was significantly lower among individuals with higher concentrations of CSF urate - the HR comparing subjects in the top to those in the bottom quintile of CSF urate - was 0.65 (95% CI: 0.44 to 0.96; p= 0.03); that associated with a 1 standard deviation increase in CSF urate was 0.89 (p = 0.09) (Table 4). Results were not significantly different by sex, although a strong interaction was found between α-tocopherol assignment and CSF urate (p for interaction = 0.009; figure 1.B). As for serum urate, a significant decrease in the HRs for the primary endpoint with increasing CSF urate was only observed among subjects not receiving α-tocopherol. The HR corresponding to 1 SD increase in CSF urate was 0.77 (0.62 to 0.96; p = 0.02) among men not treated with α-tocopherol, and 1.10 (0.90 to 1.34; p = 0.34) among those receiving α-tocopherol. In women, the corresponding HRs were 0.64 (0.40 to 1.03; p = 0.07) for subjects not assigned to α-tocopherol, and 0.77 (0.43 to 1.37; p = 0.37) for subjects treated with α-tocopherol. No significant interaction was found between serum urate and deprenyl treatment(supplementary Table 3s).
Table 4.
DATATOP Hazard ratios (HR)† for reaching the endpoint according to common quintiles of baseline CSF urate, or corresponding to 1 standard deviation (SD = 0.16 mg/dL) increase in CSF urate
| CSFuratequintile | ALL (n=713) | Men (n=473) | Women (n=240) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CSFuratemg/dL | HR (95% CI) | pvalue | n | HR (95% CI) | pvalue | n | HR (95% CI) | pvalue | |
| 1 | <0.24 | 1.00 (Ref) | - | 38 | 1.00 (Ref) | - | 105 | 1.00 (Ref) | - |
| 2 | 0.24–0.32 | 0.78 (0.55 – 1.10) | 0.16 | 81 | 1.05 (0.61 – 1.81) | 0.85 | 62 | 0.65 (0.38 – 1.11) | 0.11 |
| 3 | 0.32–0.39 | 0.70 (0.48 – 1.01) | 0.06 | 109 | 0.96 (0.57 – 1.63) | 0.89 | 35 | 0.51 (0.26 – 1.03) | 0.06 |
| 4 | 0.39–0.50 | 0.84 (0.58 – 1.22) | 0.36 | 117 | 1.08 (0.64 – 1.80) | 0.78 | 25 | 0.72 (0.34 – 1.55) | 0.40 |
| 5 | >0.50 | 0.65 (0.44 – 0.96) | 0.03 | 128 | 0.85 (0.51 – 1.43) | 0.54 | 13 | 0.47 (0.16 – 1.39) | 0.17 |
| CSF urate, 1 SD | 0.89 (0.79 – 1.02) | 0.09 | 0.93 (0.80 – 1.07) | 0.28 | 0.79 (0.57 – 1.10) | 0.17 |
Figure 1.B. Hazard ratio (HR) for reaching endpoint according to assignment to α-tocopherol (vitamin E) and quintile of baseline CSF urate.
(referenced to placebo-treated subjects in the lowest quintile). Bars indicate 95% confidence intervals for HRs, adjusted for age, gender, and treatment group (deprenyl or placebo)
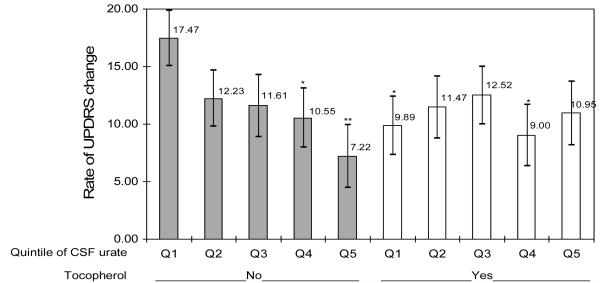
The change in UPDRS between baseline and either the time of primary endpoint or the end of follow-up was available for 702 of the 713 subjects with baseline CSF urate. Overall, the rate of UPDRS change was not related significantly to CSF urate. As observed for serum urate, however, the relation between CSF urate and the rate of UPDRS change was modified by α-tocopherol treatment (p for interaction = 0.04) (figure 2.B). Among subjects not treated with α-tocopherol the rate of UPDRS change declined with increasing CSF urate concentrations (p for trend = 0.05). No significant interaction was found between serum urate and deprenyl treatment (supplementary Table 4s).
Figure 2.B. Annualized rate of UPDRS change according to assignment to α-tocopherol (vitamin E) and quintile of baseline CSF urate.
Bars indicate standard errors of the mean, Adjusted for age, gender, and treatment group (deprenyl or placebo).
Significantly different from lowest quintile of urate placebo-treated group: *p<0.05; **p<0.01
Discussion
Among subjects with early PD participating in a large randomized trial we found that both serum urate and CSF urate, measured at baseline, were inversely related to clinical progression. The internal consistency of the results across the primary and secondary endpoints supports their validity. These findings, like data from a similar early PD trial (the PRECEPT study),10 demonstrate a robust link between blood urate concentrations and the rate of clinical progression in PD. In addition, the association of CSF urate with disease progression strengthens the possibility that brain urate (or its determinants) might protect against the neurodegeneration of PD. Taken together, these data establish urate as the first molecular predictor of clinical progression in PD and provide a rationale for investigating the possibility that a therapeutic increase of urate in patients with PD might act favorably to slow the disease course. Interestingly, the inverse relation between urate and clinical progression was not observed among patients randomized to α-tocopherol 2000 IU daily, suggesting that there may be an interaction between these antioxidants.
There is strong evidence that oxidative and nitrative stress are major pathogenetic mechanisms in PD.10,19,20 Urate is an effective antioxidant,1 peroxynitrate scavenger,21,22 iron chelator23 and ascorbate stabilizer.24 In models of induced neurodegeneration, urate can reduce oxidative stress, mitochondrial dysfunction, and cell death of cultured neurons and human dopaminergic cells exposed to the pesticide rotenone, MPP+, glutamate, and iron ions.25,26 Although urate appears to have the potential for neuroprotection, it is possible that the predictive association between urate and PD progression reflects instead the effect of a urate precursor, such as adenosine or inosine, or another determinant of systemic urate concentrations.
As compared to serum urate, the weaker association of CSF urate with respect to clinical progression of PD may seem at odds with the hypothesis that urate (or its metabolic precursors) exert a beneficial effect through CNS presence. CSF urate concentrations, however, display a strong caudo-rostral gradient from the lumbar space, with lumbar region values approximately 50% higher than those arising at the cisterna magna (brainstem) level.27,28 Although we consistently used CSF aliquots obtained from the 18 to 20th mL of CSF flow, variations in CSF circulation patterns between patients29 - along with freezer storage for 20 years - may have contributed to reduce the accuracy of this measure compared to assays of freshly collected serum samples. In addition to technical variability, substantial biological differences between the urate in CSF sampled from the subarachnoid space and that in the degenerating neurons themselves may lessen the strength of a CSF urate-clinical correlation in PD. CSF may also have limited value as a measure of brain urate actions and interactions.
The finding that the inverse relation between urate and clinical progression of PD was modified by α-tocopherol treatment was unforeseen because, as originally reported, no favorable effect of α-tocopherol on PD progression was found among study participants in DATATOP.13 The mechanisms for a possible interaction between urate and α-tocopherol remain uncertain. Although hydrophilic (e.g., urate) and hydrophobic (e.g., α-tocopherol) antioxidants target different subcellular compartments, their functional interactions are well known.30,31 Further, α-tocopherol at doses commonly used in vitamin E supplements may reduce concentrations of other endogenous antioxidants,32,33 and at high doses, may have pro-oxidant rather than antioxidant effects.34,35 Alternatively, a simple competitive interaction or ‘ceiling effect’ may have contributed to the observed lack of α-tocopheral benefits among PD patients with higher urate concentrations, as well as to the loss of the inverse association between urate and PD progression among those receiving supplemental α-tocopherol. Regardless of the mechanism for a possible interaction between α-tocopherol and urate, our results raise the possibility that such an interaction may have obscured a protective effect of α-tocopherol among those subjects with low baseline concentrations of urate in the DATATOP trial. Further investigations are therefore needed to consider the possibility that α-tocopherol supplementation may be beneficial in individuals with low urate.
Serum urate may also affect the progression of cognitive impairment, in that higher concentrations seem to be associated with slower rates of cognitive decline and lower risk of dementia.36-38 As in the present study, among participants in a randomized trial, this association was observed in patients treated with placebo, but not in those treated with α-tocopherol.37 Higher serum urate concentration has also been linked to a lower rate of worsening in Huntington disease.39 Although each of these neurodegenerative disorders differs greatly from PD, the relationships between urate and these disorders may be indicative of a more general influence of urate (or its precursors) on neuronal cell death.
The main results of the present study are strikingly consistent with those recently reported from the PRECEPT study.10 Although the overall inverse relation between serum urate and the clinical progression of PD was greater in PRECEPT than in DATATOP, results among subjects in DATATOP not assigned to α-tocopherol were virtually identical to those observed in PRECEPT (which did not include an α-tocopherol treatment arm). In both trials, hazard ratios for risk of disability progression showed a decline in patients whose values were above the median concentration but still within the normal range of serum urate. Moreover, in both trials, the concentration-dependent inverse relationship was robust in men, but weak and non-significant among women. This consistent difference between men and women could result in part from a biological effect of sex on urate mechanisms in PD,40 or else could reflect the small number of women with urate concentration high enough to impart slowing of disease progression.
A potentially therapeutic effect of elevating serum urate warrants consideration. Urate levels can be elevated by dietary means, including increased intake of fructose41,42,43 or purines,44 or by pharmacological means. The latter may include administration of the purine metabolite and urate precursor inosine, which is being investigated as a therapy for multiple sclerosis in a phase II randomized clinical trial.45,46 The potential benefit of elevating urate in individuals with PD, however, has to be weighed against possible adverse effects, which may include an increased risk of hypertension, coronary heart disease and stroke,6,47-49 in addition to the known risks of gout and urolithiasis. Available data are therefore insufficient to support a therapeutic recommendation.
The discovery of a urate link to PD progression was achieved through additional analyses of two rigorously conducted clinical trials whose databases were made available to test an unforeseen hypothesis months50 or decades15,16 after conclusion of the primary investigations. These latent insights highlight a broader opportunity to achieve further advances through explorations of the growing repository of high quality data collected from neuroprotection trials of PD and other neurodegenerative disorders.
Supplementary Material
Supplement. Data
Acknowledgment
We thank Eilis O’Reilly for conducting an expert secondary review of statistical programs and reported results, Andrew McAleavey for excellent technical assistance in neurochemical analyses, and Leslie Unger for technical assistance in preparing the manuscript.
Funding/Support: Supported by NIH NS24778, NS27892, NS048517, NS054978 and NS060991, DOD W81XWH-04-1-0881, and a Data-Mining Research award from the Parkinson Disease Foundation and the Parkinson Study Group.
Footnotes
Financial disclosures: None.
Role of the Sponsors: None.
References
- 1.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alho H, Leinonen JS, Erhola M, Lonnrot K, Aejmelaeus R. Assay of antioxidant capacity of human plasma and CSF in aging and disease. Restor Neurol Neurosci. 1998;12:159–165. [PubMed] [Google Scholar]
- 3.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt CR, Weber HK. Parkinson’s disease: a chronic, low-grade antioxidant deficiency? Med Hypotheses. 1994;43:111–114. doi: 10.1016/0306-9877(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 6.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 7.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 8.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson’s disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx J Ame Society Experiment NeuroThera. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkinson Study Group DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Arch Neurol. 1989;46:1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson Study Group Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson Study Group Effect of deprenyl on the progression of disability in early parkinson’s disease. N Engl J Med. 1989;321:1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson Study Group Cerebrospinal fluid homovanillic acid in the DATATOP study on Parkinson’s disease. Arch Neurol. 1995;52:237–245. doi: 10.1001/archneur.1995.00540270025015. [DOI] [PubMed] [Google Scholar]
- 16.LeWitt PA, Galloway MP, Matson W, et al. Markers of dopamine metabolism in Parkinson’s disease. Neurology. 1992;42:2111–2117. doi: 10.1212/wnl.42.11.2111. [DOI] [PubMed] [Google Scholar]
- 17.Marras C, McDermott MP, Rochon PA, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64:87–93. doi: 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 18.Niklasson F, Agren H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol Psychiatry. 1984;19:1183–1206. [PubMed] [Google Scholar]
- 19.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 20.Jenner P, Olanow CW. The pathogenesis of cell death in Parkinson’s disease. Neurology. 2006;66(10 Suppl 4):S24–36. doi: 10.1212/wnl.66.10_suppl_4.s24. [DOI] [PubMed] [Google Scholar]
- 21.Squadrito GL, Cueto R, Splenser AE, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. 2000;376:333–337. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]
- 22.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann N Y Acad Sci. 2002;962:242–259. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 23.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stocker R, Frei B. Endogenous antioxidant defences in human blood plasma. In: Sies H, editor. Oxidative Stress: Oxidants and Antioxidants. Academic Press; San Diego: 1991. pp. 213–243. [Google Scholar]
- 25.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 26.Haberman F, Tang SC, Arumugam TV, et al. Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice. Neuromolecular Med. 2007;9:315–323. doi: 10.1007/s12017-007-8010-1. [DOI] [PubMed] [Google Scholar]
- 27.Niklasson F, Hetta J, Degrell I. Hypoxanthine, xanthine, urate and creatinine concentration gradients in cerebrospinal fluid. Ups J Med Sci. 1988;93:225–232. doi: 10.3109/03009738809178548. [DOI] [PubMed] [Google Scholar]
- 28.Degrell I, Nagy E. Concentration gradients for HVA, 5-HIAA, ascorbic acid, and uric acid in cerebrospinal fluid. Biol Psychiatry. 1990;27:891–896. doi: 10.1016/0006-3223(90)90470-m. [DOI] [PubMed] [Google Scholar]
- 29.Quencer RM, Post MJ, Hinks RS. Cine MR in the evaluation of normal and abnormal CSF flow: intracranial and intraspinal studies. Neuroradiology. 1990;32:371–391. doi: 10.1007/BF00588471. [DOI] [PubMed] [Google Scholar]
- 30.Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004;430:97–103. doi: 10.1016/j.abb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr. 1995;62(suppl):1322S–13226S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 33.Huang HY, Appel LJ. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J Nutr. 2003;133:3137–3140. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- 34.Bowry VW, Stocker R. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. Journal of the American Chemistry Society. 1993;115:6029–6044. [Google Scholar]
- 35.Abudu N, Miller JJ, Attaelmannan M, Levinson SS. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin Chim Acta. 2004;339:11–25. doi: 10.1016/j.cccn.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 37.Irizarry MC, Raman R, Schwarzschild MA, et al. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6:23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2008 Nov 26; doi: 10.1093/brain/awn316. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington’s disease progression (abstract) Mov Disord. 2008;23(Suppl 1):S187. doi: 10.1002/mds.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 41.Hallfrisch J. Metabolic effects of dietary fructose. Faseb J. 1990;4:2652–2660. doi: 10.1096/fasebj.4.9.2189777. [DOI] [PubMed] [Google Scholar]
- 42.Gao X, Qi L, Qiao N, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50:306–312. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 43.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2008;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 44.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 45.Spitsin S, Hooper DC, Leist T, Streletz LJ, Mikheeva T, Koprowskil H. Inactivation of peroxynitrite in multiple sclerosis patients after oral administration of inosine may suggest possible approaches to therapy of the disease. Mult Scler. 2001;7:313–319. doi: 10.1177/135245850100700507. [DOI] [PubMed] [Google Scholar]
- 46.Koprowski H, Spitsin SV, Hooper DC. Prospects for the treatment of multiple sclerosis by raising serum levels of uric acid, a scavenger of peroxynitrite. Ann Neurol. 2001;49:139. doi: 10.1002/1531-8249(200101)49:1<139::aid-ana28>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric Acid Is a Risk Factor for Myocardial Infarction and Stroke. The Rotterdam Study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler JG, Juzwishin KD, Eiriksdottir G, Gudnason V, Danesh J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS Med. 2005;2:e76. doi: 10.1371/journal.pmed.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18:287–292. doi: 10.1681/ASN.2006080865. [DOI] [PubMed] [Google Scholar]
- 50.The Parkinson Study Group PRECEPT Investigators Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 51.Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 52.De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum. 2008;59:1549–1554. doi: 10.1002/art.24193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement. Data