Differential Neuroprotective Effects of Carnosine, Anserine, and N-Acetyl Carnosine against Permanent Focal Ischemia (original) (raw)
. Author manuscript; available in PMC: 2010 Jan 12.
Published in final edited form as: J Neurosci Res. 2008 Oct;86(13):2984–2991. doi: 10.1002/jnr.21744
Abstract
Carnosine (β-alanyl-L-histidine) has been shown to exhibit neuroprotection in rodent models of cerebral ischemia. In the present study, we further characterized the effects of carnosine treatment in a mouse model of permanent focal cerebral ischemia and compared them with its related peptides anserine and N-acetylated carnosine. We also evaluated the efficacy of bestatin, a carnosinase inhibitor, in ameliorating ischemic brain damage. Permanent focal cerebral ischemia was induced by occlusion of the middle cerebral artery (pMCAO). Mice were subsequently randomly assigned to receive an intraperitoneal injection of vehicle (0.9% saline), carnosine, N-acetyl carnosine, anserine, bestatin alone, or bestatin with carnosine. Infarct size was examined using 2,3,5-triphenyltetrazolium chloride staining 1, 3, and 7 days following pMCAO, and neurological function was evaluated using an 18-point-based scale. Brain levels of carnosine were measured in treated mice using high-performance liquid chromatography 1 day following pMCAO. We demonstrated that treatment with carnosine, but not its analogues, was able to significantly reduce infarct volume and improve neurological function compared with those in vehicle-treated mice. These beneficial effects were maintained for 7 days post-pMCAO. In contrast, compared with the vehicle-treated group, bestatin-treated mice displayed an increase in the severity of ischemic lesion, which was prevented by the addition of carnosine. These new data further characterize the neuroprotective effects of carnosine and suggest that carnosine may be an attractive candidate for testing as a stroke therapy.
Keywords: carnosine, anserine, N-acetyl carnosine, bestatin, permanent cerebral focal ischemia
Ischemic damage to the brain is a multifactorial process that involves excitotoxicity, oxidative stress, breakdown of the blood–brain barrier, inflammation, and apoptosis (Lo et al., 2003; Mitsios et al., 2006; Durukan and Tatlisumak, 2007). Tissue plasminogen activator is the only approved therapy for the treatment of ischemic stroke. However, it needs to be administered within 3 hr of the onset of symptoms, and only a small minority of stroke patients are eligible for treatment. Therefore, there is an urgent need for additional therapies.
Carnosine is an amino acid dipeptide made of histidine and alanine that was first isolated from meat by Gulewitsch and Amiradzibi (1900). This naturally occurring peptide is present in a variety of organs, including the brain, where it is primarily found in glial and ependymal cells (Biffo et al., 1990; De Marchis et al., 1997; Bonfanti et al., 1999). It is a well tolerated and is available as a dietary supplement with no known side effects or identified adverse drug interactions. The recent focus of attention on carnosine as a potential therapeutic agent for stroke emerged from the demonstration of its neuroprotective capabilities. Previous in vitro and in vivo studies have shown that carnosine has antioxidant properties, cytosolic buffering functions, and metal/ion chelating capabilities and protects against glutamate-induced toxicity (Boldyrev et al., 1999, 2004; Bonfanti et al., 1999; Gariballa and Sinclair, 2000; Horning et al., 2000; Trombley et al., 2000). Furthermore, exogenously administered carnosine is capable of crossing the blood–brain barrier (Jin et al., 2005). Thus, carnosine appeared to offer pharmacological potentials essential to improving neurological outcome following cerebral ischemia. In recent years, a solid core of evidence has accumulated demonstrating that, indeed, carnosine is protective against ischemic brain injury. For example, carnosine has been shown to protect cultured neurons from oxygen glucose deprivation and to exhibit neuroprotective properties in animal models of global and cerebral ischemia (Kohen et al., 1988; Boldyrev et al., 1999; Gallant et al., 2000; Stvolinsky and Dobrota, 2000; Tabakman et al., 2002; Dobrota et al., 2005; Rajanikant et al., 2007). We previously demonstrated that carnosine protects the brain against cerebral ischemia via various mechanisms, such as decreased levels of reactive oxygen species and metalloproteinase proteins (Rajanikant et al., 2007).
Methylation and acetylation of carnosine result in the formation of anserine and _N_-acetyl carnosine, respectively (Boldyrev and Abe, 1999; Bonfanti et al., 1999). Anserine and _N_-acetyl carnosine are present in the central nervous system of a variety of vertebrates (O’Dowd et al., 1990; Bonfanti et al., 1999). Interestingly, like carnosine, they also exhibit antioxidant, proton-buffering, and metal-chelating capabilities (Boldyrev et al., 1995; Boldyrev and Abe, 1999), suggesting that such compounds might also have a protective role in the ischemic brain.
In the present study, we examined and compared the effects of carnosine, anserine, and _N_-acetyl carnosine in a mouse model of permanent cerebral focal ischemia. Here, we show that of carnosine, _N_-acetyl carnosine, and anserine, carnosine is the most effective at protecting the brain following ischemia-induced brain injury. Furthermore, we demonstrate that administration of the carnosinase inhibitor bestatin worsens the severity of the cerebral ischemic insult, whereas coadministration of carnosine rescues this effect. Taken together, our data emphasize that carnosine is a promising candidate for stroke therapy.
MATERIALS AND METHODS
Focal Cerebral Ischemia
All experimental procedures were approved by the All-University Institutional Animal Care and Use and performed in accordance with the Guide for the Care and Use of Laboratory Animals. Permanent focal cerebral ischemia was induced by permanent middle cerebral artery occlusion (pMCAO) in C57BL/6 mice (22–27 g; Charles River Laboratory), as previously described (Liu et al., 2002; Rajanikant et al., 2007). Mice were anesthetized with halothane, and body temperature was maintained at 37°C by a homeothermic blanket feedback system connected to temperature probes. A 1-cm skin incision was made between the left eye and ear to create a small subtemporal craniectomy and expose the left middle cerebral artery (MCA). The MCA was then occluded using a bipolar coagulator. To verify the completeness of the occlusion, a Perimed PF-3 laser Doppler perfusion monitor (Järfalla, Sweden) was used to measure regional blood flow before and after the surgical procedure. Animals with less than 75% reduction in cerebral blood flow were excluded from the study. Finally, the incision sites were sutured, and the mice were allowed to recover from anesthesia.
Drug Administration
Drugs were dissolved in 0.9% normal saline and were intraperitoneally administered to mice 30 min before induction of pMCAO.
Experimental Design 1
To evaluate the long-term neuroprotective effects of carnosine, experimental mice were randomly distributed into two groups and treated with either vehicle (same amount of saline as chemical-treated animals; n = 21) or 1,000 mg/kg carnosine (n = 20). Animals were euthanized 1, 3, and 7 days following pMCAO.
Experimental Design 2
To assess the potential effects of carnosine-related peptides and bestatin, experimental mice were randomly distributed in six treated groups: vehicle (same amount of saline as chemical-treated animals; n = 11), 1,000 mg/kg carnosine (n = 10), 1,000 mg/kg _N_-acetyl carnosine (n = 10), 1,000 mg/kg anserine (n = 11), 100 mg/kg bestatin (n = 9; Abe et al., 1989; Botbol and Scornik, 1997), or a combination of 1,000 mg/kg carnosine and 100 mg/kg bestatin (n = 9). Animals were analyzed 1 day following pMCAO.
Evaluation of Neurological Deficits
Neurological assessment of experimental mice was performed according to an 18-point-based scale (Garcia et al., 1995) 1 hr before induction of pMCAO and 1, 3, and 7 days following surgery. This 18-point-based scale includes the following six tests (maximum of 3 points per test): spontaneous activity, symmetry of movements, symmetry of forelimbs, climbing, reaction to touch on either side of trunk, and response to vibrissae touch. Final scoring was obtained by summing the scores recorded in each individual test (maximum score of 18).
Quantification of Infarct Volume
Experimental animals were euthanized 1, 3, and 7 days following pMCAO. Brains were immediately removed, rinsed in cold saline solution, cut into 1-mm-thick coronal sections, and processed for 2% 2,3,5-triphenylterazolium chloride (TTC) staining as previously described (Liu et al., 2002). Computer images of stained slices were generated using an HP Scanjet 4470c scanner, and infarct area was measured using NIH Images/J analysis software (version 1.37). The infarct volume of each slice was calculated by taking the average of the infarct area on both sides of the slice and multiplying it by the section thickness. The infarct volumes of individual sections were then summed to determine the total brain infarct volume and adjusted for edema (corrected infarct volume; Swanson et al., 1990).
Measurements of Intracerebral Carnosine Level
High-performance liquid chromatography (HPLC) was used to measure the endogenous level of carnosine in the mouse brain 1 day following intraperitoneal injection of vehicle (n = 3), carnosine (n = 3), bestatin (n = 3), or a combination of carnosine and bestatin (n = 4). HPLC analysis was performed on a Hitachi ModelL-8800 Amino Acid Analyzer as previously described (Dunnett and Harris, 1997). Following euthanasia, the brains were rapidly removed, rinsed with saline, and a longitudinal strip of tissue was dissected from one hemisphere. The dissected tissue was weighed, placed in a 200-μL vial, and homogenized at 4°C for 15 min with vortexing in 100 μL of methanol–0.4M borate buffer [75:25 (v/v), pH 9.65]. Following centrifugation, the supernatant was removed, dried under vacuum, reconstituted in borate buffer, and derivatized with 6-aminoquinolyl-_N_-hydroxysuccinimidyl carbamate (AccQflour reagent, Waters). The derivatized carnosine was run on a modified buffer system in the physiological mode. The detection limit for this technique is 25 pmol, and the average coefficient of variation is 1%–3%.
Statistical Analysis
All data are expressed as means ± SEMs. The degree of statistical significance between groups was determined on the basis of the Student t test and one-way analysis of variance, followed by the post hoc Fisher LSD test and the Kruskal-Wallis rank test. Linear regression modeling was used to compare parameters of infarct volume and neurological scores. SigmaStat 3.0 software (SYSTAT Software Inc., Point Richmond, CA) and SPSS 11.5 software (SPSS Inc., Chicago, IL) were applied to carry out the analysis. Statistical significance was defined as P < 0.05.
RESULTS
Comparative Effects of Carnosine and Its Analogues Anserine and _N_-Acetyl Carnosine on Permanent Cerebral Focal Ischemia
We have previously shown that, when administered 30 min prior to induction of pMCAO, carnosine exhibited robust neuroprotective properties 1 day following injury (Rajanikant et al., 2007). Anserine and _N_-acetyl carnosine, two carnosine-related peptides, have been shown to elicit antioxidant and anti-free-radical properties (Kohen et al., 1988; Boldyrev et al., 1997, 2004). Consequently, we sought to investigate the potential effects of anserine and _N_-acetyl carnosine in ameliorating pMCAO-induced cerebral damage.
Physiological parameters of all experimental mice were recorded during the surgical procedure. A significant reduction in regional cerebral blood perfusion (CBF) was observed in the lesioned cerebral hemisphere in all ischemic mice following pMCAO (Table I). However, there were not differences in either CBF reduction or rectal temperature among the experimental groups when examined right after surgery (Table I).
TABLE I.
General Characteristics in Mice with Cerebral Focal Ischemia Measured Right after Surgery
| Vehicle (n = 11) | Anserine (n = 11) | N-Acetyl carnosine (n = 10) | Carnosine (n = 10) | |
|---|---|---|---|---|
| Body weight (g) | 23.1 ± 0.3 | 23.5 ± 0.2 | 23.3 ± 0.3 | 23.9 ± 0.2 |
| Temperature (°C) | 37.6 ± 0.1 | 37.6 ± 0.1 | 37.7 ± 0.1 | 37.7 ± 0.1 |
| CBF (pre-pMCAO) | 286.4 ± 16 | 288.2 ± 18 | 311.0 ± 14 | 263.0 ± 15 |
| CBF (post-pMCAO) | 67.3 ± 3.6 | 69.6 ± 5.3 | 73.0 ± 3.9 | 61.8 ± 4.9 |
| Reduction of CBF (%) | 75.7 ± 2.2 | 75.7 ± 1.2 | 76.4 ± 1.2 | 76.52 ± 1.2 |
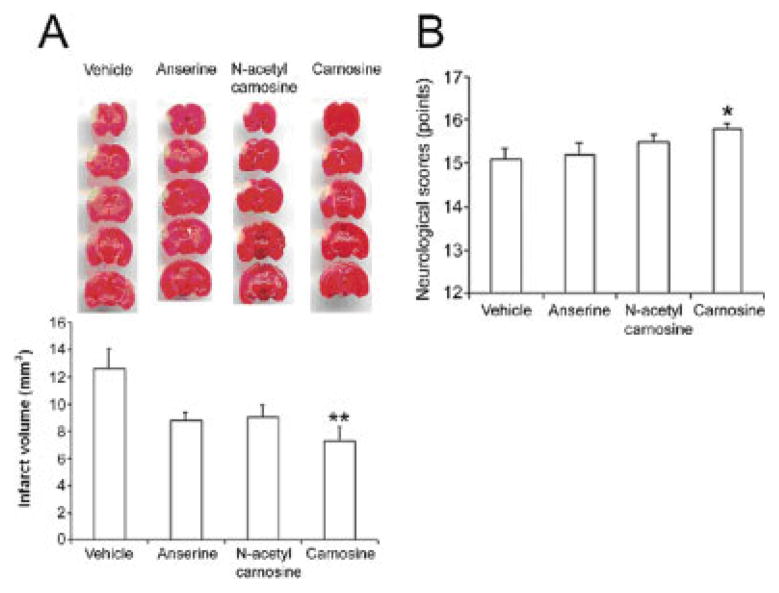
As assessed by TTC staining 1 day following pMCAO and confirmed by previous results (Rajanikant et al., 2007), treatment with carnosine significantly reduced infarct volume, by 42.5%, compared with the vehicle (7.24 ± 1.04 versus 12.60 ± 1.44 mm3, P = 0.008; Fig. 1A). Following treatment with carnosine analogues, a trend toward a decrease in infarct size that did not reach statistical significance was observed for both anserine (30.6%, 8.74 ± 0.60 versus 12.60 ± 1.44 mm3) and _N_-acetyl carnosine (28.3%, 9.03 ± 0.92 versus 12.60 ± 1.44 mm3; Fig. 1A).
Fig. 1.
Effects of pretreatment with carnosine, anserine, and N_-acetyl carnosine on infarct size and neurological function in mice 1 day after pMCAO. A: Top, TTC-stained sections showing the volume of the infarct; bottom, quantification of the infarct volume (mm3) for the various groups of treated-mice. The graph indicates that administration of carnosine was most effective at reducing infarct size compared with the vehicle-treated mice. B: Effects of pretreatment with carnosine, anserine, and N_-acetyl carnosine on neurological function. Neurological deficits were evaluated before and 1 day after surgery. Histogram values represent means ± SEM (★_P < 0.05, ★★_P < 0.01 versus vehicle).
To compare the effects of carnosine and its analogues on neurological functional outcome, neurological evaluations were carried out before surgery and 1 day following pMCAO using the 18-point-based scale of Garcia et al. (1995). Neurological scores were normal (18 points) in all vehicle- and chemical-treated mice before onset of pMCAO (data not shown). One day following surgery, vehicle-treated mice exhibited a significant decrease in neurological scores (15.1 ± 0.25 versus 18, P < 0.001). In contrast, treatment with carnosine resulted in a statistically significant improvement in neurological performance compared with that of vehicle-treated mice (15.8 ± 0.13 versus 15.09 ± 0.25, P = 0.03; Fig. 1B). Regression analysis showed weak correlation between infarct volume and neurological score, (carnosine-treated mice compared with vehicle-treated mice), but this did not reach statistical significance (Fig. 4A).
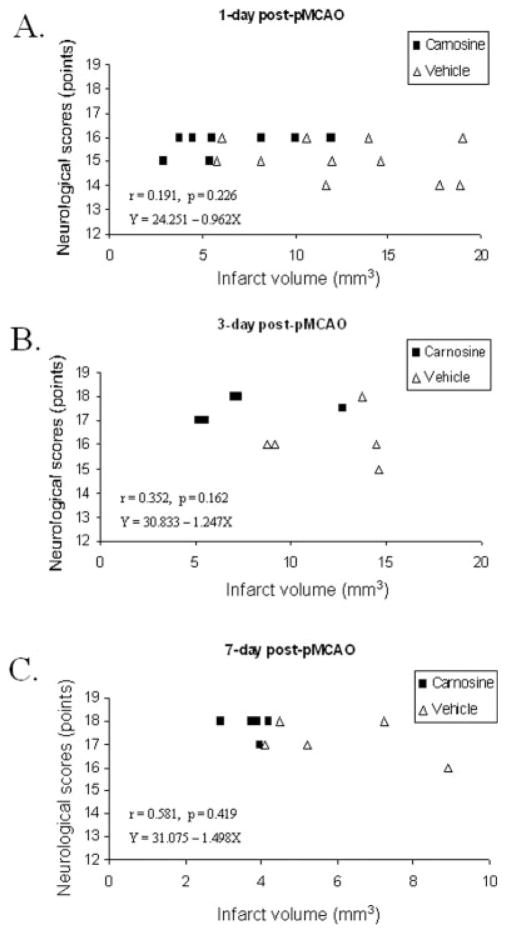
Fig. 4.
Linear regression study of infarct volume and neurological scores in mice with pMCAO. Analyses were performed 1 day (A), 3 days (B), and 7 days (C) after pMCAO treated with carnosine or vehicle. This correlation study demonstrated a weak association between infarct size and neurological score at the different times observed.
In accordance with the TTC observations, no statistically significant amelioration of neurological function was observed 1 day following pretreatment with either anserine (15.18 ± 0.30 versus 15.09 ± 0.25) or _N_-acetyl carnosine (15.5 ± 0.17 versus 15.09 ± 0.25), as shown in Figure 1B.
Long-Term Effects of Carnosine on Permanent Cerebral Focal Ischemia
To determine whether the neuroprotective effects of carnosine are sustained, we investigated the effects of carnosine treatment on infarct size and neurological function 1, 3, and 7 days following induction of pMCAO.
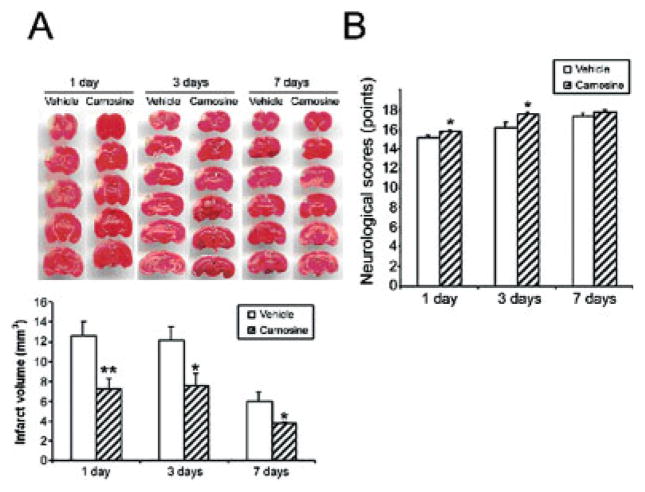
As illustrated in Figure 2A, treatment with carnosine significantly decreased the infarct volume at all times examined compared with that in vehicle-treated mice. Infarct size was reduced by 42.5% 1 day following induction of permanent focal ischemia (7.24 ± 1.04 versus 12.59 ± 1.44 mm3, P = 0.008), 38.2% 3 days following induction of ischemia (7.51 ± 3.04 versus 12.15 ± 2.95 mm3, P = 0.043), and 37.5% 7 days following induction of ischemia (3.74 ± 0.21 versus 5.99 ± 0.91 mm3, P = 0.04).
Fig. 2.
Effects of pretreatment with carnosine on infarct size and neurological function in mice, 1 day, 3 days, and 7 days following pMCAO. A: Top, TTC-stained sections showing volume of the infarct; bottom, quantification of the infarct volume (mm3) at the various points examined. Compared with vehicle-treated mice, carnosine significantly reduced the infarct size at the different observed times following pMCAO. B: Effects of pretreatment with carnosine on neurological function. Histogram values represent means ± SEMs (★P < 0.05, ★★P < 0.01, carnosine versus vehicle at the same survival point).
We then sought to determine whether the carnosine-induced reduction in infarct size would translate into functional recovery by carrying out neurological evaluations according to the 18-point-based scale of Garcia et al. (1995). Neurological evaluation performed right before surgery indicated that neurological scores were normal (18 points) in all animals treated with either vehicle or carnosine (data not shown). Vehicle-treated mice exhibited a significant decrease in neurological scores 1 day (15.1 ± 0.25 versus 18, P < 0.001) and 3 days (16.2 ± 0.49 versus 18, _P_ = 0.014) following surgery, but not 7 days (17.2 ± 0.37 versus 18, _P_ > 0.05) following surgery (Fig. 2B). However, this reduction in neurological function was distinctly lessening with increasing time (Fig. 2B). In contrast, a statistically significant improvement in neurological performances was noticeable in carnosine-treated mice 1 day (15.8 ± 0.13 versus 15.09 ± 0.25, P = 0.03) and 3 days (17.5 ± 0.22 versus 16.2 ± 0.49, P = 0.04) following permanent cerebral ischemia. A non–statistically significant trend was observed 7 days following pMCAO between vehicle- and carnosine-treated mice (17.8 ± 0.2 versus 17.2 ± 0.37, P > 0.05; Fig. 2B). We then conducted a linear regression analysis to determine whether there was a correlation between infarct size and neurological function. No significant correlation was found between those two parameters 3 days (Fig. 4B) and 7 days (Fig. 4C) after pMCAO and treatment.
Effects of Bestatin on Permanent Cerebral Focal Ischemia
In a previous study, we have shown a naturally occurring increase in immunoreactivity for carnosine in mouse brains 1 day following permanent focal ischemia (Rajanikant et al., 2007). The observation that exogenously administered carnosine is neuroprotective in the ischemic brain led us to suggest that the administration of a compound that could increase endogenous carnosine levels in the brain might be an effective strategy to alleviate pMCAO-related brain injury. To test this hypothesis, we analyzed the effects of pretreatment with the aminopeptidase inhibitor bestatin, an agent known to block the activity of carnosine degradation enzymes (Kunze et al., 1986; Otani et al., 2005).
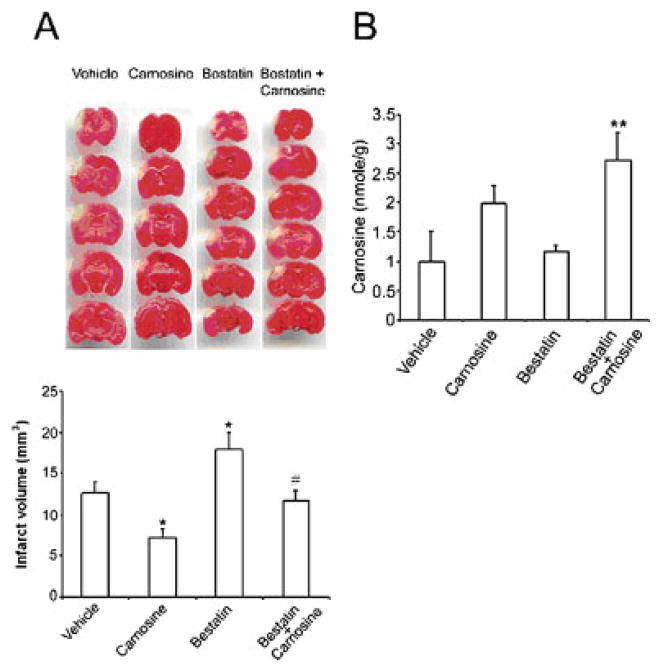
As illustrated in Figure 3A, pretreatment with bestatin surprisingly resulted in a 49% statistically significant increase in the infarct size 1 day following pMCAO compared with that in the vehicle (17.97 ± 2.08 versus 12.06 ± 1.64 mm3, P = 0.026). This effect was fully reversed when carnosine was administered with bestatin, so that the infarct size in treated mice was not different from that observed in vehicle-treated animals (11.71 ± 1.20 versus 12.06 ± 1.64 mm3).
Fig. 3.
Effects of bestatin or a combination of bestatin and carnosine pretreatment on infarct size and endogenous carnosine level in the mouse brain 1 day following chemical injection. A: Top, TTC-stained sections showing the volume of the infarct; bottom, quantification of the infarct volume (mm3) at the various points examined. Compared with vehicle-treated mice, bestatin significantly increased the volume of the infarction. B: HPLC measurements of endogenous cerebral levels of carnosine 1 day following treatments. Histogram values represent means ± SEMs (★P < 0.05, carnosine versus vehicle; ★★P < 0.05, bestatin + carnosine versus vehicle; #P < 0.05, bestatin versus vehicle).
To gain a better understanding of the deleterious effects that bestatin pretreatment had on infarct volume, we used HPLC to measure the amount of carnosine in intact mouse brain 1 day following the administration of carnosine, bestatin, or a combination of bestatin and carnosine. An average of 1 nmol/g of carnosine was detected in the brains of vehicle-treated mice (Fig. 3B). Compared with the vehicle, a slight but not significant increase in cerebral carnosine level was observed 1 day following pretreatment with bestatin (1.17 ± 0.06 versus 1.00 ± 0.5 nmol/g; Fig. 3B). In contrast, administration of both carnosine and bestatin resulted in a statistically significant increase in carnosine level in the brains of treated mice compared with that in the vehicle group (2.72 ± 0.41 versus 1.00 ± 0.5 nmol/g, P = 0.016). Although not reaching statistical significance, this increase in carnosine level was more pronounced than that observed following administration of carnosine alone (2.72 ± 0.41 versus 1.98 ± 0.30 nmol/g).
DISCUSSION
The key findings from the present study are that (1) the efficacy of carnosine, anserine (its methylated form), and _N_-acetyl carnosine (its acetylated form) in protecting the brain against permanent focal cerebral ischemia differs; (2) treatment with carnosine induces long-term protection for at least 7 days following pMCAO; and (3) treatment with bestatin, an inhibitor of a carnosine-degrading enzyme, enhances injury after focal ischemia.
We have shown that anserine and _N_-acetyl carnosine were not as effective as carnosine in protecting the brain against pMCAO. A significant reduction in infarct size and improvement in neurological function were observed 1 day post-pMCAO in mice treated with carnosine. In contrast, anserine and _N_-acetyl carnosine, administered in the same dose regimen, elicited a reduction in infarct size, but it failed to reach statistical significance. Differences in antioxidant properties may account for the disparity between carnosine and its derivatives in protecting the ischemic brain. Indeed, Boldyrev (2000) demonstrated that _N_-acetyl carnosine was the least effective at protecting human serum lipoproteins from hydroxyl radical–induced oxidation. Similarly, carnosine was more effective at suppressing myeloperoxidase reaction on rabbit leukocytes than was its acetylated form, _N_-acetyl carnosine (Boldyrev et al., 1995). Furthermore, in an in vitro model of NMDA-induced neuronal excitotoxicity, _N_-acetyl carnosine was weak at reducing the levels of reactive oxygen species (Boldyrev et al., 2004). In contrast, anserine was reported to have greater antioxidant properties than carnosine in homogeneous and liposome systems (Kohen et al., 1988; Boldyrev, 2000), as well as in cultured neuronal cells or isolated rabbit leukocytes (Boldyrev et al., 2003, 2004). We have previously shown that carnosine plays its neuroprotective role not only through the decrease in reactive oxygen species levels but also via its ability to reduce matrix metalloproteinase protein (MMP) levels and preserve normal glutathione levels. The role that anserine and _N_-acetyl carnosine play in regulating MMP and glutathione levels in the brain has yet to be determined and might be different from that described for carnosine. A dose of 1,000 mg/kg, used in this study to assess the potential roles of anserine and _N_-acetyl carnosine in permanent focal cerebral ischemia, was chosen on the basis of our previous work with carnosine, which showed that it was the dose most effective at reducing the infarct size following pMCAO (Rajanikant et al., 2007).
The present data also provide new information that the neuroprotective effect of carnosine was maintained for at least 7 days following pMCAO. Compared with in the vehicle group, carnosine pretreatment was able to significantly lessen infarct size with time, suggesting that carnosine did not simply delay the onset of ischemia but rather truly protected the brain.
Following permanent focal ischemia, rodents exhibited impaired neurological functions (Phipps, 1991). Using the 18-point-based scale from Garcia et al. (1995), deficits in neurological function were observed in all vehicle-treated mice 1 and 3 days following pMCAO-induced ischemia. Corroborating previous studies (Wishcamper et al., 2003; Wang et al., 2007), infarct volume in the vehicle-treated group progressively decreased over time, so that by 7 days post-pMCAO, infarct size was decreased by almost half, and no significant neurological deficits were observed. In contrast, treatment with carnosine significantly improved neurological performance 1 and 3 days following ischemic injury. As expected, anserine and _N_-acetyl carnosine did not exhibit any statistically significant improvement in neurological scores. Further study revealed no correlation between infarct size and improvement in neurological function in carnosine-treated ischemic mice. Such an absence of correlation has been previously reported (Reese et al., 2000; DeVries et al., 2001). These data suggest that not only is neurological outcome associated with infarct size but also that the location of the cerebral ischemic lesion and/or other downstream signals may play a significant role.
Our HPLC data suggest that exogenously administered carnosine results in increased cerebral levels. Indeed, we have previously demonstrated that in response to pMCAO, carnosine immunoreactivity is enhanced in the brain. Therefore, increasing carnosine levels in brain tissue through the use of pharmacological agents might be a valuable alternative approach to protecting the ischemic brain. Carnosine level is regulated by carnosine synthetase (synthesizing enzyme), and carnosinases, which are degradation enzymes (Kunze et al., 1986). In mammals, serum and cytosolic types of carnosinases have been identified (Margolis et al., 1979; Lenney et al., 1982; Kunze et al., 1986; Teufel et al., 2003; Otani et al., 2005). Bestatin is an antitumor dipeptide that inhibits aminopeptidases B/M, and has been shown to inhibit carnosinase activity (Teufel et al., 2003; Otani et al., 2005). We hypothesized that pretreatment with bestatin might increase cerebral levels of carnosine by interfering with its degradation process. Surprisingly, compared with the vehicle-treated group, not only did bestatin not increase intracerebral carnosine levels significantly, but it also dramatically increased the volume of the infarct. In in vitro and in vivo tumor systems, bestatin has been shown to activate macrophages and the subsequent release of cytokines, resulting into cytotoxicity (Schorlemmer, 1983; Ino et al., 1996). This bestatin-related amplification of inflammatory cascades in acute focal ischemia may increase the severity of brain tissue damage. Furthermore, bestatin has been reported in leukemic cell lines to activate caspase 3 and DNA fragmentation, two markers of apoptosis (Sekine et al., 1999). Finally, as shown in the case of enkephalins, because of bestatin’s ability to inhibit a variety of peptidases, its delivery may interfere with the metabolism of a variety of peptides, resulting in their accumulation. Such an increase in peptide content may worsen the deleterious effects of ischemic injury. All these factors combined might explain why pretreatment with bestatin increased the severity of brain damage in ischemic mice and did not raise endogenous carnosine levels in the intact brain. Interestingly, coinjection of both carnosine and bestatin prevented bestatin-related brain damage following pMCAO and resulted in a significant increase in carnosine cerebral levels under normal conditions. In the brain, aminopeptidase M is localized in blood vessels (Hersh et al., 1987). Because bestatin is an aminopeptidase M inhibitor, the higher cerebral carnosine levels observed following coinjection of bestatin with carnosine might have resulted from changes in the dynamics of the blood–brain barrier, subsequently leading to greater exogenous carnosine entry.
In summary, the present study has shown for the first time that carnosine and its analogues anserine and _N_-acetyl carnosine have different neuroprotective efficacies in a mouse model of permanent focal cerebral ischemia. We have shown that carnosine is the most effective in reducing infarct size and ameliorating neurological function. Furthermore, the neuroprotective effects are sustained for at least 7 days. These neuroprotective properties of carnosine combined with its minimal side effects make it an attractive candidate for further evaluation as a treatment for cerebral ischemia.
References
- Abe F, Schneider M, Black PL, Talmadge JE. Chemoimmunotheray with cyclophosphamide and bestatin in experimental metastasis in mice. Cancer Immunol Immunother. 1989;29:231–236. doi: 10.1007/BF00199209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffo S, DeLucia R, Mulatero B, Margolis F, Fasolo A. Carnosine-, calcitonin gene-related peptide- and tyrosine hydroxylase-immunoreactivity in the mouse olfactory bulb following peripheral denervation. Brain Res. 1990;528:353–357. doi: 10.1016/0006-8993(90)91682-7. [DOI] [PubMed] [Google Scholar]
- Boldyrev A, Abe H, Stvolinsky S, Tyulina O. Effects of carnosine and related compounds on generation of free oxygen species: a comparative study. Comp Biochem Physiol. 1995;112B:481–485. doi: 10.1016/0305-0491(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Stvolinsky SL, Tyulina OV, Koshelev VB, Hori N, Carpenter DO. Biochemical and physiological evidence that carnosine is an endogenous neuroprotector against free radicals. Cell Mol Neurobiol. 1997;17:259–271. doi: 10.1023/A:1026374114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev A, Song R, Lawrence D, Carpenter DO. Carnosine protects against excitotoxic cell death independently of effects on reactive oxygen species. Neuroscience. 1999;94:571–577. doi: 10.1016/s0306-4522(99)00273-0. [DOI] [PubMed] [Google Scholar]
- Boldyrev A, Abe H. Metabolic transformation of neuropeptide carnosine modifies its biological activity. Cell Mol Neurobiol. 1999;19:163–175. doi: 10.1023/A:1006933028389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev AA. Problems and perspective in studying the biological role of carnosine. Biochem (Mosc) 2000;65:884–890. [PubMed] [Google Scholar]
- Boldyrev A, Bulygina E, Leinsoo T, Petrushanko I, Tsubone S, Abe H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp Biochem Physiol. 2004;137:81–88. doi: 10.1016/j.cbpc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P, Marchis SD, Fasolo A. Carnosine-related dipeptides in the mammalian brain. Prog Neurobiol. 1999;59:333–353. doi: 10.1016/s0301-0082(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Botbol V, Scornik OA. Measurement of muscle protein degradation in live mice by accumulation of bestatin-induced peptides. Am J Physiol Endocrinol Metab. 1997;273:E1149–E1157. doi: 10.1152/ajpendo.1997.273.6.E1149. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Melcangi RC, Modena C, Cavaretta I, Peretto P, Agresti C, Fasolo A. Identification of the glial cell types containing carnosine-related peptides in the rat brains. Neurosci Lett. 1997;237:37–40. doi: 10.1016/s0304-3940(97)00800-8. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci Biobehav Rev. 25:326–342. doi: 10.1016/s0149-7634(01)00017-3. [DOI] [PubMed] [Google Scholar]
- Dobrota D, Fedorova T, Stvolinsky S, Babusikova E, Likavcanova K, Drgova A, Strapkova A, Boldyrev A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res. 2005;30:1283–1288. doi: 10.1007/s11064-005-8799-7. [DOI] [PubMed] [Google Scholar]
- Dunnett M, Harris RC. High-performance liquid chromatographic determination of imidazole dipeptides, histidine, 1-methylhistidine and 3-methylhistidine in equine and camel muscle and individual muscle fibres. J Chromatography B. 1997;688:47–55. doi: 10.1016/s0378-4347(97)88054-1. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behavior. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry (Mosc) 2000;65:866–868. [PubMed] [Google Scholar]
- Garcia J, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995;26:627–635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Gariballa SE, Sinclair AJ. Carnosine: physiological properties and therapeutic potential. Age Ageing. 2000;29:207–210. doi: 10.1093/ageing/29.3.207. [DOI] [PubMed] [Google Scholar]
- Gulewitsch WS, Amiradzibi S. Uber das carnosine, eine neue organische Bases des Fleischextraktes. Ber Disch Ges. 1900;33:1902–1904. [Google Scholar]
- Hersh LB, Aboukhair N, Watson S. Immunohistochemical localization of aminopeptidase M in rat brain and periphery: relationship of enzyme localization and enkephalin metabolism. Peptides. 1987;8:523–532. doi: 10.1016/0196-9781(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Horning MS, Blakemore LJ, Trombely PQ. Endogenous mechanisms of neuroprotection: role of zinc, copper, and carnosine. Brain Res. 2000;852:56–61. doi: 10.1016/s0006-8993(99)02215-5. [DOI] [PubMed] [Google Scholar]
- Ino K, Bierman PJ, Varney ML, Heimann DG, Kuszynski CA, Walker SA, Talmadge JE. Monocyte activation by an oral immunomodulator (bestatin) in lymphoma patients following autologous bone marrow transplantation. Cancer Immunol Immunother. 1996;43:206–212. doi: 10.1007/s002620050323. [DOI] [PubMed] [Google Scholar]
- Jin CL, Yang LX, Wu XH, Li Q, Ding MP, Fan YY, Zhang WP, Luo JH, Chen Z. Effects of carnosine on amygdaloid-kindled seizures in Sprague-Dawley rats. Neuroscience. 2005;135:930–947. doi: 10.1016/j.neuroscience.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Kohen R, Yamamoto Y, Cundy K, Ames BN. Antioxidant activity of carnosine, homocarnosine and anserine present in muscle and brain. Proc Natl Acad Sci USA. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze N, Kleinkauf H, Bauer K. Characterization of two carnosine-degrading enzymes from rat brain: Partial purification and characterization of a carnosinase and a B-alanyl-arginine hydrolase. Eur J Biochem. 1986;160:605–613. doi: 10.1111/j.1432-1033.1986.tb10081.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome C release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Lenney JF, George RP, Weiss AM, Kucera CM, Chan PW, Rinzler GS. Human serum carnosinase: characterization, distinction from cellular carnosinase, and activation by cadmium. Clin Chim Acta. 1982;123:221–231. doi: 10.1016/0009-8981(82)90166-8. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Margolis FL, Grillo M, Brown CE, Williams TH, Pitcher RG, Flgar GJ. Enzymatic and immunological evidence for two forms of carnosinase in the mouse. Biochim Biophy Acta. 1979;570:311–323. doi: 10.1016/0005-2744(79)90151-7. [DOI] [PubMed] [Google Scholar]
- Mitsios N, Gaffney J, Kumar P, Krupinski J, Kumar S, Slevin M. Pathophysiology of acute ischaemic stroke: An analysis of common signalling mechanisms and Identification of new molecular targets. Pathobiology. 2006;73:159–175. doi: 10.1159/000096017. [DOI] [PubMed] [Google Scholar]
- O’Dowd JJ, Cairns MT, Trainor M, Robins DJ, Miller DJ. Analysis of carnosine, homocarnosine, and other histidyl derivatives in rat brain. J Neurochem. 1990;55:446–452. doi: 10.1111/j.1471-4159.1990.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Otani H, Okumura N, Hashida-Okumura A, Nagai K. Identification and characterization of a mouse dipeptidase that hydrolyzes L-carnosine. J Biochem (Tokyo) 2005;137:167–175. doi: 10.1093/jb/mvi016. [DOI] [PubMed] [Google Scholar]
- Peppers SC, Lenney JF. Bestatin inhibition of human tissue carnosinase, a non-specific cytosolic dipeptidase. Biol Chem Hoppe Seyler. 1988;369:1281–1286. doi: 10.1515/bchm3.1988.369.2.1281. [DOI] [PubMed] [Google Scholar]
- Phipps MA. Assessment of neurological deficits in stroke. Acute-care and rehabilitation implication. Nurs Clin North Am. 1991;26:957–910. [PubMed] [Google Scholar]
- Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, Majid A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007;38:3023–3031. doi: 10.1161/STROKEAHA.107.488502. [DOI] [PubMed] [Google Scholar]
- Reese T, Pórszász P, Baumann D, Bochelen D, Boumezbeur F, McAllister KH, Sauter A, Bjelke B, Rudin M. Cytoprotection does not preserve brain functionality in rats during the acute post-stroke phase despite evidence of non-infarction provided by MRI. NMR Biomed. 2000;13:361–370. doi: 10.1002/1099-1492(200010)13:6<361::aid-nbm654>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Schorlemmer HM, Bosslet K, Sedlacek HH. Ability of the immunomodulating dipeptide bestatin to activate cytotoxic mononuclear phagocytes. Cancer Res. 1983;43:4148–4153. [PubMed] [Google Scholar]
- Sekine K, Fujii H, Abe F. Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines. Leukemia. 1999;13:729–734. doi: 10.1038/sj.leu.2401388. [DOI] [PubMed] [Google Scholar]
- Stvolinsky SL, Dobrota D. Anti-ischemic activity of carnosine. Biochemistry. 2000;65:998–1005. [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tabakman R, Lazarovici P, Kohen R. Neuroprotective effects of carnosine and homocarnosine on pheochromocytoma PC12 cells exposed to ischemia. J Neurosci Res. 2002;68:463–469. doi: 10.1002/jnr.10228. [DOI] [PubMed] [Google Scholar]
- Teufel M, Saudek V, Ledig JP, Bernhard A, Boularand S, Carreau A, Cairns NJ, Carter C, Cowley DJ, Duverger D, Ganzhorn AJ, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem. 2003;278:6521–6531. doi: 10.1074/jbc.M209764200. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Horning MS, Blakemore LJ. Interactions between carnosine and zinc and copper: implications for neuromodulation and neuroprotection. Biochemistry (Mosc) 2000;65:807–816. [PubMed] [Google Scholar]
- Wishcamper CA, Brooks DM, Douglas CJ, Lurie DI. Focal cerebral ischemia upregulates SHP-1 in reactive astrocytes in juvenile mice. Brain Res. 2003;974:88–98. doi: 10.1016/s0006-8993(03)02564-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]