Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 1.
Abstract
Objective
The development of brain inflammation largely contributes to neonatal brain injury that may lead to a lifetime of neurologic deficits. The present study was designed to investigate whether inhibition of cyclooxygenase-2 (COX-2), a critical component of the inflammatory pathway, is neuroprotective in a neonatal rat model of cerebral hypoxia-ischemia (HI).
Design
Laboratory investigation.
Setting
University research laboratory.
Subjects
Postnatal day-10 Sprague-Dawley rats.
Interventions
Neonatal HI was induced by ligation of the right common carotid artery followed by two hours of hypoxia (8% O2). The pups in treatment groups were administered 10mg/kg (low dose) or 30mg/kg (high dose) of a known selective COX-2 inhibitor (NS398). Animals were euthanized at three time points: 72hrs, 2wks, or 6wks. Inflammation outcomes were assessed at 72hrs; brain damage was assessed at 2- and 6wks along with other organs (heart, spleen). Detailed neurobehavioral examination was performed at 6wks.
Measurements and Main Results
Pharmacological inhibition of COX-2 markedly increased survivability within the first 72hrs compared to untreated rats (100% vs. 72%). Low- and high-dose NS398 significantly attenuated the loss of brain and body weights observed after HI. Neurobehavioral outcomes were significantly improved in some parameters with low dose treatment; while, high dose treatment consistently improved all neurological deficits. Immunohistochemical results showed a marked decrease in macrophage, microglial, and neutrophil abundance in ipsilateral brain of NS398 treated group along with a reduction in interleukin-6 expression.
Conclusions
Selective COX-2 inhibition protected neonatal rats against death, progression of brain injury, growth retardation, and neurobehavioral deficits after a hypoxic-ischemic insult.
Keywords: inflammation, cyclooxygenase-2, neonatal, neuroprotection, stroke, cerebral ischemia
Introduction
A hypoxic-ischemic insult to neonates results not only in brain damage, but is also associated with increased mortality and somatic growth retardation (1, 2). There is no effective treatment for neonatal hypoxia-ischemia (HI). Long-term effects of HI for the survivors may include motor disability, cognitive dysfunction, and problems in learning and behavior (3). The inflammatory response is particularly detrimental in the immature brain, serving a key component in the progression of neonatal encephalopathy (4).
Cyclooxygenase-2 (COX-2), the inducible form of the enzyme and a key mediator of inflammation, is critical in different forms of brain injury such as excitotoxic brain injury, cerebral ischemia, traumatic brain injury, and neurodegenerative disorders (5). Cerebrospinal fluid concentrations of prostaglandins such as PGE2 and PGI2, which are downstream effectors of COX-2 enzyme, have been reported to be significantly higher in children with perinatal hypoxia (6). Moreover, pharmacological inhibition of COX-2 has been shown to be beneficial in brain and spinal cord-related injuries in adults (7, 8). To date, no study has examined the effects of COX-2 inhibition against neonatal HI-induced brain injury.
Accordingly, we hypothesized that inhibition of COX-2-induced-inflammation will reduce brain injury; improve neurological outcomes, and somatic and systemic organ growth following HI in neonates. We examined the lasting effects of COX-2 inhibition using two different doses of a selective COX-2 inhibitor, NS398, in a well established neonatal HI model in rats. We also confirmed the presence of COX-2 in neuronal cells and used the pro-inflammatory cytokine, interleukin-6 (IL-6), as an endpoint for inflammation.
Materials and Methods
Animal Groups and Operative Procedure
This study was in accordance with the National Institutes of Health guidelines for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Timed pregnant Sprague-Dawley rats were housed with food and water available ad libitum. Postnatal day-10 pups were randomly assigned to the following groups: sham, HI [Vehicle], HI+10mg/kg NS398 [NS-10], or HI+30mg/kg NS398 [NS-30]. Each litter consisted of all groups. Pups were placed on a surgical table maintained at 37°C and anesthetized by inhalation with isoflurane (3% in mixed air and oxygen). HI-groups had right common carotid artery permanently ligated. After 1.5hours (hrs) of recovery, pups were placed in a glass jar (submerged in a water bath maintained at 37°C) perfused with 8% oxygen for 2hrs. Rats were euthanized under general anesthesia [ketamine (80mg/kg)/xylazine (10mg/kg)] by decapitation at 72hrs, 2- and 6weeks (wks) post-HI.
Treatment Method
Some pups were treated intraperitoneally with a COX-2 inhibitor (NS398) at either 10mg/kg or 30mg/kg dosage (Cayman Chemical, Ann Arbor, MI) diluted in 10% dimethylsulfoxide (DMSO) and saline. Treatment consisted of six injections (1, 6, 24, 36, 48, and 60hrs) after hypoxia. Vehicle pups followed same injection regimen and methodology, but administered 10% DMSO in saline.
Evaluation of Brain Damage
Hemispheric weight loss has been used as the primary variable to estimate brain damage in this animal model (9). At 2- and 6wks, the brain was removed, without prior perfusion, and the hemispheres were separated by a midline incision and weighed on a high-precision balance (sensitivity ±0.001g).
Measurement of Organ Weight
After removal of brain, the spleen and heart were isolated and detached from surrounding tissue and vessels and weighed at the 6wk interval.
Assessment of Neurobehavioral Deficits
Rats were tested at 6wks and scored accordingly: 0 for immediate and correct placement; 1 for delayed and/or incomplete placement; 2 for no placement. Scores corresponded to raw values: 0 = raw value of 100; 1 = raw value of 50; 2 = raw value of 0. Methodology was as previously described (10) for the first six tests:
- Postural Reflex: Assessed upper body posture and symmetry in forelimb extension (11). Rat was held by tail and lowered to 10cm above table top.
- Proprioceptive Limb Placing: Rat's head was tilted 45° upwards to avoid visual and tactile contact with table. Dorsum of paw was pushed against table edge to stimulate limb muscles and joints for forelimb placement onto table top.
- Back Pressure Towards Edge: Rat was moved from behind toward table edge for assessment of forward limb placement.
- Lateral Pressure Towards Edge: Rat was moved from ipsilateral or contralateral side toward table edge for assessment of lateral and forward limb placement.
- Forelimb Placement: Rat was held facing table edge with visual and tactile (whisker) contact with table, and assessed for forward limb placement onto table top.
- Lateral Limb Placement: Rat was held parallel to table edge with tactile contact, and assessed for lateral limb abduction onto table top.
- T-Maze: Assessed short-term or working memory (12). Rat was placed in the stem (40×10cm) of maze and allowed to explore until an arm (46×10cm) of maze was chosen. The sequence (10 trials) of left and right arm choices was expressed as rate of spontaneous alternation (0%=no alternation, 100%=alternation at each trial).
- Foot-fault: Assessed placement dysfunction of forepaws and motor coordination, and is reliable in differentiating between ischemic and normal rats (11, 13). Rat was placed onto an elevated wire grid floor (20×40cm) for 2 minutes. Foot-faults were when a complete paw fell through openings.
Immunohistochemistry with DAB Staining
Animals (n=5/group) were perfused with 0.1M phosphate buffered saline (PBS) and fixed with 10% paraformaldehyde (formalin) diluted in PBS, via trans-cardiac approach 72hrs post-insult. The sectioned brain volume (5mm) encompassed dorsal hippocampus. Every fifth section of the tissue block was collected, and from this set, 6 random, non-adjacent sections were stained and then observed. Special care was taken to analyze sections from the same levels of sectioning in different animals. Antigen retrieval was done on slices (10μm) by microwave irradiation in 0.1M sodium citrate (pH=6) for 10 minutes. Diaminobenzidine (DAB) staining method (ABC Staining Kit, Santa Cruz Biotech, Santa Cruz, CA) was implemented as previously described (14) for detection of COX-2 expression in the ipsilateral cortex and CA1 region of hippocampus. Antibodies included goat anti-COX-2 (1:100) and donkey anti-goat secondary antibody (1:200). All antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA), unless otherwise stated. Controls for non-specific immunohistochemical staining were done with omission of the primary antibodies.
Western Blotting of COX-2
Animals (n=5/group) were perfused (0.1M PBS) at 72hrs post-HI. Ipsilateral hemisphere was isolated then snap-frozen and kept at -80°C until analysis. Samples in (300mg/mL) extraction buffer (50mM Tris-HCl buffer [pH 7.4] with 150mM NaCl, 1% Nomide P40, 0.1% sodium dodecyl sulfate (SDS), 0.1% deoxycholic acid, and 1% PMSF) and 1% protease inhibitor were homogenized with a tissue homogenizer for a total of 60 seconds (20× 3 sec pulses). The homogenate was centrifuged (15000 g for 20 min), the supernatant of the extract was collected, and the concentration of the protein samples was determined by Bradford assay (BioRad, Hercules, CA). All procedures were performed at 4°C. 40μg sample of extracted protein with 2× loading buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 25% glycerol, 0.01% Bromophenol Blue, and 5% β-mercaptoethanol) were subjected to electrophoresis on 10% polyacrylamide SDS gel (BioRad, Hercules, CA). Procedures were as previously described (15). Primary antibodies were goat anti-COX-2 and rabbit anti-β-actin. Incubation with donkey anti-goat and donkey anti-rabbit secondary antibodies was done, respectively. Bands were detected by chemiluminescent kit (Amersham Bioscience, Piscataway, NJ) on X-ray film (Kodak, Rochester, NY). Optical density was determined using NIH Image J software and expressed relative to β-actin then to sham group.
Triple Fluorescent Labeling
Cerebral tissue was perfused (0.1M PBS), fixed (10% formalin), and sectioned (5mm volume) at 72hrs for use in triple-fluorescent labeling. Primary antibodies were goat anti-COX-2 (1:50), rabbit anti-IL-6 (1:50) with mouse anti-NeuN (1:100; Millipore Corp., Billerica, MA). Tissue slices (10μm) were blocked with 5% donkey serum in PBS at room temperature for 2hrs. Slices were incubated overnight at 4°C, followed with respective donkey secondary antibodies conjugated with fluorescent dyes for 2hrs at room temperature in the dark, as previously described (16). Between incubations, three washes of 5min were performed at room temperature with 0.01 M PBS (pH 7.4). Controls for non-specific immunofluorescence staining were done with omission of the primary antibodies. To test for tissue autofluorescence, some sections were processed without the secondary antibodies. The ipsilateral cortex and CA1 region of hippocampus (n=5/group) were analyzed using a fluorescent microscope with digital camera (OLYMPUS BX51, Melville, NY).
Inflammatory Cell Infiltration
Animals (n=5/group) were perfused (0.1M PBS) at 72hrs post-HI. Ipsilateral hemisphere was isolated then snap-frozen and kept at -80°C until analysis of interleukin-6 (IL-6) concentration by ELISA technique (Invitrogen Corp., Carlsbad, CA) and expressed as picogram per milligram protein. Samples in (300mg/mL) extraction buffer (50mM Tris-HCl buffer [pH 7.4] with 0.6M NaCl, 0.2% Triton X-100, 10μl aprotinin, 1μg/mL leupeptin, and 1mM PMSF) and 1% protease inhibitor were homogenized, the supernatant of the extract was collected, and the protein samples were assayed in duplicate. Cerebral tissue was perfused (0.1M PBS), fixed (10% formalin), and sectioned (5mm volume) for detection of inflammatory cell infiltration into ipsilateral cortex (n=5/group). Rabbit anti-Iba1 (1:100; Wako Chemicals USA Inc., Richmond, VA), mouse anti-CD68 (1:100; Millipore Corp., Billerica, MA), or goat anti-MPO (1:100) primary antibodies (Abcam Inc., Cambridge, MA) were added to sections (10μm). Incubation methodology was same as in triple-fluorescent labeling. An estimation of the amount of positive cells were defined as being low (<10 postive cells/per high power visual field) or high (>10 positive cells/per high power visual field). As with all staining, differences in levels of infiltrating cell markers were noted between groups by an experimenter blinded to the treatment group of each section/slide observed.
Data analysis
Data was expressed as mean ± SEM. One-way ANOVA and Tukey test were used to determine significance in differences between means. Neurological scores were analyzed using Means of Dunn Method (except T-Maze and Foot-fault tests); mortality rates using chi square test; and assays using Dunnett's post hoc test with vehicle designated as control. Significance was accepted at p < .05.
Results
NS398 protects against HI-related lethality
Treatment with NS398 completely abolished the mortality evidenced in the untreated group (0% vs. 28.12%). While nine vehicle pups died within the first 72hrs following hypoxia; none of the NS398-treated pups died at any time during the experiment, indicating that survival was specifically related to COX-2 inhibition. No sham-operated pups died.
Cyclooxygenase-2 blockade maintains body weight after HI
Growth retardation is evident in both patients and experimental studies as a result of hypoxia-ischemia (2, 17). Accordingly, somatic growth retardation was apparent in vehicle rats at 2-, 3-, and 6wks (Fig. 1A) compared to sham. NS-30 significantly improved body weight at the 6wk time-point. Drastic differences in fur texture and appearance were detected as early as 2wks between treated and untreated rats (Fig. 1, B and C).
Figure 1.
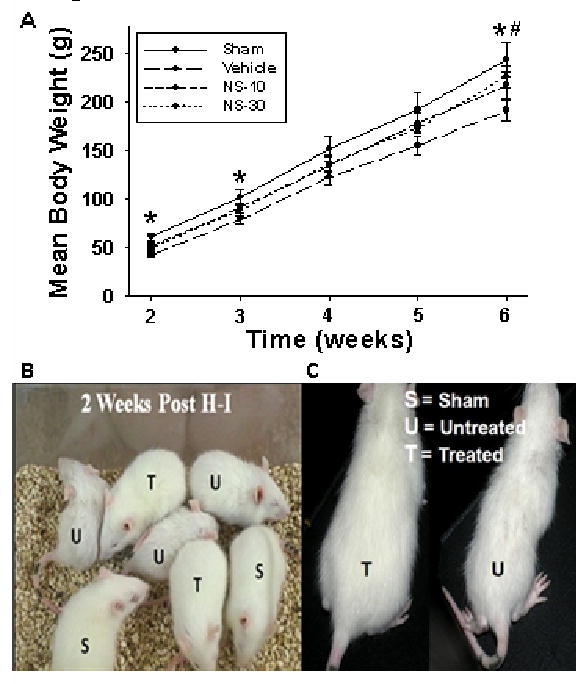
Long-term effects of NS398 on body weight. Postnatal day-10 rats were induced with a hypoxic-ischemic (HI) event: ligation of right common carotid artery and 2hrs hypoxia (8% O2) [Vehicle], then treated with 6 intraperitoneal injections (1, 6, 24, 36, 48, and 60hrs post-hypoxia) of a selective cyclooxygenase-2 (COX-2) inhibitor at either 10mg/kg [NS-10] or 30mg/kg [NS-30] dosage. Sham animals had same anesthesia and surgical procedure, except that the common carotid artery was not ligated. A, Vehicle animals had a significantly lower mean body weight at 2-, 3- and 6wks, compared to sham (*p < .05). NS-30 had long-term lasting effects as it maintained body weight compared to vehicle 6wks after HI (#p < .05). Only mean body weights of animals kept through the 6wk time-point are represented (n = 9/group). B, Differences in fur texture and appearance were detected as early as 2wks between treated (T) and untreated rats (U). C, Is a close-up picture demonstrating the somatic differences between treated and untreated rats at 2wks.
NS398 provides neuroprotection by maintaining brain weight at two time intervals
Hemispheric weight loss is an established estimate of brain damage in this animal model (9). Severe brain atrophy, marked by a reduction in right to left hemispheric weight ratio, was seen in vehicle rats at 2- and 6wks post-HI (Fig. 2, A and B). Blockade of COX-2 protected rats at both time-points.
Figure 2.
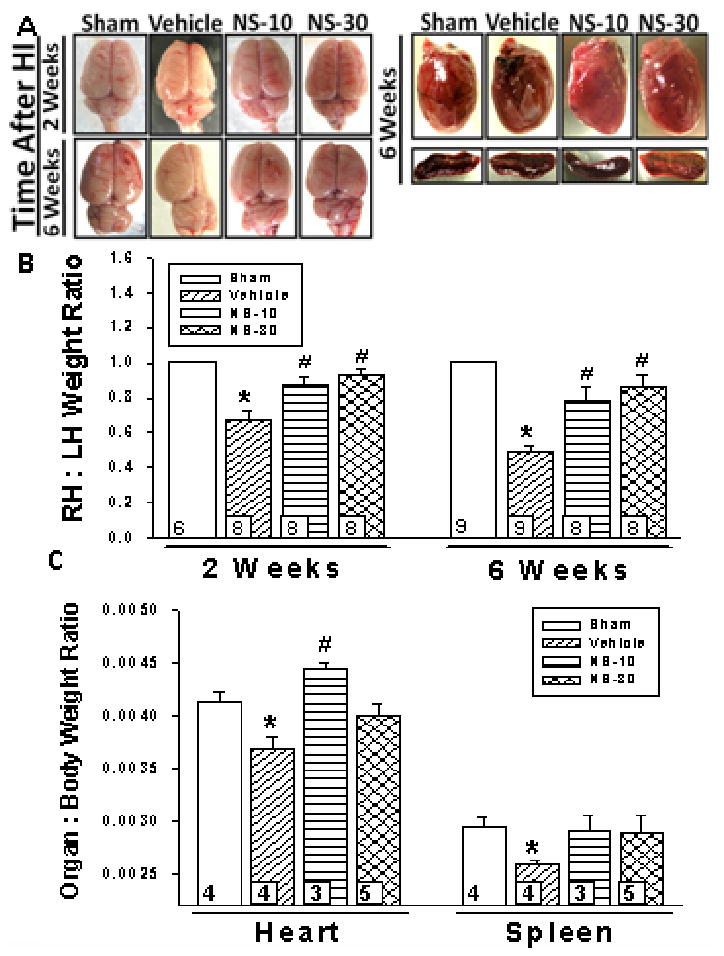
Dose-dependent effect of NS398. Brain and organ tissue of postnatal day-10 rats induced with a hypoxic-ischemic (HI) event [Vehicle], then treated with a selective cyclooxygenase-2 (COX-2) inhibitor at either 10mg/kg [NS-10] or 30mg/kg [NS-30] dosage were examined. Sham animals were used as control. A, NS-10 and NS-30 maintained the gross morphology of the rat brains at 2- and 6wks post-HI. Representative pictures of heart and spleen are shown of all groups. B, Right to left hemispheric (RH:LH) weight ratio is representative of brain atrophy. At 2wks post-insult, vehicle rats had a significantly reduced RH:LH ratio compared to sham (0.67 ± .05 vs. 1.00 ± .01). This was attenuated by NS-10 (0.88 ± .04) and NS-30 (0.93 ± .03). At 6wks post-insult, vehicle rats had a significantly reduced RH:LH ratio compared to sham (0.49 ± .04 vs. 1.00). This was attenuated by treatment (NS-10:0.78 ± .08; NS-30:0.87 ± .06). C, Vehicle rats had a significantly reduced heart to body weight ratio (0.0037 ± .0001 vs. 0.0041 ± .0001) and spleen to body weight ratio (0.0026 vs. 0.0030 ± .0001) as compared to sham, at 6wks post-insult. Treatment increased the heart to body weight ratio (NS-10:0.0045 ± .0001; NS-30:0.0040 ± .0001) and the spleen to body weight ratio (NS-10: 0.0029 ± .0002; NS-30: 0.0029 ± .0002) at 6wks post-insult. Data represent mean ± SEM; *p < .05 versus sham, #p < .05 versus vehicle. Numbers in bars indicate animals/group.
NS398 prevents HI-related systemic organ atrophy
A reduction in spleen size following middle cerebral artery occlusion (MCAO) model of stroke (18) is correlated with the extent of brain damage (19). Our experiments supported this observation in the HI neonatal model; with vehicle having a reduced spleen to body weight ratio (Fig. 2, A and C). Treatment groups demonstrated a trend towards maintaining spleen weight; however, no statistical significance was reached. Heart to body weight ratio decreased in vehicle pups and was attenuated by NS-10 (Fig. 2, A and C). Although NS-30 demonstrated a trend towards maintaining heart weight, no significance was reached.
NS398 prevents neurobehavior deficits
Motor, cognitive, and behavioral deficits can be a consequence of perinatal stroke and may last a lifetime (20). Numbers in parenthesis denote mean raw score. Sham demonstrated a successful performance (91.67) in postural reflex test (Fig. 3A); treatment (78.57) significantly improved the gross asymmetry in posture and extension of forelimbs seen in vehicle rats (8.33). In proprioceptive limb placing test, vehicle was delayed or unable to place forelimbs onto table top (25.0). NS-30 entirely ameliorated the deficit (100.0). In back pressure towards edge test, vehicle was delayed or unable to place limbs forward (25.0); in contrast, NS-30 rats showed immediate placement (92.86). In lateral pressure towards edge test, sham (91.67) had immediate lateral and forward limb placement. This response was delayed or not present in vehicle (25.0), but markedly improved by NS-30 (85.71). In forelimb placement test, vehicle was delayed or unable to place limbs forward when allowed visual and tactile contact (33.33); NS-30 corrected performance (92.86). In lateral limb placement test, vehicle rats were unable to abduct limbs (8.33), a deficit improved by NS-30 (71.3). In T-maze, untreated animals showed a significant decline in memory (reduced %alternations) that was improved by NS-10 and more so by NS-30 (Fig. 3B). Treatment also significantly attenuated the increased number of foot-faults in brain injured rats (Fig. 3C).
Figure 3.
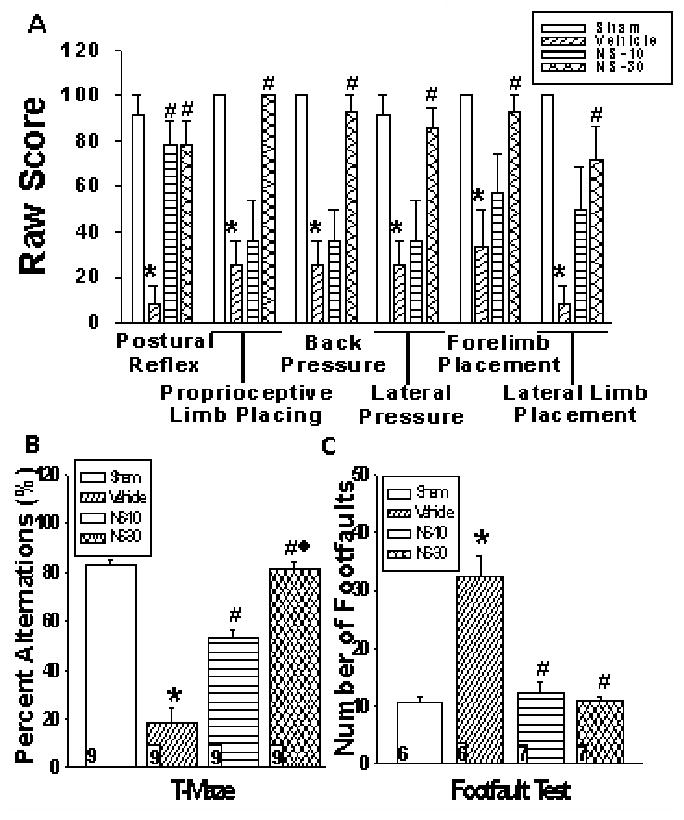
Long-term neurobehavioral effects of postnatal day-10 rats induced with a hypoxic-ischemic (HI) event [Vehicle] then treated with a selective cyclooxygenase-2 (COX-2) inhibitor at either 10mg/kg [NS-10] or 30mg/kg [NS-30] dosage were examined at 6wks post-insult. Sham animals were used as control. A, Rats received a raw score of 100 for immediate and correct placement; 50 for delayed and/or incomplete placement; 0 for no placement (n = 9/group). Administration of NS-30 significantly improved all assessed behavior deficits; while, NS-10 significantly improved deficits associated with the postural reflex test. Data represent *p < .05 versus sham, #p < .05 versus vehicle. B, Vehicle rats alternated significantly less between the two arms of the maze as compared to sham (18.52% ± 5.56 vs. 82.72% ± 2.69). Percent alternations significantly rose with administration of NS-10 (53.09% ± 3.09) and more so by NS-30 (81.48% ± 2.62). Data represent mean ± SEM; *p < .05 versus sham, #p < .05 versus vehicle, ◆p < 0.05 versus NS-10. C, Vehicle rats averaged the greatest number of foot-faults (32.33 ± 3.49), while treatment significantly reduced the deficit (NS-10:12.29 ± 1.70; NS-30:10.86 ± .86). Sham animals had an average of 10.67 ± 1.05 foot-faults. Data represent mean ± SEM; *p < .05 versus sham, #p < .05 versus vehicle. Numbers in bars indicate animals/group.
NS398 effectively reduces cyclooxygenase-2 expression
Positive staining for COX-2 was detected by DAB stain (Fig. 4A). The negative control showed no positive staining indicating that the primary antibody, and not non-specific immunohistochemical staining, was responsible for the positive signal. NS-30 qualitatively reduced post-HI COX-2 expression in both examined regions of the brain. Higher magnification confirmed COX-2 localization in the cytoplasm of the cell. Western blotting of COX-2 quantitatively supported significant differences between treated and vehicle groups (Fig. 4B).
Figure 4.
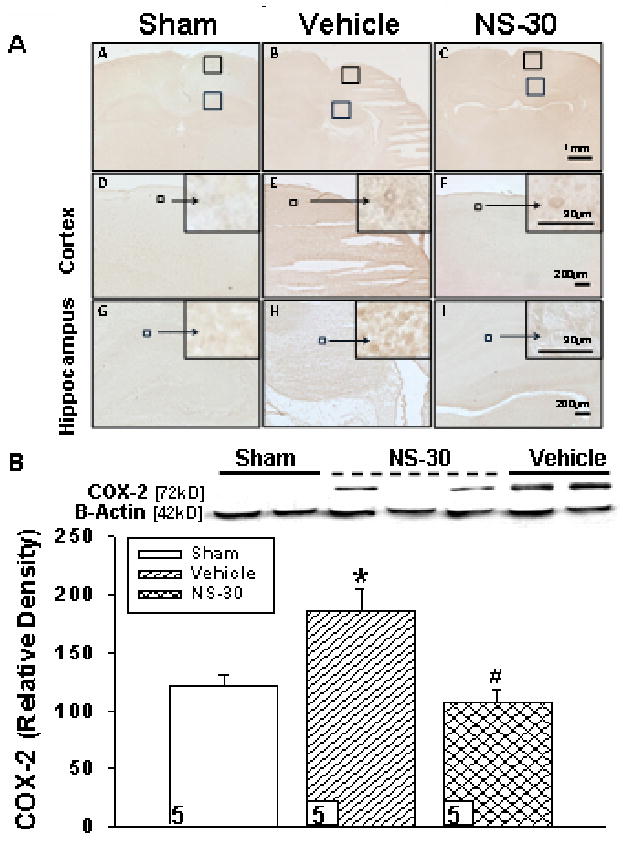
Cyclooygenase-2 (COX-2) expression in postnatal day-10 rats induced with a hypoxic-ischemic (HI) event [Vehicle], then treated with a selective COX-2 inhibitor at 30mg/kg [NS-30] dosage were examined at 72hrs post-insult. Sham animals were used as control. A, Diaminobenzidine (DAB) stain for COX-2 in ipsilateral cerebral cortex (D-F) and CA1 region of hippocampus (G-I) qualitatively appears less in sham (A,D,G) and NS-30 (C,F,I), as compared to vehicle (B,E,H). Six non-adjacent coronal sections per brain (n = 5/group) were analyzed. B, Western blotting supported a statistically significant reduction in COX-2 expression in the ipsilateral hemisphere of NS-30 compared to vehicle (106.92 ± 10.48 vs. 185.20 ± 19.54). Sham (120.88 ± 9.30) also had significantly less COX-2 expression than the vehicle group. Data represent *p < .05 versus sham, #p < .05 versus vehicle. Numbers in bars indicate animals/group.
NS398 reduces cytokine expression in the brain
In the hippocampus and cerebral cortex, there was strong neuronal signal for COX-2 and IL-6 in vehicle (Fig. 5, A and B). The opposite was seen in NS-30: marked suppression of immunoreactive COX-2 and IL-6; while fluorescence was strong for neurons.
Figure 5.
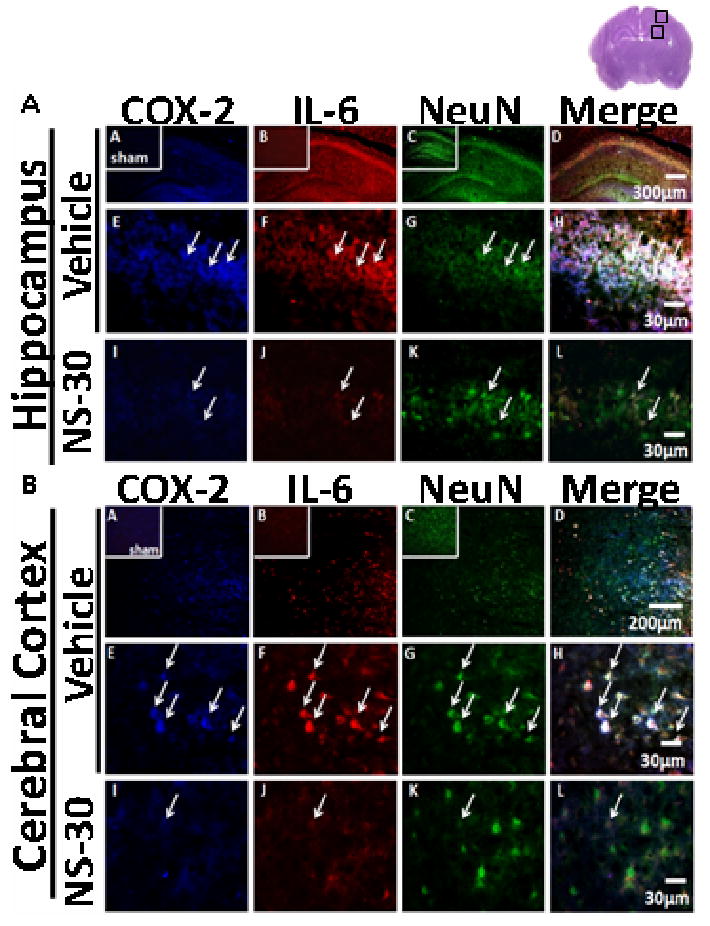
Co-localization of cyclooxygenase-2 (COX-2) and interleukin-6 (IL-6) in neuronal cells (NeuN) of postnatal day-10 rats induced with a hypoxic-ischemic (HI) event [Vehicle], then treated with a selective COX-2 inhibitor at 30mg/kg [NS-30] dosage were examined at 72hrs post-insult. Sham animals were used as control. Six non-adjacent coronal sections per brain (n = 5/group) were analyzed. Brain slice in upper right corner of figure denotes the specific cortical and hippocampal area the immunofluorescent pictures represent. A, Immunoreactivity is shown of COX-2, IL-6 and NeuN in ipsilateral CA1 region of hippocampus. Vehicle (A-H) demonstrated strong neuronal fluorescence for COX-2 and IL-6; NS-30 (I-L) demonstrated strong fluorescence for NeuN, but weak fluorescence for COX-2 and IL-6. Sham animals are shown in subsets of (A-C). B, Immunoreactivity is shown of COX-2, IL-6 and NeuN in ipsilateral cerebral cortex. Vehicle (A-H) demonstrated a strong co-localization of COX-2, IL-6, and NeuN. NS-30 (I-L) treatment reduced signals of COX-2 and IL-6. Sham animals are shown in subsets of (A-C).
COX-2 inhibition reduces inflammatory infiltration
IL-6 protein level was significantly increased in vehicle and substantially reduced by NS-30 (Fig. 6A). Single-immunofluorescent labeling demonstrated a qualitative increase in expression of microglia (Iba1), macrophages (CD68), and neutrophils (MPO) after HI (Fig. 6B); COX-2 blockade reduced expression of these cell-types.
Figure 6.
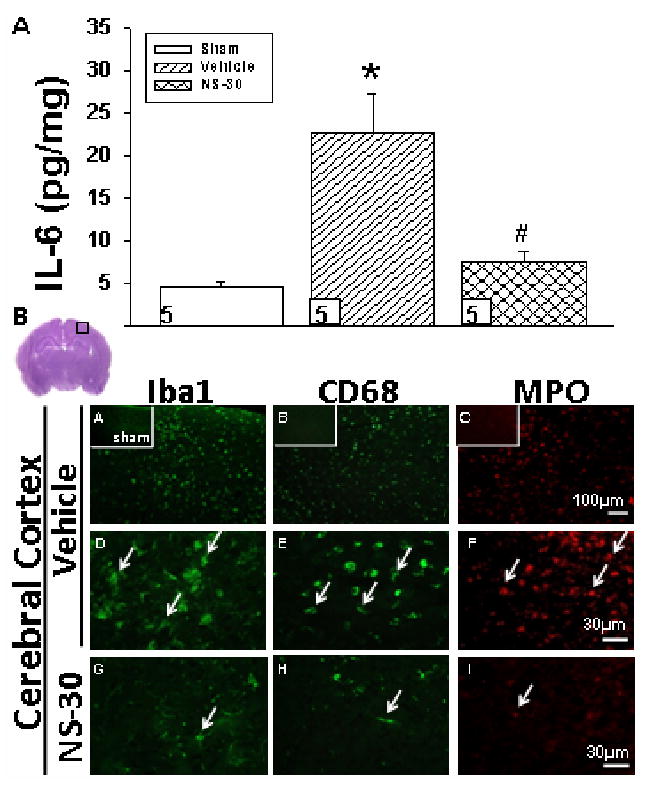
Interleukin-6 (IL-6) protein levels and inflammatory cell infiltration in the ipsilateral brain of postnatal day-10 rats induced with a hypoxic-ischemic (HI) event [Vehicle], then treated with a selective COX-2 inhibitor at 30mg/kg [NS-30] dosage were examined at 72hrs post-insult. Sham animals were used as control. A, Analysis by ELISA technique showed significant increase of IL-6 in the ipsilateral cerebral hemisphere of vehicle rats as compared to sham (22.61pg/mg ± 4.60 vs. 4.54pg/mg ± .77). Treatment with NS-30 markedly reduced IL-6 concentration (7.42pg/mg ± 1.44). Data represent *p < .05 versus sham, #p < .05 versus vehicle. Numbers in bars indicate animals/group. B, Vehicle (A-F) pups showed marked activation of microglia (Iba1; A and D), and infiltration of macrophages (CD68; B and E) and neutrophils (MPO; C and F) in the ipsilateral cerebral cortex. NS-30 qualitatively reduced expression of all three cell markers of inflammation (G-I). Sham animals are shown in subsets of (A-C). Six non-adjacent coronal sections per brain (n = 5/group) were analyzed. Brain slice in upper left corner of Fig. 3B denotes the specific cortical area the immunofluorescent pictures represent.
Discussion
In the present study, we tested whether multiple treatments of low-dose or high-dose COX-2 inhibitor, over the first few days following brain insult, can reduce the neurodevelopmental and/or somatic consequences of the injury. We showed for the first time that COX-2 inhibition limited morphologic damage, improved long-term functional deficits, reversed somatic growth retardation and lowered mortality rates after a hypoxic-ischemic injury.
NS398 is a well known selective COX-2 inhibitor shown to have neuroprotective effects in adult rat CNS injury models (7, 8). The dosage and treatment frequency (b.i.d) of NS398 was adopted from cerebral ischemia studies in adult rats (7, 21); however, the high dose used in this study is slightly higher but comparable with that used in adult rat models (10-20mg/kg). Both, NS-10 and NS-30 decreased the brain damage, as assessed by brain weight, at both 2- and 6wks following brain injury. Trends suggested an improvement in spleen weight following NS398 treatment, and significant improvement in heart weight. Similarly, NS-30 consistently improved neurological deficits 6wks post-insult. This was an important finding, considering past therapeutic modalities in neonatology have resulted in unforeseen side effects (22, 23). The partial protective effects of NS-10 may be due to the developmental physiological status of the neonates. Some clinical studies have shown that currently used COX-2 inhibitors such as celecoxib are rapidly cleared (twice as fast) in children as compared to adults (24). Thus, the pharmacokinetics of NS398 needs to be determined in neonatal experimental models.
High dose of NS398 also showed an improvement in body weight and other somatic characteristics such as fur growth and quality. Clinical and experimental studies have shown that neonatal HI not only causes brain damage and neurological deficits but also decreased somatic growth (2). The exact mechanism of how COX-2 inhibition affects somatic growth after neonatal HI remains to be determined.
The anti-inflammatory properties of COX-2 inhibition attenuated brain injury after neonatal HI. Over-expression of IL-6 in premature neonates is associated with severe cerebral injury (25). Studies have shown an IL-6-mediated activation of microglia around site of brain lesion; and a marked reduction of these effects in IL-6 deficient mice (26). IL-6 is also associated with increased mortality, and is an independent predictor of neurological deterioration following ischemic stroke (27, 28). Inhibition of COX-2 significantly reduced the expression of IL-6; as well as showed a marked reduction in infiltration of inflammatory cells such as macrophages and neutrophils and decreased activation of microglia in the affected brain tissue. Thus, the neuroprotective effects and increase in survivability demonstrated by COX-2 inhibition may be mediated by a reduction in IL-6 and the subsequent inflammatory response.
Recently, selective COX-2 inhibitors have come under progressively intense scrutiny due to an increased incidence of cardiovascular events among general populations treated with COX-2 inhibitors. But, the evidence-at-large remains contradictory and a host of studies both affirm and refute the putative cardiovascular harms of COX-2 inhibitors, as reviewed by Salinas G, et al. Furthermore, the underlying disease process and the type of cells involved may be pertinent factors in the overall effect produced by inhibition of COX-2 (29, 30). These selective inhibitors were commonly prescribed as a chronic regimen for patients with inflammatory arthritis and may thus produce entirely different effects when administered as acute regimens. Until more definitive evidence is available, strong judgments about the harm-to-benefit ratio of COX-2 inhibitors should be withheld, as these may pre-empt valuable research into untapped benefits for the general population. As evidenced in this study, selective inhibition of COX-2 may be effective at protecting the injured neonatal brain, and be a promising therapeutic option as acute treatment after stroke with lasting beneficial effects.
Conclusions
These results suggest that inhibition of COX-2 may provide major benefits for brain and systemic organ integrity, neurobehavioral deficits, and survival after neonatal hypoxia-ischemia. Collectively, these data provide a foundation for an investigation of COX-2 inhibitors as treatment for neonatal encephalopathy in the clinical setting.
Acknowledgments
Source of Funding: NIH grants HD43120 and NS54695 to JHZ
Footnotes
Conflict of Interest: None
References
- 1.Wagner BP, Nedelcu J, Martin E. Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr Res. 2002;51:354–360. doi: 10.1203/00006450-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Lubics A, Reglodi D, Tamas A, et al. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–165. doi: 10.1016/j.bbr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Shaywitz BA, Fletcher JM. Neurological, cognitive, and behavioral sequelae of hypoxic-ischemic encephalopathy. Semin Perinatol. 1993;17:357–366. [PubMed] [Google Scholar]
- 4.Calvert JW, Zhang JH. Pathophysiology of an hypoxic-ischemic insult during the perinatal period. Neurol Res. 2005;27:246–260. doi: 10.1179/016164105X25216. [DOI] [PubMed] [Google Scholar]
- 5.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 6.Sumanovic-Glamuzina D, Culo F, Culo MI, et al. Vasodilatory prostaglandins in perinatal hypoxic brain damage. Coll Antropol. 2008;32 1:183–187. [PubMed] [Google Scholar]
- 7.Nagayama M, Niwa K, Nagayama T, et al. The cyclooxygenase-2 inhibitor NS-398 ameliorates ischemic brain injury in wild-type mice but not in mice with deletion of the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab. 1999;19:1213–1219. doi: 10.1097/00004647-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Resnick DK, Graham SH, Dixon CE, et al. Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma. 1998;15:1005–13. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- 9.Andine P, Thordstein M, Kjellmer I, et al. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–260. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]
- 10.De Ryck M, Van Reempts J, Borgers M, et al. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- 11.Yager JY, Wright S, Armstrong EA, et al. The influence of aging on recovery following ischemic brain damage. Behav Brain Res. 2006;173:171–180. doi: 10.1016/j.bbr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Bona E, Johansson BB, Hagberg H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr Res. 1997;42:678–683. doi: 10.1203/00006450-199711000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Yamaguchi M, Kusaka G, et al. Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:419–431. doi: 10.1097/00004647-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Zhou C, Polk P, et al. Mechanisms of erythropoietin-induced brain protection in neonatal hypoxia-ischemia rat model. J Cereb Blood Flow Metab. 2004;24:259–270. doi: 10.1097/01.WCB.0000110049.43905.AC. [DOI] [PubMed] [Google Scholar]
- 16.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 17.Chiswick ML. Intrauterine growth retardation. Br Med J (Clin Res Ed) 1985;291:845–848. doi: 10.1136/bmj.291.6499.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gendron A, Teitelbaum J, Cossette C, et al. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 2002;955:85–97. doi: 10.1016/s0006-8993(02)03368-1. [DOI] [PubMed] [Google Scholar]
- 19.Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Balduini W, De AV, Mazzoni E, et al. Simvastatin protects against long-lasting behavioral and morphological consequences of neonatal hypoxic/ischemic brain injury. Stroke. 2001;32:2185–2191. doi: 10.1161/hs0901.094287. [DOI] [PubMed] [Google Scholar]
- 21.Kunz A, Anrather J, Zhou P, et al. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- 22.Halliday HL. Use of steroids in the perinatal period. Paediatr Respir Rev. 2004;5 A:S321–S327. doi: 10.1016/s1526-0542(04)90057-7. [DOI] [PubMed] [Google Scholar]
- 23.James S, Lanman JT. History of oxygen therapy and retrolental fibroplasia. Prepared by the American Academy of Pediatrics, Committee on Fetus and Newborn with the collaboration of special consultants. Pediatrics. 1976;57:591–642. [PubMed] [Google Scholar]
- 24.Stempak D, Gammon J, Klein J, et al. Single-dose and steady-state pharmacokinetics of celecoxib in children. Clin Pharmacol Ther. 2002;72:490–497. doi: 10.1067/mcp.2002.129322. [DOI] [PubMed] [Google Scholar]
- 25.Harding DR, Dhamrait S, Whitelaw A, et al. Does interleukin-6 genotype influence cerebral injury or developmental progress after preterm birth? Pediatrics. 2004;114:941–947. doi: 10.1542/peds.2003-0494-F. [DOI] [PubMed] [Google Scholar]
- 26.Penkowa M, Moos T, Carrasco J, et al. Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia. 1999;25:343–357. [PubMed] [Google Scholar]
- 27.Rallidis LS, Vikelis M, Panagiotakos DB, et al. Inflammatory markers and in-hospital mortality in acute ischaemic stroke. Atherosclerosis. 2006;189:193–197. doi: 10.1016/j.atherosclerosis.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Vila N, Castillo J, Davalos A, et al. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 29.Salinas G, Rangasetty UC, Uretsky BF, et al. The cycloxygenase 2 (COX-2) story: it's time to explain, not inflame. J Cardiovasc Pharmacol Ther. 2007;12:98–111. doi: 10.1177/1074248407301172. [DOI] [PubMed] [Google Scholar]
- 30.LaPointe MC, Mendez M, Leung A, et al. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol. 2004;286:H1416–H1424. doi: 10.1152/ajpheart.00136.2003. [DOI] [PubMed] [Google Scholar]