Localization of Zip1 and Zip4 mRNA in the Adult Rat Brain (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 1.
Published in final edited form as: J Neurosci Res. 2009 Nov 1;87(14):3221–3230. doi: 10.1002/jnr.22144
Abstract
The localization of two members of the Slc39a (zip1 and zip4) family of zinc transporters was examined in the brains of adult mice. Zip1 was highly enriched in brain regions with high densities of neuronal cell bodies, including the hippocampus, thalamus, and perifontal cortex. Zip1 was also expressed in commissural fiber tracts such as the corpus callosum and anterior commissure, but little was found in the internal and external capsules. Also, very low amounts of zip1 mRNA were detected in resting astrocytes and reactive astrocytes that were examined at 14 days after inflicting a stab wound. Zip1 mRNA was detected in ependymal cells lining the third and lateral ventricles and epithelium cells in the choroid plexus. Interestingly, zip4 mRNA was detected in the choroid plexus but not in the ependymal cells or other neural elements. Zip4 mRNA was also detected in brain capillaries, but zip1 mRNA was not. In zip4 knockout heterozygotes that express green fluorescent protein regulated by the zip4 promoter, green fluorescent protein was detected in brain capillaries. Because zip4 levels are regulated by dietary Zn, our studies suggest that the brain has the potential of adapting to changes in Zn status.
Keywords: zinc, transporter, brain, nutrition
Zinc-binding proteins account for nearly half of the transcription regulatory proteins in the human genome and are the most abundant class of proteins in the human proteome (Coleman, 1998; Nyborg and Peersen, 2004). Interestingly, the concentration of zinc in bacteria, yeast, and mammals is similar, approximately 0.1–0.5 M. Very likely these similarities reflect the requirement for zinc for the basic biological mechanisms. In mammals, however, specialized functions performed by the prostate (Hu and Friede, 1968; Apgar, 1985) and brain require higher levels. In the forebrain, for example, a subset of glutamatergic neurons displays a pool of Zn that is sequestered in synaptic vesicles (Wenzel et al., 1997; Frederickson, 1989; Slomianka, 1992), whereas neurons displaying synaptic zinc in the cerebellum and spinal cord are γ-aminobutyric acid (GABA)-ergic (Birinyi et al., 2001; Wang et al., 2001a,b). Zinc is released upon synaptic activity and is a potent inhibitor of N-methyl-D-aspartate (Li et al., 2001a,b; Qian and Noebels, 2005) and GABAA receptor subtypes (Hosie et al., 2006). At glycine receptors, Zn potentiates at submicromolar concentrations and inhibits at submillimolor concentrations (Bloomenthal et al., 1994; Laube et al., 1995). Zn has also been shown to inhibit noncompetitively excitatory amino acid transporters 1 (Vandenberg et al., 1998) and 4 (Mitrovic et al., 2001) and has a distinctive role in myelination by stabilizing interactions between lipids and myelin basic protein (Earl et al., 1988; Nuzzo et al., 2002). Mild zinc deficiency impairs cognitive development in rats (Massaro et al., 1982; Keller et al., 2000) and probably in humans (Bhatnagar and Taneja, 2001). In neurological diseases and experimental models, Zn has been shown to be a mediator of toxicity. For example, the number of dying neurons is reduced with a Zn chelator in a brain trauma model and in a brain ischemic model in rats (Suh et al., 2000a; Lee et al., 2002). The involvement of Zn in Alzheimer's disease was suggested because Zn was shown to promote aggregation of β-amyloid in vitro, and high amounts of Zn have been measured in plaques in Alzheimer's disease (Lee et al., 1999; Suh et al., 2000b; Bush, 2002).
Zinc homeostasis is accomplished predominantly by members of two families of zinc transporters. Member of the Slc30a family (also referred to as ZnT or the cation diffusion family) mediate Zn efflux, and members of the Slc39a family (also referred to as zip) mediate Zn influx. Members of both families are located in different tissues and in different cellular organelles. ZnT1, for example, is expressed in neurons in several brain regions, including cerebellum, cerebral cortex, and olfactory bulb (Sekler et al., 2002). ZnT3 is highly specific and is located in nerve terminals that display vesicular Zn, such as mossy fibers boutons of the hippocampus (Wenzel et al., 1997). In ZnT3 knockout mice, vesicular Zn is lost, which suggests that ZnT3 regulates vesicle Zn (Cole et al., 1999).
Less is know about the 14 members of the SLC39 family (Eide, 2003). Zip1 mRNA has been found in almost all tissues (Dufner-Beattie et al., 2003a), and zip1 protein mediates Zn uptake in prostate cells (Franklin et al., 2003) and the K562 erythroleukemic cells line (Gaither and Eide, 2001). Zip4 mediates uptake of Zn, but its expression is highly restricted to the intestine, pancreatic islets, and visceral yolk (Dufner-Beattie et al., 2004; Kim et al., 2004). In the intestine, zip4 mediates uptake of Zn at the luminal surface and is up-regulated within days of feeding rodents a Zn-deficient diet (Dufner-Beattie et al., 2003b; Liuzzi et al., 2004).
To gain a better understanding of Zn homeostasis in the brain, we examined the regional and cellular expression of zip1 and zip4 mRNA in rat brain. Zip1 mRNA was located in all identified brain regions with high densities of neuronal cell bodies and in some white matter tracts, ventricles, and choroid plexus, although little was found in normal or reactive astrocytes or in brain capillaries. Interestingly, zip4 mRNA was identified in the brain but was restricted to choroid plexus and brain capillaries.
Materials and Methods
Animals
Rats were purchased from Charles River. Zip4 heterozygous knockouts were generated as previously described (Dufner-Beattie et al., 2007).
In Situ Hybridization
Rats were anesthetized with xylaket and perfused with fixative (4% paraformaldehyde in 0.15 M phosphate buffer, pH 7.2) through the heart. Brains were excised and placed in fixative for 72 hr and incubated for 2 days at 4°C in 30% sucrose in PBS. Sections were cut at 25 μm with a cryostat and dried. Sections were hybridized with sense and antisense digoxygenin-labeled riboprobes. The vectors for making the probes were gift from Dr. Eide, University of Wisconsin. After hybridization, slides were washed twice in 50% formamide, 5× SSC (pH 4.5), and 1% SDS for 30 min at 70°C, and then twice in 50% formamide, 2× SSC (pH 4.5) for 30 min at 65°C. Sections were incubated overnight at 4°C with anti-DIG antibody conjugated to alkaline phosphatase (AP; Boehringer) at a 1:2,000 dilution. After extensive washing steps in washing buffer (100 mM Tris, 25 mM MgCl2, 150 mM NaCl), detection of AP activity was performed using an NBT (4-nitroblue tetrazolium chloride)-based assay (Boehringer).
Stab Wound
Adult F-344 rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (100 mg/kg) and xylazine (5 mg/kg). Rats were fixed on a stereotactic frame, and a 1-cm-long incision was made on the head skin with a scalpel. A 3-mm burr hole was drilled lateral to the bregma in the skull, and an 18-gauge needle was inserted 4.5 mm deep in the striatum under stereotactic control. At 14 days after the wound was placed, rats were euthanized by asphyxiation and processed for in situ hybridization.
Immunocytochemistry
The localization of zip4 was accomplished with a mouse strain expressing green fluorescent protein driven by the zip4 promoter sequence (Dufner-Beattie et al., 2007). To generate the strain, mice with a targeted disruption of the Zip4 gene were generated by homologous recombination in embryonic stem cells. The targeting construct fused the initiator methionine codon of Zip4 with the open reading frame of the enhanced green fluorescent protein (EGFP) reporter followed by several stop codons. This disrupted the protein-coding sequence of Zip4 and deleted the remaining codons in exon 1. The remainder of the gene was not altered. This allowed for EGFP expression that was driven by the Zip4 promoter in targeted cells with a targeted disruption in the zip4 gene. To stain for green fluorescent protein and laminin, sections were incubated in five changes in CitroSol for 2 min each, dehydrated in buffers with decreasing concentrations of ethanol for 2 min, and incubated for 15 min in warm 10 mM sodium citrate, pH 6.0. Sections were digested in 25% pepsin in 0.01 N HCl at room temperature for 15 min, washed in PBS for 5 min, and blocked with 5% normal goat serum with 2% Triton X-100 in PBS. Sections were incubated with antibody against laminin at 1:300 at 4°C overnight, washed three times in PBS, and incubated in secondary antibody at 1:250 for hours. Finally, sections were washed three times in PBS, and coverslips were placed over section with Prolong (contains an antifading chemical and DAPI).
Results
Zip1 mRNA is expressed in several brain regions. Previous studies found high levels of zip1 mRNA expression in whole brain (Dufner-Beattie et al., 2003a) To determine whether expression was specific to different regions or expressed throughout the brain, a series of coronal sections was hybridized with a zip1 cDNA probe. Zip1 mRNA was found in several regions, including hippocampus, cerebellum, thalamus, and piriform cortex (Fig. 1). The pattern of staining is very similar to that of Nissl staining, which indicates that much of the hybridization signal is in regions enriched with neuronal cell bodies.
Fig. 1.
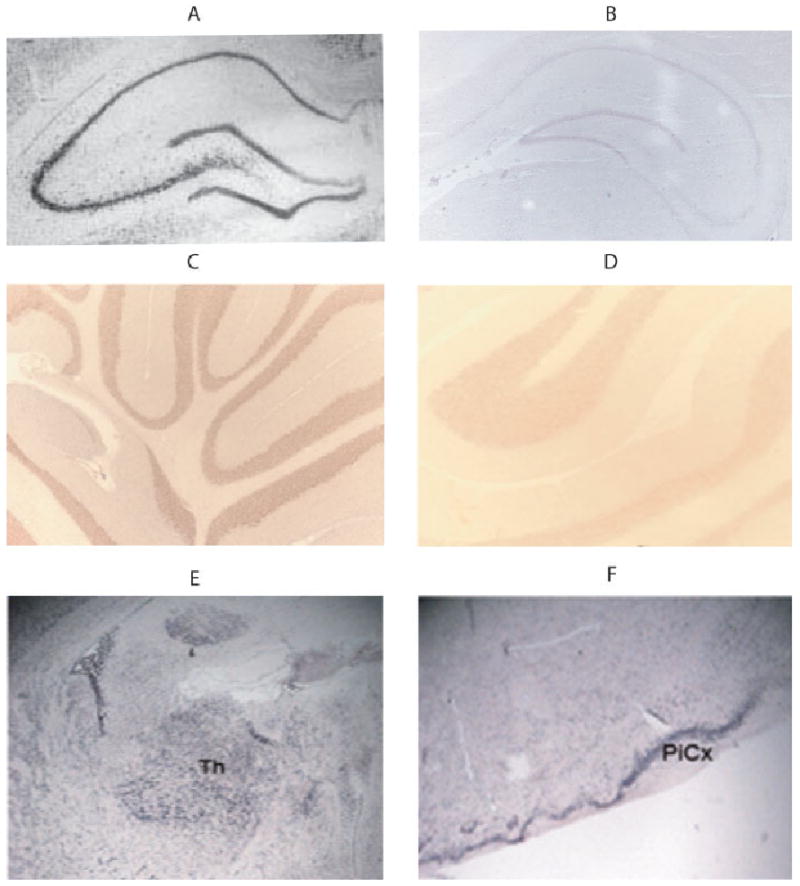
Zip1 mRNA broadly expressed in neuronal cell bodies in different brain regions. In situ hybridization with the antisense zip1-specific probe was performed on coronal sections of hippocampus (A,B), cerebellum (C,D), thalamus (E), and piriform cortex (F) from rats that were 70 days old. Hybridization with a probe in the antisense (A,C,E,F) sense (B,D) directions were examined. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Zip1 mRNA Is Expressed in Oligodendrocytes
Because the strong hybridization signals were found in regions with high densities of neuronal cell bodies, we determined whether zip1 mRNA was associated with oligodendrocytes by examining white matter tracts. Curiously, the expression of zip1 mRNA was observed in some but not all. Commissural tracts such as the corpus callosum and the anterior commissure displayed very intense staining, but almost no staining was observed in projecting tracts such as the internal and external capsules. Hybridization was also weak in the optic chiasm (Fig. 2).
Fig. 2.
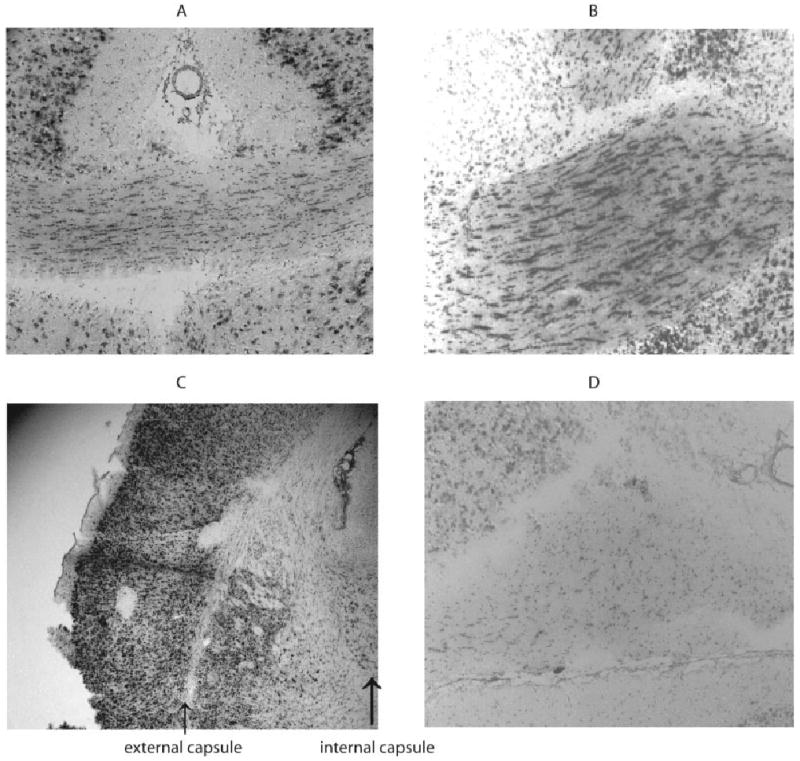
Zip1 mRNA expressed in some white matter tracts. In situ hybridization with antisense zip1-specific probe was performed on coronal sections of corpus callosum (A), anterior commisure (B), internal and external commissure (C), and optic chiasm (D) from rats that were 70 days old.
Astrocytes Express Less Zip1 mRNA
A closer examination of the cingulum revealed distinct differences in the cell types expressing zip1 mRNA. The cingulum is a white matter tract and expressed zip1 mRNA, which is similar to our observations for the corpus callosum. There is also a population of astrocytes (GFAP+) immediately adjacent to the cingulum that expressed very little, if any, zip1 mRNA (Fig. 3). To learn more about astroglial expression of zip1 mRNA, a model of gliosis was employed. To induce gliosis, a mechanical injury was given by inserting an 18-gauge needle into the cerebrum of anesthetized rats. Reactive astrocytes were observed at 14 days after injury as shown by intense GFAP staining (Fig. 3). Similar to the observations of resting astrocytes in the cingulum, the expression of zip1 mRNA was much lower in the areas with astrocytes.
Fig. 3.
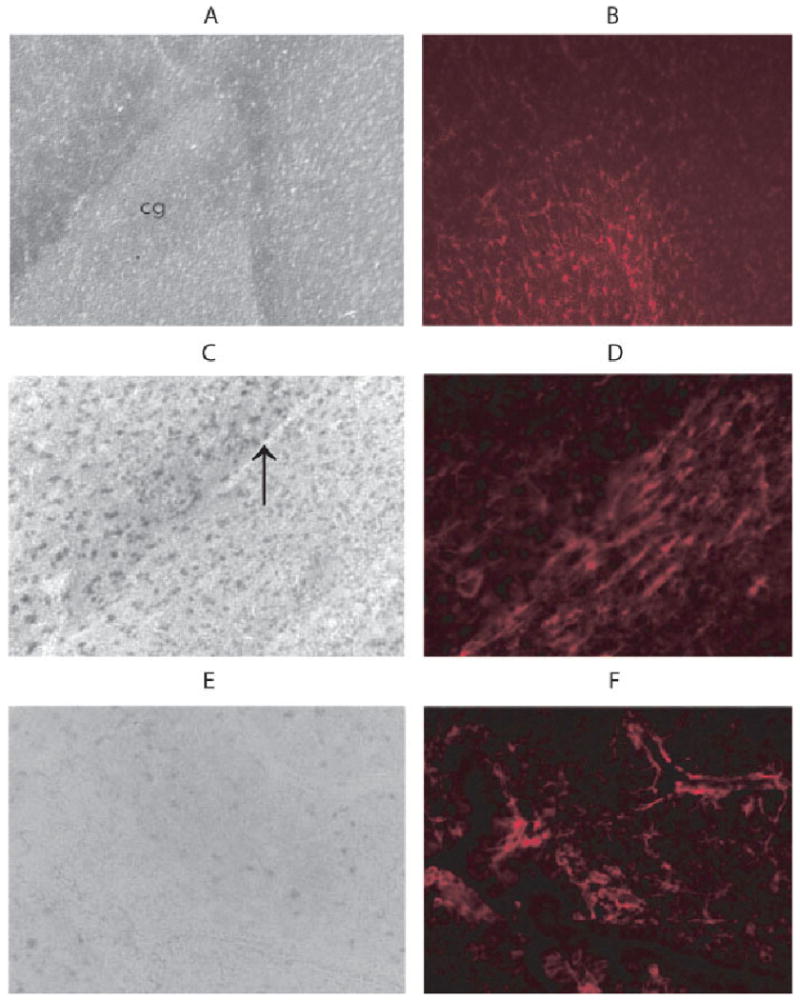
Zip1 mRNA and GFAP expression in cingulum. Coronal sections of the cingulum were first hybridized with the antisense zip1-specific probe (A) and then stained with rabbit antibody against GFAP, followed by Texas-red conjugated anti-rabbit IgG (B). A stab wound was inflicted in rats as described in Materials and Methods, and coronal sections were prepared 14 days later at the site of injury. Sections were prepared from the injured area and hybridized with the antisense zip1-specific probe (C,E), followed by staining with rabbit antibody against GFAP (D,F) as described. An arrow indicates the stab wound. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Expression of Zip1 and Zip4 mRNA in the Choroid Plexus and Ventricles
Because Zn is both required and potentially toxic in the brain, mechanisms should be needed for zinc homeostasis. Intense staining for zip1 mRNA was observed in the cells lining the third ventricle as well as cells lining the lateral ventricles, which indicates expression in ependymal cells (Fig. 4A). Furthermore, the choroid plexus also displayed high amounts of zip1 mRNA (Fig. 4B).
Fig. 4.
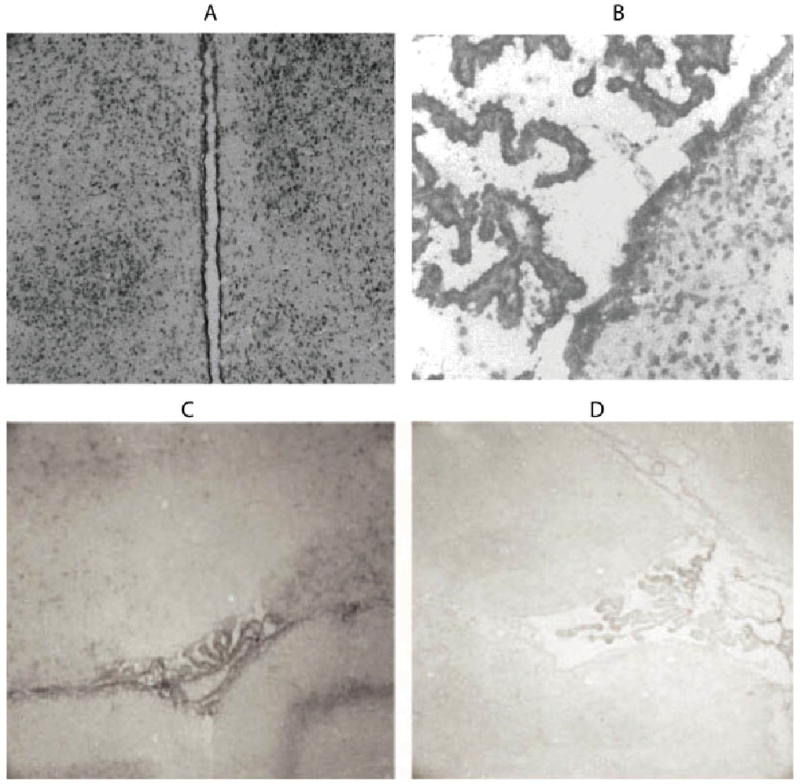
Zip1 and zip4 mRNA expression in ependyma and choroid plexus. Coronal sections of ventricles (A) and choroid plexus (B) were hybridized with antisense zip1 probe. Choroid plexus was also hybridized with a zip4 antisense (C) and sense probes (D). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Zip4 mRNA was also examined because a recent study reported severe neurodevelopmental problems in heterozygotic zip4 knockouts. These problems might be due to nutritional Zn deficiency. Additionally, zip4 might be more directly involved in zinc homeostasis in the brain. To examine the expression of zip4 mRNA in the brain, in situ hybridization was performed as for zip1. Expression was observed in the choroid plexus epithelium but not the ependyma (Fig. 4C,D). It thus appears that the choroid plexus expresses three members of the SLC39A family, zip1, zip4 (both shown here), and zip6 (Chowanadisai et al., 2005).
Expression of Zip1 and Zip4 mRNA in Brain Capillaries
Brain capillaries, unlike most other capillaries in other tissues, display properties similar to epithelial cells and express transporters to bring nutrients into the brain. The staining for zip1 mRNA was barely detected in capillary structures (Fig. 5A,B), which is different from the epithelial cells of the choroid plexus. Interestingly, capillaries expressed zip4 mRNA (Fig. 5C,D). To verify this observation, the heterozygotic zip4 knockout mouse strain was used because it expresses green fluorescent protein regulated by the zip4 promoter. The location of cells expressing zip4 was examined by double antibody staining with an antibody against green fluorescent protein and an antibody against laminin for detecting brain capillaries. In the brain, antibody against laminin is used to identify capillaries (Eriksdotter-Nelsson et al., 1986; Jucker et al., 1996) Brains from wild-type animals did not display staining for GFP, as expected, though many capillaries were detected (Fig. 6). GFP-positive staining was observed in the zip4 knockouts in capillaries.
Fig. 5.
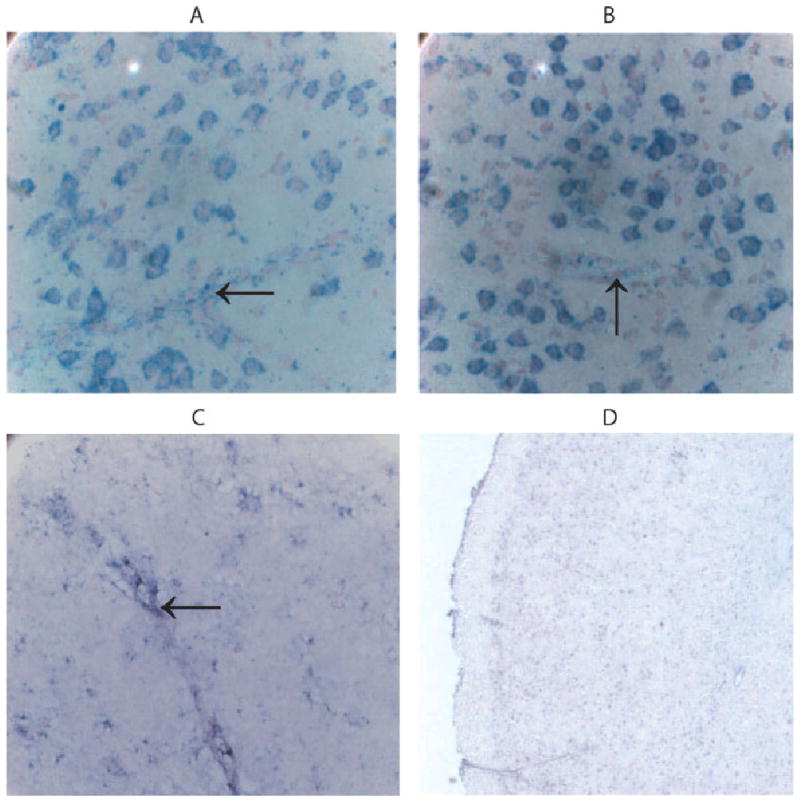
Zip4 mRNA not zip1 mRNA is located in capillaries. Coronal sections were prepared from cerebral cortex and hybridized with antisense zip1-specific probe (A,B) or with a zip4 antisense-specific probe (C). Sections were also hybridized with a zip4 sense probe (D). Arrows point to microvessels. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 6.
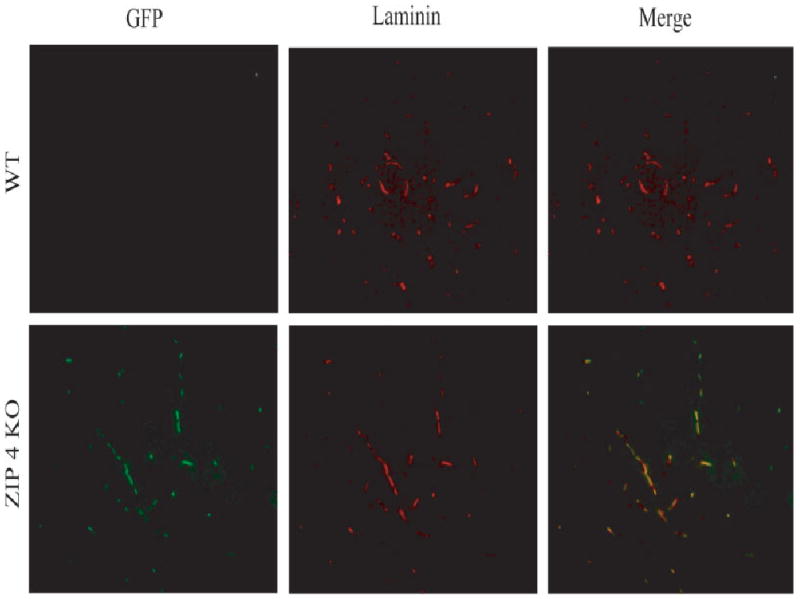
Localization of zip4 promoter-driven expression of zip4 and green fluorescent protein and laminin in brain capillaries. Coronal sections from cerebral cortex from wild-type (WT) and zip4 heterozygous knockout mice (zip4 KO) were stained with antibodies against green fluorescent protein (GFP) and laminin as described in Materials and Methods. GFP is a reporter gene for zip4 expression and laminin stains for capillaries. Micrograph merging both images are shown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
Our study is the first extensive study examining the location of zip1 and zip4 in the rat brain. Zip1 was examined because its mRNA is highly expressed in whole brain compared with the other members of the Slc39a family (Dufner-Beattie et al., 2003a). The high levels of zip1 likely are due to its expression in neurons. All the brain regions with high numbers of neurons expressed high levels of zip1 mRNA. Furthermore, astrocytes did not express zip1 mRNA. Because other studies found zip1 as a plasma membrane zinc transporter, very likely zip1 is a plasma membrane zinc transporter for neurons. A recent study also found expression of zip6 (LIV-1) in hippocampal and cortical neurons, but other brain regions were not described (Chowanadisai et al., 2005). Zip6 (Chowanadisai et al., 2008) and zip1 (Gaither and Eide, 2001; Franklin et al., 2003) are both plasma membrane transporters, which raises the possibility that the same cells displays more than one plasma membrane zinc transporter. One possible explanation is that transporters display different locations to achieve different functions. For example, a transporter at the neuronal cell body might ensure proper zinc uptake to synthesize transcription factors, whereas zinc transporters at the presynaptic and postsynaptic terminals would satisfy the need for synaptic function. Indeed, a specialized member of the Slc30a family, ZnT-3, is expressed only at synaptic vesicles (Palmiter et al., 1996).
The expression of zip4 mRNA in choroid plexus and brain capillaries was quite intriguing, because it was not found previously in whole brain by RT-PCR (Dufner-Beattie et al., 2003a). Very likely, expression was not observed because the concentration of zip4 mRNA was too low. Accordingly, zip4 might be expressed in other organs not yet identified but restricted to specific cell types. Zip4 mRNA is most abundant in tissues involved in zinc homeostasis, such as the intestine, visceral endoderm, pancreatic islets, and yolk sac (Dufner-Beattie et al., 2004). Several members of the Slc39a family respond to changes in extracellular zinc at the protein level, but zip4 is the only member whose level of mRNA and protein increases (Dufner-Beattie et al., 2003b). The explanation for why these cells express zip4 is that they are involved in nutrient uptake and zinc homeostasis. In the same way, capillaries might be involved in similar processes in the brain. Indeed, brain capillaries express many transporters for nutrients. For example, brain capillaries are involved in iron homeostasis as indicated by their expression of the transferrin receptor and divalent metal transporter 1 (Moos, 1996). Similarly, iron deficiency increases transferrin receptor, divalent metal transport 1, and iron uptake (Taylor et al., 1991; Burdo et al., 2004; Garcia et al., 2007), although these increases vary and depend on rat strain, length of time, and level of iron in the diet. The presence of zip4 in brain capillaries suggests that the brain is capable of adapting to changes in serum zinc that could come from the diet.
Metallothionein expression is induced by Zn and has been suggested to be involved in zinc homeostasis (Maret, 2000; Lee et al., 2003). In the brain, astrocytes are the major cell type expressing metallothionein (Penkowa et al., 1999; Hidalgo et al., 2001), which suggests that they might have an important role in Zn homeostasis in the brain. Therefore, it was surprisingly that resting astrocytes express little zip1 mRNA and even more surprising that higher levels of zip1 were not found in reactive astrocytes, which express even more metallothionein than resting astrocytes (Penkowa et al., 1997; Chung et al., 2008). One possibility is that astrocytes express a different member of the Slc39a family. Zip6 is the only member of the Slc39a family examined in the brain in situ, but its location in astrocytes was not indicated. Zip8 and Zip14 are other candidate astroglial zinc transporters because their mRNA has been observed in brain (Girijashanker et al., 2008). Another possibility, however, is that the higher levels of metallothionein in astrocytes are due to Zn taken up from other types of transporters, for example, voltage-dependent calcium channels. Astrocytes express the L-type voltage-dependent calcium channel, which was shown to mediate zinc uptake in astrocytes (Sheline et al., 2002) and pancreas islet cells (Gyulkhandanyan et al., 2006). The authors of the latter study suggest that the L-voltage-dependent calcium channel mediates fast zinc transport, whereas members of the SLC39 family mediate slower Zn transport. Accordingly, astrocytes might express metallothionein as a result of taking up Zn because of synaptic activity rather than storing Zn from a nutritional transporter.
Evidence was also presented demonstrating the expression of zip1 mRNA in oligodendrocytes in some white matter tracts but not others. Corpus callosum and anterior commissure displayed much more zip1 mRNA than the internal and external capsules or the optic chiasm. In our review of the literature, an explanation for these differences is not apparent. The differences were so striking that they likely were not due to differences in cell numbers. Similar to our suggestions for astrocytes, the white matter tracts that do not express zip1 mRNA might express a different transporter that is due to special Zn requirements. In any event, our data support the concept of heterogeneity in expression of SLC39 family members in white matter tracts in the brain.
In summary, the data presented demonstrate that zip1 is expressed in neurons and in a subpopulation of oligodendrocytes, with little in astrocytes. The expression of zip4 in brain capillaries and choroid plexus suggests that the brain expresses homeostatic mechanisms for adapting to changes in zinc status.
Acknowledgments
Contract grant sponsor: Gerber Foundation.
References
- Apgar J. Zinc and reproduction. Annu Rev Nutr. 1985;5:43–68. doi: 10.1146/annurev.nu.05.070185.000355. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Taneja S. Zinc and cognitive development. Br J Nutr. 2001;85(Suppl 2):S139–S145. doi: 10.1079/bjn2000306. [DOI] [PubMed] [Google Scholar]
- Birinyi A, Parker D, Antal M, Shupliakov O. Zinc co-localizes with GABA and glycine in synapses in the lamprey spinal cord. J Comp Neurol. 2001;433:208–221. doi: 10.1002/cne.1136. [DOI] [PubMed] [Google Scholar]
- Bloomenthal AB, Goldwater E, et al. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- Burdo JR, Simpson IA, et al. Regulation of the profile of iron-management proteins in brain microvasculature. J Cereb Blood Flow Metab. 2004;24:67–74. doi: 10.1097/01.WCB.0000095800.98378.03. [DOI] [PubMed] [Google Scholar]
- Bush AI. Metal complexing agents as therapies for Alzheimer's disease. Neurobiol Aging. 2002;23:1031–1038. doi: 10.1016/s0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- Chowanadisai W, Kelleher SL, et al. Zinc deficiency is associated with increased brain zinc import and LIV-1 expression and decreased ZnT-1 expression in neonatal rats. J Nutr. 2005;135:1002–1007. doi: 10.1093/jn/135.5.1002. [DOI] [PubMed] [Google Scholar]
- Chowanadisai W, Lonnerdal B, et al. Zip6 (LIV-1) regulates zinc uptake in neuroblastoma cells under resting but not depolarizing conditions. Brain Res. 2008 doi: 10.1016/j.brainres.2008.01.015. in press. [DOI] [PubMed] [Google Scholar]
- Chung RS, Hidalgo J, et al. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J Neurochem. 2008;104:14–20. doi: 10.1111/j.1471-4159.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, et al. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE. Zinc enzymes. Curr Opin Chem Biol. 1998;2:222–234. doi: 10.1016/s1367-5931(98)80064-1. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Langmade SJ, et al. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem. 2003a;278:50142–50150. doi: 10.1074/jbc.M304163200. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Wang F, et al. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003b;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Kuo YM, et al. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem. 2004;279:49082–49090. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Weaver BP, et al. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- Earl C, Chantry A, et al. Zinc ions stabilise the association of basic protein with brain myelin membranes. J Neurochem. 1988;51:718–724. doi: 10.1111/j.1471-4159.1988.tb01803.x. [DOI] [PubMed] [Google Scholar]
- Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2003 doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- Eriksdotter-Nelsson M, Bjorklund H, et al. Laminin immunohistochemistry: a simple method to visualize and quantitate vascular structures in the mammalian brain. J Neurosci Methods. 1986;17:275–285. doi: 10.1016/0165-0270(86)90128-7. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Ma J, et al. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96:435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J Biol Chem. 2001;276:22258–22264. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, et al. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci. 2007;95:205–214. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Girijashanker K, He L, et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyulkhandanyan AV, Lee SC, et al. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J Biol Chem. 2006;281:9361–9372. doi: 10.1074/jbc.M508542200. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Aschner M, et al. Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull. 2001;55:133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Buckingham SD, et al. Replacement of asparagine with arginine at the extracellular end of the second transmembrane (M2) region of insect GABA receptors increases sensitivity to penicillin G. Invert Neurosci. 2006;6:75–79. doi: 10.1007/s10158-006-0020-4. [DOI] [PubMed] [Google Scholar]
- Hu KH, Friede RL. Topographic determination of zinc in human brain by atomic absorption spectrophotometry. J Neurochem. 1968;15:677–685. doi: 10.1111/j.1471-4159.1968.tb08967.x. [DOI] [PubMed] [Google Scholar]
- Jucker M, Tian M, et al. Laminin alpha 2 is a component of brain capillary basement membrane: reduced expression in dystrophic dy mice. Neuroscience. 1996;71:1153–1161. doi: 10.1016/0306-4522(95)00496-3. [DOI] [PubMed] [Google Scholar]
- Keller KA, Chu Y, et al. Supplementation with L-histidine during dietary zinc repletion improves short-term memory in zinc-restricted young adult male rats. J Nutr. 2000;130:1633–1640. doi: 10.1093/jn/130.6.1633. [DOI] [PubMed] [Google Scholar]
- Kim BE, Wang K, et al. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, et al. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol. 1995;483:613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Geiser J, et al. Pancreatic metallothionein-I may play a role in zinc homeostasis during maternal dietary zinc deficiency in mice. J Nutr. 2003;133:45–50. doi: 10.1093/jn/133.1.45. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, et al. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–878. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Lee JY, Mook-Jung I, et al. Histochemically reactive zinc in plaques of the Swedish mutant beta-amyloid precursor protein transgenic mice. J Neurosci. 1999;19:RC10. doi: 10.1523/JNEUROSCI.19-11-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hough CJ, et al. Induction of mossy fiber→Ca3 long-term potentiation requires translocation of synaptically released Zn2+ J Neurosci. 2001a;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hough CJ, et al. Rapid translocation of Zn2+ from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol. 2001b;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Bobo JA, et al. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci U S A. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000;130(Suppl):1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- Massaro TF, Mohs M, et al. Effects of moderate zinc deficiency on cognitive performance in young adult rats. Physiol Behav. 1982;29:117–121. doi: 10.1016/0031-9384(82)90374-2. [DOI] [PubMed] [Google Scholar]
- Mitrovic AD, Plesko F, et al. Zn2+ inhibits the anion conductance of the glutamate transporter EEAT4. J Biol Chem. 2001;276:26071–26076. doi: 10.1074/jbc.M011318200. [DOI] [PubMed] [Google Scholar]
- Moos T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol. 1996;375:675–692. doi: 10.1002/(SICI)1096-9861(19961125)375:4<675::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nuzzo S, Meneghini C, et al. An x-ray absorption spectroscopy study of the zinc environment in Langmuir-Blodgett phospholipid multilayers. Biophys J. 2002;83:3507–3512. doi: 10.1016/S0006-3495(02)75350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg JK, Peersen OB. That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J. 2004;381:e3–e4. doi: 10.1042/BJ20041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, et al. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Hidalgo J, et al. Increased astrocytic expression of metallothioneins I + II in brainstem of adult rats treated with 6-aminonicotinamide. Brain Res. 1997;774:256–259. doi: 10.1016/s0006-8993(97)81716-7. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Nielsen H, et al. Distribution of metallothionein I + II and vesicular zinc in the developing central nervous system: correlative study in the rat. J Comp Neurol. 1999;412:303–318. doi: 10.1002/(sici)1096-9861(19990920)412:2<303::aid-cne9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekler I, Moran A, et al. Distribution of the zinc transporter ZnT-1 in comparison with chelatable zinc in the mouse brain. J Comp Neurol. 2002;447:201–209. doi: 10.1002/cne.10224. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Ying HS, et al. Depolarization-induced 65zinc influx into cultured cortical neurons. Neurobiol Dis. 2002;10:41–53. doi: 10.1006/nbdi.2002.0497. [DOI] [PubMed] [Google Scholar]
- Slomianka L. Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience. 1992;48:325–352. doi: 10.1016/0306-4522(92)90494-m. [DOI] [PubMed] [Google Scholar]
- Suh SW, Chen JW, et al. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000a;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- Suh SW, Jensen KB, et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 2000b;852:274–278. doi: 10.1016/s0006-8993(99)02096-x. [DOI] [PubMed] [Google Scholar]
- Taylor EM, Crowe A, et al. Transferrin and iron uptake by the brain: effects of altered iron status. J Neurochem. 1991;57:1584–1592. doi: 10.1111/j.1471-4159.1991.tb06355.x. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Mitrovic AD, et al. Molecular basis for differential inhibition of glutamate transporter subtypes by zinc ions. Mol Pharmacol. 1998;54:189–196. doi: 10.1124/mol.54.1.189. [DOI] [PubMed] [Google Scholar]
- Wang Z, Danscher G, et al. Retrograde tracing of zinc-enriched (ZEN) neuronal somata in rat spinal cord. Brain Res. 2001a;900:80–87. doi: 10.1016/s0006-8993(01)02261-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li JY, et al. Zinc-enriched GABAergic terminals in mouse spinal cord. Brain Res. 2001b;921:165–172. doi: 10.1016/s0006-8993(01)03114-6. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, et al. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]