The 22q11.2 Deletion in African-American Patients: An Underdiagnosed Population? (original) (raw)
. Author manuscript; available in PMC: 2010 Jan 25.
Published in final edited form as: Am J Med Genet A. 2005 Apr 30;134(3):242–246. doi: 10.1002/ajmg.a.30069
Abstract
Findings associated with the 22q11.2 deletion often include congenital heart malformations, palatal anomalies, immunodeficiency, hypocalcemia, and developmental delay or learning disabilities. Often the clinical suspicion of the diagnosis in a patient with one or more of these findings is heightened based on the presence of a characteristic facial appearance. In our large cohort of 370 patients with the 22q11.2 deletion, we report the under-representation of African-Americans in our group, as well as, the paucity of craniofacial dysmorphism in these patients. We note that the absence of the typical facial features may result in decreased ascertainment in this population and, furthermore, may delay the implementation of palliative care, cognitive remediation, and recurrence risk counseling. We, therefore, suggest that the clinician’s threshold of suspicion should be lower in African-American patients.
Keywords: 22q11.2 deletion syndrome, DiGeorge syndrome, velocardiofacial syndrome
INTRODUCTION
The 22q11.2 deletion has been identified in the majority of patients with DiGeorge anomalad, velocardiofacial syndrome, and conotruncal anomaly face syndrome, and in some cases of autosomal dominant Opitz G/BBB syndrome and Cayler cardiofacial syndrome [Driscoll et al., 1992a,b, 1993; Burn et al., 1993; Giannotti et al., 1994; Matsouka et al., 1994; McDonald-McGinn et al., 1995; Fryburg et al., 1996; LaCassie and Arriaza, 1996].
The list of findings associated with the deletion is extensive and variable [McDonald-McGinn et al., 1997, 1999]. Some patients present with classic symptoms of DiGeorge anomalad including a conotruncal cardiac defect, hypocalcemia, and immune deficiency, others have the learning disability and velopharyngeal incompetence described in velocardiofacial syndrome. Additional patients present with only minimal findings of the deletion but gain clinical attention when these findings are coupled with the characteristic craniofacial features. These typical facies include: hooded eyelids, a prominent nasal root with a bulbous nasal tip, hypoplastic alae nasae, and auricular abnormalities [McDonald-McGinn et al., 1997, 1999]. It has been our experience that there is a paucity of the latter findings in our African-American patients [McDonald-McGinn et al., 1996]. Here we examine the dysmorphisms in both Caucasians and non-Caucasian patients with a 22q11.2 deletion, discuss how they differ and compare the incidence of non-Caucasians in our cohort of patients with a 22q11.2 deletion to our hospital based population.
MATERIALS AND METHODS
Three hundred and seventy patients with a 22q11.2 deletion were ascertained through The Children’s Hospital of Philadelphia’s multidisciplinary 22q11.2 deletion clinic. The laboratory diagnosis was made using fluorescence in situ hybridization with the commercially available N25 (D22S75) or TUPLE 1 probes. Patients ranged in age from birth to 52 years; 52% were female. Anthropometric measures including height, head circumference, and inter-pupillary distance were available for review on a total of 255 of the 370 patients. Of this group, 204 were Caucasian, 33 were African-American, 11 were Hispanic, four were Asian, and three were of mixed racial/ethnic background. Measurements were plotted on standard growth curves from the National Center for Health Statistics and particular attention was paid to the craniofacial features associated with the 22q11.2 deletion [Feingold and Bossert, 1974; McDonald-McGinn et al., 1997, 1999; National Center for Health Statistics, 1997]. Findings in non-Caucasians were compared to the Caucasian population.
RESULTS
Of the total group of 370 patients with a 22q11.2 deletion, 294 were Caucasian (79.5%), 45 (12%) were African-American, 15 (4%) were Hispanic, four (1%) were Asian, and the remaining 12 (3.5%) were of mixed racial/ethnic background. The racial/ethnicity was determined by full family history taken at clinic. These numbers are compared to our hospital based population figures which reveals a significant under representation of African-American patients in our cohort (Table I).
TABLE I.
The Children’s Hospital of Philadelphia: Frequency by Race/Ethnicity
| Patients with a22q11.2 deletion(N = 370) (%) | Hospitalpopulation(%)a | |
|---|---|---|
| Caucasian (N = 294) | 79.5 | 50 |
| African-American (N = 45) | 12 | 42 |
| Hispanic (N = 15) | 4 | 1 |
| Asian (N = 4) | 1 | 1 |
| Other (N = 12) | 3.5 | 6 |
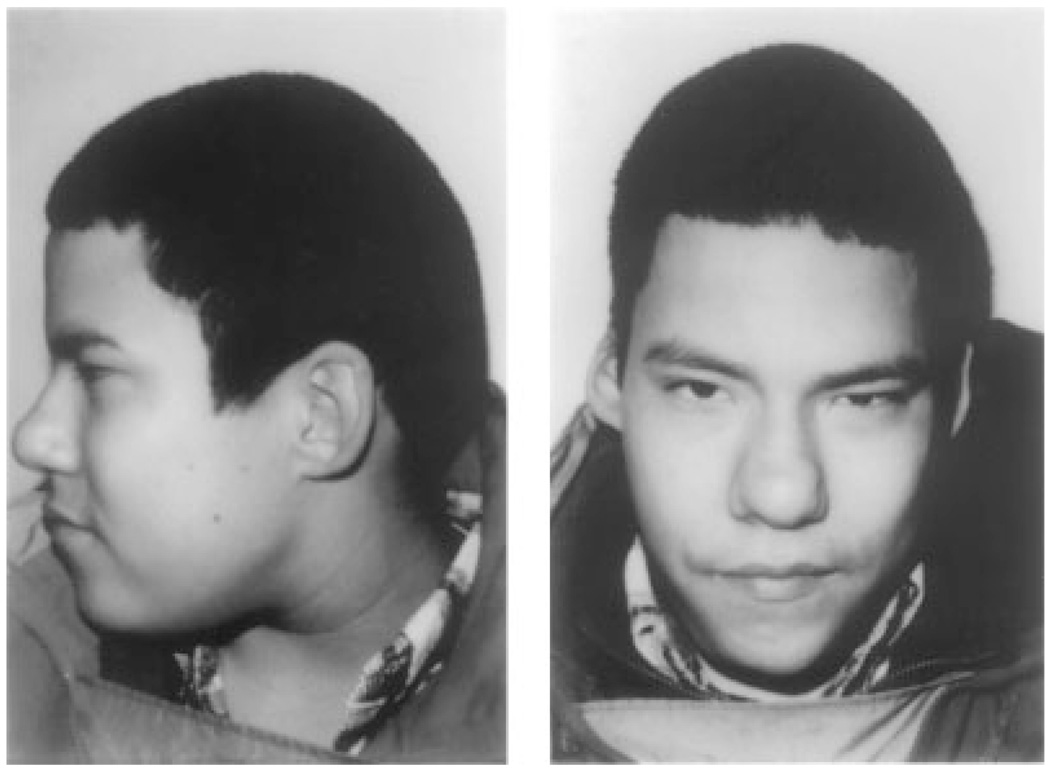
Of the 45 African-American patients, five were adults; four of whom were ascertained through an affected child. The remaining adult was diagnosed with VCFS clinically, as a teenager through the Cleft Palate Clinic, and subsequently reached adulthood and had confirmatory deletion studies. The remaining 40 patients were children. Twenty-five of the children were identified in infancy solely on the basis of their cardiac defect as part of our conotruncal cardiac anomaly screening program; seven children came to the attention of Clinical Genetics in infancy because of their cardiac defects and dysmorphic features; four children met criteria for a clinical diagnosis of DiGeorge anomaly; two children were offspring of affected adults, both of whom were diagnosed prenatally with the deletion; one child was identified following referral from a behavioral intervention unit with a history of a learning disability; and one child was diagnosed following referral because of a history of hypocalcemia in infancy and a non-verbal learning disability consistent with the 22q11.2 deletion (Fig. 1) [Goldmuntz et al., 1993; Moss et al., 1999].
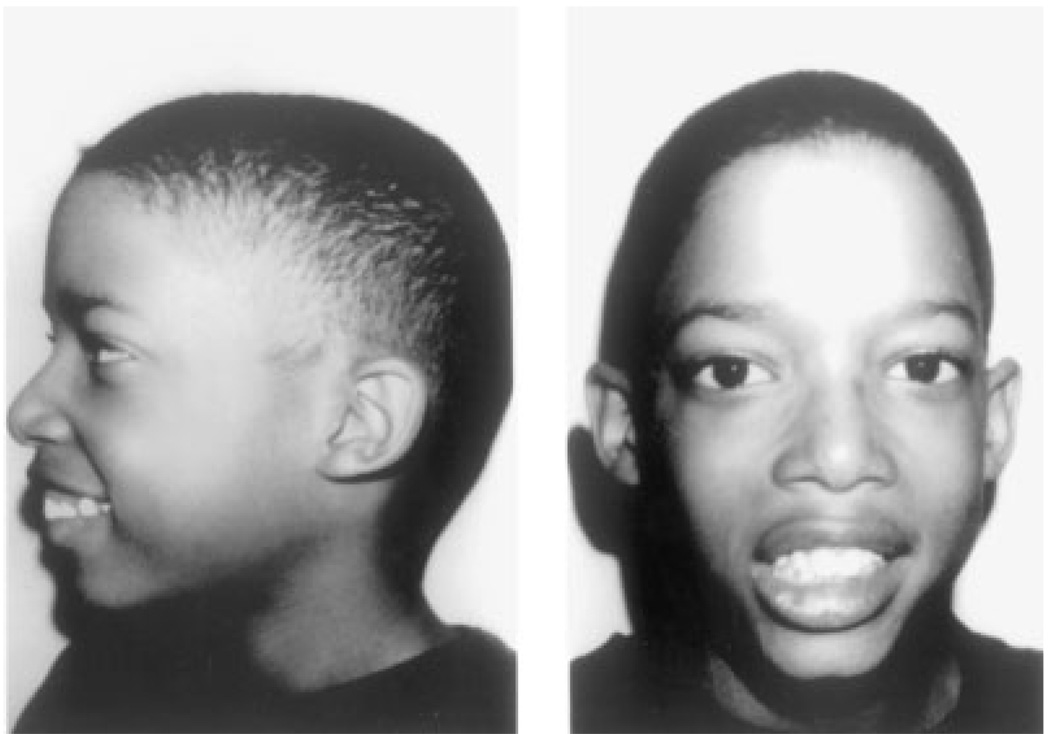
Fig. 1.
Nine-year-old non-dysmorphic male with a 22q11.2 deletion referred with a history of hypocalcemia in infancy and a non-verbal learning disability.
Review of the clinical genetics evaluation performed in a subset of patients (Table II) revealed that seven of 33 (21.2%) African-American patients had none of the classic facial characteristics of the deletion and only three of 33 (9.1%) had three of the four typical characteristics including a bulbous nasal tip, prominent nasal root, hooded eyelids, and auricular abnormalities (Fig. 2 and Fig 3). This is compared to a similar group of Caucasian patients where 11/204 (5.4%) had no typical facial features and 63/204 (30.9%) had three of the four characteristics (Fig. 4). Eighteen African-American patients had auricular abnormalities (Fig. 5) as their only dysmorphism. Atypical findings noted in the African-American cohort included extra nuchal skin in two non-cardiac patients and ptosis in another. In addition, we identified several anthropometric measures, which were found to be variant from the norm (two standard deviations below the mean) including: midfacial depth, lower facial depth, nasal breadth, and auricular height and breadth [Minugh-Purvis et al., 2000].
TABLE II.
Patients With a 22q11.2 Deletion and Frequency of Facial Features by Race/Ethnicity (N = 255)
| Nodysmorphisms(%) | Three or morefeatures of thedeletion (%)a | Bulbousnasal tip (%) | Prominentnasal root (%) | Hooding ofeyelids (%) | Auricularanomalies (%) | |
|---|---|---|---|---|---|---|
| Caucasian (N = 204) | 5.4 | 30.9 | 62.3 | 25 | 25.5 | 83.3 |
| African-American (N = 33) | 21.2 | 9.1 | 12.1 | 15.2 | 6.1 | 78.8 |
| Hispanic (N = 11) | 0 | 36.4 | 81.8 | 9.1 | 36.4 | 90.9 |
| Asian (N = 4) | 25 | 25 | 25 | 50 | 0 | 75 |
| Other (N = 3) | 0 | 0 | 33.3 | 0 | 0 | 100 |
Fig. 2.
Mother and daughter with the 22q11.2 deletion. Neither had dysmorphia consistent with the deletion.
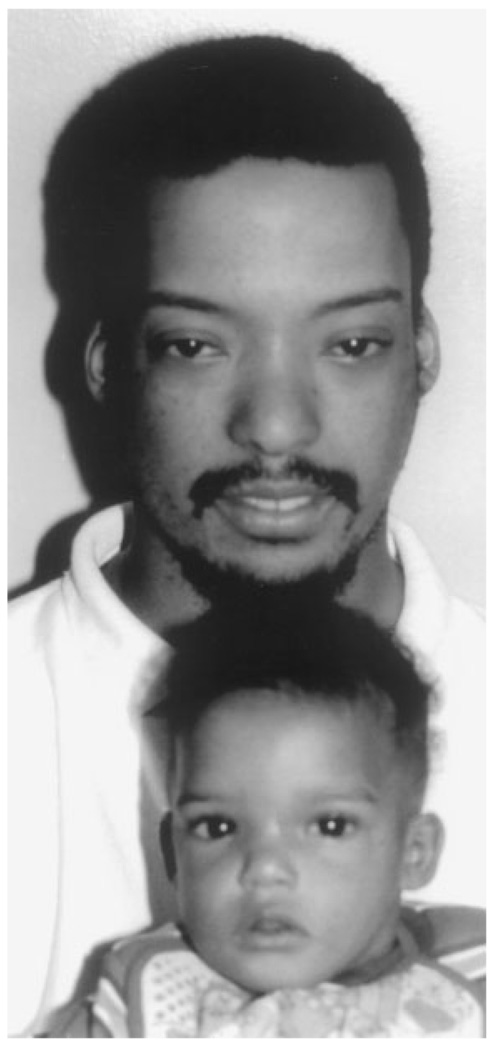
Fig. 3.
Father and son with the 22q11.2 deletion. Both were hyperteloric. In addition, the father had upslanting palpebral fissures, a bulbous nasal tip, hypoplastic alae nasae, and small ears with overfolded helices.
Fig. 4.
Caucasian patient demonstrating the typical hooded eyelids, bulbous nasal tip and hypoplastic alae nasae.
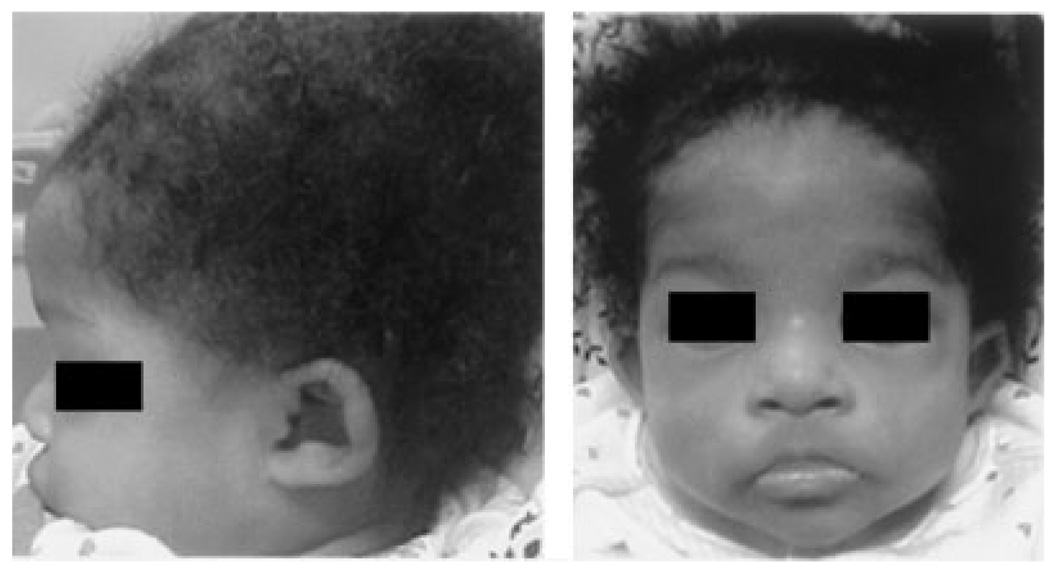
Fig. 5.
African-American patient with auricular abnormalities as the only typical dysmorphism.
Of the four Asian patients, one child (Fig. 6) with cleft palate and growth hormone deficiency was ascertained through the endocrinology clinic following our report of the association of growth hormone deficiency and the 22q11.2 deletion; and one was diagnosed in infancy with congenital heart disease, cleft palate, and myelomeningocele [Nickel et al., 1993, 1994; Nickel and Magenis, 1996; Weinzimer et al., 1998]. Another child was ascertained through Child Development with a history of cleft palate and a learning disability and the fourth child was self-referred following the a diagnosis of the 22q11.2 deletion with a history of congenital heart disease, cleft palate, and a terminal transverse defect of one upper extremity. One of the four children had no dysmorphic features and only one was found to have three of the four classic craniofacial characteristics (Table II).
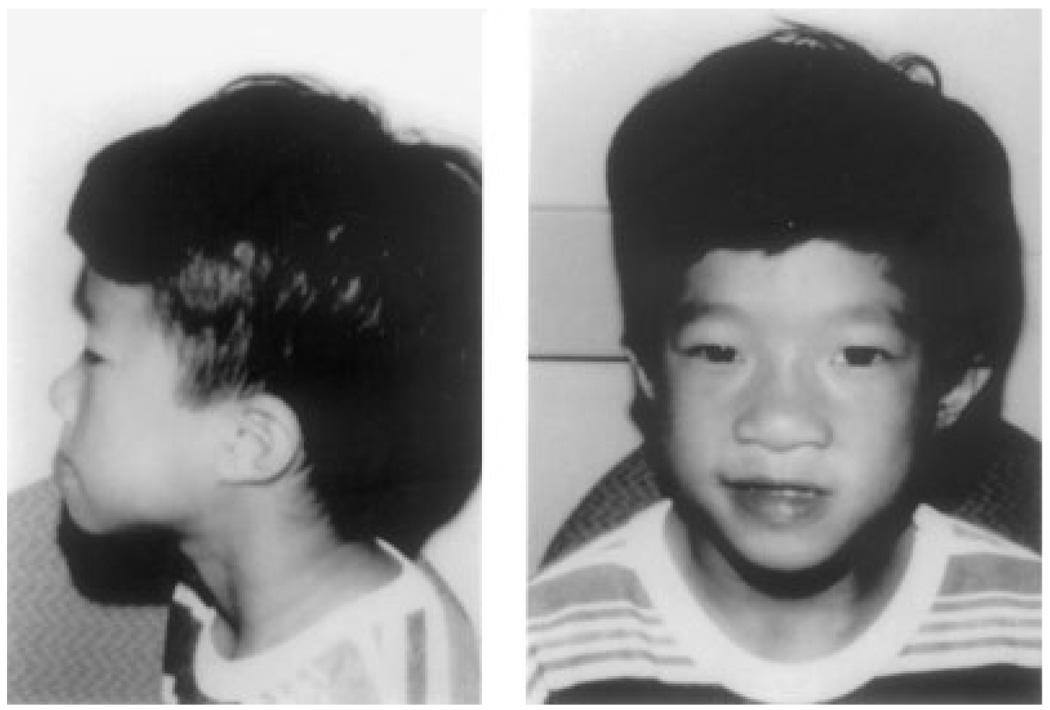
Fig. 6.
Vietnamese patient ascertained with a history of cleft palate and growth hormone deficiency. He was noted to be hyperteloric with hooding of eyelids and mild overfolding of his helix.
Of the 11 Hispanic patients several had typical dysmorphic features more closely resembling those of the Caucasian patients (Fig. 7). Whereas, the three patients of mixed ethnicity had variable facial features.
Fig. 7.
Hispanic child demonstrating hooded eyelids, auricular anomalies, prominent nasal root, bulbous nasal tip, and hypoplastic alae nasae.
Growth parameters, including head circumference and interpupillary distance revealed only minor differences between Caucasians and non-Caucasians. However, short stature was notably more common among Caucasians, Hispanics, and Asians as compared to African-Americans (significance > P = 0.05 using χ2 analysis) (Table III).
TABLE III.
Patients With a 22q11.2 Deletion and Selected Features by Race/Ethnicity (N = 255)
| Microcephalic(<5%) (%) | Macrocephalic(>95%) (%) | Hyperteloric(>95%) (%) | Short stature(<5%) (%) | |
|---|---|---|---|---|
| Caucasian (N = 204) | 19.1 | 2.0 | 13.2 | 35.8 |
| African-American (N = 33) | 9.1 | 3.0 | 15.2 | 15.2 |
| Hispanic (N = 11) | 27.3 | 0 | 45.5 | 36.4 |
| Asian (N = 4) | 0 | 0 | 25 | 50 |
| Other (N = 3) | 33.3 | 0 | 0 | 0 |
DISCUSSION
We report our anthropometric and craniofacial findings in both Caucasian and non-Caucasian patients with the 22q11.2 deletion. In looking at our large cohort of 370 patients, only12% are African-American. This is in contrast to our hospital’s population of patients where 42% are African-American. To explain this discrepancy, one might speculate that the deletion is less common in certain ethnic groups. Based on our own cohort, however, we found no difference in the numbers of Hispanic, Asians, and patients of mixed background as compared to our hospital’s overall population (Table I). Therefore, the best method of answering this question would be by performing a newborn screening study. However, with the exception of auricular anomalies, we did find a paucity of the typical craniofacial features associated with the 22q11.2 deletion in African-American patients. Thus, this may offer an explanation for the under-representation of this group in our cohort. In particular, we found a lack of noticeable nasal differences amongst the African-American cohort including a dearth of patients with hypoplastic alae nasae, a prominent nasal root or a bulbous nasal tip. In addition, we found hooding of the eyelids in only 6% of African-American patients as compared to 26% of Caucasians. This may be explained in part by the underlying influence of ethnic and racial background on craniofacial development.
In comparison, Hispanics were likely to have dysmorphisms which were similar to their Caucasian counterparts (Table II). In looking at our Asian subgroup, the numbers were too small to make a reliable assessment. Head circumference, height, and interpupillary distance were not contributory in making the diagnosis in any category.
The paucity of dysmorphic features among African-American patients has been noted in other syndromic diagnoses. Hudgins et al. [1998] reported that the typical facial characteristics in African-American patients with Prader–Willi syndrome were lacking and suggested that the “clinicians threshold of suspicion of Prader–Willi syndrome should be lower in African-American individuals with hypotonia and developmental delays.”
We would echo these sentiments as regards the 22q11.2 deletion. Furthermore, we would suggest microdeletion studies in non-Caucasian patients who present with even minor symptomatology of the 22q11.2 deletion regardless of their facial appearance in an effort to provide appropriate clinical management, cognitive remediation, and genetic counseling in a timely manner for these patients.
ACKNOWLEDGMENTS
We gratefully acknowledge the help of the Clinical Genetics Fellows and Genetic Counselors; the technical expertise of the Clinical Cytogenetics Laboratory and the Research Laboratory; most importantly, we thank the patients and their families for continuing to participate in these studies and the many clinicians who have made referrals to our center. These studies were supported by funds from the Velocardiofacial syndrome: Molecular and Clinical Studies Research Grant Number DC02027 from the National Institutes of Health and NIH/NCRR grant M01-RR00240.
Grant sponsor: National Institute of Deafness and Communication Disorders; Grant number: DC02027; Grant sponsor: GCRC; Grant number: MO1-RR00240.
REFERENCES
- Burn J, Takao A, Wilson D, Cross I, Momma K, Wadey R, Scambler P, Goodship J. Conotruncal anomaly face syndrome is associated with a deletion within chromosome 22. J Med Genet. 1993;30:822–824. doi: 10.1136/jmg.30.10.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Budarf ML, Emanuel BS. A genetic etiology for DiGeorge syndrome: Consistent deletions and microdeletions of 22q11. Am J Hum Genet. 1992a;50:924–933. [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Spinner NB, Budarf ML, McDonald-McGinn DM, Zackai EH, Goldberg RB, Shprintzen FJ, Saal HM, Zonana J, Jones MC, Mascarello JT, Emanuel BS. Deletions and microdeletions of 22q11.2 in Velo–Cardio–Facial syndrome. Am J Med Genet. 1992b;44:261–268. doi: 10.1002/ajmg.1320440237. [DOI] [PubMed] [Google Scholar]
- Driscoll DA, Salvin J, Sellinger B, McDonald-McGinn D, Zackai EH, Emanuel BS. Prevalence of 22q11 microdeletions in DGS and VCFS: Implications for genetic counseling and prenatal diagnosis. J Med Genet. 1993;30:813–817. doi: 10.1136/jmg.30.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold M, Bossert WH. Normal values for selected physical parameters: An aid to syndrome delineation. Birth Defects: Original Article Series 10. 1974 [PubMed] [Google Scholar]
- Fryburg JS, Lin KY, Golden EF. Chromosome 22q11.2 deletion in a boy with Opitz oculo–genito–laryngeal syndrome. Am J Med Genet. 1996;62:274–275. doi: 10.1002/(SICI)1096-8628(19960329)62:3<274::AID-AJMG13>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Giannotti A, Diglio MC, Marino B, Mingarelli R, Dallapiccola B. Cayler cardiofacial syndrome and del 22q11: Part of the CATCH22 phenotype. Am J Med Genet. 1994;30:807–812. doi: 10.1002/ajmg.1320530320. [DOI] [PubMed] [Google Scholar]
- Goldmuntz E, Driscoll D, Budarf ML, Zackai EH, McDonald-McGinn DM, Biegel JA, Emanuel BS. Microdeletions of chromosomal region 22q11 in patients with congenital conotruncal cardiac defects. J Med Genet. 1993;30:807–812. doi: 10.1136/jmg.30.10.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins L, Geer JS, Cassidy SB. Phenotypic differences in African Americans with Prader–Willi syndrome. Genet Med. 1998;1:49. doi: 10.1097/00125817-199811000-00010. [DOI] [PubMed] [Google Scholar]
- LaCassie Y, Arriaza MI. Letter to the Editor: Opitz GBBB syndrome and the 22q11.2 deletion syndrome. Am J Med Genet. 1996;62:318. doi: 10.1002/(SICI)1096-8628(19960329)62:3<318::AID-AJMG21>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Matsouka R, Takao A, Kimura M, Imamura S-I, Kondo C, Joh-O K, Ikeda K, Nishibatake M, Ando M, Momma K. Confirmation that the conotruncal anomaly face syndrome is associated with a deletion within 22q11.2. Am J Med Genet. 1994;53:285–289. doi: 10.1002/ajmg.1320530314. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Driscoll DA, Bason L, Christensen K, Lynch D, Sullivan K, Canning D, Zavod W, Quinn N, Rome J, Paris Y, Weinberg P, Clark BJ, Emanuel BS, Zackai EH. Autosomal dominant Opitz GBBB syndrome due to a 22q11.2 deletion. Am J Med Genet. 1995;59:103–113. doi: 10.1002/ajmg.1320590122. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Driscoll DA, Emanuel BS, Zackai EH. The 22q11.2 deletion in African-American patients: An underdiagnosed population. Am J Hum Genet. 1996;59:A90. doi: 10.1002/ajmg.a.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, LaRossa D, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, Moss E, Wang P, Solot C, Schultz P, Lynch D, Bingham P, Keenan G, Weinzimer S, Ming JE, Driscoll D, Clark BJ, III, Markowitz R, Cohen A, Moshang T, Pasquariello P, Randall P, Emanuel BS, Zackai EH. The 22q11.2 deletion: Screening, diagnostic workup, and outcome of results: Report on 181 patients. Genet Testing. 1997;1:99–108. doi: 10.1089/gte.1997.1.99. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Kirschner R, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, Moss E, Solot C, Wang P, Jacobs I, Handler S, Knightly C, Heher K, Wilson M, Ming JE, Grace K, Driscoll D, Pasquariello P, Randall P, LaRossa D, Emanuel BS, Zackai EH. The Philadelphia story: The 22q11.2 deletion: Report on 250 patients. Genet Couns. 1999;10:11–24. [PubMed] [Google Scholar]
- Minugh-Purvis N, Kirschner R, LaRossa D, McDonald-McGinn D, Zackai E. Cephalofacial morphometrics in children with the 22q11.2 deletion. Presentation, bringing the 22q11.2 deletion into the 21st Century, Second International Conference for Families and Professionals; Philadelphia. June.2000. [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, Emanuel BS, Zackai EH, Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. J Pediatr. 1999;134:193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. NCHS growth curves for children, birth-18 years, United States. Vital and Health Statistics. Series 11, No. 165. DHEW Publication No. Public Health Service. Washington: US: Government Printing Office 78–7650; 1997. [PubMed] [Google Scholar]
- Nickel RE, Magenis RE. Neural tube defects and deletions of 22q11. Am J Med Genet. 1996;66:25–27. doi: 10.1002/(SICI)1096-8628(19961202)66:1<25::AID-AJMG6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Nickel RE, Pillers DM, Merkens M, Magenis RE, Driscoll DA, Emanuel BS, Zonana J. Velocardiofacial and DiGeorge syndromes with meningomyelocele and deletions of the 22q11 region. Eur J Pediatr Surg. 1993;3:27–28. [PubMed] [Google Scholar]
- Nickel RE, Pillers DA, Merkens M, Magenis RE, Driscoll DA, Emanuel BS, Zonana J. Velo–cardio–facial syndrome and DiGeorge sequence with meningomyelocele and deletions of the 22q11 region. Am J Med Genet. 1994;52:445–449. doi: 10.1002/ajmg.1320520410. [DOI] [PubMed] [Google Scholar]
- Weinzimer SA, McDonald-McGinn DM, Driscoll DA, Emanuel BS, Zackai EH, Moshang T., Jr Growth hormone deficiency in patients with 22q11.2 deletion: Expanding the phenotype. Pediatr. 1998;101:929–932. doi: 10.1542/peds.101.5.929. [DOI] [PubMed] [Google Scholar]