B cell–intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans (original) (raw)
Abstract
Engagement of cytokine receptors by specific ligands activate Janus kinase–signal transducer and activator of transcription (STAT) signaling pathways. The exact roles of STATs in human lymphocyte behavior remain incompletely defined. Interleukin (IL)-21 activates STAT1 and STAT3 and has emerged as a potent regulator of B cell differentiation. We have studied patients with inactivating mutations in STAT1 or STAT3 to dissect their contribution to B cell function in vivo and in response to IL-21 in vitro. STAT3 mutations dramatically reduced the number of functional, antigen (Ag)-specific memory B cells and abolished the ability of IL-21 to induce naive B cells to differentiate into plasma cells (PCs). This resulted from impaired activation of the molecular machinery required for PC generation. In contrast, STAT1 deficiency had no effect on memory B cell formation in vivo or IL-21–induced immunoglobulin secretion in vitro. Thus, STAT3 plays a critical role in generating effector B cells from naive precursors in humans. STAT3-activating cytokines such as IL-21 thus underpin Ag-specific humoral immune responses and provide a mechanism for the functional antibody deficit in STAT3-deficient patients.
Human antibody (Ab) deficiency disorders are the most common form of primary immunodeficiency (Cunningham-Rundles and Ponda, 2005; Fischer, 2007). Many of these patients have absolute deficiency of serum Ig and are diagnosed with common variable immunodeficiency (CVID). Interestingly, individuals with normal serum IgG can nonetheless experience recurrent invasive infections with encapsulated organisms or have defects in establishing antigen (Ag)-specific Ab responses (Cunningham-Rundles and Ponda, 2005; Fischer, 2007). These clinical observations indicate that intrinsic functional B cell defects have significant consequences for host defense, even though they may not manifest as hypogammaglobulinemia. With the exception of the hyper-IgM syndromes caused by mutations in CD40, AICDA, and UNG (Cunningham-Rundles and Ponda, 2005), we have very little insight into the mechanisms underlying these intrinsic B cell defects.
Differentiation of naive lymphocytes into effector cells is regulated in part by signals delivered through cytokine receptors and subsequent activation of JAK–STAT signaling pathways (Akira, 1999; Shuai and Liu, 2003; O'Shea and Murray, 2008). Four JAK and seven STAT proteins have been identified, and germline or conditional deletion has revealed important roles for specific JAK–STAT pathways in the development and differentiation of multiple cell lineages (Akira, 1999; Shuai and Liu, 2003; O'Shea and Murray, 2008). Furthermore, mutations in JAK3, TYK2, STAT1, and STAT5B are associated with human immunodeficiencies (Macchi et al., 1995; Dupuis et al., 2003; Bernasconi et al., 2006; Minegishi et al., 2006; Fischer, 2007), underscoring the fundamental role of these molecules in immune regulation.
Recently, heterozygous mutations in STAT3 have been found to cause ~60% of cases of autosomal dominant hyper-IgE syndrome (AD-HIES; Holland et al., 2007; Minegishi et al., 2007). Most mutations act in a dominant-negative (DN) manner, reducing the number of functional STAT3 dimers by 75% (Minegishi et al., 2007). In contrast to all other STATs, germline deletion of Stat3 is embryonically lethal (Akira, 1999). Thus, although residual functional STAT3 dimers in AD-HIES allow placental development, they are insufficient to prevent disease. Because STAT3 is widely expressed and activated by >25 cytokines (O'Shea and Murray, 2008; Tangye et al., 2009), it is not surprising that AD-HIES is a multisystem disease affecting the immune and musculoskeletal systems. Immunological defects include skin lesions (eczema, boils), recurrent invasive mucocutaneous and lung infections with Staphylococcus aureus and Candida albicans, and high levels of IgE (Davis et al., 1966; Grimbacher et al., 2005; Tangye et al., 2009). The dysregulation underlying increased serum IgE in AD-HIES patients remains to be established. It does not appear to result from an intrinsic B cell defect that results in enhanced switching to IgE (Avery et al., 2008b) but may reflect altered production of regulatory cytokines by CD4+ T cells (Ma et al., 2008) and defects at epithelial surfaces (Grimbacher et al., 2005; Tangye et al., 2009). Although AD-HIES patients have normal serum levels of IgM, IgG, and IgA, many exhibit poor Ag-specific Ab responses after immunization with T cell–dependent (TD) Ag (Dreskin et al., 1985; Leung et al., 1988; Sheerin and Buckley, 1991). Consequently, they suffer from an increased incidence of infections with encapsulated organisms (Streptococcus pneumoniae, Haemophilus influenzae), a scenario consistent with a B cell defect manifesting as functional Ab deficiency (Dreskin et al., 1985; Leung et al., 1988; Sheerin and Buckley, 1991).
The mechanisms underlying some of the clinical features of AD-HIES are being unraveled. Elucidation of the T cell–intrinsic actions of STAT3 revealed that the unique susceptibility of these patients to infections at epithelial surfaces is caused by a deficiency of Th17 cells (de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008; Renner et al., 2008; Minegishi et al., 2009). These studies highlighted the utility of analyzing lymphocyte differentiation in patients with defined molecular lesions as a strategy of understanding key clinical features of immunodeficient conditions. Despite this progress, the mechanism underlying reduced Ag-specific Ab responses, despite normal serum Ig levels, in patients with STAT3 mutations is unknown. Thus, it is of considerable interest to understand the B cell–intrinsic function of STAT3.
Studies of gene-modified mice and of human B cells in vitro have revealed that IL-21 is a key cytokine for establishing, maintaining, and regulating the quality of Ab responses (Ozaki et al., 2002; Ozaki et al., 2004; Pène et al., 2004; Bryant et al., 2007; Avery et al., 2008a,b; Ettinger et al., 2008; Nurieva et al., 2008; Vogelzang et al., 2008). IL-10 is also capable of inducing Ig secretion from human B cells, albeit to a much lesser extent than IL-21 (Rousset et al., 1992; Brière et al., 1994; Bryant et al., 2007). Importantly, these cytokines are capable of activating STAT3 (Asao et al., 2001; Habib et al., 2002; Zeng et al., 2007; Diehl et al., 2008). Based on this, we hypothesized that impaired responsiveness to IL-10 and IL-21 may account for the functional Ab deficiency in AD-HIES patients. We found that STAT3 plays a critical role in generating memory cells and plasma cells (PCs) from naive precursors in vivo and in vitro in response to IL-10 and IL-21. This function of STAT3 is nonredundant because similar defects were not observed in the context of mutations in STAT1. This reveals that STAT3-activating cytokines, predominantly IL-21, are key mediators of human B cell effector function and humoral immune responses, and importantly, provides a mechanism for the functional Ab deficit in AD-HIES patients.
RESULTS
IL-21 predominantly activates STAT1 and STAT3 in primary human B cells
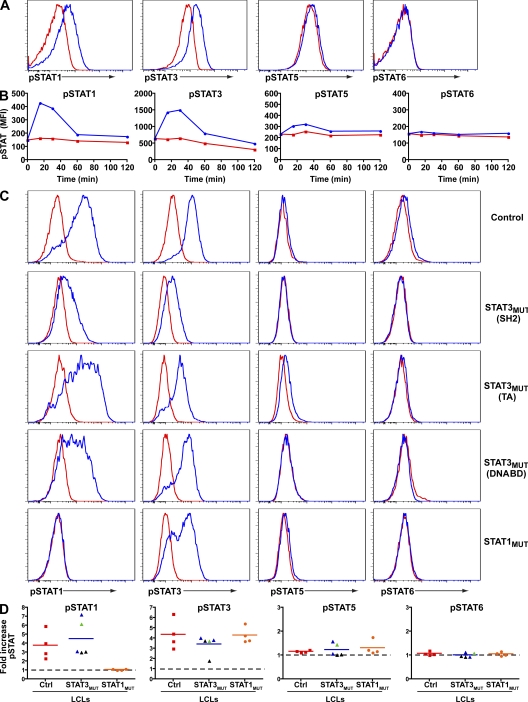
IL-21 can activate STAT1, STAT3, and STAT5, and the mitogen-activated protein kinase and Akt pathways (Asao et al., 2001; Zeng et al., 2007; Diehl et al., 2008). To extend these studies, human splenic B cells were stimulated with CD40L and anti-Ig for ~24 h and then exposed to IL-21. STAT1 and STAT3 became maximally phosphorylated within 15–30 min of IL-21 exposure and returned to basal levels by 60 min (Fig. 1, A and B). In contrast, there was only a low level of phospho-STAT5 detected in IL-21–stimulated human B cells and no difference in the phosphorylation status of STAT6 in B cells cultured with or without IL-21 (Fig. 1, A and B). Thus, IL-21 predominantly activates STAT1 and STAT3 in human B cells.
Figure 1.
IL-21 predominantly activates STAT1 and STAT3 in primary human B cells. (A and B) Human splenic B cells were cultured for ~24 h with CD40L plus anti-Ig and then cultured in the absence (red) or presence (blue) of IL-21. Phosphorylation of STAT1, 3, 5, and 6 was determined by intracellular staining. (A) Expression of phospho-STATs after 30 min. (B) Kinetics of IL-21–induced STAT phosphorylation in primary human B cells. These results are representative of two (STAT5 and 6) or three (STAT1 and 3) experiments. (C) Expression of phospho-STATs in EBV-LCLs from normal controls or patients with mutations in STAT3 or STAT1 that were cultured without (red) or with (blue) IL-21 for 30 min. (D) Induction of phospho-STATs in cell lines examined from the indicated number of normal donors (red squares) and STAT3MUT (black triangles, Src homology 2 domain mutations; blue triangles, DNA-binding domain mutations; green triangles, transactivation domain mutations) and STAT1MUT patients (orange circles) in response to IL-21. The values represent the fold increase in mean fluorescence intensity of cells stimulated with IL-21 for 30 min over unstimulated cells. The symbols correspond to the mean derived from one to four experiments performed on each cell line; the horizontal bars represent means. The dashed lines indicate a fold change of 1, which represents no change in expression of phospho-STAT proteins in the presence of IL-21. DNABD, DNA-binding domain; MFI, mean fluorescence intensity; SH2, Src homology 2 domain; TA, transactivation domain.
STAT1 and STAT3 mutant patients
To dissect the molecular mechanism whereby IL-21 activates human B cells, we analyzed B cell development and effector function in individuals with mutations in STAT1 or STAT3. We studied 10 patients with STAT3 mutations. All had aberrantly high levels of serum IgE, a Th17 deficiency, and heterozygous mutations in the DNA-binding, Src homology 2, or transactivation domains of STAT3 (Table S1; Ma et al., 2008). We also examined six patients who had either homozygous (Chapgier et al., 2006b; Chapgier et al., 2009) or heterozygous DN mutations (Dupuis et al., 2001; Chapgier et al., 2006a) in STAT1 (Table S2).
We examined IL-21–induced STAT phosphorylation in EBV-transformed lymphoblastoid cell lines (EBV-LCLs) from patients with heterozygous mutations in STAT3 (n = 5), complete STAT1 deficiency (n = 3; Dupuis et al., 2003; Chapgier et al., 2006b), or partial STAT1 deficiency with homozygous (n = 1; Chapgier et al., 2009) or heterozygous (n = 4; Dupuis et al., 2001; Chapgier et al., 2006a) STAT1 mutations. Stimulation of normal LCLs with IL-21 resulted in robust phosphorylation of both STAT1 and STAT3 (Fig. 1, C and D). STAT1 and STAT3 were also phosphorylated in IL-21–stimulated LCLs from STAT3 mutant (STAT3MUT) patients, irrespective of the location of the STAT3 mutation, or patients with DN STAT1 mutations, with the levels falling within the ranges established for normal LCLs (Fig. 1 D and Fig. S1). IL-21 also activated STAT3 in LCLs from patients who lacked STAT1 (Fig. 1, C and D). Because of the deficiency of STAT1 in these cell lines, no phospho-STAT1 was detected. IL-21 induced only modest phospho-STAT5 in normal and STATMUT LCLs, whereas it did not activate STAT6 in any of these LCLs (Fig. 1 C, D). Thus, consistent with the findings by us (Fig. 1, A and B) and others (Diehl et al., 2008) for primary human B cells, IL-21 predominantly activates STAT1 and STAT3 even in the presence of mutations in these signaling molecules.
STAT3 deficiency impairs the in vivo generation of human memory B cells in AD-HIES patients
The ability of IL-21 to activate STAT1 and STAT3, and the important role of IL-21 in regulating B cell responses (Ozaki et al., 2002; Pène et al., 2004; Bryant et al., 2007; Avery et al., 2008a,b; Diehl et al., 2008; Ettinger et al., 2008) suggested that patients with STAT1 or STAT3 mutations may have defects in B cell differentiation in vivo. Thus, we examined the generation of memory cells in these patients.
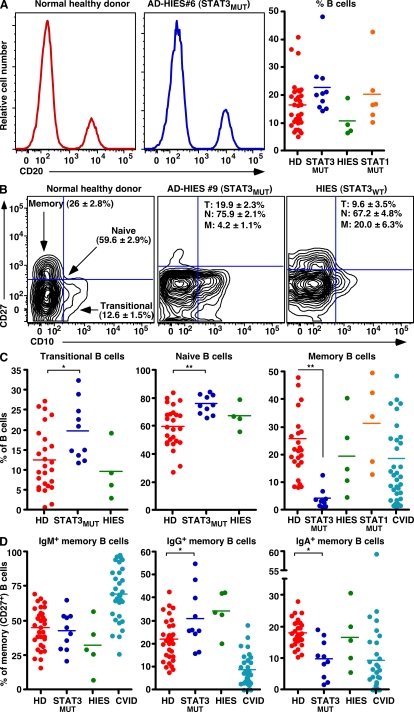
Human B cells can be resolved into subsets of transitional, naïve, and memory cells by the differential expression of CD10 and CD27. In normal donors, ~15% of peripheral blood (PB) lymphocytes are B cells; ~10% of these are transitional (CD10+CD27−), whereas ~25–35% are memory (CD27+; Fig. 2, A–C; Klein et al., 1998; Tangye et al., 1998; Wardemann et al., 2003; Ma et al., 2005; Ma et al., 2006; Avery et al., 2008b). Within the CD27+ B cell population of normal donors (5–65 yr old), ~50, 25, and 20% of them express IgM, IgG, or IgA, respectively (Fig. 2 D; Ma et al., 2005; Ma et al., 2006). Analysis of 10 STAT3MUT and 6 STAT1MUT patients demonstrated no significant difference (P > 0.05) in the frequencies of total PB B cells compared with normal controls (Fig. 2 A). However, the frequencies of transitional and naive cells were increased and memory cells were dramatically reduced (P < 0.01; Fig. 2, B and C) in STAT3MUT patients compared with healthy donors. The defect in memory cell generation was observed for all patients examined irrespective of their STAT3 mutation, in contrast to patients with elevated serum IgE levels but WT STAT3, or patients with STAT1 mutations—who had normal frequencies of B cell subsets (Fig. 2, A–C)—and a large cohort of CVID patients, of whom ~50% had a memory B cell deficit (Fig. 2 C). We also detected normal percentages of IgG+ memory cells (32.1 ± 4.7%; range: 24.5–45.4%) in STAT1MUT patients (n = 5), suggesting that memory B cell generation is normal in these individuals. Thus, intact signaling through STAT3 is required to generate the memory B cell pool, whereas signaling through STAT1 is dispensable for memory B cell formation.
Figure 2.
Mutations in STAT3 severely compromise the generation of memory B cells. (A–C) The frequencies of total B cells (A) or transitional (CD20+CD10+CD27−), naive (CD20+CD10−CD27−), and memory (CD20+CD10−CD27+) B cells (B and C) in healthy donors (HD; n = 26–32), STAT3MUT patients (n = 10), patients with AD-HIES but WT STAT3 alleles (HIES; n = 4–5), STAT1MUT patients (n = 5–6), or CVID patients (n = 36) were determined by flow cytometry. The values in B correspond to the frequencies (means ± SEM) of transitional (T), naive (N), and memory (M) B cells in the indicated donor populations. (D) memory (CD20+CD10−CD27+) B cells from healthy donors (HD; n = 32), STAT3MUT patients (n = 10), WT STAT3 AD-HIES patients (n = 5), and CVID patients (n = 31) were labeled with mAbs specific for IgM, IgG, or IgA. The frequency of memory B cells that were IgM+, IgG+, or IgA+ was then determined. The plots in A and B are from representative healthy donors and patients; the graphs in A, C, and D show data points for all donors and patients examined, and the horizontal bars represent means. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Notably, the distribution of nonswitched and switched B cells within the CD27+ subset from normal donors and STAT3MUT patients was similar, with ~50% expressing IgM and ~40% having undergone switching to express IgG or IgA (Fig. 2 D). There was a significant increase and decrease in the frequencies of IgG+ and IgA+ memory cells, respectively, in STAT3MUT patients compared with healthy controls (Fig. 2 D). Because B cells need to undergo greater rounds of proliferation to switch to IgA than they do for IgG (Deenick et al., 1999; Avery et al., 2008a), these differences in frequencies of IgG+ and IgA+ memory B cells may reflect in vivo defects in the proliferation of STAT3MUT B cells (see Proliferation and survival induced by…). These phenotypic features of memory B cells from STAT3MUT patients starkly contrast patients with mutations in CD40LG, CD40, IKBKG, ICOS, or SH2D1A, whose few memory B cells fail to undergo isotype switching (Tangye and Tarlinton, 2009), and CVID patients who display great heterogeneity in the nature of the Ig isotype expressed by their cells (Fig. 2 D).
STAT3MUT AD-HIES patients fail to generate Ag-specific effector B cells
The importance of STAT3 in generating memory B cells is consistent with previous findings of defective functional Ab responses in AD-HIES patients (Dreskin et al., 1985; Leung et al., 1988; Sheerin and Buckley, 1991). One caveat of these studies is that they predated the discovery that STAT3 mutations caused this condition. Thus, the molecular lesion in the patients studied was unknown. This is particularly relevant because STAT3 mutations cause AD-HIES in only ~60% of patients (Minegishi et al., 2007). Moreover, there is some controversy regarding the appropriate criteria by which human B cells are categorized as memory cells (Tangye and Tarlinton, 2009). Thus, to confirm a role for STAT3 mutations in causing a functional memory B cell and Ab deficiency, we assessed Ag-specific B cell responses in our cohort of STAT3MUT patients by adopting an approach that made no assumptions about the phenotype of memory cells.
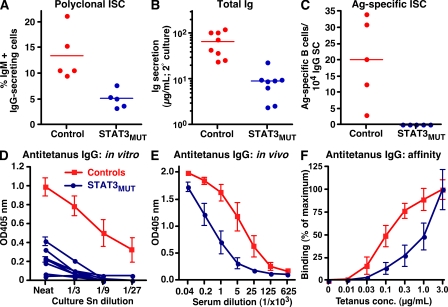
Total B cells were cultured with CD40L, CpG, S. aureus Cowan I (SAC; to engage the BCR), IL-10, and IL-21 for 4 d and recultured for another 3 d. The frequencies of cells secreting IgM, IgG, or antitetanus IgG were then determined. This assay relies on the in vitro differentiation of Ag-specific memory B cells into Ig-secreting cells (ISCs). Under these conditions, ~10–15% of control B cells differentiated into ISCs (Fig. 3 A) and produced substantial quantities of Ig during the secondary culture (Fig. 3 B). Furthermore, the IgG-ISCs contained a detectable population of tetanus-specific B cells (Fig. 3 C). Although STAT3MUT B cells also yielded polyclonal ISCs, the frequency and amount of Ig secreted was approximately three- to sixfold less than control B cells (Fig. 3, A and B). Moreover, we were unable to detect Ag-specific cells in any of the STAT3MUT patients examined (Fig. 3 C). We also measured antitetanus IgG secreted by cultured B cells. The levels of tetanus-specific IgG produced by control B cells exceeded STAT3MUT B cells by >30-fold (Fig. 3 D). This correlated with reduced levels of these Abs in the serum of STAT3MUT patients (Fig. 3 E). When the relative affinity of antitetanus IgG in the sera of controls and STAT3MUT patients was compared, the half-maximal binding of control sera to immobilized tetanus occurred at ~100 ng/ml tetanus, whereas STAT3MUT sera required at least 10-fold more specific Ag (Fig. 3 F). Thus, production of high titer and high-affinity Ag-specific Ig requires intact signaling through STAT3.
Figure 3.
STAT3 deficiency impairs the generation of Ag-specific memory B cells in vivo. B cells from healthy controls or STAT3MUT patients were cultured with CD40L, CpG, SAC, IL-10, and IL-21 for 4 d and were recultured for 3 d. The proportion of cells secreting (A) IgM+IgG or (C) antitetanus IgG was determined by ELISPOT. (B) Levels of total Ig (IgM+IgG+IgA) secreted during the secondary culture were determined by ELISAs; the results are expressed as micrograms of Ig secreted per 105 input cells in the secondary culture. Each symbol represents the response from an individual control or patient; the horizontal bars represent means. (D and E) Levels of antitetanus IgG in culture supernatants (Sn; D) or serum (E) from normal controls or STAT3MUT patients were determined by ELISA. (F) Sera from controls or STAT3MUT patients (1:40 dilution) were incubated with decreasing amounts of immobilized tetanus toxoid. Binding of antitetanus IgG was determined by ELISA. The results are expressed as the percentage of maximum binding, which is defined as the binding to the highest concentration of tetanus (i.e., 3 μg/ml). In D, the data for the controls represent means ± SEM from eight control donors; each blue line represents data from an individual STAT3MUT patient (n = 8). The data in E and F represent means ± SEM from four control donors and five STAT3MUT patients.
Proliferation and survival induced by IL-10 and IL-21 is compromised in STAT3MUT naive B cells
To better understand how mutations in STAT3, but not STAT1, might compromise the generation of both memory cells and PCs, the functional consequences of such loss-of-function mutations on the responses of human B cells in vitro were investigated. We first examined the ability of IL-21 to augment proliferation of naive control, STAT3MUT, and STAT1MUT B cells induced by CD40L, as revealed by CFSE dilution over a 5-d culture. We also investigated responses to IL-10, because it activates STAT3 and enhances human B cell proliferation and differentiation, although to a much lesser extent than IL-21 (Fig. S1; Rousset et al., 1992; Leonard, 2001; Bryant et al., 2007; Ettinger et al., 2008; O'Shea and Murray, 2008). IL-4 was also examined as it predominantly activates STAT6 (Leonard, 2001).
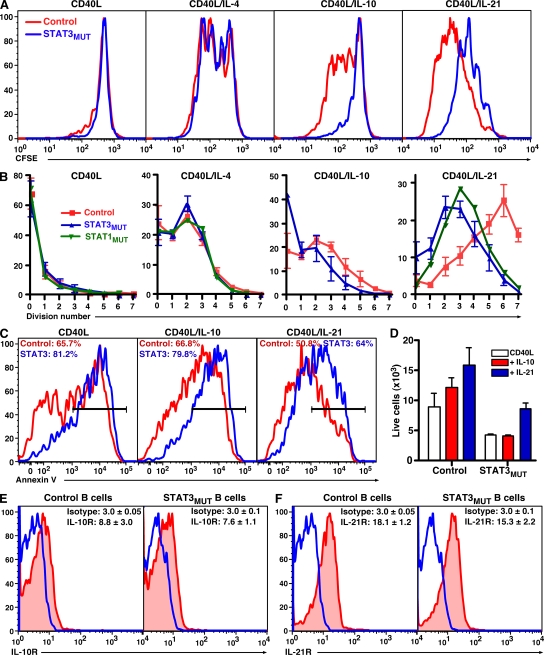
Naive B cells from healthy donors and STAT3MUT and STAT1MUT patients exhibited identical proliferative responses after stimulation with CD40L alone or CD40L/IL-4 (Fig. 4, A and B). In contrast, expansion of CD40L-stimulated STAT3MUT and STAT1MUT B cells to IL-10 and/or IL-21 was reduced (Fig. 4, A and B). Despite the reduced proliferation of STAT3MUT and STAT1MUT B cells to IL-10 or IL-21, the extent of cell division induced by these cytokines exceeded that of CD40L alone. Thus, loss of STAT1 and STAT3 function compromises the proliferation-enhancing effects of IL-10 and IL-21 on CD40L-stimulated B cells.
Figure 4.
Mutations in STAT3 or STAT1 partially reduce IL-10– and IL-21–mediated proliferation and survival of CD40L-activated B cells. (A and B) Naive B cells were sort purified from healthy controls, STAT3MUT patients, or a STAT1MUT patient; labeled with CFSE; and cultured for 5 d with CD40L alone or together with IL-4, IL-10, or IL-21. (A) CFSE profiles of control and STAT3MUT naive B cells stimulated under the indicated conditions. (B) Frequency of B cells in each division; the values for the control and STAT3MUT B cells represent means ± SEM from five independent experiments. Data for STAT1MUT B cells are from a single experiment. (C and D) Naive B cells from healthy controls or STAT3MUT patients were cultured for 3 d with CD40L alone or together with IL-10 or IL-21. (C) The percentage of apoptotic cells was determined by annexin V staining. The values represent the mean percentage of annexin V+ cells. (D) The number of viable cells recovered from each culture was also determined. (E and F) Expression of IL-10R (red; E) and IL-21R (red; F) on naive B cells was determined by flow cytometry. The overlays (blue) represent isotype control mAb. The values are means ± SEM (n = 4) of the fluorescence intensity of cells labeled with the isotype control or anti–IL-10R or –IL-21R mAb.
STAT3-activating cytokines can promote B cell survival in vitro (Levy and Brouet, 1994; Otero et al., 2006). Thus, we were interested in determining whether such prosurvival effects were affected by STAT3 mutations. Naive B cells were cultured with CD40L alone or together with IL-10 or IL-21, and the level of apoptosis and the number of surviving cells were determined after 3 d. Fewer STAT3MUT B cells survived in vitro in response to CD40L than normal B cells (Fig. 4, C and D). IL-10 had no effect on apoptosis (Fig. 4 C) but modestly increased the recovery of viable cells in cultures of normal, but not STAT3MUT, naive B cells (Fig. 4 D). IL-21 reduced apoptosis and improved survival of both control and STAT3MUT B cells (Fig. 4, C and D). Thus, STAT3MUT B cells have an intrinsic defect in survival irrespective of the mode of in vitro stimulation. Furthermore, IL-10 and IL-21 differ in their abilities and mechanisms to support the survival of in vitro–cultured B cells, with the effect of IL-10 but not IL-21 being STAT3 dependent.
Several cytokines regulate expression of their cognate receptor. Thus, it was possible that reduced function of IL-10 and IL-21 on human naive STAT3MUT B cells reflected differences in expression of their receptors. However, naive B cells from healthy controls and STAT3MUT patients displayed comparable levels of IL-10R and IL-21R (Fig. 4, E and F). Thus, the reduced responses of STAT3MUT B cells to the stimulatory effects of IL-21 and IL-10 reflect impaired STAT3-dependent signaling rather than reduced expression of cytokine receptors.
IL-21–induced isotype switching to IgG is unaffected by mutations in STAT3 or STAT1
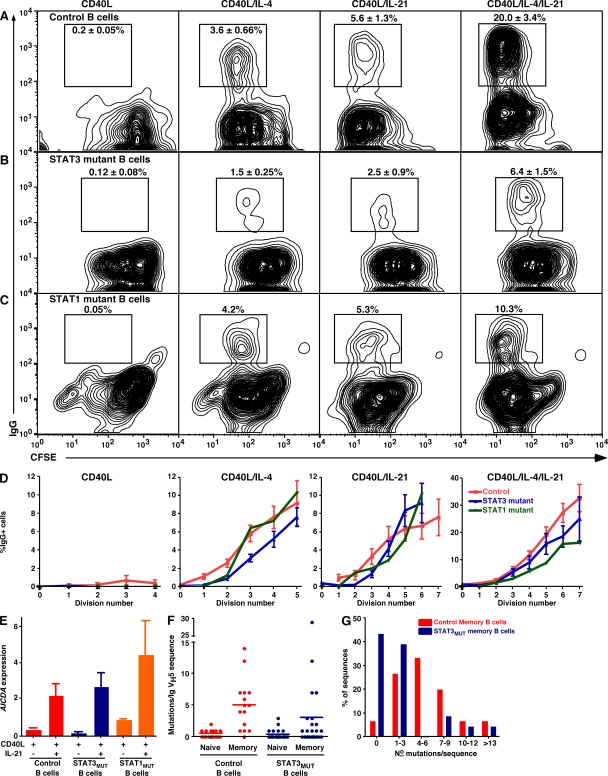
Although total memory cells were decreased in STAT3MUT patients, the finding that the proportion of IgG+ and IgA+ cells within their memory compartment approximated normal donors suggested that isotype switching occurred normally in vivo despite mutations in STAT3. Because IL-10 and IL-21 can induce switching to IgG (Brière et al., 1994; Pène et al., 2004; Avery et al., 2008a; Ettinger et al., 2008) and the effect of IL-21 is enhanced by IL-4 (Avery et al., 2008a), we tested whether naive STAT3MUT and STAT1MUT B cells could switch to IgG in response to IL-10, IL-21, and/or IL-4. Although CD40L had no effect on isotype switching by control B cells, addition of IL-4 or IL-21 generated comparable frequencies (~3–5%) of IgG+ cells, and this frequency was increased approximately four- to fivefold by the combination of IL-4 plus IL-21 (Fig. 5 A; Tangye et al., 2002; Avery et al., 2008a). CD40L-stimulated naive STAT3MUT B cells also underwent isotype switching to IL-4 and IL-21 alone or in combination (Fig. 5 B). However, approximately two- to threefold fewer IgG+ cells were generated compared with control naive B cells (Fig. 5 B). In contrast, STAT1MUT B cells yielded normal frequencies of IgG+ B cells (Fig. 5 C). IL-10 induced few normal or STAT3MUT naive B cells to switch to IgG (Fig. S2, A and B; Tangye et al., 2002). This was consistent with a lack of induction of phospho-STAT1 and reduced phospho-STAT3 (Fig. S1), and proliferation (Fig. 4, A and B) in IL-10–stimulated B cells compared with IL-21. Thus, it was difficult to ascertain whether isotype switching induced by IL-10 was compromised by STAT3 mutations.
Figure 5.
Isotype switching, AICDA expression, and SHM are unaffected by mutations in STAT3 or STAT1. Naive B cells from (A) normal controls, (B) STAT3MUT patients, or (C) a STAT1MUT patient were labeled with CFSE; cultured for 5 d with CD40L alone or together with IL-4, IL-21, or IL-4/IL-21; and analyzed for division and expression of surface IgG. A–C depict contour plots from one experiment. (D) Frequency of IgG+ (±SEM) cells in each division (control and STAT3MUT: n = 7; STAT1MUT: n = 1). (E) Naive B cells from healthy controls or STAT3MUT or STAT1MUT patients were cultured with CD40L alone or with IL-21. After 5 d, expression of AICDA was determined by quantitative PCR. The results are means ± SEM (control: n = 12; STAT3MUT: n = 7; or STAT1MUT: n = 3 experiments) and are expressed relative to GAPDH. (F and G) Naive and memory B cells were isolated from three healthy donors and three STAT3MUT patients. Ig VH5 genes were cloned and sequenced. (F) The number of mutations detected in each Ig VH5 gene isolated from normal and STAT3MUT naive and memory B cells; the horizontal bars represent means. (G) Distribution of mutations in IgV region genes in control and STAT3MUT memory B cells.
Ig isotype switching is linked to cell division (Hodgkin et al., 1996; Deenick et al., 1999; Tangye et al., 2002). Because there was a profound defect in proliferation of naive STAT3MUT B cells to IL-21 (Fig. 4, A and B), it was important to investigate isotype switching in the context of cell division. CD40L/IL-4 or CD40L/IL-4/IL-21 induced fewer (~25–40%) switched B cells per division from STAT3MUT B cells compared with normal B cells, suggesting that STAT3 plays a role in IL-4–induced switching to IgG (Fig. 5 D). This is likely because of the ability of IL-4 to activate STAT3 in human B cells via the IL-4Rα–IL-13Rα1 complex, which functions as the IL-13R as well as an alternative IL-4R (Umeshita-Suyama et al., 2000). In contrast, the division-based rate of isotype switching by STAT3MUT B cells in response to CD40L/IL-21 was similar to normal B cells (Fig. 5 D). Thus, the reduced frequency of IgG+ cells generated from STAT3MUT B cells as detected at the bulk population resulted from the cells undergoing fewer rounds of division. Not surprisingly, the division-based rate of switching of STAT1MUT B cells in response to IL-4 and IL-21 was intact (Fig. 5 D).
The observations of normal isotype switching by STAT3MUT and STAT1MUT B cells in vivo and in vitro prompted us to examine expression of AICDA, which is required for isotype switching (Muramatsu et al., 2000). IL-21 increased AICDA expression in CD40L-stimulated normal, STAT3MUT, and STAT1MUT naive B cells by ~5–10-fold compared with that induced by CD40L alone (Fig. 5 E). In contrast, IL-10 failed to induce AICDA expression in normal and STAT3MUT naive B cells (Fig. S2 C). Thus, IL-21–induced expression of AICDA and subsequent class switching can occur despite impaired signaling through STAT1 or STAT3.
STAT3MUT memory B cells undergo somatic hypermutation (SHM) in vivo
AICDA is also required for SHM (Muramatsu et al., 2000). Thus, we assessed SHM in the few memory cells detectable in STAT3MUT patients. Most Ig VH5 genes isolated from normal (18 out of 19; 94.7%) and STAT3MUT (24 out of 26; 92.3%) naive B cells contained zero to one mutations (normal: 0.52 ± 0.14; STAT3MUT: 0.35 ± 0.14; Fig. 5 F), and 14 out of 15 VH5 gene sequences from normal memory B cells harbored mutations (5.1 ± 1.04 mutations/sequence; Fig. 5, F and G). In contrast, only 13 out of 23 (56.5%) VH5 gene sequences from STAT3MUT memory B cells were mutated, resulting in a mean of 3 ± 1.33 mutations/sequence (Fig. 5, F and G). Restricting analysis to only mutated sequences revealed that the load of mutations was comparable to normal memory B cells (i.e., 5.4 ± 2.2 mutations/sequence; n = 13). Thus, although fewer memory B cells from AD-HIES patients expressed mutated Ig V region genes, SHM can occur at a normal rate. This is consistent with intact expression of AICDA and isotype switching in STAT3MUT B cells.
STAT3 plays a nonredundant role in mediating the differentiation of human naive B cells into ISCs
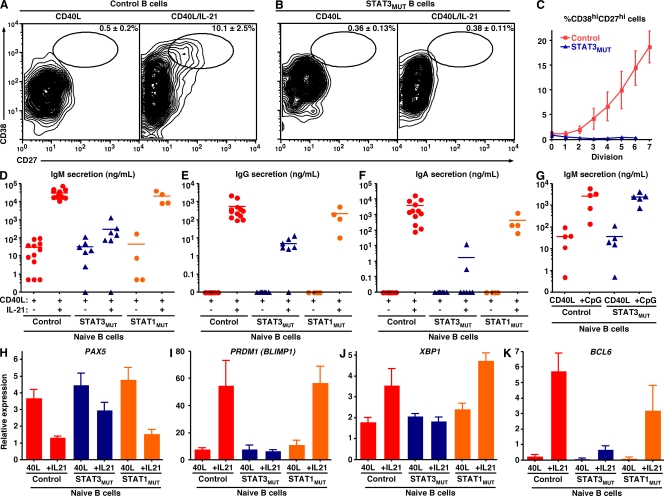
IL-10 and IL-21 can induce Ig secretion from CD40L-stimulated naive B cells, with IL-21 being 10–100 times more efficient than IL-10 (Pène et al., 2004; Bryant et al., 2007; Ettinger et al., 2008). For this reason, we next examined the consequences of STAT3 mutations on the differentiation of naive B cells into ISCs in response to these cytokines. CD40L induced few if any normal naive B cells to differentiate into ISCs (Fig. 6 A). However, in the presence of CD40L/IL-21, ~10% became ISCs as determined by acquisition of a CD38hiCD27hi phenotype (Fig. 6 A), a differentiation event that correlates with commitment of in vivo– and in vitro–activated human B cells to the PC lineage (Ellyard et al., 2004; Avery et al., 2005). In complete contrast, STAT3MUT naive B cells failed to generate ISCs after stimulation with CD40L/IL-21 (Fig. 6 B).
Figure 6.
STAT3 but not STAT1 is absolutely required for IL-21–induced differentiation of human naive B cells into ISCs. (A–C) Naive B cells from control donors (A) or STAT3MUT patients (B) were labeled with CFSE and cultured with CD40L alone or together with IL-21. After 5 d, the percentage of CD38hiCD27hi cells was determined. (C) Percentage of CD38hiCD27hi cells (mean ± SEM; n = 5) generated per division from control and STAT3MUT naive B cells stimulated with CD40L/IL-21. (D–G) Naive B cells from healthy controls (D–F: n = 12; G: n = 5), STAT3MUT patients (D–F: n = 7; G: n = 5), or STAT1MUT patients (n = 4) were cultured with CD40L alone or together with IL-21 (D–F) or CpG (G). The levels of secreted IgM (D and G), IgG (E), and IgA (F) were determined by ELISA after 12 d. Each symbol represents Ig secretion by naive B cells from an individual normal donor or patient; the horizontal bars represent means. (H–K) Naive B cells from healthy controls or STAT3MUT or STAT1MUT patients were cultured with CD40L alone (40L) or together with IL-21 (+IL21) for 5 d. Expression of PAX5 (H), PRDM1 (I), XBP1 (J), and BCL6 (K) was determined by quantitative PCR. The results are means ± SEM (control: n = 12; STAT3MUT: n = 7; or STAT1MUT: n = 3 experiments).
Differentiation into ISCs is also linked to cell division (Hodgkin et al., 1996; Tangye et al., 2003; Bryant et al., 2007). Although the frequency of normal naive B cells that became CD38hiCD27hi in response to CD40L/IL-21 increased with division, STAT3MUT naive B cells failed to generate any ISCs irrespective of division history (Fig. 6 C). Secretion of IgM by normal naive B cells stimulated with CD40L/IL-21 is increased ~1,000-fold over that induced by CD40L alone, whereas CD40L/IL-21 induced these cells to secrete substantial amounts of IgG and IgA (Table I; and Fig. 6, D–F). Consistent with the inability of STAT3MUT naive B cells to differentiate into phenotypic ISCs in response to CD40L/IL-21, their Ig secretion was 100–1,000-fold lower than that of corresponding normal naive B cells (Table I; Fig. 6, D–F; and Fig. S3). STAT3MUT naive B cells were also refractory to the effects of IL-10 (Table I). These findings were specific for naive B cells from patients with STAT3 mutations, because there was no significant difference in secretion of IgM, IgG, and IgA by STAT1MUT (n = 4) and normal naive B cells in response to CD40L/IL-21 (Table I; Fig. 6, D–F; and Table S3). Similarly, naive B cells from individuals with high serum IgE levels but WT STAT3 alleles (n = 4) secreted 10–200-fold more Ig in response to CD40L/IL-21 than STAT3MUT naive B cells (Table I), whereas CD40L/IL-21 induced normal Ig production by B cells from CVID patients (n = 11; Fig. S3).
Table I.
Impaired Ig secretion by STAT3MUT naive B cells in response to IL-10 and IL-21
| Ig secretion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgA | ||||||||
| CD40L | +IL-21 | +IL-10 | +CpG | CD40L | +IL-21 | +IL-10 | CD40L | +IL-21 | +IL-10 | |
| ng/ml | ng/ml | ng/ml | ng/ml | ng/ml | ng/ml | ng/ml | ng/ml | ng/ml | ng/ml | |
| Control (n = 12) | 30 ± 10 | 30,646 ± 5,344 | 640 ± 213 | 3,223 ± 1,023 | <0.1 | 538 ± 193 | 3.8 ± 2.5 | <0.1 | 3,750 ± 1,440 | 117 ± 81 |
| STAT3MUT (n = 7) | 33 ± 15 | 299 ± 168 | 47 ± 20 | 2,382 ± 546 | <0.1 | 4.9 ± 1.6 | <0.1 | <0.1 | 1.8 ± 1.7 | 7.5 ± 7.5 |
| HIES STAT3WT (n = 4) | 25 ± 13 | 4,125 ± 955 | ND | ND | <0.1 | 113 ± 67 | ND | <0.1 | 785 ± 484 | ND |
| STAT1MUT (n = 4) | 45 ± 43 | 20,706 ± 7,569 | 715 ± 555 | 5,314 ± 3,399 | <0.1 | 214 ± 114 | ND | <0.1 | 432 ± 275 | ND |
The inability of STAT3MUT naive B cells to become ISCs in vitro in response to CD40L plus IL-10 or IL-21 was not caused by a general defect in Ig secretion, because they produced normal levels of IgM in response to CD40L/CpG, which increases IgM secretion induced by CD40L alone by 50–100-fold (Fig. 6 G and Table I). Thus, unlike isotype switching, STAT3 is absolutely required for IL-10– and IL-21–mediated differentiation of naive B cells into ISCs in vitro. Furthermore, because the response of STAT1MUT B cells was normal, STAT1 is unlikely to make a major contribution to this process, highlighting the necessary role of STAT3.
The failure of STAT3MUT naive B cells to differentiate into ISCs in response to IL-21 or IL-10 is caused by an inability to undergo the requisite molecular changes for commitment to the PC lineage
Commitment of B cells to the PC lineage involves the coordinated actions of several transcription factors. It is initiated by the loss of PAX5 (Kallies et al., 2007), followed by induction of BLIMP-1 and XBP-1, which are required for repression of the “B cell program” and enforcement of the “PC program” of gene expression (Kallies et al., 2007; Tangye and Tarlinton, 2009). To elucidate a molecular mechanism for the inability of STAT3MUT naive B cells to become ISCs, we assessed changes in the expression of PAX5, PRDM1 (encoding BLIMP-1), and XBP1 in activated B cells by Q-PCR. Expression of PAX5 was reduced ~3-fold, whereas PRDM1 and XBP1 were increased 2–10-fold in normal naive B cells stimulated with CD40L/IL-21 compared with the expression by B cells cultured with CD40L alone (Fig. 6, H–J). In contrast, STAT3MUT B cells only modestly down-regulated PAX5 but completely failed to acquire PRDM1 and XBP1 after culture with CD40L/IL-21 (Fig. 6, H–J), demonstrating an inability to undergo the transcriptional changes necessary for the generation of ISCs. Similar results were observed for normal and STAT3MUT B cells cultured with IL-10 (Fig. S2, D and E). In complete contrast to STAT3MUT B cells, IL-21 caused naive STAT1MUT B cells to down-regulate PAX5 and increase PRDM1 and XBP1 to a comparable extent as normal naive B cells (Fig. 6, H–J), confirming further that STAT1 is dispensable for this process.
Memory B cell development largely occurs within germinal centers (GCs; Tangye and Tarlinton, 2009). The paucity of functional memory B cells in STAT3MUT patients (Fig. 2, B and C) together with the finding that ~50% of STAT3MUT memory B cells express unmutated Ig V region genes (Fig. 5, F and G) were suggestive of suboptimal GC formation in cases of STAT3 mutations. BCL-6 is required for GC formation in vivo (Tangye and Tarlinton, 2009) and can be induced in B cells by IL-21 (Ettinger et al., 2008). Thus, we also examined BCL6 expression. IL-21 induced substantial and comparable expression of BCL6 in CD40L-stimulated naive normal and STAT1MUT B cells. Although IL-21 could induce BCL6 in STAT3MUT naive B cells, it was 10-fold less than that observed for normal and STAT1MUT naive B cells (Fig. 6 K). Unlike IL-21, IL-10 did not induce BCL6 in naive B cells (Fig. S2, D and E). Thus, the inability to generate a sufficient pool of memory B cells in AD-HIES may result from inefficient GC reactions.
DISCUSSION
Although it has been appreciated for many years that cytokines can activate specific JAK–STAT signaling pathways in B cells (Leonard, 2001; Murray, 2007), contributions of individual STATs to the development and effector function of primary human B cells have not been established. IL-21 has emerged as a key cytokine for establishing, maintaining, and regulating the quality of Ab responses (Ozaki et al., 2002; Pène et al., 2004; Bryant et al., 2007; Avery et al., 2008a,b; Ettinger et al., 2008; Nurieva et al., 2008; Vogelzang et al., 2008). IL-10 can also induce modest isotype switching and Ig secretion from human B cells (Rousset et al., 1992; Brière et al., 1994; Bryant et al., 2007). Our detailed analysis of IL-10– and IL-21–mediated signaling in human naive B cells demonstrated that although these cytokines predominantly activate STAT1 and STAT3, B cell–intrinsic STAT3 is necessary and sufficient, whereas STAT1 is dispensable, for their establishment of long-lived, protective Ag-specific Ab responses.
Intact signaling through STAT3 is required to generate the memory B cell pool (Fig. 7 B). In contrast, STAT1MUT patients could generate normal numbers of memory B cells. Studies of female carriers of X-linked severe combined immunodeficiency indicated that naive B cells expressed both the WT and mutant alleles of IL2RG/common γ chain (γc), whereas memory B cells expressed only the WT allele (Conley et al., 1988). This suggested that although signals through γc were dispensable for B cell development, they were necessary for the generation of memory B cells (Conley et al., 1988). In light of the findings that memory B cells are dramatically reduced in STAT3MUT patients, that IL-21 activates STAT3, and that γc is a component of the IL-21R (Asao et al., 2001; Habib et al., 2002), we propose that IL-21 is a major contributor to memory B cell formation and that this occurs through a γc/STAT3-dependent mechanism.
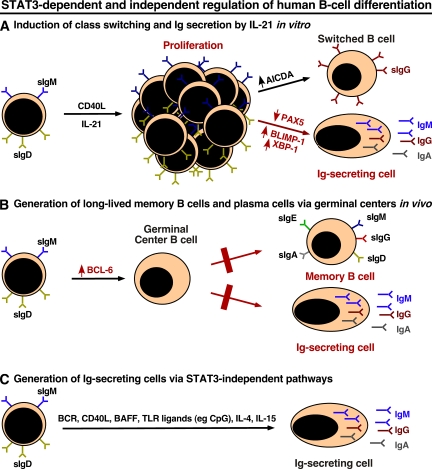
Figure 7.
Differential requirement for STAT3 in regulating human B cell differentiation. Naive B cells can be induced to undergo proliferation, isotype switching, and differentiation to the PC lineage via distinct pathways: (A) in vitro in response to TD stimulation (CD40L plus IL-21), (B) in vivo in response to TD Ag and subsequent GC formation, and (C) in response to TD and T cell–independent stimuli in the form of CD40L and/or BAFF, APRIL, TLR ligands (e.g., viral double-stranded RNA [TLR3] or CpG [TLR9]), and cytokines. Mutations in STAT3 differentially affect each of these pathways (red text). By impairing the up-regulation of PRDM1 (BLIMP-1) and XBP1, STAT3MUT B cells are unable to become ISCs in vitro; in contrast, induction of AICDA and Ig isotype switching are intact (A). Although GCs can be detected in STAT3MUT patients, the output of memory B cells and efficient PCs, as assessed by reduced numbers of CD27+ B cells and serum Ab titers against specific Ag, respectively, is impaired. This may result from reduced induction of BCL6 in response to IL-21. Ig production by STAT3MUT B cells in response to STAT3-independent TD and T cell–independent pathways is intact; this may explain the normal serum Ig levels in these patients (C).
Although IL-21 activates both STAT1 and STAT3, the consequences of DN mutations in STAT3, which reduce function by 75%, are much more severe than STAT1 deficiency. Such reduced STAT3 function completely abolished IL-21–mediated maturation of naive B cells into ISCs in vitro (Fig. 7 A). In contrast, these processes were intact in STAT1MUT B cells. These findings mirrored the impaired generation of Ag-specific Ab responses noted in AD-HIES patients (Dreskin et al., 1985; Leung et al., 1988; Sheerin and Buckley, 1991) and the intact Ab responses in cases of STAT1 deficiency after standard vaccinations or natural infection (Chapgier et al., 2006a,b). Our results complement a recent study that demonstrated overexpression of STAT3 in human B cells induced PC differentiation (Diehl et al., 2008), and therefore highlight a critical role for IL-21 and STAT3 in initiating and/or maintaining serological immunity by generating Ag-specific effector B cells from naive precursors in vivo. Our data also suggest that IL-10 may contribute to this process. However, given the differences in the magnitude of the responses induced by IL-10 and IL-21, the effect of IL-10 in vivo is likely to be proportionally less than IL-21. IL-6 also activates STAT3 (Leonard, 2001) and supports Ig secretion by human PCs via their heightened expression of the IL-6R (Ellyard et al., 2004; Good et al., 2006; Ettinger et al., 2008). Thus, it is plausible that IL-6 also contributes to STAT3-mediated regulation of human B cell function in vivo.
Induction of AICDA and isotype switching could occur despite mutations in STAT1 or STAT3, implying that (a) isotype switching is either mediated by residual STAT3 function in STAT3MUT B cells, (b) there is functional redundancy between STAT1 and STAT3, or (c) isotype switching is independent of both STAT1 and STAT3 and involves pathways such as Akt or mitogen-activated protein kinase (Fig. 7 A; Zeng et al., 2007). Irrespective of the mechanism, it is clear that Ig isotype switching is unaffected by STAT3 deficiency, which contrasts with the dependency of the generation of memory B cells and PCs on intact STAT3 function (Fig. 7, A and B).
Germline deletion of Stat3 in mice is embryonically lethal (Akira, 1999; Tangye et al., 2009). However, insights into STAT3 function in vivo have been provided by generating Stat3flox/floxCd19cre mice (Fornek et al., 2006). Some aspects of the phenotype of these mice are similar to STAT3MUT patients, as both have normal levels of total serum IgM, IgG, and IgA but a paucity of Ag-specific Ab (Dreskin et al., 1985; Leung et al., 1988; Sheerin and Buckley, 1991; Fornek et al., 2006). In Stat3flox/floxCd19cre mice, this resulted from an inability to generate Ag-specific IgG-secreting PCs (Fornek et al., 2006). Interestingly, _Il-21r_-deficient mice also have a phenotype that resembles Stat3flox/floxCd19cre mice and STAT3MUT humans: normal levels of serum IgM, IgA, and IgG (although IgG1 is variably reduced under some experimental conditions) but impressive reductions in Ag-specific serum IgG1 titers and splenic PCs (Ozaki et al., 2002). It was unclear from this study (Ozaki et al., 2002) whether the humoral immune defect resulted from an intrinsic B cell defect and/or an extrinsic defect because of impaired generation of T follicular helper cells (Nurieva et al., 2008; Vogelzang et al., 2008). By using a well-established model of B cell differentiation (Brink et al., 2008), we found that the Ag-specific (i.e., hen egg lysozyme) response of IL-21R–deficient B cells was drastically diminished compared with WT B cells, as indicated by a 10–20-fold reduction in the production of hen egg lysozyme–specific PCs and IgM and IgG (Fig. S4, A and B). This analysis of in vivo responses of Il21r_−/_− B cells strongly suggests that the key cytokine acting upstream of STAT3 in mouse B cells required for intact humoral immune responses to TD Ag is IL-21. Together with our in vitro analysis of STAT3MUT human B cells, this is further evidence of a pivotal role of the IL-21R–STAT3 axis in regulating humoral immunity to TD Ag in humans.
Despite these similarities between Stat3flox/floxCd19cre mice and STAT3MUT patients, the latter also have reductions in the numbers of circulating memory B cells, as well as the levels of serum and salivary Ag-specific IgA (Dreskin et al., 1985), whereas Stat3flox/floxCd19cre B cells could apparently produce Ag-specific IgM and IgA and generate IgG+ memory B cells (Fornek et al., 2006). One explanation for these differences is that STAT3 deficiency has a more dramatic effect on human compared with mouse B cells, which is reminiscent of more severe phenotypes in humans with mutations in TYK2, BTK, and BLNK compared with gene-targeted mice (Conley et al., 2000; Minegishi et al., 2006). Alternatively, these differences may reflect the fact that all cells are STAT3 deficient in AD-HIES, whereas in Stat3flox/floxCd19cre mice, only the B cells lack STAT3. Thus, B cell help afforded by other cells (CD4+ T cells) may also be impaired in STAT3MUT patients, and this may manifest as a more severe defect in humoral immunity in humans compared with mice.
The mechanism underlying impaired humoral immune responses in STAT3MUT patients was an inability of their B cells to activate the molecular machinery necessary for the generation of ISCs from naive precursors in response to IL-21 (Fig. 7 A). It is important to appreciate that STAT3MUT patients do not lack PCs, as they have normal serum Ig levels, and we could detect PCs in their lymphoid tissues (Fig. S5 A). Thus, STAT3-dependent signaling is crucial for generating effective Ag-specific Ab responses but not for maintaining total serum Ig levels, which are presumably regulated by STAT3-independent pathways such as those downstream of BCR, CD40, BAFF-R/TACI, Toll-like receptors (TLRs), and specific cytokine receptors (Fig. 7 C). This is supported by the ability of human STAT3MUT B cells to isotype switch to IL-4 and/or IL-21, and Ig secretion to CpG, and the well-established roles of TLRs and BAFF/APRIL, together with IL-4 or IL-15, in directing Ig isotype switching and secretion by human B cells (Litinskiy et al., 2002; Xu et al., 2008).
In contrast to the functional Ab deficit, the explanation for the memory B cell deficiency in STAT3MUT patients is less clear. Most memory B cells are generated in GCs (Tangye and Tarlinton, 2009). Because Stat3flox/floxCd19cre mice can form GCs in response to TD Ag (Fornek et al., 2006) and GC-like structures were detected in the lymph node of STAT3MUT patients (Fig. S5 B), a lack of GCs cannot explain the paucity of memory B cells in these patients. One possibility is that STAT3 is required for the survival of memory B cells generated from a GC, which is consistent with a requirement for STAT3 in protecting mouse B cells from apoptosis in vivo (Otero et al., 2006), and that STAT3MUT B cells appeared to be less responsive to the prosurvival effects of IL-10 than control B cells. Alternatively, STAT3 mutations may result in qualitatively inefficient GC reactions that are unable to generate memory B cells and only yield low-affinity PCs (Fig. 7 B). This is supported by our findings that STAT3MUT memory B cells have a reduced load of SHM, Ag-specific IgG produced by STAT3MUT patients was of reduced affinity, and expression of BCL6 by STAT3MUT naive B cells in response to IL-21 in vitro was only ~10% of that observed for normal naive B cells.
Although STAT3MUT patients have reduced levels of Ag-specific IgG and IgA, they have aberrantly high IgE (Grimbacher et al., 2005). Several studies have reported that the IgE in AD-HIES patients is Ag specific (Schopfer et al., 1979; Berger et al., 1980). Although our findings do not provide a conclusive explanation for the high level of serum IgE conferred by STAT3 mutations, they point to fundamental differences in the STAT3 dependence of production/maintenance of Ag-specific IgM, IgG, and IgA compared with Ag-specific IgE. Similarly, our findings indicate that STAT3 is crucial for generating high-affinity Ag-specific Ab but not for maintaining total serum Ig (Fig. 7). We infer from this that STAT3 is important for the formation and/or survival of memory B cells and PC-producing IgM, IgG, and IgA, and our data represent evidence of the importance of IL-21 signaling via STAT3 for the differentiation of GC B cells into these effector cells. The corollary is that there is a significant contribution to IgE responses from pathways outside the GC, which are STAT3 independent. Indeed, Erazo et al. (2007) noted that although IgG+ PCs arise from GCs, IgE+ PCs were excluded from GCs and localized to extrafollicular areas. Furthermore, IgE variable region genes exhibit significantly fewer somatic mutations than IgG variable region genes (Dahlke et al., 2006), which suggests that IgE+ B cells are not selected within GCs. These observations are consistent with the ability of BCL-6 (expressed by GC B cells) to inhibit IL-4/STAT6–mediated differentiation of naive B cells into IgE+ B cells (Harris et al., 1999). Interestingly, _Bcl6_-deficient mice fail to form GCs yet have elevated levels of IgE because of aberrant generation of IgE-PCs resulting from dysregulated IL-4 secretion and signaling via STAT6 (Harris et al., 1999; Tangye and Tarlinton, 2009). Thus, reduced expression of BCL6 but not AICDA in STAT3MUT B cells and potentially inefficient GC reactions in STAT3MUT patients may remove a regulatory circuit that ordinarily controls IL-4/STAT6–induced IgE production, thereby manifesting as hyper-IgE.
Overall, our data identify STAT3 as the key signaling component downstream of the IL-21R necessary for the generation of human memory B cells and ISCs, and thus, humoral immune responses. These findings identify a pathway that results in a clinical phenotype of specific Ab deficiency, and implicate B cell–intrinsic defects in components of the IL-21R–STAT3 pathway as candidates underlying impaired Ab formation in other immunodeficient conditions.
MATERIALS AND METHODS
mAbs and reagents.
The following mAbs were used: FITC–anti-CD20 and allophycocyanin (APC)–anti-CD10 (BD); biotinylated anti–human IgM, IgG, and IgA; PE–anti-CD27 and anti–phospho-STAT3; APC–anti-IgG and anti-CD38; streptavidin-PerCp; PE–anti–IL-10R, Alexa Fluor 488–anti–phospho-STAT5, Alexa Fluor 647–anti–phospho-STAT1, and anti–phospho-STAT6 (BD); biotin– and APC–anti-CD27 (eBioscience); and PE–anti–IL-21R (R&D Systems).
Human blood and tissue samples.
Buffy coats from healthy donors and spleens from organ donors were provided by the Australian Red Cross Blood Service. PB was collected from patients with STAT3 mutations, patients with mutations in STAT1, or patients with CVID or with elevated levels of serum IgE but WT STAT3 alleles. The mutations and clinical details of the STAT1MUT patients (Dupuis et al., 2001; Chapgier et al., 2006a,b; Chapgier et al., 2009), some of the AD-HIES patients (patients 1–5), and the STAT3 genotyping protocol (Ma et al., 2008) have been previously described; those of AD-HIES patients 6–10 are listed in Table S1. All human experiments were approved by jurisdictional ethics committees in Canberra, Sydney, Melbourne, Adelaide, Brisbane, and Perth, as well as by the institutional review boards of the Necker Medical School and the Rockefeller University.
B cell phenotyping and isolation.
PBMCs were incubated with mAb to CD20, CD10, and CD27. The frequencies of transitional (CD20+CD10+CD27−), naive (CD20+CD10−CD27−), and memory (CD20+CD10−CD27+) B cells were then determined (Wardemann et al., 2003; Avery et al., 2008b). Expression of Ig isotypes on memory B cells and cytokine receptors on naive B cells was determined as previously described (Ma et al., 2005; Good et al., 2006; Ma et al., 2006; Avery et al., 2008b). B cells were isolated with a Negative Isolation kit (Invitrogen). Naive PB B cells were purified by labeling total B cells with mAbs against CD20, CD27, and IgG and sorting CD20+CD27−IgG− cells (FACSAria; BD). The purity of the recovered naive population was typically >98%.
Expression of phospho-STATs.
Human splenic B cells were cultured with CD40L (Tangye et al., 2002) plus F(ab′)2 fragments of goat anti–human Ig (Jackson ImmunoResearch Laboratories, Inc.) for ~24 h, washed, and recultured in the absence or presence of 100 ng/ml IL-21 for different periods of time before being fixed, permeabilized, and labeled with anti–phospho-STAT1, -STAT3, -STAT5, and -STAT6 mAbs (Avery et al., 2008b). EBV-LCLs established from normal healthy donors and STAT3MUT and STAT1MUT patients were stimulated with or without IL-10 or IL-21 for 30 min, and expression of phospho-STATs was then determined.
Detecting Ag-specific B cells.
Total B cells (1–2 × 105 cells/400 μl) were cultured with CD40L, 1 μg/ml CpG 2006 (Sigma-Aldrich), 0.01% SAC (EMD), 100 U/ml IL-10 (provided by R. de Waal Malefyt, DNAX Research, Palo Alto, CA), and 50 ng/ml IL-21 (PeproTech). After 4 d, the cells were washed and recultured (1–2 × 105 cells/500 μl) with CD40L, CpG, IL-10, and IL-21 for a further 3 d. The activated B cells were then incubated in ELISPOT plates (Millipore) precoated with goat anti–human IgM and IgG antisera (SouthernBiotech) or tetanus toxoid (Sigma-Aldrich). The percentage of cells secreting polyclonal IgM, IgG, or tetanus-specific IgG was determined (Tangye et al., 2003). Levels of antitetanus IgG in culture supernatants and serum were determined by performing titrations in ELISA plates precoated with tetanus and then detecting bound IgG.
In vitro activation of naive B cells.
Purified B cells were cultured with CD40L alone or together with 100 U/ml IL-4 (provided by R. de Waal Malefyt), 100 U/ml IL-10, 50 ng/ml IL-21, IL-4 and IL-21, or 1 μg/ml CpG 2006. To investigate survival, the cells were cultured for 72 h, and the frequency of apoptotic and the number of viable cells were determined by annexin V binding and cell counting, respectively. For phenotypic analysis, the cells were first labeled with CFSE (Invitrogen; Hodgkin et al., 1996; Tangye et al., 2002) and then cultured in 48-well plates (~1.25–2 × 105 cells/400 μl/well; BD) for 5 d. In vitro–activated naive B cells were harvested from culture wells and incubated with isotype control or mAb specific for IgG, CD27, and CD38. Cells present in different divisions were characterized by “division slicing” (Hodgkin et al., 1996). The frequency of cells expressing IgG or the phenotype of ISCs (CD27hiCD38hi) was determined by analyzing the differently divided populations as defined by CFSE dilution (Tangye et al., 2002; Avery et al., 2005) using FlowJo software (Tree Star, Inc.). Ig secretion was determined by ELISA after culture of naive B cells (~10–20 × 103 cells/200 μl/well; BD) for 10–12 d (Ellyard et al., 2004; Bryant et al., 2007).
Sequence analysis of Ig VH genes.
The VH5 genes were amplified from cDNA isolated from sort-purified naive and memory B cells by nested PCR using Pfu DNA polymerase (Ellyard et al., 2004; Ma et al., 2006). Nucleotide sequences were analyzed using the Sequencher program (Gene Codes) and compared with germline Ig VH5 region genes (available from IMGT/GENE-DB under accession nos. M99686 and M18806). Ambiguous sequences were excluded from the analysis.
Quantitative PCR for expression of B cell transcription factors.
Naive B cells isolated from normal donors or STAT3MUT or STAT1MUT patients were cultured with CD40L alone or together with IL-10 or IL-21 for 5 d. Expression of BCL6 (forward, 5′-GAGCTCTGTTGATTCTTAGAACTGG-3′; reverse, 5′-GCCTTGCTTCACAGTCCAA-3′), AICDA (forward, 5′-GACTTTGGTTATCTTCGCAATAAGA-3′; reverse, 5′-AGGTCCCAGTCCGAGATGTA-3′), PAX5 (forward, 5′-ACGCTGACAGGGATGGTG-3′; reverse, 5′-CCTCCAGGAGTCGTTGTACG-3′), PRDM1/BLIMP-1 (forward, 5′-AACGTGTGGGTACGACCTTG-3′; reverse, 5′-ATTTTCATGGTCCCCTTGGT-3′), and XBP1 (forward, 5′-CCGCAGCACTCAGACTACG-3′; reverse, 5′-TGCCCAACAGGATATCAGACT-3′) was then determined by real-time PCR using the LightCycler 480 Probe Master Mix and System (Roche). All real-time PCR primers were from Integrated DNA Technologies. All reactions were standardized to the expression of GAPDH (forward, 5′-CTCTGCTCCTCCTGTTCGAC-3′; reverse, 5′-ACGACCAAATCCGTTGACTC-3′).
Online supplemental material.
Table S1 lists features of AD-HIES patients 6–10 (patients 1–5 have been described previously; Ma et al., 2008). Table S2 lists features of the patients with mutations in STAT1 used in this study. Table S3 lists the level of statistical significance for Ig secretion by STAT3MUT and STAT1MUT naive B cells relative to normal naive B cells. Fig. S1 compares the effect of IL-10 and IL-21 on induction of phospho-STATs in LCLs from normal donors and STAT3MUT and STAT1MUT patients. Fig. S2 shows the effect of IL-10 on induction of IgG isotype switching and expression of AICDA, PAX5, PRDM1, and BCL6 by CD40L-stimulated normal and STAT3MUT naive B cells. Fig. S3 shows Ig secretion by CD40L- and CD40L/IL-21–stimulated B cells from normal donors and CVID patients. Fig. S4 shows impaired responses of _Il21r_-deficient mouse B cells in vivo after challenge with specific Ag. Fig. S5 shows the presence of PCs and GC-like structures in lymphoid tissues of AD-HIES patients. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091706/DC1.
Acknowledgments
We thank the Garvan Flow Facility for cell sorting; A. Kan for performing the histology (Fig. S5); R. Daly, T. Phan, and T. Basten for critically reviewing this manuscript; and the patients and their families for their willingness to be involved in this study.
This work was funded by grants and fellowships awarded by the Australian National Health and Medical Research Council to S.G. Tangye, E.K. Deenick, C.S. Ma, S. Suranyi, R. Brink, D.A. Fulcher, and M.C. Cook. J.-L. Casanova is supported by Fondation BNP Paribas, Fondation Schlumberger, Institut Universitaire de France, the March of Dimes, the Dana Foundation, the Agence Nationale pour la Recherche, and the Howard Hughes Medical Institute.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
Ab
antibody
AD-HIES
autosomal dominant hyper-IgE syndrome
Ag
antigen
CVID
common variable immunodeficiency
DN
dominant negative
γc
common γ chain
GC
germinal center
ISC
Ig-secreting cell
LCL
lymphoblastoid cell line
PB
peripheral blood
PC
plasma cell
SAC
Staphylococcus aureus Cowan I
SHM
somatic hypermutation
TD
T cell dependent
TLR
Toll-like receptor
References
- Akira S. 1999. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 17:138–146 10.1002/stem.170138 [DOI] [PubMed] [Google Scholar]
- Asao H., Okuyama C., Kumaki S., Ishii N., Tsuchiya S., Foster D., Sugamura K. 2001. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167:1–5 [DOI] [PubMed] [Google Scholar]
- Avery D.T., Ellyard J.I., Mackay F., Corcoran L.M., Hodgkin P.D., Tangye S.G. 2005. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J. Immunol. 174:4034–4042 [DOI] [PubMed] [Google Scholar]
- Avery D.T., Bryant V.L., Ma C.S., de Waal Malefyt R., Tangye S.G. 2008a. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 181:1767–1779 [DOI] [PubMed] [Google Scholar]
- Avery D.T., Ma C.S., Bryant V.L., Santner-Nanan B., Nanan R., Wong M., Fulcher D.A., Cook M.C., Tangye S.G. 2008b. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood. 112:1784–1793 10.1182/blood-2008-02-142745 [DOI] [PubMed] [Google Scholar]
- Berger M., Kirkpatrick C.H., Goldsmith P.K., Gallin J.I. 1980. IgE antibodies to Staphylococcus aureus and Candida albicans in patients with the syndrome of hyperimmunoglobulin E and recurrent infections. J. Immunol. 125:2437–2443 [PubMed] [Google Scholar]
- Bernasconi A., Marino R., Ribas A., Rossi J., Ciaccio M., Oleastro M., Ornani A., Paz R., Rivarola M.A., Zelazko M., Belgorosky A. 2006. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 118:e1584–e1592 10.1542/peds.2005-2882 [DOI] [PubMed] [Google Scholar]
- Brière F., Servet-Delprat C., Bridon J.M., Saint-Remy J.M., Banchereau J. 1994. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J. Exp. Med. 179:757–762 10.1084/jem.179.2.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink R., Phan T.G., Paus D., Chan T.D. 2008. Visualizing the effects of antigen affinity on T-dependent B-cell differentiation. Immunol. Cell Biol. 86:31–39 10.1038/sj.icb.7100143 [DOI] [PubMed] [Google Scholar]
- Bryant V.L., Ma C.S., Avery D.T., Li Y., Good K.L., Corcoran L.M., de Waal Malefyt R., Tangye S.G. 2007. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179:8180–8190 [DOI] [PubMed] [Google Scholar]
- Chapgier A., Boisson-Dupuis S., Jouanguy E., Vogt G., Feinberg J., Prochnicka-Chalufour A., Casrouge A., Yang K., Soudais C., Fieschi C., et al. 2006a. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genet. 2:e131 10.1371/journal.pgen.0020131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapgier A., Wynn R.F., Jouanguy E., Filipe-Santos O., Zhang S., Feinberg J., Hawkins K., Casanova J.L., Arkwright P.D. 2006b. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J. Immunol. 176:5078–5083 [DOI] [PubMed] [Google Scholar]
- Chapgier A., Kong X.F., Boisson-Dupuis S., Jouanguy E., Averbuch D., Feinberg J., Zhang S.Y., Bustamante J., Vogt G., Lejeune J., et al. 2009. A partial form of recessive STAT1 deficiency in humans. J. Clin. Invest. 119:1502–1514 10.1172/JCI37083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M.E., Lavoie A., Briggs C., Brown P., Guerra C., Puck J.M. 1988. Nonrandom X chromosome inactivation in B cells from carriers of X chromosome-linked severe combined immunodeficiency. Proc. Natl. Acad. Sci. USA. 85:3090–3094 10.1073/pnas.85.9.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M.E., Rohrer J., Rapalus L., Boylin E.C., Minegishi Y. 2000. Defects in early B-cell development: comparing the consequences of abnormalities in pre-BCR signaling in the human and the mouse. Immunol. Rev. 178:75–90 10.1034/j.1600-065X.2000.17809.x [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Ponda P.P. 2005. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat. Rev. Immunol. 5:880–892 10.1038/nri1713 [DOI] [PubMed] [Google Scholar]
- Dahlke I., Nott D.J., Ruhno J., Sewell W.A., Collins A.M. 2006. Antigen selection in the IgE response of allergic and nonallergic individuals. J. Allergy Clin. Immunol. 117:1477–1483 10.1016/j.jaci.2005.12.1359 [DOI] [PubMed] [Google Scholar]
- Davis S.D., Schaller J., Wedgwood R.J. 1966. Job's Syndrome: Recurrent, "cold", staphylococcal abscesses. Lancet. 287:1013–1015 10.1016/S0140-6736(66)90119-X [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Jannière L., et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17–producing T cells. J. Exp. Med. 205:1543–1550 10.1084/jem.20080321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick E.K., Hasbold J., Hodgkin P.D. 1999. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J. Immunol. 163:4707–4714 [PubMed] [Google Scholar]
- Diehl S.A., Schmidlin H., Nagasawa M., van Haren S.D., Kwakkenbos M.J., Yasuda E., Beaumont T., Scheeren F.A., Spits H. 2008. STAT3-mediated up-regulation of BLIMP1 is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J. Immunol. 180:4805–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreskin S.C., Goldsmith P.K., Gallin J.I. 1985. Immunoglobulins in the hyperimmunoglobulin E and recurrent infection (Job's) syndrome. Deficiency of anti-Staphylococcus aureus immunoglobulin A. J. Clin. Invest. 75:26–34 10.1172/JCI111683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis S., Dargemont C., Fieschi C., Thomassin N., Rosenzweig S., Harris J., Holland S.M., Schreiber R.D., Casanova J.L. 2001. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 293:300–303 10.1126/science.1061154 [DOI] [PubMed] [Google Scholar]
- Dupuis S., Jouanguy E., Al-Hajjar S., Fieschi C., Al-Mohsen I.Z., Al-Jumaah S., Yang K., Chapgier A., Eidenschenk C., Eid P., et al. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388–391 10.1038/ng1097 [DOI] [PubMed] [Google Scholar]
- Ellyard J.I., Avery D.T., Phan T.G., Hare N.J., Hodgkin P.D., Tangye S.G. 2004. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood. 103:3805–3812 10.1182/blood-2003-09-3109 [DOI] [PubMed] [Google Scholar]
- Erazo A., Kutchukhidze N., Leung M., Christ A.P., Urban J.F., Jr., Curotto de Lafaille M.A., Lafaille J.J. 2007. Unique maturation program of the IgE response in vivo. Immunity. 26:191–203 10.1016/j.immuni.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R., Kuchen S., Lipsky P.E. 2008. The role of IL-21 in regulating B-cell function in health and disease. Immunol. Rev. 223:60–86 10.1111/j.1600-065X.2008.00631.x [DOI] [PubMed] [Google Scholar]
- Fischer A. 2007. Human primary immunodeficiency diseases. Immunity. 27:835–845 10.1016/j.immuni.2007.11.012 [DOI] [PubMed] [Google Scholar]
- Fornek J.L., Tygrett L.T., Waldschmidt T.J., Poli V., Rickert R.C., Kansas G.S. 2006. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 107:1085–1091 10.1182/blood-2005-07-2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K.L., Bryant V.L., Tangye S.G. 2006. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J. Immunol. 177:5236–5247 [DOI] [PubMed] [Google Scholar]
- Grimbacher B., Holland S.M., Puck J.M. 2005. Hyper-IgE syndromes. Immunol. Rev. 203:244–250 10.1111/j.0105-2896.2005.00228.x [DOI] [PubMed] [Google Scholar]
- Habib T., Senadheera S., Weinberg K., Kaushansky K. 2002. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 41:8725–8731 10.1021/bi0202023 [DOI] [PubMed] [Google Scholar]
- Harris M.B., Chang C.C., Berton M.T., Danial N.N., Zhang J., Kuehner D., Ye B.H., Kvatyuk M., Pandolfi P.P., Cattoretti G., et al. 1999. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: specific regulation of iepsilon transcription and immunoglobulin E switching. Mol. Cell. Biol. 19:7264–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin P.D., Lee J.H., Lyons A.B. 1996. B cell differentiation and isotype switching is related to division cycle number. J. Exp. Med. 184:277–281 10.1084/jem.184.1.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.M., DeLeo F.R., Elloumi H.Z., Hsu A.P., Uzel G., Brodsky N., Freeman A.F., Demidowich A., Davis J., Turner M.L., et al. 2007. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 357:1608–1619 10.1056/NEJMoa073687 [DOI] [PubMed] [Google Scholar]
- Kallies A., Hasbold J., Fairfax K., Pridans C., Emslie D., McKenzie B.S., Lew A.M., Corcoran L.M., Hodgkin P.D., Tarlinton D.M., Nutt S.L. 2007. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 26:555–566 10.1016/j.immuni.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Klein U., Rajewsky K., Küppers R. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689 10.1084/jem.188.9.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W.J. 2001. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 1:200–208 10.1038/35105066 [DOI] [PubMed] [Google Scholar]
- Leung D.Y., Ambrosino D.M., Arbeit R.D., Newton J.L., Geha R.S. 1988. Impaired antibody responses in the hyperimmunoglobulin E syndrome. J. Allergy Clin. Immunol. 81:1082–1087 10.1016/0091-6749(88)90873-1 [DOI] [PubMed] [Google Scholar]
- Levy Y., Brouet J.C. 1994. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J. Clin. Invest. 93:424–428 10.1172/JCI116977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinskiy M.B., Nardelli B., Hilbert D.M., He B., Schaffer A., Casali P., Cerutti A. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829 10.1038/ni829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Hare N.J., Nichols K.E., Dupré L., Andolfi G., Roncarolo M.G., Adelstein S., Hodgkin P.D., Tangye S.G. 2005. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J. Clin. Invest. 115:1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Pittaluga S., Avery D.T., Hare N.J., Maric I., Klion A.D., Nichols K.E., Tangye S.G. 2006. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J. Clin. Invest. 116:322–333 10.1172/JCI25720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551–1557 10.1084/jem.20080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi P., Villa A., Giliani S., Sacco M.G., Frattini A., Porta F., Ugazio A.G., Johnston J.A., Candotti F., O'Shea J.J., et al. 1995. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 377:65–68 10.1038/377065a0 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 452:773–776 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Morio T., Watanabe K., Agematsu K., Tsuchiya S., Takada H., Hara T., Kawamura N., Ariga T., et al. 2006. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 25:745–755 10.1016/j.immuni.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., et al. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 448:1058–1062 10.1038/nature06096 [DOI] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Nagasawa M., Takada H., Hara T., Tsuchiya S., Agematsu K., Yamada M., Kawamura N., Ariga T., et al. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 206:1291–1301 10.1084/jem.20082767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563 10.1016/S0092-8674(00)00078-7 [DOI] [PubMed] [Google Scholar]
- Murray P.J. 2007. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178:2623–2629 [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29:138–149 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J.J., Murray P.J. 2008. Cytokine signaling modules in inflammatory responses. Immunity. 28:477–487 10.1016/j.immuni.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero D.C., Poli V., David M., Rickert R.C. 2006. Cutting edge: inherent and acquired resistance to radiation-induced apoptosis in B cells: a pivotal role for STAT3. J. Immunol. 177:6593–6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Feng C.G., Qi C.F., Cheng J., Sher A., Morse H.C., III, Liu C., Schwartzberg P.L., Leonard W.J. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science. 298:1630–1634 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Ettinger R., Kim H.P., Wang G., Qi C.F., Hwu P., Shaffer D.J., Akilesh S., Roopenian D.C., et al. 2004. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173:5361–5371 [DOI] [PubMed] [Google Scholar]
- Pène J., Gauchat J.F., Lécart S., Drouet E., Guglielmi P., Boulay V., Delwail A., Foster D., Lecron J.C., Yssel H. 2004. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172:5154–5157 [DOI] [PubMed] [Google Scholar]
- Renner E.D., Rylaarsdam S., Anover-Sombke S., Rack A.L., Reichenbach J., Carey J.C., Zhu Q., Jansson A.F., Barboza J., Schimke L.F., et al. 2008. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J. Allergy Clin. Immunol. 122:181–187 10.1016/j.jaci.2008.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D.H., Kastelein R., Moore K.W., Banchereau J. 1992. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA. 89:1890–1893 10.1073/pnas.89.5.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer K., Baerlocher K., Price P., Krech U., Quie P.G., Douglas S.D. 1979. Staphylococcal IgE antibodies, hyperimmunoglobulinemia E and Staphylococcus aureus infections. N. Engl. J. Med. 300:835–838 [DOI] [PubMed] [Google Scholar]
- Sheerin K.A., Buckley R.H. 1991. Antibody responses to protein, polysaccharide, and phi X174 antigens in the hyperimmunoglobulinemia E (hyper-IgE) syndrome. J. Allergy Clin. Immunol. 87:803–811 10.1016/0091-6749(91)90126-9 [DOI] [PubMed] [Google Scholar]
- Shuai K., Liu B. 2003. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3:900–911 10.1038/nri1226 [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Tarlinton D.M. 2009. Memory B cells: effectors of long-lived immune responses. Eur. J. Immunol. 39:2065–2075 10.1002/eji.200939531 [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Liu Y.J., Aversa G., Phillips J.H., de Vries J.E. 1998. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J. Exp. Med. 188:1691–1703 10.1084/jem.188.9.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye S.G., Ferguson A., Avery D.T., Ma C.S., Hodgkin P.D. 2002. Isotype switching by human B cells is division-associated and regulated by cytokines. J. Immunol. 169:4298–4306 [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Avery D.T., Hodgkin P.D. 2003. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J. Immunol. 170:261–269 [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Cook M.C., Fulcher D.A. 2009. Insights into the role of STAT3 in human lymphocyte differentiation as revealed by the hyper-IgE syndrome. J. Immunol. 182:21–28 [DOI] [PubMed] [Google Scholar]
- Umeshita-Suyama R., Sugimoto R., Akaiwa M., Arima K., Yu B., Wada M., Kuwano M., Nakajima K., Hamasaki N., Izuhara K. 2000. Characterization of IL-4 and IL-13 signals dependent on the human IL-13 receptor alpha chain 1: redundancy of requirement of tyrosine residue for STAT3 activation. Int. Immunol. 12:1499–1509 10.1093/intimm/12.11.1499 [DOI] [PubMed] [Google Scholar]
- Vogelzang A., McGuire H.M., Yu D., Sprent J., Mackay C.R., King C. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 29:127–137 10.1016/j.immuni.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377 10.1126/science.1086907 [DOI] [PubMed] [Google Scholar]
- Xu W., Santini P.A., Matthews A.J., Chiu A., Plebani A., He B., Chen K., Cerutti A. 2008. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J. Immunol. 181:276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R., Spolski R., Casas E., Zhu W., Levy D.E., Leonard W.J. 2007. The molecular basis of IL-21-mediated proliferation. Blood. 109:4135–4142 10.1182/blood-2006-10-054973 [DOI] [PMC free article] [PubMed] [Google Scholar]