Velopharyngeal Anatomy in 22q11.2 Deletion Syndrome: A Three-Dimensional Cephalometric Analysis (original) (raw)
. Author manuscript; available in PMC: 2010 Jan 28.
Published in final edited form as: Cleft Palate Craniofac J. 2006 Jul;43(4):446. doi: 10.1597/04-193R.1
Abstract
Objective
22q11.2 deletion syndrome is the most common genetic cause of velopharyngeal dysfunction (VPD). Magnetic resonance imaging (MRI) is a promising method for noninvasive, three-dimensional (3D) assessment of velopharyngeal (VP) anatomy. The purpose of this study was to assess VP structure in patients with 22q11.2 deletion syndrome by using 3D MRI analysis.
Design
This was a retrospective analysis of magnetic resonance images obtained in patients with VPD associated with a 22q11.2 deletion compared with a normal control group.
Setting
This study was conducted at The Children’s Hospital of Philadelphia, a pediatric tertiary care center.
Patients, Participants
The study group consisted of 5 children between the ages of 2.9 and 7.9 years, with 22q11.2 deletion syndrome confirmed by fluorescence in situ hybridization analysis. All had VPD confirmed by nasendoscopy or videofluoroscopy. The control population consisted of 123 unaffected patients who underwent MRI for reasons other than VP assessment.
Interventions
Axial and sagittal T1- and T2-weighted magnetic resonance images with 3-mm slice thickness were obtained from the orbit to the larynx in all patients by using a 1.5T Siemens Visions system.
Outcome Measures
Linear, angular, and volumetric measurements of VP structures were obtained from the magnetic resonance images with VIDA image- processing software.
Results
The study group demonstrated greater anterior and posterior cranial base and atlanto-dental angles. They also demonstrated greater pharyngeal cavity volume and width and lesser tonsillar and adenoid volumes.
Conclusion
Patients with a 22q11.2 deletion demonstrate significant alterations in VP anatomy that may contribute to VPD.
Keywords: 22q11.2 deletion syndrome, magnetic resonance imaging, velopharyngeal dysfunction
Normal velopharyngeal (VP) function is dependent upon multiple factors. Neuromuscular function, velar anatomy, and overall morphology of the VP port all contribute to the process of VP valving. The structural and functional integrity of each of these factors ultimately determines the adequacy of the VP mechanism as a whole. The VP port is composed of soft tissues (e.g., adenoids, lateral and posterior pharyngeal walls, velum) and their underlying bony structures (e.g., maxilla, basicranium, upper cervical spine). Alterations in one or more of these structures may be a feature of some craniofacial syndromes and may play a critical role in the pathogenesis of associated VP dysfunction (VPD).
With an estimated incidence of 1 in 4000 births, the 22q11.2 deletion syndrome, also known as velocardiofacial syndrome (VCFS), is the most common genetic cause of VPD (McDonald-McGinn et al., 1999). Affected patients present with a spectrum of functional and anatomic abnormalities that may contribute to alterations in VP valving, including cleft palate or submucosal cleft palate, palatopharyngeal hypotonia, platybasia, adenoid hypoplasia, and cervical spine abnormalities (Havkin et al., 2000). To date, however, the relative contribution of each of these abnormalities to the pathogenesis of VPD in 22q11.2 deletion syndrome remains uncertain.
Traditional methods used to study VP structure and function, including cephalometry, videofluoroscopy, and nasendoscopy, provide only a limited view of the structures that determine VP port anatomy. Magnetic resonance imaging (MRI) has recently emerged as a promising modality of VP assessment, one that avoids exposing young patients to ionizing radiation and that provides an accurate, reproducible, and quantifiable assessment of VP structures in three dimensions. The aim of this study was to use this three-dimensional (3D) MRI to compare VP anatomy in patients diagnosed with a 22q11.2 deletion and VPD with that in normal controls. We hypothesized that MRI analysis would demonstrate anatomic differences between the two groups that may contribute to VPD by increasing the dimensions of the VP port in patients with a 22q11.2 deletion.
METHODS
Subjects
Five children (2 boys, 3 girls) between the ages of 2.9 and 7.9 years (mean age 5.25 years) were included in the study group. Each child was diagnosed with a 22q11.2 deletion as confirmed by fluorescence in situ hybridization analysis and had VPD confirmed by either videofluoroscopy or nasendoscopy after a comprehensive speech evaluation. All patients underwent MRI before surgical management of the velopharynx. None had undergone tonsillectomy or adenoidectomy.
The control group was composed of 123 children (74 boys, 49 girls) between the ages of 4 and 7 years (mean age 5.94 years) without a history of VPD. These children underwent MRI of the head and neck at The Children’s Hospital of Philadelphia for reasons other than VP assessment (i.e., trauma, headaches, or seizures). All control-group children exhibited normal growth and development and were not excluded based on the following exclusion criteria: (1) presence of obstructive sleep apnea (OSA) symptoms; (2) history of tonsillectomy or adenoidectomy; (3) evidence of brain tumor, brain anomaly, or seizure disorder; (4) genetic disorders associated with any craniofacial anomaly; and (5) chronic respiratory disease (Arens et al., 2001).
MRI
All MRI procedures were conducted at The Children’s Hospital of Philadelphia in the Department of Radiology. Sedation was administered in all cases. Pentobarbital was infused intravenously at increments of 2 mg/kg until the endpoint of sleep was achieved. Up to three doses to a maximum of 200 mg was administered. Each patient was continuously monitored via pulse oximetry and observed for approximately 1 hour after completion of the study until fully recovered.
A 1.5T Siemens Vision system (Iselin, NJ) was used to perform the MRI in each group. A commercially available anterior-posterior volume head coil was used to acquire images. Each patient was placed in the standard supine position with his or her head perpendicular to the table. Images initially included a rapid spin echo sagittal localizing scan to confirm that the field of view and centering were accurate for the analysis described below. Sequential T1-weighted spin echo sagittal sections were obtained bilaterally from the determined midsagittal section, maintaining a 3-mm slice thickness throughout. Axial sections were collected from the orbital cavity to the larynx, again maintaining a 3-mm slice thickness.
Image Analysis
T1-weighted axial images were used to calculate the pharyngeal airway volume (AirV) measurements, whereas T2-weighted images were used to analyze tonsillar volumes (TVs) and airway volumes and the linear measurements taken in the axial plane.
The linear, angular, and volumetric measurements were obtained by VIDA (Volumetric Image Display and Analysis, Department of Radiology, University of Iowa, Iowa City, IA) imaging software. Three-dimensional soft tissue and airway reconstruction was performed by 3DVIEWNIX software (Udupa et al., 1994; Arens et al., 2003).
Linear measurements (mm), volumetric analyses (mm3), and angular measurements (°) were defined. Linear and angular measurements were all made manually. Volumes were determined from adjacent axial slices after the specified anatomic structure was manually traced. Tonsillar volumes included the sum of both tonsils as measured on each slice. Final volumes were calculated by the VIDA image software (Uong et al., 2001).
Accuracy of measurements was previously determined by using a set of commercial phantoms for lengths, areas, and volumes spanning the measurements used in the study. Intra-class correlation coefficient was computed to assess the reliability of MRI measurements (Landis and Koch, 1977; Fleiss, 1986; Arens et al., 2001).
All sagittal measurements were obtained from the midsagittal section of each patient (Fig. 1). These linear measurements included (1) hard palate length (HPL: anterior nasal spine to posterior nasal spine), (2) velar length (VL: posterior nasal spine to the tip of the velum), (3) velar thickness (VT: the thickness of the velum taken from the centroid of the velum as determined by VIDA imaging software), (4) osseous pharyngeal depth (OPD: posterior nasal spine to the anterior body of C1), and (5) depth:length ratio (OPD:VL).
FIGURE 1.
Measurements obtained from midsagittal magnetic resonance images: HPL (ANS-PNS), VL (PNS-V), OPD (PNS-AA), ACBA (Na-S-Ba), PCBA (S-Ba-M), and ADA (AA-PA/SO-IO).
The angular measurements were also obtained on midsagittal section and included (1) anterior cranial base angle (ACBA: nasion to sella to basion), (2) posterior cranial base angle (PCBA: sella to basion to foramen magnum), and (3) atlanto-dental angle (ADA: the angle of the superior-anterior quadrant subtended by the intersection of the horizontal plane of the atlas and the vertical plane of the odontoid process of the axis).
Linear axial measurements taken from T2-weighted images included (1) VP width (VPW: distance between the innermost aspects of the lateral pharyngeal walls at the level of the anterior prominence of C1) and (2) medial pterygoid width (MPW: distance between the innermost aspects of the medial pterygoid plates). Tonsillar volume and adenoid volume (AdV) were measured on axial T2-weighted images because lymphoid tissue is more readily identified in this view. The AirVs were measured based on axial T1-weighted images. The airway volume was determined from the base of the orbital cavities to the epiglottis.
Data Analysis
The various dimensions measured on the MRI were compared by using two sample two-tailed t tests. All measurements had an approximately normal distribution in the control group. Therefore, we did not use transformations to the data. A two-sample Wilcoxon rank sum test was also performed to confirm the results of the t tests. These results are not shown. There were 14 outcome measurements in this study, which were most likely correlated. Therefore, significance was defined based on the Tukey, Ciminera, and Heyse adjustment to the critical value (Zhang et al., 1997). The adjustment required rejecting the null hypothesis of equality at the 1−(1−0.05)**1/14=0.014 level if the basic type I error is set to be acceptable at the 0.05 level. The sample size of the study was large enough to detect a difference of 1.5 standard deviation score between the two groups with 80% power while controlling for α = 0.014. Therefore, only large differences between controls and patients with 22q11.2 deletion syndrome were detected by this study.
RESULTS
Sagittal Measurements
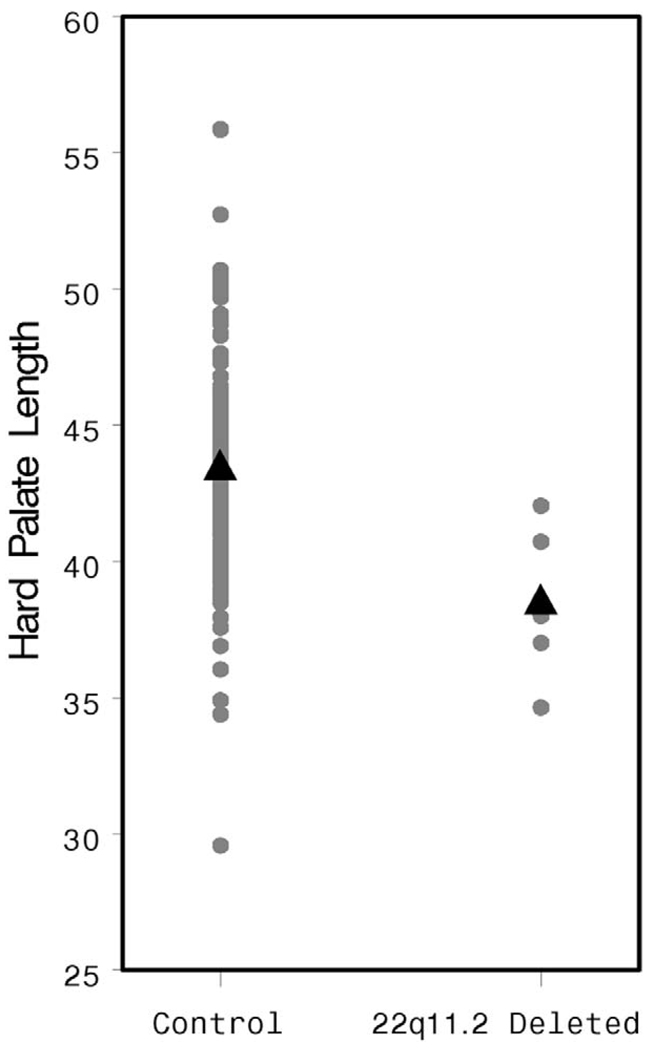
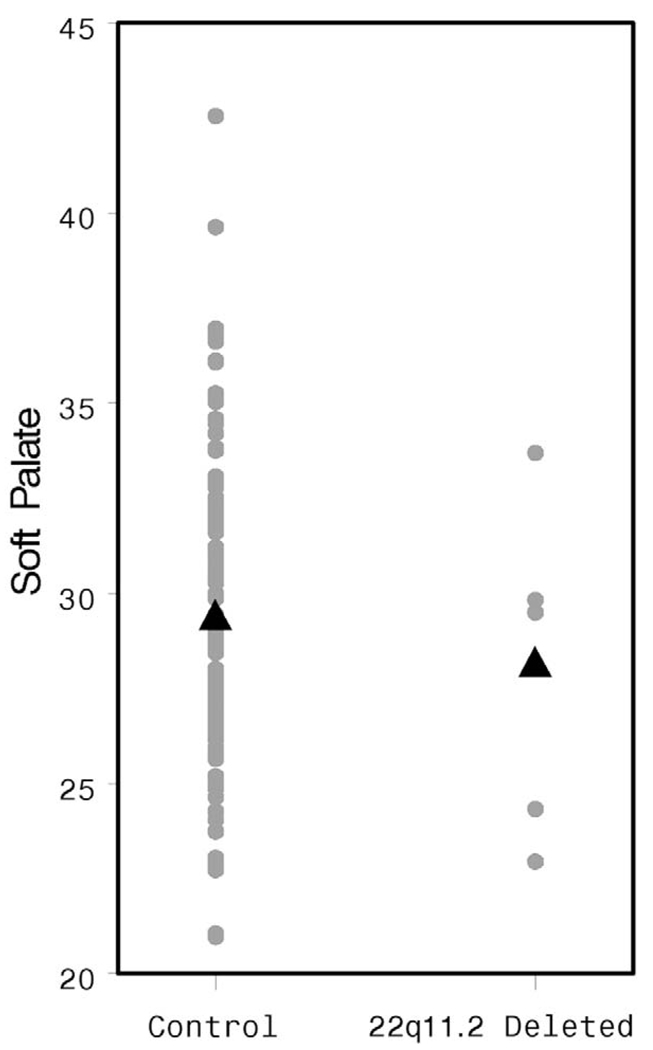
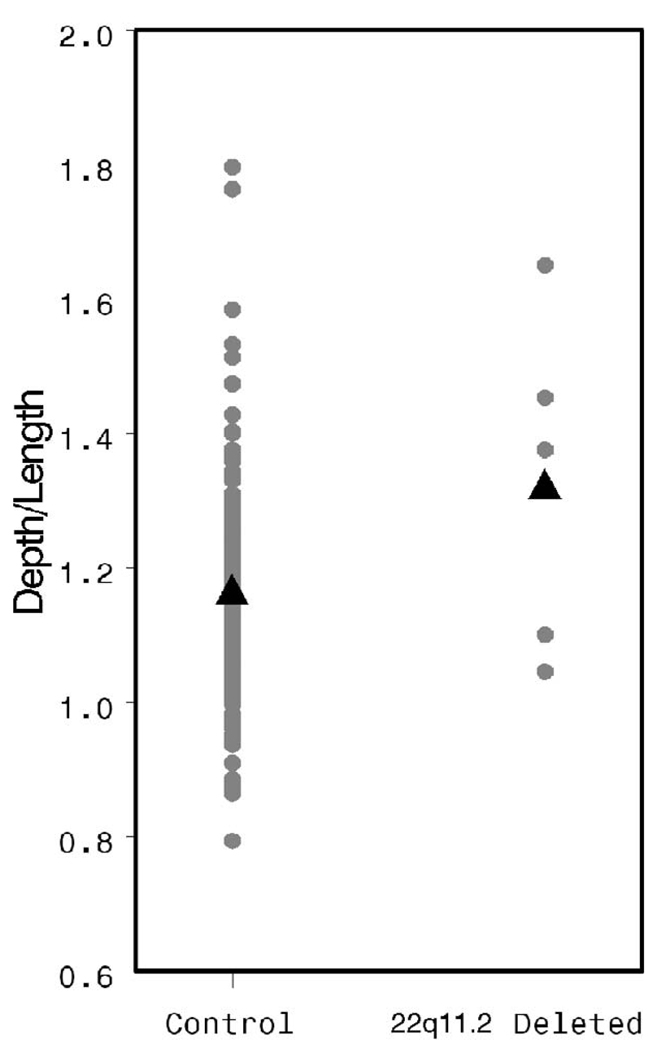
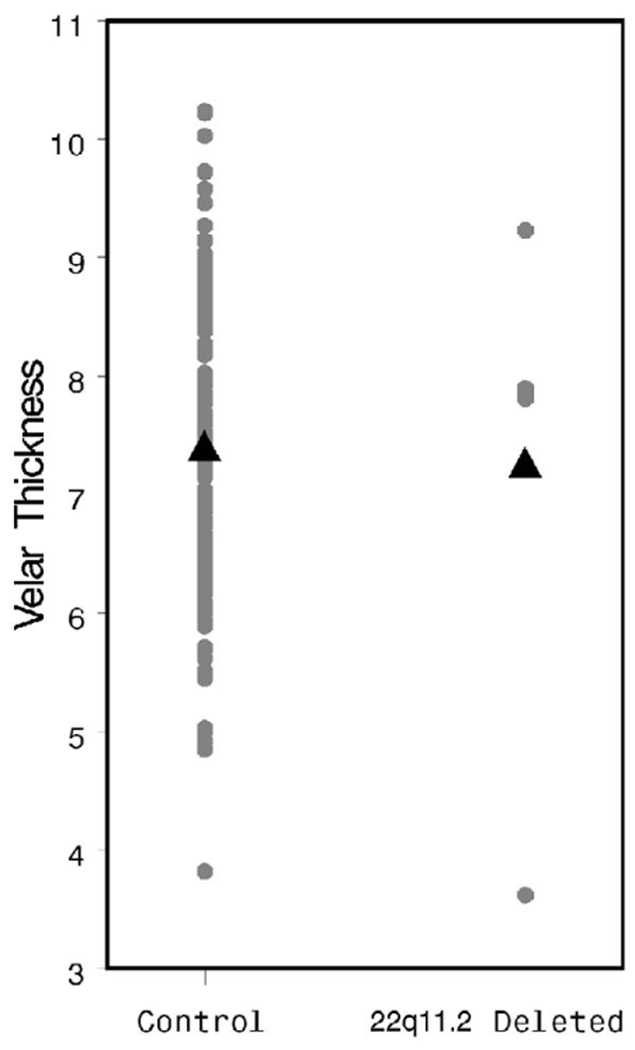
The HPL was significantly shorter in the study population than in controls (38.49 versus 43.59 mm, p = .007, Fig. 2), whereas the soft palate was of similar length in both groups (28.06 versus 29.34 mm, p = .47, Fig. 3). Osseous pharyngeal depth was comparable in both groups (36.76 versus 33.76 mm, p = .10, Fig. 4). The OPD:VL ratio was greater in the patients with 22q11.2 deletion syndrome, although this difference did not reach statistical significance (1.33 versus 1.16 mm, p < .04, Fig. 5). Velar thickness was similar in the two groups (p = .81, Fig. 6).
FIGURE 2.
Individual and mean HPL values by patient group.
FIGURE 3.
Individual and mean VL values by patient group.
FIGURE 4.
Individual and mean OPD values by patient group.
FIGURE 5.
Individual and mean OPD:VL values by patient group.
FIGURE 6.
Individual and mean VT values by patient group.
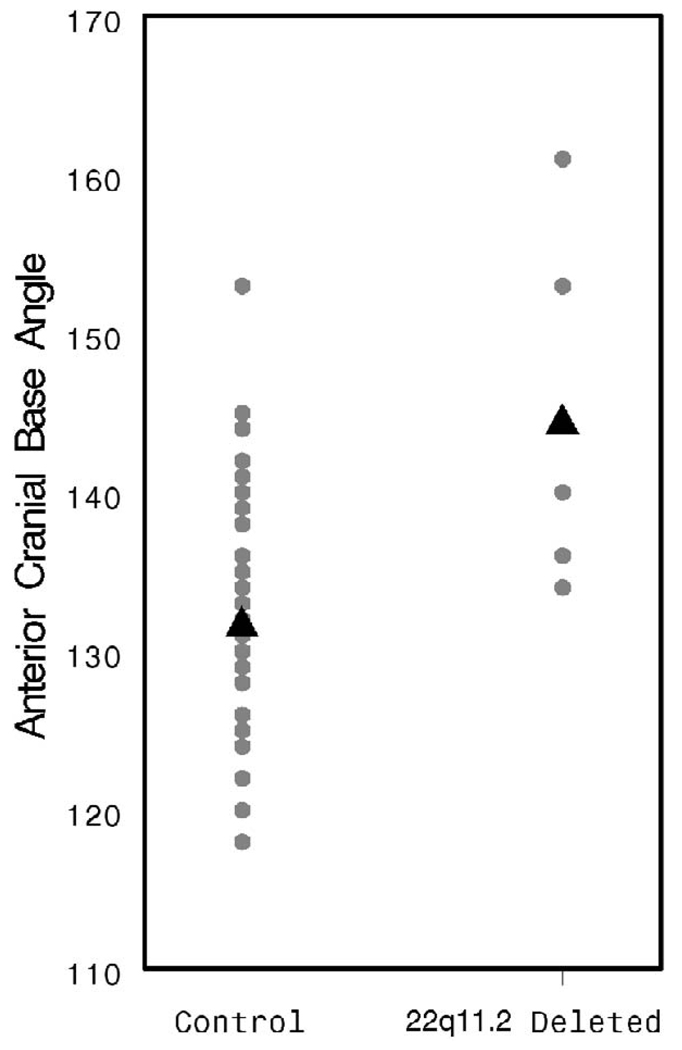
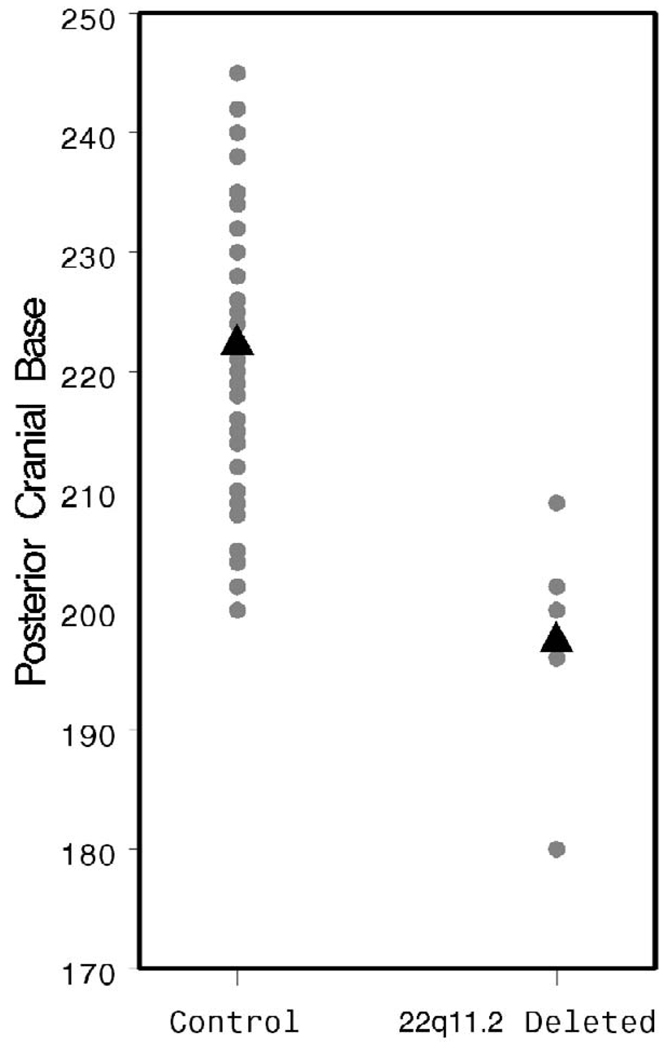
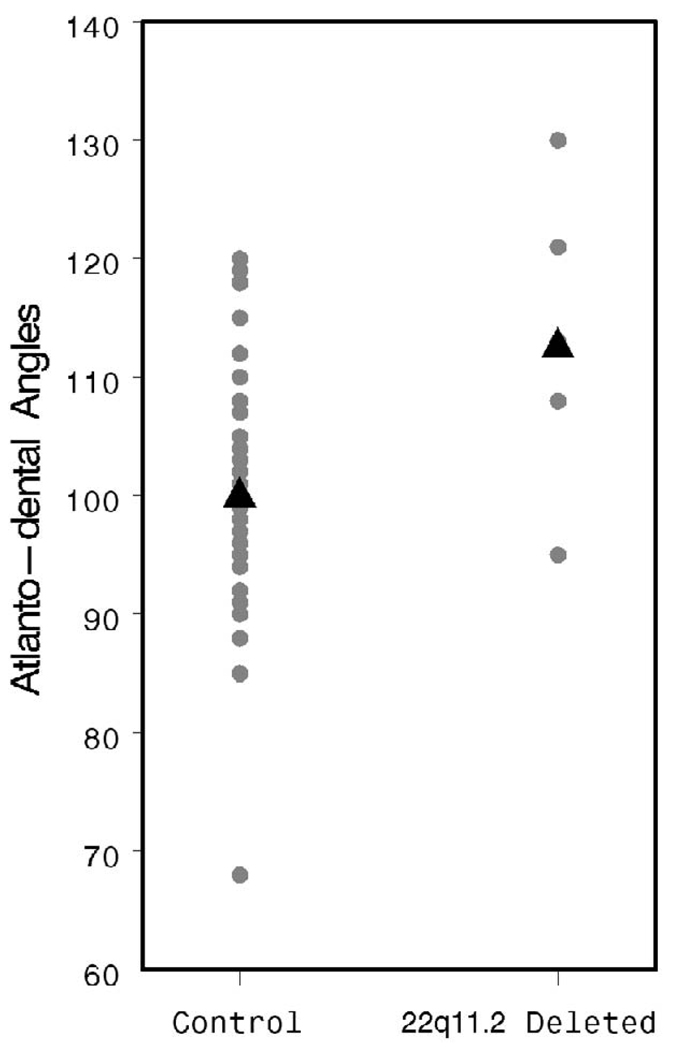
The ACBA was found to be significantly more obtuse in patients with a 22q11.2 deletion (144.8 versus 131.6°, p < .001; Fig. 7), supporting the observation of others that this group of patients tends toward platybasia. The PCBA and ADA were also more obtuse in the patients with 22q11.2 deletion syndrome (PCBA: 162.6 versus 136.9°, p < .001, Fig. 8; ADA: 113.4 versus 100.3°, p = .002, Fig. 9). As discussed above, patients with 22q11.2 deletion syndrome often manifest a variety of upper cervical spine abnormalities. These data indicate that such cervical abnormalities may be associated with significant flattening and lengthening of the cranial base, perhaps contributing to VPD by increasing the OPD:VL ratio.
FIGURE 7.
Individual and mean ACBA values by patient group.
FIGURE 8.
Individual and mean PCBA values by patient group.
FIGURE 9.
Individual and mean ADA values by patient group.
Axial Measurements
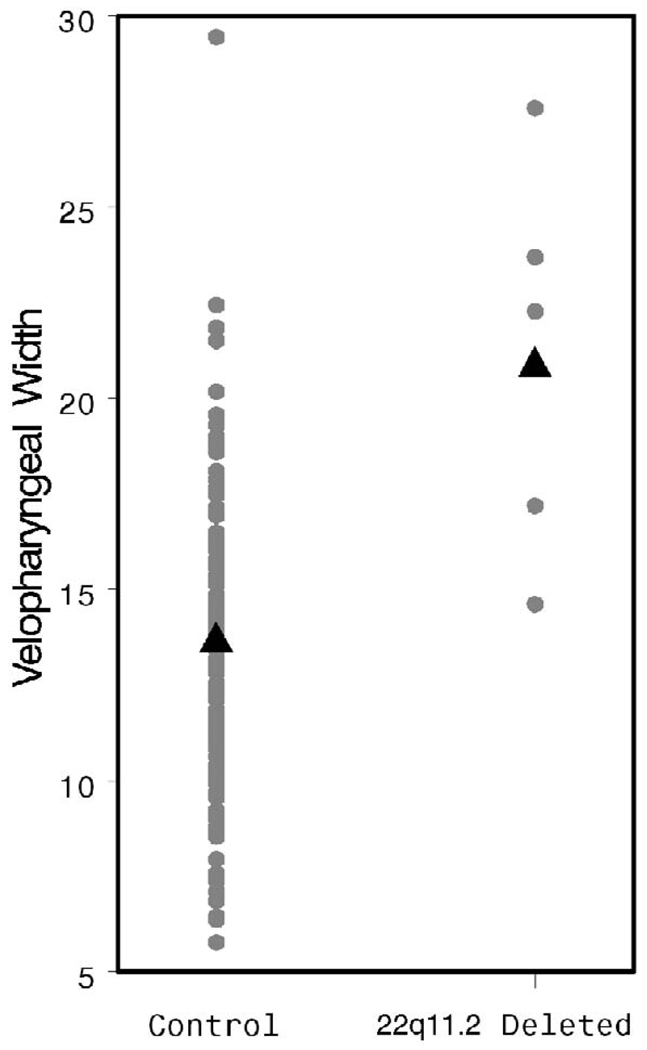
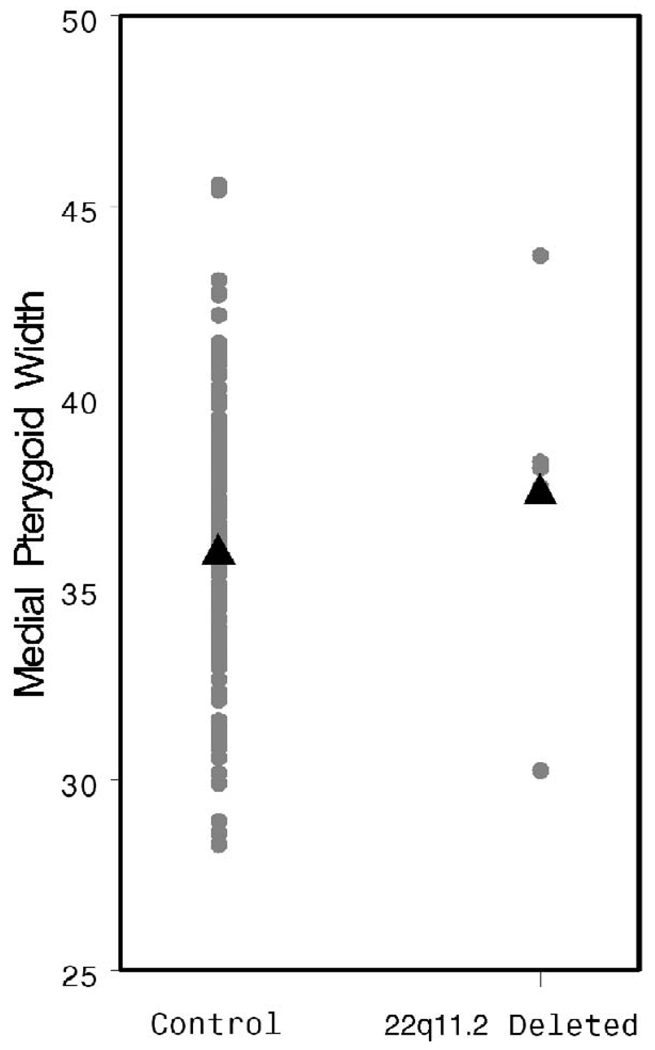
Velopharyngeal width was measured at the level of C1. A significant increase was observed in the study population when compared with the controls (21.07 versus 13.64 mm, p < .001, Fig. 10). There was no difference in MPW between the patients and the control group (p = .31, Fig. 11).
FIGURE 10.
Individual and mean VPW values by patient group.
FIGURE 11.
Individual and mean MPW values by patient group.
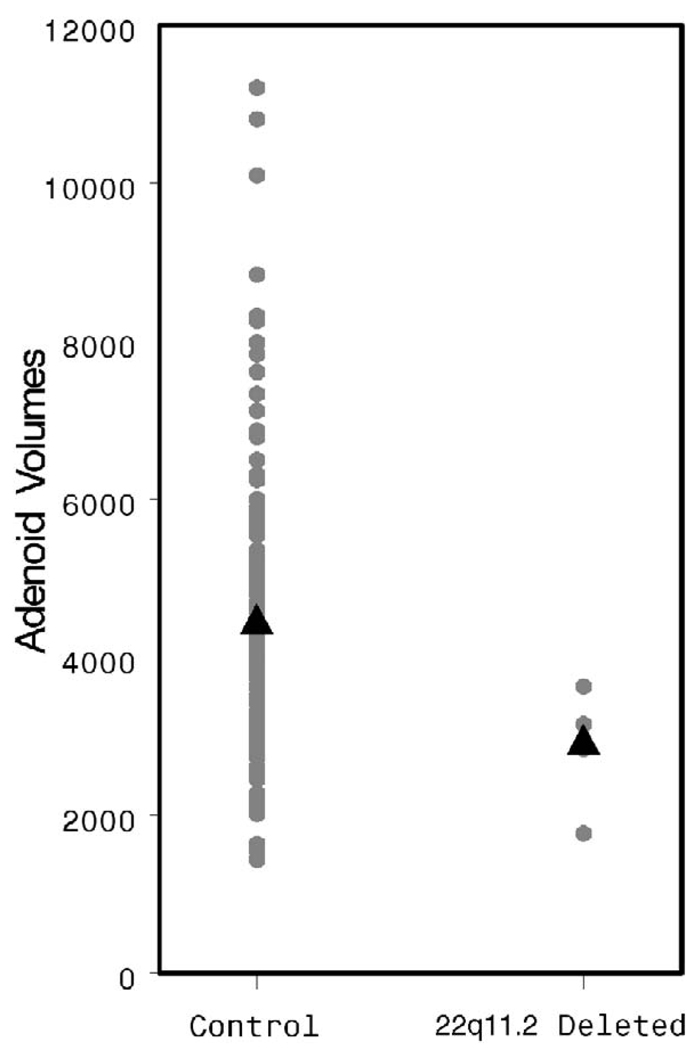
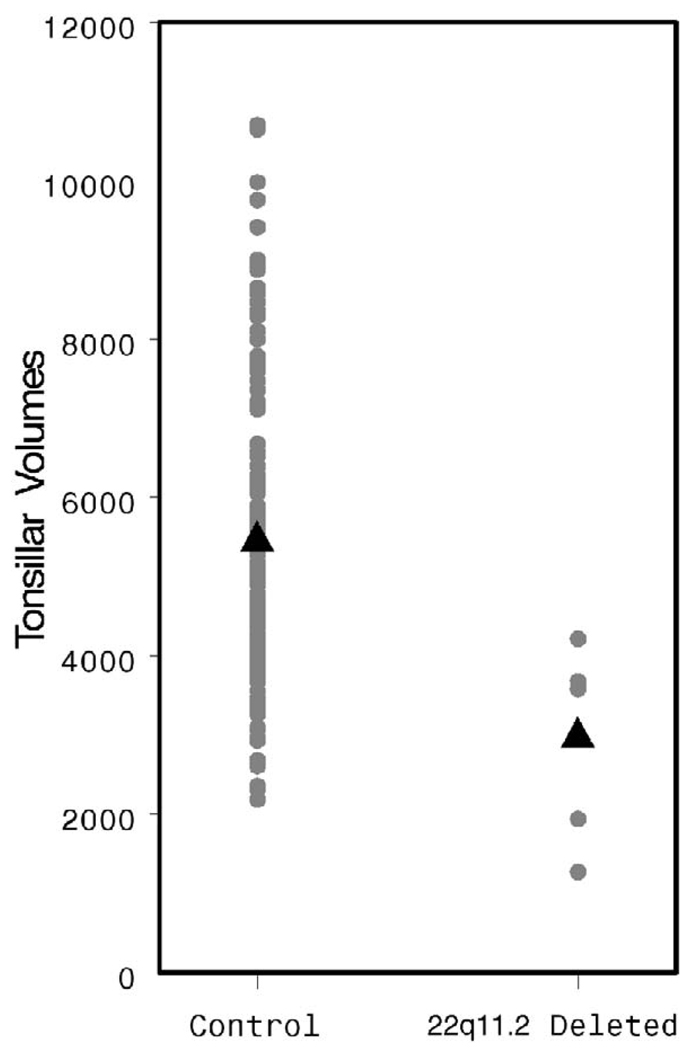
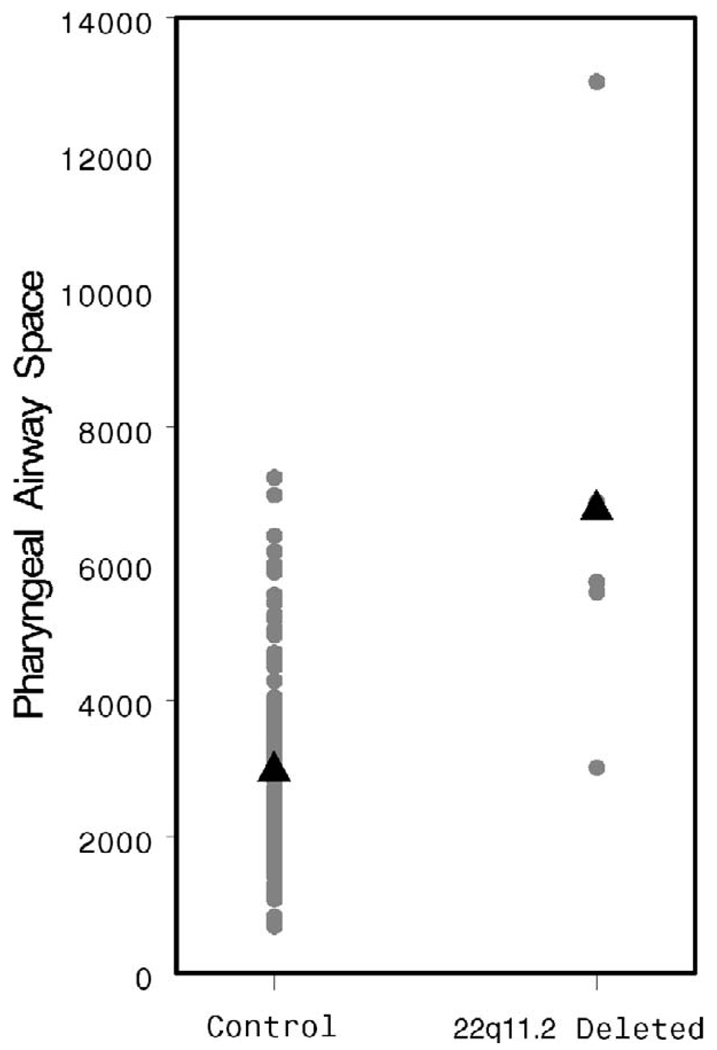
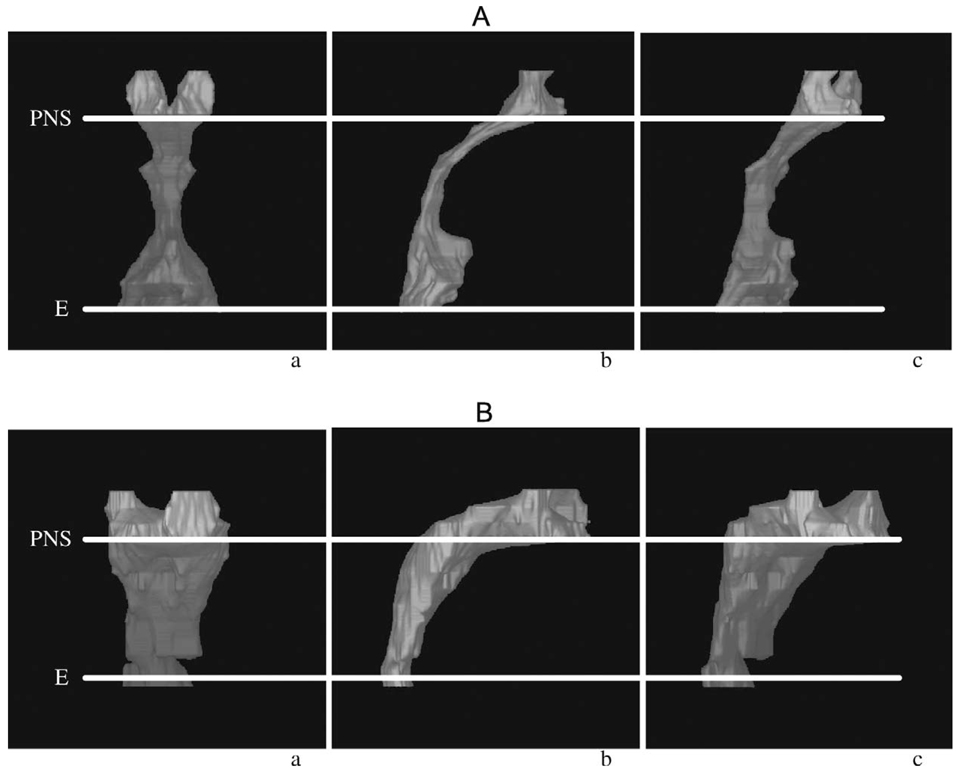
The AdVs appear smaller in the study population, although this difference does not achieve statistical significance (2862 versus 4434 mm3, p = .06, Fig. 12). The TVs are quite clearly smaller (2938 versus 5492 mm3, p = .003, Fig. 13). The patients with 22q11.2 deletion syndrome demonstrated a dramatically larger pharyngeal airway space when compared with controls (6855 versus 2901 mm3, p < .001, Fig. 14). A representative 3D reconstruction of the pharyngeal airway of patients in the control and study groups is shown in Figure 15A and 15B.
FIGURE 12.
Individual and mean AdV values by patient group.
FIGURE 13.
Individual and mean TV values by patient group.
FIGURE 14.
Individual and mean AirV values by patient group.
FIGURE 15.
AP (anterior-posterior) (a), lateral (b), and oblique (c) 3D MRI reconstruction of the pharyngeal airway. PNS = posterior nasal spine; E = epiglottis. A: Control subject. B: Patient with a 22q11.2 deletion.
Summary of Results
Patients with a 22q11.2 deletion and VPD demonstrated multiple anatomic differences when compared with controls. These patients had a significantly wider velopharynx, a shorter hard palate, and a larger OPD:VL ratio. Their entire cranial base was flattened, making it longer and the pharynx deeper. Finally, the 3DVIEWNIX 3D images of the VP airway revealed that the patients studied had a significantly more obtuse and voluminous airway (Fig. 15A and 15B).
DISCUSSION
Using 3D MRI, we have shown that children with 22q11.2 deletion syndrome demonstrate multiple anatomic differences that may contribute to VPD by significantly enlarging the VP port.
Microdeletions of chromosome 22q11.2 are currently the most common genetic cause of VPD (Goldberg et al., 1993; Kirschner, 2005). Moreover, up to 37.5% of cases of apparently isolated VPD have been reported to be associated with a 22q11.2 deletion (Zori et al., 1998). Impaired VP valving is among the most common clinical features of the disorder, having been demonstrated in nearly 75% of patients with a 22q11.2 deletion (McDonald-McGinn et al., 1999; Kirschner, 2005). Often it is this observation alone that leads to the work-up and eventual diagnosis of 22q11.2 deletion syndrome in young people. Speech in affected patients may be characterized by hypernasal resonance and by the presence of articulation errors, including glottal stop substitutions, which develop in an effort to compensate for inadequate VP valving. There are multiple anatomical features of 22q11.2 deletion syndrome that may contribute to VPD, including cleft palate and sub-mucosal cleft palate, VP hypotonia, adenoid hypoplasia, platybasia, and upper cervical spine abnormalities. Cranial nerve defects have been observed in about 30% of patients, with the vagal nerve most often involved. Vagal involvement may explain the velar paresis observed in many of these patients with otherwise normal anatomy (Hultman et al., 2000). Abnormalities of the soft tissues, such as the soft palate, adenoids, and pharyngeal walls, and of the underlying skeletal structures, including the basicranium and upper cervical spine, may lead to VP disproportion in affected individuals (Havkin et al., 2000). In an MRI study, Punjabi et al. (2000) reported that the levator veli palatini muscle appeared to be thin and hypoplastic in three of their five subjects with VCFS.
As with other clinical features of 22q11.2 deletion syndrome, there is considerable variability in the speech manifestations of the disorder. A minority of patients may present with normal resonance, whereas others present with hypernasality that may range from mild to severe. Most patients with VPD associated with a 22q11.2 deletion are candidates for surgical intervention. The outcomes of physical management of VPD in these patients, however, have been reported to be poorer than those achieved in patients with VP valving disorders of other etiologies (i.e., nonsyndromic cleft palate). It is likely that the constellation of functional and anatomic abnormalities associated with microdeletions of 22q11.2 contributes to the difficulty in optimizing surgical outcome. Accordingly, a thorough understanding of the function and the anatomy of the velopharynx is essential to the accurate diagnosis and management of affected patients. By tailoring intervention strategies to the particular derangements in VP valving diagnosed in individual patients, speech outcomes may be optimized.
Treatment of VPD requires precision in diagnosis, surgical management, and rehabilitation. The successful management of VPD, therefore, is wholly dependent upon a comprehensive assessment of VP anatomy and function in affected patients. Techniques for the study and assessment of VP function have evolved to allow us to understand VP physiology and to recognize that integrity of the mechanism is essential for normal speech. Nevertheless, our views of both normal and abnormal VP function and of the correlates between VPD and speech have remained somewhat simplistic. It is widely believed that alterations in VP anatomy play a significant role in congenital VPD and in the response of such to surgical management. With advances in imaging technology, it is now possible to obtain high-quality, 3D images of head and neck anatomy that will greatly enhance our understanding of VP structure and function and that will improve both clinical assessment and management of patients with VPD. Recent experience at our institution with MRI and novel computer reconstruction algorithms for quantitative 3D assessment of the soft tissue and skeletal structures of the upper airway has provided important information regarding anatomical risk factors for OSA in children (Schwab et al., 1995; Uong et al., 2001; Arens et al., 2001, 2003). These studies have led to enhanced understanding of the pathogenesis of OSA and allow investigators to study the mechanisms underlying the efficacy of therapeutic interventions for the disorder. This experience suggests that this technique can provide important information about VP anatomy in patients with both normal and abnormal VP function and about the efficacy of management strategies for VP valving disorders.
Velopharyngeal closure is dependent not only upon adequate neuromuscular function of the velum and synergistic musculature but also upon the morphology of the VP port (McWilliams et al., 1990). The size and shape of the velopharynx is determined by the skeletal structure of the maxilla, cranial base, and upper cervical spine and by the overlying soft tissues, such as the adenoids, pharyngeal walls, and velum. Radiographic studies have established that certain consistent relationships exist between anatomic structures of the velopharynx and sound production and that significant anatomic derangements of these structures are present in patients with craniofacial anomalies (Calnan, 1956; Jakhi and Karjodkar, 1990; Wu et al., 1996). For example, several studies have demonstrated that the transverse dimension of the pharynx is greater in patients with palatal clefts. Calnan (1958) used cephalometric techniques to study 41 patients without clefts with hypernasal speech. His studies revealed that such patients had significantly greater pharyngeal dimensions than did normal controls. Our results indicate that 22q11.2 deletions are associated with significant increases in VP port size. Patients with a 22q deletion and VPD demonstrated increases in VP depth and width. This, along with a decrease in AdV, results in dramatic increases in the volume of the pharyngeal cavity.
The importance of the upper cervical vertebrae on VP closure has been discussed in the literature but has received relatively little attention. Osborne (1968) and Osborne and colleagues (1971) reported that upper cervical spine abnormalities, the prevalence of which is higher in patients with congenital craniofacial anomalies, may increase osseous pharyngeal depth and thereby predispose affected patients to VPD. In both studies, 18.8% of patients with congenital VPD demonstrated abnormalities of the upper cervical vertebrae and skull base on lateral cephalometric studies, including fusion of C2–C3, occipitalization of the atlas, atlanto-axial subluxation, and hypoplasia of the anterior arch of the atlas. Patients with congenital VPD with abnormalities of the cervical spine demonstrated significantly greater osseous pharyngeal depth than did matched controls. Hoenig and Schoener (1992) studied lateral cephalometric radiographs of 30 patients with cleft lip and palate and noted a significantly greater number of upper cervical spine abnormalities than in controls. Patients with vertebral anomalies were also found to demonstrate significantly increased osseous nasopharyngeal depth, suggesting that such anomalies may have a direct effect on VP closure.
Patients with a 22q11.2 deletion may demonstrate multiple cervical spine abnormalities, including an abnormally shaped dens, an upswept C2, a hypoplastic or anomalous C1, posterior element fusion with block vertebrae, posterior element fusion without block vertebrae, and occipitalization of the atlas (McDonald-McGinn et al., 2003). The present study indicates that such cervical abnormalities may be associated with a significant increase in the ADA, an alteration that may increase VP depth.
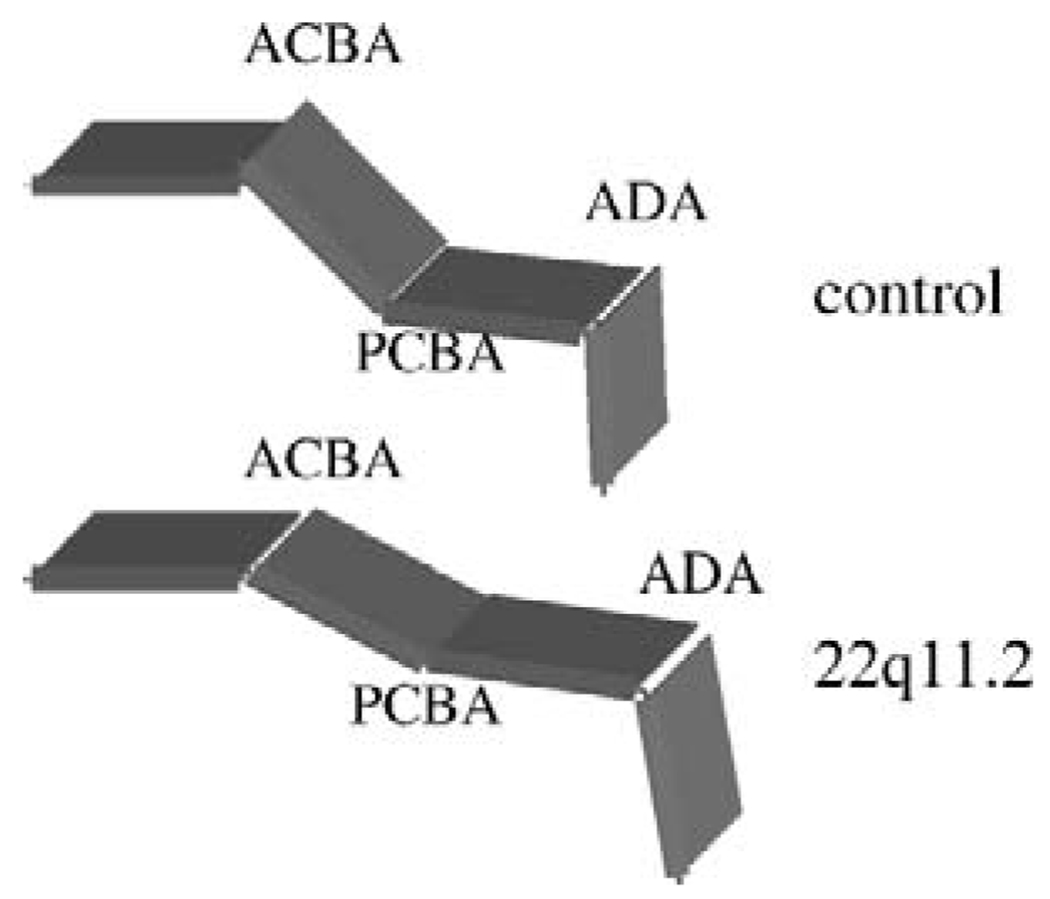
Patients with a 22q11.2 deletion have been shown to demonstrate a more obtuse ACBA than do normal controls (Arvystas and Shprintzen, 1984). Our results confirm this finding and indicate that affected patients also demonstrate a significantly greater PCBA. The resulting platybasia, along with changes in angulation of the upper cervical spine, effectively deepens the velopharynx and increases the need ratio in affected patients (Fig. 16). Thus, alterations in cranial-base anatomy may be the single most important determinant of VPD in 22q11.2 deletion syndrome.
FIGURE 16.
Composite rendering of mean cranial base angle and ADA. The patients with 22q11.2 deletion syndrome demonstrate platybasia and lengthening of the cranial base.
Current techniques for the assessment of VPD in patients with a 22q11.2 deletion seek to quantify the degree of hypernasality and nasal air escape, to determine VP gap size, and to characterize VP closure patterns. Although commonly used imaging modalities can provide useful information, they possess several limitations (Witt et al., 2000). Lateral cephalometric radiographs were formerly the standard for VP assessment. Cephalograms provide standardized, quantitative information regarding the size of and the relationship among VP structures. However, they provide only a static two-dimensional view of a dynamic 3D structure (Wu et al., 1996). Multiview videofluoroscopy provides a real-time, multidimensional assessment of VP structure and function. Fluoroscopic images, however, may be difficult to interpret. Overlying shadows often prevent clear visualization of the VP mechanism, making its evaluation challenging. Patient cooperation may be difficult to achieve, with the injection of intranasal contrast and awkward positioning limiting its use in some younger patients. In addition, both cephalometry and videofluoroscopy expose patients to ionizing radiation. Nasopharyngoscopy, the current “gold standard” for assessment of VP function, is limited by its inability to provide truly quantitative information and to provide a 3D view of VP structures. Nasendoscopy provides a real-time, direct view of the VP port during speech, thus allowing us to define closure patterns and possibly locate carotid displacement. Although it also has the benefit of being free of ionizing radiation, it limits the evaluator to a nonquantitative, single view that prevents examination of the tissues below the level of the velum. Visualization of the port may also be obscured by the surrounding tissue or by the angle of the scope, resulting in a distorted image and faulty reporting of results. The invasiveness of the procedure limits its use in younger patients, and the presence of the scope as well as the anesthesia required for its placement both may interfere with speech production.
Magnetic resonance imaging has emerged as a promising method for noninvasive, 3D assessment of the velopharynx (McGowan et al., 1992; Akgüner, 1999; Vadodaria et al., 2000; Kuehn et al., 2001, 2004; Ettema et al., 2002; Kane et al., 2002; Ruotolo et al., 2003). Reports on the use of MRI for VP imaging, however, have thus far provided only rudimentary data. McGowan et al. (1992) established the application of MRI to the visualization of palatal motion during sustained phonation. They compared midsagittal images of normal subjects to those who had undergone cleft palate repair and evaluated the position of the velum relative to the posterior pharyngeal wall. They concluded that dynamic magnetic resonance images may be used postoperatively in evaluations to help guide speech therapy options and preoperatively when planning a palate repair or treating VPD. Vadodaria et al. (2000) compared several measurements (e.g., VL, VT, area of the nasopharynx, and angle of the levator sling) determined by nasendoscopy, videofluoroscopy, and MRI. Although the magnetic resonance images were static, the authors concluded that MRI is superior to the more traditional imaging modalities. Magnetic resonance imaging proved to be a reliable, quantitative study with strong potential for evaluating VP function and planning surgical repair. Multiple quantitative measurements were possible: VP closure, posterior and lateral wall motion, and velar stretch and lift. With the development of gated-MRI technology, we will likely be able to assess the anatomy of the VP port during specific phonation exercises (Kane et al., 2002; Shinagawa, 2004).
Magnetic resonance imaging provides clinicians with a non-invasive technology that is able to visualize soft tissue with greater contrast compared with other imaging procedures and without exposure to radiation. The mismatch, however, between the acquisition time required for detailed images and the rapid motion resulting from speech tasks has forced clinicians to limit the use of MRI to images at rest or during sustained phonation. The development of faster MRI will address this current limitation. With current technology, however, resolution diminishes significantly with faster image acquisition (Kane et al., 2002). Similar to more traditional methods of VP assessment, MRI also has its disadvantages. Most important is that its cost is often prohibitive, but this is likely to change as this technology becomes more mainstream. The effect of gravity on image assessment secondary to standard supine positioning must be considered. The scanner tends to be quite noisy, and MRI requires a relatively long acquisition time. Therefore, sedation becomes essential in the younger population. Sedation itself can have an effect on muscle tone of the airway and pharynx and may have affected some of the measurements in our study. If our aim is to conduct gated and rapid acquisition MRI during phonation, full cooperation of each patient is essential, and sedation may have to be modified or eliminated altogether.
CONCLUSION
Patients diagnosed with 22q11.2 deletion syndrome demonstrate multiple anatomic differences when compared with the normal population that may contribute to their high incidence of VPD. Recent advances in MRI have allowed for accurate assessment of the anatomy of the velopharynx, providing us with a quantitative, 3D, noninvasive technique for VP imaging. The future of MRI technology, with gating and more rapid image acquisition, may allow us to similarly evaluate the VP valve during phonation and may eventually serve as a powerful tool for assessment and treatment planning in these and other patients with VPD.
Footnotes
This paper was presented at the 60th Annual Meeting of the American Cleft Palate–Craniofacial Association in Asheville, North Carolina, April 11, 2003.
REFERENCES
- Akgüner M. Velopharyngeal anthropometric analysis with MRI in normal subjects. Ann Plast Surg. 1999;43:142–147. [PubMed] [Google Scholar]
- Arens R, McDonough JM, Corbin AM, Rubin NK, Carroll ME, Pack AI, Liu J, Udupa JK. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2003;167:65–70. doi: 10.1164/rccm.200206-613OC. [DOI] [PubMed] [Google Scholar]
- Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- Arvystas M, Shprintzen RJ. Craniofacial morphology in the velo-cardio-facial syndrome. J Craniofac Genet Dev Biol. 1984;4:39–45. [PubMed] [Google Scholar]
- Calnan J. Diagnosis, prognosis and treatment of palatopharyngeal incompetence with special reference to radiographic investigation. Br J Plast Surg. 1956;8:256. doi: 10.1016/s0007-1226(55)80047-1. [DOI] [PubMed] [Google Scholar]
- Calnan J. Investigations of children with speech defects with particular reference to nasality. Br Med J. 1958;1:737. doi: 10.1136/bmj.1.5073.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema SL, Keuhn DP, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. doi: 10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Fleiss JK. The Design and Analysis of Clinical Experiments. New York: J Wiley & Sons; 1986. chapter 1. [Google Scholar]
- Goldberg R, Motzkin B, Marion R, Scambler PJ, Shprintzen RJ. Velo-cardio-facial syndrome: a review of 120 patients. Am J Med Genet. 1993;45:313–319. doi: 10.1002/ajmg.1320450307. [DOI] [PubMed] [Google Scholar]
- Havkin N, Tatum SA, III, Shprintzen RJ. Velopharyngeal insufficiency and articulation impairment in velocardiofacial syndrome: the influence of adenoids on phonemic development. Int J Pediatr Otorhinolaryngol. 2000;54:103–110. doi: 10.1016/s0165-5876(00)00350-5. [DOI] [PubMed] [Google Scholar]
- Hoenig JF, Schoener WF. Radiological survey of the cervical spine in cleft lip and palate. Dentomaxillofac Radiol. 1992;21:36–39. doi: 10.1259/dmfr.21.1.1397450. [DOI] [PubMed] [Google Scholar]
- Hultman CS, Riski JE, Cohen SR, Burstein FD, Boydston WR, Hudgins RF, Grattan-Smith D, Uhas K, Simms C. Chiari malformation, cervical spine anomalies, and neurologic deficits in velocardiofacial syndrome. Plast Reconstr Surg. 2000;106:16–24. doi: 10.1097/00006534-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Jakhi SA, Karjodkar FR. Use of cephalometry in diagnosing resonance disorders. Am J Orthod Dentofacial Orthop. 1990;98:323–332. doi: 10.1016/S0889-5406(05)81489-1. [DOI] [PubMed] [Google Scholar]
- Kane AA, Butman JA, Mullick R, Skopec M, Choyke P. A new method for the study of velopharyngeal function using gated magnetic resonance imaging. Plast Reconstr Surg. 2002;109:472–481. doi: 10.1097/00006534-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Kirschner RE. Palatal anomalies and velopharyngeal dysfunction associated with chromosome 22q11.2 microdeletions. In: Murphy K, Scamble P, editors. VCFS: Understanding Microdeletion Disorders. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC. Magnetic resonance imaging of the levator veli palatini muscle before and after primary rhino-plasty. Cleft Palate Craniofacial J. 2004;41:584–592. doi: 10.1597/03-060.1. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC, Wachtel JM. Magnetic resonance imaging in the evaluation of occult submucous cleft palate. Cleft Palate Craniofacial J. 2001;38:421–431. doi: 10.1597/1545-1569_2001_038_0421_mriite_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- McDonald-McGinn DM, Catanzaro J, Drummond D, Jacobs I, Kirschner RE, LaRossa D, Russell K, Schiffman P, Simon E, States L, Tamai J, Tonneson M, Zackai EH. Cervical spine instablitly in patients with the 22q11.2 deletion. Abstract presented at the 60th Annual Meeting of the American Cleft Palate–Craniofacial Association; April 2003; Asheville, North Carolina. [Google Scholar]
- McDonald-McGinn DM, Kirschner R, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, Moss E, Solot C, Wang P, Jacobs I, Handler S, Knightly C, Heher K, Wilson M, Ming JE, Grace K, Driscoll D, Pasquariello P, Randall P, LaRossa D, Emanuel BS, Zackai EH. The Philadelphia story: the 22q11.2 deletion: report on 250 patients. Genet Couns. 1999;10:11–24. [PubMed] [Google Scholar]
- McGowan JC, III, Hatabu H, Yousem DM, Randall P, Kressel HY. Evaluation of soft palate function with MRI: application to the cleft palate patient. J Comput Assist Tomogr. 1992;16:877–882. doi: 10.1097/00004728-199211000-00009. [DOI] [PubMed] [Google Scholar]
- McWilliams BJ, Morris HL, Shelton RL. Cleft Palate Speech. 2nd ed. Philadelphia: BC Decker; 1990. [Google Scholar]
- Osborne GS. Ph.D. thesis. Carbondale, IL: Southern Illinois University; 1968. The prevalence of anomalies of the upper cervical vertebrae in patients with craniofacial malformations, and their effects on osseous nasopharynx depth. [Google Scholar]
- Osborne GS, Pruzansky S, Koepp-Baker H. Upper cervical spine anomalies and osseous nasopharyngeal depth. J Speech Hear Res. 1971;14:14–22. doi: 10.1044/jshr.1401.14. [DOI] [PubMed] [Google Scholar]
- Punjabi AP, Holshouser BA, D’Antonio LL, Kuehn DP. Magnetic resonance imaging of the velopharynx in patients with velocardiofacial syndrome. Paper presented at annual meeting of the American Cleft Palate–Craniofacial Association; May 3, 2002; Seattle, Washington. [Google Scholar]
- Ruotolo RA, Veitia N, Corbin A, McDonough J, Solot CB, McDonald-McGinn DM, Zackai EH, Emanuel BS, LaRossa D, Arens R, Kirschner RE. Velopharyngeal anatomy in 22q11.2 deletion syndrome: a three-dimensional cephalometric analysis. Presented at the annual meeting of the American Cleft Palate–Craniofacial Association; April 11, 2003; Asheville, North Carolina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab RJ, Gupta KB, Gefter WB, Hoffman EA, Pack AI. Upper airway soft tissue anatomy in normals and patients with sleep disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- Shinagawa H. Imaging of oral and maxillofacial function: new perspectives for neuroradiology. J Oral Biosci. 2004;47:177–190. [Google Scholar]
- Udupa JK, Odhner D, Samarasekera S, Goncalves R, Iyer K, Venugopal K, Furuie S. 3DVIEWNIX: an open transportable, multidimensional, multimodality, multiparametric imaging software system. SPIE Proc. 1994;2164:58–73. [Google Scholar]
- Uong EC, McDonough JM, Tayag-Kier CE, Zhao H, Haselgrove J, Mahboubi S, Schwab RJ, Pack AI, Arens R. Magnetic resonance imaging of the upper airway in children with Down syndrome. Am J Respir Crit Care Med. 2001;163:731–736. doi: 10.1164/ajrccm.163.3.2004231. [DOI] [PubMed] [Google Scholar]
- Vadodaria S, Goodacre TEE, Anslow P. Does MRI contribute to the investigation of palatal function? Br J Plast Surg. 2000;53:191–199. doi: 10.1054/bjps.1999.3308. [DOI] [PubMed] [Google Scholar]
- Witt PD, Marsh JL, McFarland EG, Riski JE. The evolution of velopharyngeal imaging. Ann Plast Surg. 2000;45:665–673. doi: 10.1097/00000637-200045060-00019. [DOI] [PubMed] [Google Scholar]
- Wu JT, Huang GF, Huang CS, Noordhoff MS. Nasopharyngoscopic evaluation and cephalometric analysis of velopharynx in normal and cleft palate patients. Ann Plast Surg. 1996;36:117–123. doi: 10.1097/00000637-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang J, Quan H, Ng J, Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Control Clin Trials. 1997;18:204–221. doi: 10.1016/s0197-2456(96)00129-8. [DOI] [PubMed] [Google Scholar]
- Zori RT, Boyar FZ, Williams WN, Gray BA, Bent-Williams A, Stalker HJ, Rimer LA, Nackashi JA, Driscoll DJ, Rasmussen SA, Dixon-Wood V, Williams CA. Prevalence of 22q11 region deletions in patients with velopharyngeal insufficiency. Am J Med Genet. 1998;77:8–11. [PubMed] [Google Scholar]