Familial Aggregation of Quantitative Autistic Traits in Multiplex versus Simplex Autism (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 5.
Published in final edited form as: Am J Med Genet B Neuropsychiatr Genet. 2009 Apr 5;150B(3):328–334. doi: 10.1002/ajmg.b.30810
Abstract
Objective
Recent research has suggested that the mode of inheritance for simplex autism (SA, one individual in the family affected) may be distinct from that for multiplex autism (MA, two or more individuals affected). Since sub clinical autistic traits have been observed in “unaffected” relatives of children with autism, we explored whether the distributions of such traits in families supported differential modes of genetic transmission for SA and MA autism.
Methods
We measured patterns of familial aggregation of quantitative autistic traits (QAT) in children and parents in 80 SA families and 210 MA families, using the Social Responsiveness Scale.
Results
When considering all SA and MA siblings who scored below a uniform quantitative (clinical-level) severity threshold, MA brothers exhibited a distinct pathological shift in the distribution, compared to SA brothers (p<.0001). Such aggregation of QAT was also observed in fathers but not among females in MA families. Significant spousal correlations for QAT—suggestive of assortative mating—were observed in both SA and MA families, but neither group was characterized by a greater-than-chance level of concordant elevation among spousal pairs in this volunteer sample.
Conclusions
Among male first degree relatives, there exist distinct patterns of QAT manifestation for simplex versus multiplex autism. These findings are consistent with the results of molecular genetic studies that have suggested differential modes of intergenerational transmission for SA and MA. Characterization of QAT and other endophenotypes among close relatives may be useful for reducing sample heterogeneity in future genetic and neurobiologic studies of autism.
Keywords: Pervasive Developmental Disorders, Genetics, Social Responsiveness Scale, Family Studies, Complex Disease
INTRODUCTION
Recent studies of idiopathic autism spectrum disorders (ASD) have implicated disparate genetic pathways for families in which ASD affects only one individual in the family (“simplex” autism, SA) versus multiple siblings in the family (familial or “multiplex” autism, MA). For the former (SA), a variety of relatively rare single gene mutations, each of large effect, have been associated with sporadically-occurring autistic syndromes (Sebat et al., 2007; Weiss et al., 2008). For the latter (MA), interactive effects of multiple common susceptibility alleles (each of minor influence when operating independently), have long been hypothesized as causes of familial autistic syndromes (Pickles et al., 1995; Risch et al., 1999). In support of differential patterns of inheritance for SA versus MA, the first common allele to be identified as a risk gene for autism (a variant of the MET gene) was recently reported to confer susceptibility among MA families only (Campbell et al., 2006). In contrast, a variety of rare copy number variations have been associated with ASD, but these have been observed more commonly in SA (where they account for up to 10 per cent of cases) than in MA (where they account for 1–2 per cent of cases) (Sebat et al., 2007, Weiss et al., 2008). Furthermore, Miles et al. (2005) observed that in a series of autism cases complicated by dysmorphism and mental retardation, nearly all were SA.
There are other variables that may distinguish MA families from SA families and that might provide important clues to both mechanisms of inheritance and to the neurobiologic underpinnings of the autism. Sub clinical behavioral features of the autistic syndrome have been observed in the unaffected relatives of children with autism (Piven et al., 1997; Szatmari et al., 2000; Constantino et al., 2006), as have event-related potential (ERP) abnormalities (Dawson et al., 2005), functional magnetic resonance imaging (fMRI) variations (Dalton et al., 2007), and a variety of psychological and personality traits that are similar in nature to the phenotypic characteristics of autism (Murphy et al., 2000; Losh et al., 2007). Losh, Piven and colleagues (2007) recently observed that parents of children in MA families (n=25) more commonly exhibited social behavioral characteristics akin to autism than did parents of children in SA families (n=40), when examined with standard psychological and personality measures. Similarly, Szatmari et al. (2000) screened 1362 biological relatives of 92 ASD probands (34 of whom were multiplex) for presence versus absence of ASD-like traits and observed that they were more prevalent in multiplex families.
This study is an attempt to replicate and advance these findings, with a focus on quantitative characterization of first degree relatives of a larger number of ASD probands (n=290); it is the first study of its kind to use a validated quantitative measure of phenotypic characteristics known to aggregate specifically in autism families. The study explores how such traits are distributed in those autism families in which they aggregate, with the hope of identifying ways in which phenotypic characterization might elucidate specific differences in the manner in which autism is inherited in multiplex versus simplex families.
The issue of whether the family of an autistic individual should be considered MA or SA is not always straightforward. Given a generally-reported sibling recurrence risk on the order of 10 per cent, and a 4:1 ratio of subsequently-affected males to females, families with only a single affected child might ultimately prove to be MA if more children (especially males) were born into the family. Furthermore, the phenotypic severity of autistic impairment can be highly variable in multiple-incidence pedigrees (Pickles et al., 2000), such that it becomes unclear whether or not to include a mildly-affected individual in the family as a “co-affected” family member, unless an unambiguous clinical cutoff is established. This has been a critical issue in establishing concordance rates in twins (LeCouteur et al., 1996) and is especially relevant when considering the fact that autistic symptoms are essentially continuously distributed in the general population (Constantino and Todd, 2003), in which case it becomes arbitrary where to place a “cutoff” point below which to designate an individual as “unaffected.”
For these reasons, we adopted a relatively conservative approach to the inclusion of SA families for this study. All SA families were required to have a single-affected proband, a minimum of one male sibling, and all siblings of the proband unaffected by ASD on the basis of the impressions of the children’s pediatricians. In addition, families were excluded from the SA group if a sibling manifested clinical-level QAT (in spite of the absence of a clinical diagnosis)—this resulted in the exclusion of some families who were originally presumed simplex but might more appropriately be categorized as multiplex (see Methods below).
In a recent study involving males only (from 89 multiplex families and 60 simplex families considered together) we demonstrated that QATs as measured by the Social Responsiveness Scale (SRS) preferentially aggregate in the male siblings of ASD probands in comparison to non-autistic child psychiatric controls (Constantino et al., 2006). As further evidence that QAT elevation represents an autism endopheynotype, we recently observed a linkage signal approaching genome-wide significance for the trait characterized by the SRS when incorporating data from all children (not just affected sibling pairs) in multiplex autism families (Duvall et al., 2007).
In this study we attempted a) to explore a larger sample of autism families (n=290) than was available at the time of our original report; b) to differentiate patterns of familial aggregation between MA and SA families; and c) to extend the exploration of familial aggregation to both female siblings (in MA families) and parents (in respective sub groups of MA and SA families). The goals were to clarify patterns of familial aggregation as discussed above, to refine our understanding of the level of aggregation of QAT (if any) that occurs in simplex families, and to determine whether quantitative methods for differentiating SA from MA might be more precise in distinguishing biologically-meaningful subgroups within autism. The latter is particularly important for research on the genetic and neurobiologic causes of autism, which may be confounded by the inadvertent mixing of causally-distinct sub groups within study samples.
MATERIALS AND METHODS
Sample
This study involved two separate samples of ASD-affected families: 1) multiplex ASD (MA) families, n=210, recruited from the Autism Genetic Resource Exchange (AGRE); and 2) conservatively-defined simplex ASD families (SA) recruited for a longitudinal study of male sibling pairs at Washington University (WU). In this study, a simplex family was defined as one in which all of the following conditions were met: a) the nuclear family contained a single ASD-affected proband; b) the family additionally was comprised of at least one full biological male sibling of that proband—this criterion was chosen because sibling recurrence is 4 times more common in male siblings than in female siblings of an ASD proband; c) all siblings in the family were free of categorical designations of affected status on the basis of the respective impressions of the children’s pediatricians; and furthermore d) all male siblings scored below an average (multi-informant) T_-score of 65 on the Social Responsiveness Scale (SRS, see below)—this was derived by calculating the mean of parent- and teacher-report T_-scores for each subject. This conservative multi-informant cutoff—which is slightly above the published threshold of 60T for specific ascertainment of clinically-significant symptomatology (Constantino & Gruber 2005; Constantino et al., 2007)—was chosen to avoid overexclusion of families in which there may have been modest disagreement between parent and teacher reports about whether a child exceeded the 60_T threshold. We originally recruited and enrolled 89 families presumed simplex on the basis of the impression of each child’s pediatrician and parents at time of enrollment, but the SA sample was subsequently winnowed to 80 families by excluding 9 in whom one or more presumed-unaffected male siblings of the proband were found to score at or above the 65_T multi-informant cutoff.
The diagnosis of all ASD probands was confirmed by the Autism Diagnostic Interview-Revised; for the MA sample 89% of the affected children met full criteria for Autistic Disorder, the remainder for Broader Autism Spectrum Disorder (as designated in the AGRE database, http://www.agre.org); for the SA sample 69% of probands met ADI-R criteria for Autistic Disorder, the remainder for Broader Autism Spectrum Disorder. Both samples were predominantly Caucasian (SA 92%; MA 75%). The mean family size (number of full biological siblings in nuclear family) was 2.2 for SA families, 2.7 for MA families. NVIQ of MA probands estimated from the Raven’s Progressive Matrices averaged a standardized score of 96. Full scale IQ of SA probands averaged 92; IQ scores for SA probands were obtained from formal psychoeducational assessments performed by the school districts in which the subjects resided. Thus, WU data on full scale IQ was derived from a variety of tests as deemed most appropriate by the respective school psychologists for testing individual subjects.
For each male child in the SA sample, and each child of either gender in the MA sample, quantitative autistic trait (QAT) measurements using the Social Responsiveness Scale (SRS, see below) were requested to be completed by one parent (in most cases mother) and one teacher (who had known the child for at least 2 months). Completion rates for the assessments and mean ages for each study group are provided in Table 1.
Table I.
Selected Sample Characteristics
| Mean Age | Gender | # with parent-report SRS | # with teacher-report SRS | |
|---|---|---|---|---|
| Affected Children, Simplex Families (n=80) | 8.2 (SD=4.0) | Male | 80 | 80 |
| Unaffected Children, Simplex Families (n=97) | 7.8 (SD=3.8) | Male | 95 | 89 |
| Affected Children, Multiplex Families (n=393, from 210 families) | 10.6 (SD=3.6) | 312 male81 female | 333 | 343 |
| Unaffected Children, Multiplex Families (n=85) | 10.9 (SD=4.2) | 40 male45 female | 77 | 74 |
Following the acquisition of SRS data on the children in the study, SRS reports were subsequently obtained on parents (completed by spouse report) in a sub sample comprised of 41 of the SA families and 58 of the MA families (in which one or more of the affected children were male). We note that some families are either averse to or protected from requests for supplemental data collection, and this was respected to avoid excessive research burden on longitudinal study subjects. We note also that it is a matter of policy within the AGRE registry that subjects are ineligible for remuneration for research participation, and this may have compromised the acquisition of parental data from a higher proportion of AGRE families, since the families may have viewed this supplemental data collection as non-essential to the primary mission of the AGRE collection. There were no statistically-significant differences between families who did versus did not participate in parental assessments, with respect to the means and distributions of SRS scores of probands or male siblings (this held true within both the SA and MA study groups).
The research protocol for this study was approved by both the Washington University Institutional Review Board and the Western Institutional Review Board (which governs AGRE research). After complete description of the study to the subjects, written informed consent was obtained.
Measure
The SRS is a normed, extensively validated (Constantino and Gruber, 2005), 65-item questionnaire that quantitatively measures severity of autistic social impairment and distinguishes ASD from other psychiatric disorders (Constantino et al., 2000). SRS scores are highly heritable, stable over time (Constantino et al., in press), continuously distributed in the general population (Constantino and Todd, 2003), and exhibit a unitary factor structure. This means that although the SRS incorporates ascertainment of symptoms across all three DSM-IV criterion domains for autism (reciprocal social behavior, language, and repetitive behavior/restricted interests), inter-individual trait correlations between these 3 symptom domains—indeed between all SRS items—have been found to be very high, in both clinical and non-clinical samples (Constantino and Gruber 2005; Constantino et al., 2004; Constantino et al., 2007), such that when statistical methods have been used to empirically derive “factors” or symptom clusters within the data, the result is a single-factor solution. In principal components analysis, items representing all 3 DSM-IV criterion domains load onto the principal factor (Constantino et al., 2004; Constantino et al., 2007). It is this unitary factor structure of the quantitative trait data derived from the SRS which supports the use of a single index score as a quantitative measure of autistic severity.
For the parents who were assessed, the adult version of the SRS was completed by spouse-report. The adult version of the SRS corresponds item-by-item to the child and adolescent version; a number of items differ moderately in wording or content, in accordance with what is most developmentally appropriate for adult behavior. SRS heritability and population distributions for adults (ascertained by spouse-report) closely match those observed for children (Constantino and Todd, 2005).
SRS _T_-scores greater than 60 (corresponding to: male raw teacher-report SRS score of 80; female raw teacher-report SRS score of 60; male raw parent-report SRS score of 55; female raw parent-report SRS score of 46) indicate a level of autistic social impairment that is clinically and functionally significant; this arbitrary “cutoff” exceeds the population mean by at least one standard deviation (across gender and informant type) and is within 2 standard deviations of the mean observed in children who carry expert-clinician diagnoses of ASD (Constantino and Gruber, 2005). Both parent- and teacher-report SRS scores have been extensively validated against, and exhibit substantial agreement with, the Autism Diagnostic Interview-Revised (ADI-R), a research standard for assessing autism (Constantino et al., 2003, Constantino et al. 2007). In unselected clinical populations, the SRS exhibits high specificity and sensitivity for an ASD diagnosis; when both teacher and parent report that a child has a _T_-score of 60 or greater, the likelihood of a correctly identified PDD designation (by ADI-R, ADOS, or clinician diagnosis) is 97% (Constantino et al., 2007).
Data Analysis
It is important to note that in this study, following the process of sample selection, a quantitative approach to the data was employed. Although multiplex families are selected on the basis of concordance in siblings (in a categorical sense) and simplex families are selected on the basis of discordance (again, in a categorical sense), the following analyses were employed to explore a) whether quantitative autistic trait (QAT) distributions in multiplex families actually support the notion of categorical differences between affected and unaffected members within those families (separately considering males and females), and b) whether familial aggregation of quantitative autistic traits that have been observed in multiplex families extends to simplex families.
We first examined the distributions of QATs (measured by the SRS) for all children for whom SRS data was available (boys in SA families, boys in MA families, girls in MA families). We selected teacher-reports as the primary data source for analyzing these distributions, given evidence from prior clinical studies (see Duvall et al., 2007) that subtle rater contrast effects can result in some parents of ASD children giving their undiagnosed children lower ratings for QAT than are evident from the objective reports of raters whose basis of comparison would not be an ASD-affected child. A recent validation study of teacher-report SRS data (Constantino et al., 2007) strongly supports this approach. We note that parent-teacher agreement for total SRS score in this sample was extremely strong, ICC = .76, p< .00001. Intraclass coefficients of correlation were also computed for male sibling pairs, one per family, involving the index case and one male sibling (selected at random within the family if there was more than a single male sib).
Next, a comparison of means exclusively involving boys whose scores fell below the clinical severity level of 60_T_ by teacher-report was conducted between siblings in simplex families and siblings in multiplex families. This analysis was then repeated after removing any multiplex sibs with a categorical designation of ASD-affected status on the ADI-R, since some of the MA boys with scores below the quantitative severity threshold had nevertheless met criteria for ASD on that instrument. The latter constitutes a stringent test of whether differences exist between unaffected male sibs in SA families and MA boys who are “unaffected” on the basis of both quantitative and categorical criteria.
Finally, we compared spouse-report adult SRS scores of the parents in MA and SA families. We also examined whether, in this clinical sample, the proportion of spousal pairs with concordant upper-quartile elevations in SRS score (as defined in the general population, Constantino and Todd, 2005) was greater than would be expected by chance.
RESULTS
1. Affected and Unaffected Children Considered Together
Distributions of teacher-report SRS scores of all children in the study are depicted as a function of gender, family type, and categorical designation of affected status in Figure 1. As expected, the distribution for all male children in SA families was bimodal (the sample was ascertained on the basis of having one affected and one unaffected male child); what was particularly notable, however, was a cluster of unaffected male siblings in SA families who were tightly grouped about a mean of 25.0. No such cluster was observed in the lower range of the MA distribution (see below). Boys in MA families exhibited a unimodal distribution in which there was no discernible severity cutoff distinguishing boys affected versus unaffected by ASD. The sibling intraclass correlation for boys in MA families was highly statistically significant (ICC = .46, p<.000001); the sibling correlation in SA families, however, was not (ICC = .00). In contrast to the continuous distribution for boys in MA families, the distribution for the girls in these families was distinctly bimodal: affected girls represented by a quantitative trait distribution that was almost entirely non-overlapping with that of unaffected girls.
Figure 1.
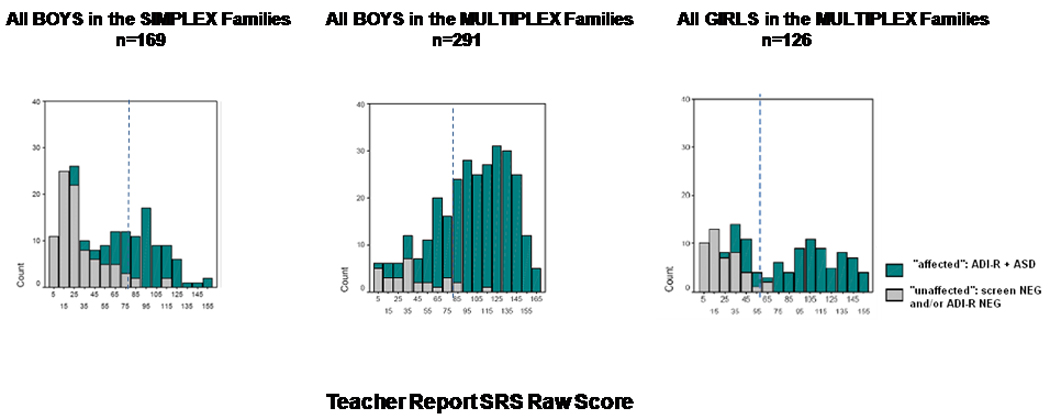
Distribution of RAW teacher-report Social Responsiveness Scale (SRS) scores for ALL assessed children in each family type (all of the boys in the simplex families, all of the boys in the multiplex families, all of the girls in the same set of multiplex families). A teacher-report SRS T-score of 60, the quantitative threshold used for comparison of less-affected / unaffected male siblings in multiplex versus simplex families (see text) is denoted by the hatched line in each panel. Each bin is color-coded to depict the respective numbers of children categorically-designated as affected or unaffected on the basis of physician screening and/or ADI-R result, as described in Methods.
2. Comparison of SA versus MA boys scoring below a uniform severity threshold (60_T_)
For all unaffected SA boys (n=113, including the low-scoring cluster and the remaining male siblings who scored below a teacher-report SRS score of 60_T_), the mean was 35.0 with a standard deviation of 22.4. This contrasts substantially with the mean and distribution for boys in MA families whose teacher-report SRS scores fell below the same severity threshold (mean = 48.8; SD = 22.2, n = 84; t= −4.3; df= 195; p<.0001). This mean difference (i.e. for boys in the two types of families scoring below the uniform severity threshold) remained highly statistically significant when considering parent-report SRS scores or averaged scores of both parents and teachers. This sub group of MA boys exhibited a steadily ascending right half of the distribution, as shown in Figure 1, which contrasts with the shape of the distribution for low-scoring SA males. Finally, when excluding from this analysis even the low-scoring MA males who nevertheless carried categorical designations of affected status on the ADI-R, the difference in mean teacher-report SRS scores for unaffected males between the two family groups remained statistically significant (p=.035). Means and standard deviations for teacher-report SRS scores as a function of gender, family type, and affected status, are presented in Table 2.
Table II.
Quantitative Autistic Traits in the first degree relatives of ASD probands. Social Responsiveness Scale Mean scores by gender, categorical “affected” status (on the basis of SRS screening, clinician diagnosis, and/or ADI-R result—see text), and family type (simplex versus multiplex autism), incorporating data from children for whom teacher-report data was available, and for the sub sample of parental pairs (all undiagnosed) assessed by spouse-report. P-values are for two-tailed test, except where indicated.
| SimplexAutismFamilies | MultiplexAutismFamilies | p Sudent’s t-test | |
|---|---|---|---|
| Affected Boys_(raw teacher-report SRS)_ | 87.8(SD=28.3)n=80 | 104.7(SD=33.8)n=262 | .0001 |
| Unaffected Boys_(raw teacher-report SRS)_ | 30.7(SD=23.5)n=89 | 40.3(SD=28.8)n=29 | .035* |
| Affected Girls_(raw teacher-report SRS)_ | 97.5(SD=36.0)n=81 | ||
| Unaffected Girls_(raw teacher-report SRS_ | 23.0(SD=16.3)n=45 | ||
| Fathers_(spouse-report SRS)_ | 23.8(SD 17.5)n=41 | 34.3(SD 23.3)n=58 | .01 |
| Mothers_(spouse-report SRS)_ | 23.9(SD 20.1)n=41 | 27.0(SD 17.8)n=58 | ns |
3. Analysis of parent-report SRS data
Means and standard deviations for the spouse-report SRS scores of parents are also presented in Table 2. MA fathers exhibited significantly higher SRS scores than SA fathers, although the effect of family type (SA versus MA) was only significant at the level of a trend in linear regression analysis controlling for father’s age, father’s educational level, and average SRS T-score of the proband. There was no appreciable difference in the SRS scores between SA mothers and MA mothers.
Finally, we examined whether spousal correlations for QATs—previously observed in the general population (Constantino and Todd, 2005) and suggestive of the presence of assortative mating—were present in the parental generation in this clinical ASD sample. For parents in all ASD families with available intergenerational data (n=99 couples), ICC was 0.26 (p<.01); there was no significant difference in spousal correlations between SA and MA families. Despite this significant level of correlation, concordantly-elevated (upper quartile) spousal pairs—whose offspring tend to exhibit elevated SRS scores in the general population (Constantino and Todd, 2005)—were no more common in this clinically-ascertained ASD research sample than would be predicted by chance: only 4 of the 58 MA couples and 2 of the 41 SA couples assessed by the SRS fell into this category.
DISCUSSION
In this first-reported study comparing quantitative autistic traits in simplex and multiplex families, we observed that the aggregation patterns of such traits in unaffected males are distinct in these two groups of families. When specifically considering the subset of boys in ASD families whose SRS scores fall below a quantitative severity threshold for level of affectedness by the independent report of a classroom teacher (in this study, SRS 60_T_), the distribution for boys in simplex autism (SA) families is characterized by a) a distinct low-scoring cluster that is not appreciable among multiplex autism (MA) boys; and b) a substantially lower mean. This observation—as well as the presence of substantial sibling correlations in MA but not SA families—provides evidence of distinctive patterns of distribution of QAT in these two types of families, consistent with the notion of distinct genetic mechanisms influencing SA versus MA autism. Quantitative autistic traits, which exhibit characteristics of an autism endophenotype, may constitute such an endophenotype exclusively among males in multiplex families. The observation in MA families that sibling QAT elevations are substantially greater than paternal elevations (not necessarily the case for other psychiatric syndromes such as bipolar disorder or schizophrenia) supports the possible contribution of interactive effects of multiple genetic loci in MA families. In other words, if specific combinations of alleles are necessary to give rise to a clinical autistic syndrome in a given child, only subsets of those allelic combinations may be present in either parent, and such subsets might be responsible for sub threshold autistic trait manifestations.
This is also the first study to examine the distribution of quantitative autistic traits in the female siblings of ASD probands. Remarkably, the aggregation of QAT that we observed in male siblings in this study and in a previously published report (Constantino et al., 2006), was not observed in female siblings; the distribution of QAT scores in MA families was distinctly bimodal, indicating that familial aggregation of QAT as measured by the SRS is restricted to males in these families. It remains possible that subtle degrees of QAT aggregation might be present in females but only appreciable in larger samples or using other methods of quantitative measurement.
This study was limited by the relatively low number of categorically-unaffected MA males (incurred by the ascertainment strategy of the AGRE sample), a relatively low number of assessed subjects in the parental generation (one which will be remedied by ongoing research), and by the unavailability of QAT measurements for female siblings in SA families (also planned for future research). The latter drawback is mitigated by the observation of a bimodal distribution for a substantial sample of females in MA families—there is little reason to suspect that females in SA families would be more likely than males to exhibit familial aggregation. Future studies involving larger numbers of families would allow greater statistical power to refine our characterization of patterns of intergenerational QAT transmission in autism-affected families, and would also allow for systematic exploration of whether more precise patterns can be identified when alternate thresholds for designation of affected versus unaffected status are considered. Although there were differences in age and proband severity between SA and MA boys in this study (higher scores for MA probands were expected on the basis of multiplex ascertainment), the age differences would have favored higher SRS scores in the SA sibs (the opposite of what was observed), and the absence of sib correlations in the SA sample suggests that the distribution for unaffected SA sibs would not have been different if the SA proband scores were high enough to match those of MA probands in this study.
Spousal correlations for QAT, consistent with assortative mating, were appreciable in both groups of autism-affected families, however ASD families did not appear to be disproportionately characterized by concordantly-elevated QAT (in both parents) in this volunteer sample. This was particularly surprising since concordant parental QAT elevations in the general population are associated with a substantial pathologic shift in the distribution of offspring QAT scores. Although these respective findings in our clinical and epidemiologic samples are somewhat difficult to reconcile, any effect of elevated adult QAT to a) inhibit childbearing; or b) reduce participation in voluntary studies, might reduce the tendency for concordant parental elevations to be observed in samples ascertained on the basis of affected children.
We note finally that in this sample, family designation based on QAT aggregation (as opposed to categorical sibling diagnosis) resulted in re-classification of 10% of families presumed simplex, into a multiple-incidence category. Such re-classification can vary as a function of where a quantitative severity threshold is set, and future research is needed to more precisely define thresholds that might optimally distinguish families with differing mechanisms of genetic transmission for autism. For now we simply conclude that if there exist non-overlapping genetic mechanisms for MA and SA families (as supported by these and other studies), research involving traditionally-defined “simplex” samples (or those involving a mix of families with and without familial aggregation) may be particularly prone to confounding influences of sample heterogeneity. Continued research on patterns of familial aggregation of core autistic traits and symptoms are warranted, especially in samples that are representative (in an epidemiologic sense) of the general population of autism-affected families, additionally incorporating (whenever possible) quantitative measurements of biological characteristics that may more closely relate to the genetic and neurobiologic causes of familial autism.
Acknowledgements
This research was supported by grants to Dr. Constantino from the National Institute of Child Health and Human Development (HD42541), the Simons Foundation, and the National Alliance for Autism Research (NAAR)/Autism Speaks (1081). We gratefully acknowledge the resources provided by the Autism Genetic Resource Exchange (AGRE) Consortium* and the participating families. The Autism Genetic Resource Exchange is a program of Autism Speaks and has been supported, in part, by grant MH64547 from the National Institute of Mental Health to Daniel H. Geschwind (PI). The authors would like to thank Jonathan Green, MD, Rosaline Newman, PhD, and two anonymous reviewers for their very helpful reviews of previous versions of this manuscript.
References
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Abbacchi AM, LaVesser PD, Reed H, Givens L, Chiang L, Gray T, Gross M, Zhang Y, Todd RD. Developmental Course of Autistic Social Impairment in Males. Dev Psychopathol. doi: 10.1017/S095457940900008X. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. J Child Psychol Psychiatry. 2004;45:719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The Social Responsiveness Scale Manual. California: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1668–1676. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Pryzbeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61(4):512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol. 2005;17:679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN, Geschwind DH. A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry. 2007;164:656–662. doi: 10.1176/ajp.2007.164.4.656. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Bailey A, Goode S, Pickles A, Robertson S, Gottesman I, Rutter M. A broader phenotype of autism: the clinical spectrum in twins. J Child Psychol Psychiatry. 1996;37:785–801. doi: 10.1111/j.1469-7610.1996.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: a comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JH, Takahashi TN, Bagby S, Sahota PK, Vaslow DF, Wang CH, Hillman RE, Farmer JE. Essential versus complex autism: definition of fundamental prognostic subtypes. Am J Med Genet A. 2005;135:171–180. doi: 10.1002/ajmg.a.30590. [DOI] [PubMed] [Google Scholar]
- Murphy M, Bolton PF, Pickles A, Fombonne E, Piven J, Rutter M. Personality traits of the relatives of autistic probands. Psychol Med. 2000;30(6):1411–1424. doi: 10.1017/s0033291799002949. [DOI] [PubMed] [Google Scholar]
- Pickles A, Bolton P, Macdonald H, Bailey A, LeCouteur A, Sim CH, Rutter M. Latent-class analysis of recurrence risks for complex phenotypes with selection and measurement error: a twin and family history study of autism. Am J Hum Genet. 1995;57(3):717–726. [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, Goodman R, Rutter M. Variable expression of the autism broader phenotype: findings from extended pedigrees. J Child Psychol Psychiatry. 2000;41(4):491–502. [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, Kalaydjieva L, McCague P, Dimiceli S, Pitts T, Nguyen L, Yang J, Harper C, Thorpe D, Vermeer S, Young H, Herbert J, Lin A, Ferguson J, Chiotti C, Wiese-Slater S, Rogers T, Salmon B, Nicholas P, Peterson PB, Pingree C, McMahon W, Wong DL, Cavalli-Sforza LL, Kraemer HC, Myers RM. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507. doi: 10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon AT, Puura K, Lehtimäki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King M, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, MacLean JE, Jones MB, Bryson SE, Zwaigenbaum L, Bartolucci G, Mahoney WJ, Tuff L. The familial aggregation of the lesser variant in biological and nonbiological relatives of PDD probands: a family history study. J Child Psychol Psychiatry. 2000;41:579–586. doi: 10.1111/1469-7610.00644. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira M, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo S, Gusella JF, Sklar P, Wu B, Daly MJ. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. the Autism Consortium. [DOI] [PubMed] [Google Scholar]