TCR-dependent regulation of lipid rafts controls naive CD8+ cell homeostasis (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 26.
SUMMARY
TCR contact with self-MHC ligands in vivo keeps T cells alive and is shown here to cause naive CD8+ cells, but not CD4+ cells, to be hypersensitive to certain γc cytokines, notably IL-2, IL-15 and IL-7. Hypersensitivity of CD8+ cells to IL-2 is dependent on a low-level TCR signal, associated with high expression of CD5 and GM1, a marker for lipid rafts, and is largely abolished by disruption of lipid rafts; by contrast, CD4+ cells express low levels of GM1 and are unresponsive to IL-2. Physiologically, sensitivity to IL-7 and IL-15 maintains survival of resting CD8+ cells. By contrast, sensitivity to IL-2 may be irrelevant for normal homeostasis but crucial for the immune response. Here, we show that TCR contact with antigen upregulates GM1 and amplifies responsiveness of naive CD8+ cells to IL-2, thereby making the cells highly sensitive to exogenous IL-2 from CD4+ T helper cells.
INTRODUCTION
Naive T cells are kept alive through continuous TCR interaction with MHC molecules complexed with various self peptides (Boyman et al., 2007; Guimond et al., 2005; Jameson, 2005). Such TCR/MHC interaction plus contact with IL-7 causes low-level signaling, which promotes long-term survival of T cells in interphase through synthesis of anti-apoptotic molecules such as Bcl-2. During lymphopenia, T cells begin to divide and differentiate into cells with features of memory cells. Such lymphopenia-induced “homeostatic” proliferation (LIP) reflects a rise in levels of IL-7 and serves to replenish the T-cell pool size.
The propensity for T cells to undergo LIP correlates with their intrinsic TCR affinity for self-MHC ligands. Thus, for CD8+ cells, naive cells from the 2C and OT-1 lines of TCR transgenic (Tg) mice have relatively high (“above-average”) affinity for self-MHC-I ligands and these cells proliferate extensively when transferred to T-deficient mice (Kieper et al., 2004; Surh and Sprent, 2000). By contrast, cells from the HY line of TCR Tg mice have very low self reactivity and fail to proliferate in lymphopenic hosts (Kieper et al., 2004; Rocha and von Boehmer, 1991). Although direct data on the extent of TCR affinity for self-MHC ligands is sparse, there appears to be a good correlation with levels of CD5. Thus, cells with relatively high self-MHC reactivity express high levels of CD5, and vice versa (Azzam et al., 2001; Azzam et al., 1998; Smith et al., 2001). This correlation may seem surprising because CD5 is generally viewed as a negative regulator of T cell function (Tarakhovsky et al., 1995). Nevertheless, expression of CD5 and various other negative regulators on T cells after positive selection is important for modulating TCR reactivity. Such TCR “tuning” is presumed to maintain the overall avidity of T cell interaction with MHC and other ligands on APC at a precise level sufficient to deliver survival signals but not to initiate entry into cell cycle, thereby preserving self-tolerance (Azzam et al., 2001; Grossman and Paul, 2001; Marquez et al., 2005; Wong et al., 2001).
IL-7-driven LIP in lymphopenic hosts is characteristically slow. Recently, a rapid form of homeostatic proliferation has been observed following T cell transfer to mice lacking components of the IL-2 receptor (Cho et al., 2007; Ramsey et al., 2008). In these hosts, naive CD8+ cells undergo massive expansion driven by the elevated concentrations of IL-15 and/or IL-2 in IL-2R-deficient hosts. Similar proliferation is induced by injection of high doses of IL-2 in normal mice (Cho et al., 2007; Kamimura and Bevan, 2007). Significantly, IL-2-induced proliferation of naive CD8+ cells is dependent on TCR/MHC interaction and is much lower with HY than 2C or OT-1 TCR Tg T cells and is substantially reduced following T cell transfer to MHC-I−/− hosts (Cho et al., 2007).
The physiological significance of naive T cell responsiveness to IL-2 and IL-15 and why such responsiveness is MHC dependent is unknown. To assess this issue we have studied stimulation of naive T cells with cytokines in vitro and examined the role of GM1-containing lipid rafts; these structures consist of cholesterol and sphingolipid-enriched microdomains on the cell membrane and serve to promote signal transduction via raft-associated receptors (Simons and Toomre, 2000). We show here that expression of lipid rafts (GM1) is especially high on CD8+ cells and correlates directly with responsiveness to cytokines and relative TCR affinity for self-MHC ligands.
RESULTS
Responses of naive T cells to cytokines in vitro
FACS-sorted naive (CD44lo) CD8+ cells prepared from lymph nodes (LN) of C57BL/6 (B6) mice (Figure S1A) were cultured in vitro with cytokines in the absence of APC. Of 11 cytokines tested, only IL-2 and IL-15 induced proliferation of CD8+ cells as defined by CFSE dilution (Figure 1A) or [3H]thymidine incorporation (Figure 1B); these cytokines were stimulatory only for CD8+ cells and not CD4+ cells (Figures 1A and 1B). Proliferation of CD44lo CD8+ cells by IL-2 or IL-15 required quite high concentrations of cytokines, i.e., 0.1–1 μg/ml, which was 10–100-fold higher than for stimulation of memory-phenotype CD44hi CD8+ cells (Figures S1B and S1C). For CD44lo cells, responses to cytokines occurred slowly and reached a peak on day 6–7 (Figure 1C and Figure S1D); viability of the cells was close to 100% (Figure 1D). In control cultures stimulated by CD3 ligation (cross-linked anti-CD3 mAb), proliferative responses were high on day 2 and then declined to low levels by day 4 (Figure 1C), in parallel with a sharp decline in cell viability (Figure 1D).
Figure 1.
Proliferation and differentiation of naive CD8+ cells exposed to cytokines in vitro. (A and B) CFSE-labeled (A) or unlabeled (B) purified naive (CD44lo) B6 CD4+ or CD8+ T cells were cultured with the indicated cytokines (all 1 μg/ml except IFNβ used at 2 × 104 units/ml) and analyzed on day 5 for proliferation by flow cytometry (A) and [3H]thymidine incorporation (B). (C and D) Proliferation kinetics (C) and viable cell counts (D) for purified B6 naive CD8+ cells cultured with cross-linked anti-CD3 mAb (5 μg/ml), IL-2 (1 μg/ml) or IL-15 (1 μg/ml) by [3H]thymidine uptake (C) and trypan blue exclusion (D). (E) Proliferation of naive B6, 2C or OT-I CD8+ cells on day 5 after culture with or without 1 μg/ml IL-2 or IL-15 (F) Expression of activation markers on B6 naive CD8+ cells stimulated with cross-linked anti-CD3 mAb (5 μg/ml) or IL-2 (1 μg/ml). (G) Intracellular cytokine production and granzyme B expression were analyzed for naive B6 CD8+ cells cultured for 3 days with the indicated stimuli (1st stimuli) as described in Supplemental Experimental Procedures. Data (A–G) are representative of at least three independent experiments (B–E; mean and SD of triplicate samples).
Proliferation of naive CD8+ cells by IL-2 vs. IL-15 was almost identical, although responses were generally slightly lower with IL-15 than for IL-2, both for polyclonal B6 as well as for OT-I and 2C TCR Tg CD8+ cells (Figure 1E). Both cytokines were used for nearly all of the experiments discussed below, with comparable results. For simplicity, only the data for IL-2 are shown.
Naive CD8+ cells stimulated with IL-2 (or IL-15) alone showed slow upregulation of a variety of typical markers found on activated CD8+ cells, including CD25, CD44 and CD69 (Figure 1F). Significantly, IL-2-stimulated CD8+ cells showed strong effector function in terms of both cytokine (IFNγ and TNFα) and granzyme B synthesis (Figure 1G). This finding was surprising because the cells were not subjected to TCR ligation. The influence of TCR signaling is discussed below.
IL-2 stimulation and the requirement for TCR/MHC interaction
Since IL-2-induced proliferation of naive CD8+ cells in vivo required TCR interaction with self-MHC-I (Cho et al., 2007), IL-2 responses by purified naive CD8+ cells in vitro might depend upon some form of T-T interaction. A requirement for cell-cell interaction via costimulatory/adhesion molecules seems unlikely because responses of 2C cells to IL-2 were as high with LFA-1−/− cells and CD28−/− cells as with normal wild-type (WT) cells (Figures S2A and S2B).
As discussed earlier, CD8+ cells from the HY TCR Tg line are thought to have much lower intrinsic TCR affinity for self-MHC-I ligands than 2C, OT-I, or P14 CD8+ cells (Ernst et al., 1999; Kieper et al., 2004; Rocha and von Boehmer, 1991). In marked contrast to B6 and P14 cells, purified CD44lo CD8+ HY cells gave negligible proliferative responses to IL-2 in vitro and failed to differentiate into effector cells (Figures 2A and 2B); by contrast, all three populations gave equivalent responses to anti-CD3 mAb (see below).
Figure 2.
Response of naive CD8+ cells to IL-2 depends on TCR/self-MHC-I interaction. (A and B) Proliferation (A) and CFSE dilution and granzyme B expression (B) of naive B6, HY (from HY.Rag−/− mice) or P14 CD8+ cells on day 6 after culture with various (0.05–1.5 μg/ml; A) or fixed (1.5 μg/ml; B) concentrations of IL-2. (C) Proliferation of purified MHC-I+/+ (from normal B6) or MHC-I−/− (from MHC-I−/−→B6 BM chimeras) naive CD8+ cells on days 3 and 6 after stimulation with IL-2. (D and E) CFSE dilution and granzyme B expression of purified MHC-I+/+ (Thy1.1; from B6) and MHC-I−/− (Thy1.2; from BM chimeras in C) naive CD8+ cells either cultured separately (D) or cocultured (E) for 5 days with IL-2. (F) Proliferation of naive CD8+ cells from B6 (MHC-I+/+), BM chimeras in C (MHC-I−/−) or MHC-I−/− mice injected with MHC-I−/− CD8+ cells (from BM chimeras in C) 3 days before (MHC-I−/− → MHC-I−/−) was analyzed on day 4 after culture with IL-2. (G) Proliferation of B6 naive CD8+ cells (Ly5.1) parked for 3 days and recovered from B6 (B6 → B6), IL-7−/− (B6 → IL-7−/−) or TAP-1−/− (B6 → TAP-1−/−) mice on day 4 after culture with IL-2 or on day 3 with cross-linked anti-CD3 mAb. Data (A–G) are representative of 2–3 experiments (C, F and G; mean ± SD of triplicate samples).
Direct evidence that IL-2 responses in vitro were MHC-I dependent came from studies with MHC-I−/− CD8+ cells, which were prepared from bone marrow (BM) chimeras (Figure S3A). As shown in Figure 2C, responses of CD44lo CD8+ cells to IL-2 were much lower for MHC-I−/− cells than for WT cells; CD3 responses were unimpaired (Figure S3B). These findings applied when the cells were cultured separately, as shown for CFSE dilution vs. granzyme B synthesis in Figure 2D. In marked contrast, both populations gave equivalent responses to IL-2 when MHC-I+/+ and MHC-I−/− cells were cocultured (Figure 2E). These findings indicated that IL-2 responses in vitro required TCR/MHC-1 interaction via cell-cell contact.
The residual response of MHC-I−/− CD8+ cells to IL-2 in vitro declined further when MHC-I−/− CD8+ cells from chimera donors were parked for 3 days in MHC-I−/− hosts, thus depriving the cells of all MHC-I contact (Figure S3C). Such short-term parking reduced IL-2 responsiveness of the transferred cells by about 4-fold relative to fresh MHC-I−/− cells from chimeras, and by 10-fold relative to cells from normal mice (Figure 2F). Further evidence on this issue came from experiments in which normal MHC-I+/+ CD8+ cells were parked for 3 days in TAP-1−/− (MHC-Ilo) mice, with normal B6 and IL-7−/− mice as controls; nonirradiated hosts were used as hosts, thus limiting the opportunity for T-T interaction between the donor cells. The notable finding was that IL-2 responsiveness was 5–10-fold lower for CD8+ cells parked in TAP-1−/− hosts than in normal B6 or IL-7−/− mice (Figure 2G); by contrast, responses to CD3 ligation were unimpaired.
The above data provide strong evidence that CD8+ cell responses to IL-2 in vitro required continuous TCR/MHC-I interaction. Preventing this interaction during culture reduced but did not abolish IL-2 responsiveness, apparently because CD8+ cells retained “memories” of the TCR signals encountered during their prior interaction with self-MHC-I ligands in vivo.
Influence of CD5 on responsiveness to cytokines
As mentioned earlier, TCR Tg CD8+ cells exhibiting poor homeostatic proliferation in T-deficient hosts have low CD5 expression, and vice versa (Kieper et al., 2004). These findings raised the possibility that the responsiveness of normal polyclonal B6 CD8+ cells to IL-2 in vitro would correlate with their relative expression of CD5. To assess this idea, CD44lo CD8+ B6 cells were FACS-sorted into CD5lo and CD5hi cells (Figure S4A). Despite giving similar responses to CD3 ligation (Figure 3A), B6 CD5lo cells gave far lower responses than CD5hi cells to IL-2 (Figure 3B) and failed to upregulate activation markers in response to IL-2 (Figure 3C). Similar findings applied to CD5lo vs. CD5hi CD8+ cells from 2C and HY Tg mice (Figure 3D and data not shown); note that the HY mice used were on a normal (not RAG−/−) background and thus contained a mixture of TCR-clonotype-negative (T3.70−) cells, nearly all CD5hi, as well as T3.70+ cells, mostly CD5lo.
Figure 3.
Levels of CD5 on naive CD8+ cells correlate with the strength of responsiveness to cytokines in vitro and in vivo. (A–D) Purified naive CD5lo and CD5hi CD8+ cells from B6 (A–D), 2C (A and D) or HY (from HY.Rag+/+ mice; A and D) mice were cultured with cross-linked anti-CD3 mAb (for 3 days; A) or IL-2 (for days 3 and 5, B; for day 5, C; and for day 6, D) and analyzed for proliferation (A, B and D) or for expression of activation markers (C). (E) CFSE-labeled purified B6 naive CD5lo (Ly5.1) and CD5hi (Thy1.1) CD8+ cells were cotransferred into irradiated B6 or RAG−/− mice and 6 days later, pooled SP and LN were analyzed for CFSE dilution (left), percentage of donor cells that underwent >3 rounds of division, and total donor cell recovery (middle and right, respectively; mean and SD of three mice per group). (F) Proliferation of CFSE-labeled B6 naive CD5lo and CD5hi CD8+ cells on day 7 after culture with IL-7 (50 ng/ml), IL-12 (50 ng/ml) or both. Data (A–F) are representative of 2–3 experiments (A, B and D; mean ± SD of triplicate samples).
As for TCR Tg lines (see above), the subset of polyclonal CD5hi naive CD8+ cells from B6 mice gave stronger homeostatic proliferation in T-depleted hosts than CD5lo cells (Figure 3E). Since proliferation in T-depleted hosts is driven by high levels of IL-7, the implication is that CD5hi cells are hypersensitive to IL-7. This question was difficult to address in vitro because IL-7 caused minimal proliferation of naive CD8+ cells in culture (Figures 1A and 1B). Nevertheless, cell-sorting studies showed that the minor subset of CD5hi CD8+ cells did give significant proliferation to IL-7 in vitro (Figure 3F). Interestingly, these responses were considerably enhanced by the addition of IL-12, perhaps mimicking the cytokine environment encountered in vivo (see Discussion); responses with CD5lo cells were much lower.
Collectively, these findings indicated that the hyperresponsiveness of CD5hi cells to cytokines applied to IL-7 as well as to IL-2. Conversely, CD5lo cells responded poorly to both cytokines, while retaining strong reactivity to TCR/CD3 ligation. Similar findings applied to IL-15. Thus, following injection into irradiated hosts, proliferative responses to IL-15 were substantially higher for CD5hi than CD5lo cells, especially in IL-7−/− hosts (Figure S4B).
GM1 and the role of lipid rafts
The simplest explanation for the hyperrsiveness of CD5hi cells to cytokines is that these cells have higher levels of cytokine receptors than CD5lo cells. However, this possibility is unlikely because the expression of CD122 (IL-2Rβ was only slightly lower on CD5lo than CD5hi cells and there was no significant difference in the expression of CD127 (IL-7Rα) or CD132 (γc) (Figures S4C and S4D). For CD122 expression, cell sorting for subsets of CD5hi and CD5lo cells that expressed the same density of CD122 did not affect the much higher response of CD5hi cells to IL-2 (Figure S4E).
In considering other possibilities, it is striking that naive CD8+ cells responded strongly to IL-2 despite only low expression of IL-2Rβ, relative to memory CD8+ cells (Zhang et al., 1998). One explanation for this paradox is that binding of IL-2 to low levels of IL-2Rβ causes this receptor to move into lipid rafts, thereby enhancing signal transduction (Simons and Toomre, 2000). To assess this idea, naive CD8+ cells were pretreated with methyl-β-cyclodextrin (MβCD) to disrupt lipid rafts before culture with IL-2. Significantly, this treatment substantially reduced proliferation to IL-2, but caused only a small decrease in CD3 responses (Figures 4A and 4B). This finding raised the possibility that the strong response of CD5hi CD8+ cells to IL-2 correlated with high expression of lipid rafts. This was indeed the case. Thus, using cholera toxin B subunit (CTB) to detect GM1 in lipid rafts, GM1 expression on CD44lo CD8+ cells was significantly higher on CD5hi cells than CD5lo cells, both for HY.Rag+/+ cells (Figure 4C and Figure S5A), OT-I and 2C cells (Figure S5B), and normal B6 cells (Figure 4D and Figure S5C). There was a close correlation between CD5 and GM1 levels, CD5hi cells being uniformly GM1hi, and CD5lo cells being GM1lo (Figures S5A-S5D). Memory CD44hi CD8+ cells were CD5hi and GM1hi (Figures 4C and 4D).
Figure 4.
GM1 expression on T cell subsets, and the effects of disrupting lipid rafts on the ability of naive CD8+ cells to respond to IL-2. (A and B) Proliferation (A) and % inhibition of proliferation (B) of MβCD-treated purified naive B6 CD8+ cells were analyzed on day 2 after culture with the indicated stimuli as described in Supplemental Experimental Procedures. Data show the mean ± SD of triplicate samples. (C) GM1 levels (MFI) on CD44hi CD8+, CD44lo CD5lo vs. CD44lo CD5hi CD8+ cells from HY.Rag+/+ mice analyzed by flow cytometry. Data show the mean and SD of five mice. (D) GM1 MFI levels on CD44lo vs. CD44hi subsets (left) or naive (CD44lo) CD5lo vs. CD5hi subsets (right) of B6 CD4+ and CD8+ cells. Each circle represents an individual mouse and the line indicates the mean. (E) Proliferation of purified B6 naive GM1lo and GM1hi CD8+ cells on day 3 after culture with IL-2 or on day 1 with cross-linked anti-CD3 mAb. Data show the mean ± SD of triplicate samples. (F) CFSE-labeled purified B6 naive GM1lo (Ly5.1) and GM1hi (Thy1.1) CD8+ cells were cotransferred into irradiated B6 mice and 7 days later, pooled SP and LN were analyzed for CFSE dilution (left), % of donor cells that underwent >4 rounds of division, and total donor cell recovery (middle and right, respectively; mean and SD of three mice). Data (A–F) are representative of 2–3 independent experiments.
For naive CD8+ cells, MβCD treatment did not affect expression of CD5, CD122 or CD132 (data not shown). Hence, IL-2 responsiveness was not the direct result of high CD5 expression per se; likewise, the effects of MβCD treatment did not reflect decreased IL-2R expression.
FACS-sorting CD44lo CD8+ cells for GM1 expression (Figure S5E) showed that sorted GM1hi cells closely resembled sorted CD5hi cells. Thus, GM1hi cells were 10-fold more sensitive to IL-2 in vitro (Figure 4E, left) and showed stronger homeostatic proliferation in T-deficient hosts (Figure 4F) than GM1lo cells; both populations gave similar responses to CD3 ligation (Figure 4E, right). GM1hi CD8+ cells also gave stronger proliferation to IL-7/IL-12 in vitro than GM1lo cells (Figure S5F). In general, results were “cleaner” with sorted CD5hi and CD5lo cells than with sorted GM1hi and GM1lo cells, reflecting better cell separation for CD5 than GM1 expression (Figure S4A and Figure S5E).
GM1 and thymic selection
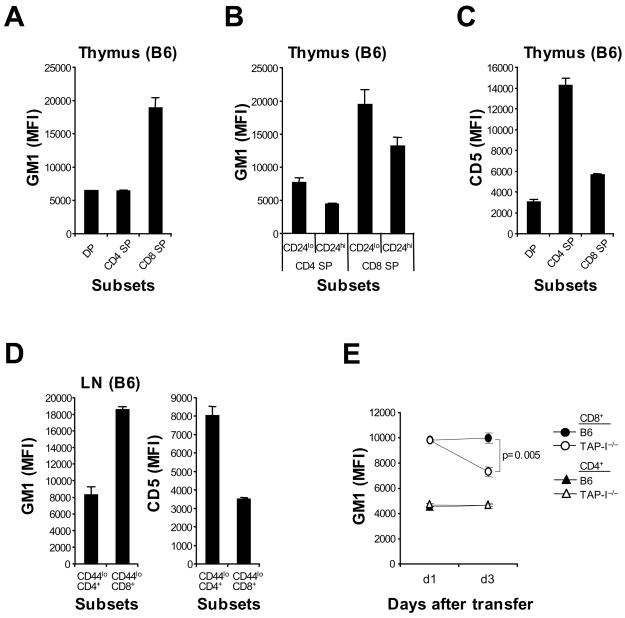
For B6 thymocytes, GM1 expression was low on “double-positive” (DP) cells but high on “single-positive” (SP) CD4− CD8+ cells (Figure 5A). For SP CD8 thymocytes, GM1 expression was higher on fully-mature CD24 (HSA)lo cells than on less-mature CD24hi cells (Figure 5B). Thus, GM1 was upregulated on CD8+ cells during positive selection and increased progressively during maturation of these cells. Likewise, as described elsewhere (Azzam et al., 1998), CD5 expression was low on DP cells and increased moderately on SP CD8 cells during their maturation into CD24lo cells (Figure 5C and data not shown). The data on CD4+ cells were quite different. Thus, differentiation of DP cells into mature SP CD4 cells led to very high expression of CD5 (much higher than on CD8+ cells) but to only a minor increase in GM1 expression (Figures 5A–5C and data not shown). This phenotype was retained by fully-mature naive LN CD4+ cells, indicating that the complete unresponsiveness of these cells to IL-2 (Figures 1A and 1B) correlated with very high CD5 expression and very low GM1 expression (Figure 5D).
Figure 5.
Expression of GM1 and CD5 on T cell subsets during ontogeny. (A–C) GM1 (A and B) and CD5 (C) MFI levels on B6 DP, SP CD4+ and SP CD8+ thymocytes (A and C) or on CD24lo vs. CD24hi subsets of SP CD4+ and CD8+ thymocytes (B). (D) GM1 (left) and CD5 (right) MFI levels on CD44lo CD4+ vs. CD44lo CD8+ cells in B6 LN. Data (A–D; mean and SD of three to five mice) are representative of 3 experiments. (E) GM1 MFI levels of B6 naive CD4+ and CD8+ cells (Ly5.1) cotransferred into normal B6 or TAP-1−/− mice 1 or 3 days before; pooled SP and LN were analyzed by flow cytometry. Data (mean ± SD of 2–3 mice per group at each time point) are representative of 3 experiments.
GM1 and MHC-I dependency
The reduction in IL-2 responsiveness that occurred when CD8+ cells were deprived of MHC-I contact (Figures 2F and 2G) correlated with a decrease in GM1 expression. Thus, parking CD44lo B6 T cells for 3 days in normal B6 vs. TAP-1−/− mice caused a significant, 30%, decrease in GM1 expression on the donor CD8+ cells, but not CD4+ cells, in TAP-1−/− hosts (Figure 5E and Figure S6). By contrast, CD5 expression remained unchanged or decreased only slightly and there was no decline in CD122 (data not shown).
Colocalization of GM1 and IL-2Rβ
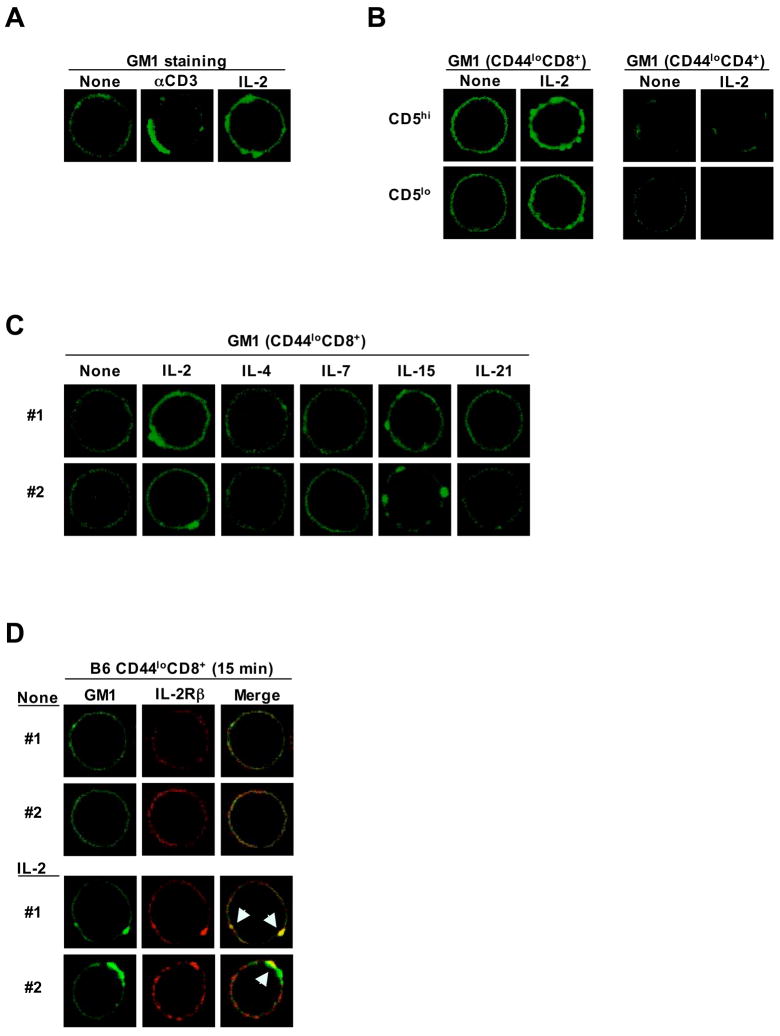
Confocal microscopy showed prominent clustering of cell-surface GM1 when naive CD8+ cells were stimulated with either IL-2 or anti-CD3 mAb (Figure 6A and Figure S7A). GM1 clustering was more noticeable with CD5hi than CD5lo CD8+ cells and, because of minimal staining, was not apparent with CD4+ cells (Figure 6B). For CD8+ cells, culture with IL-2 or IL-15 induced intense GM1 clustering on nearly all of the cells (Figure 6C and Figure S7A). Significant but weaker clustering was induced by IL-7, though only on about 20% of the cells (Figure S7B); this finding correlated with IL-7 responsiveness in vitro being restricted to the minor subset of CD5hi cells (Figure 3F). No GM1 clustering was observed with IL-4 or IL-21 (Figure 6C). Of particular interest was the finding that IL-2 stimulation caused coclustering of GM1 with IL-2Rβ (Figure 6D and Figure S7C); such coclustering was especially prominent with CD5hi cells (Figure S7D). By contrast, there was no coclustering of CD5 with either GM1 or IL-2Rβ (data not shown). With regard to the mechanisms involved, GM1 clustering was not associated with an increase in GM1 synthesis (Figure S7E), but was abolished by addition of cytochalasin D (but not by actinomycin D or cyclohexamide), suggesting that clustering was dependent on actin polymerization and redistribution of pre-existing GM1 on the plasma membrane (Figure S7F). Correlating with the extent of GM1/IL-2Rβ coclustering (Figure S7D), the proximal signaling events induced by IL-2 (phosphorylation of Stat5, ERK and AKT) were clearly more prominent in CD5hi than CD5lo cells (Figure S8).
Figure 6.
Culturing naive CD8+ cells with IL-2 induces lipid raft (GM1) clustering and colocalization of GM1 with IL-2Rβ. (A) B6 naive CD8+ cells were untreated or treated for 15 min with the indicated stimuli and analyzed for lipid raft clustering by GM1 confocal staining with FITC-conjugated CTB (green). (B) GM1 confocal staining of CD5lo vs. CD5hi subsets of B6 naive CD4+ and CD8+ cells untreated or treated for 15 min with IL-2 (1 μg/ml). (C) GM1 confocal staining of B6 naive CD8+ cells cultured for 15 min with or without the indicated γc cytokines (1 μg/ml). (D) B6 naive CD8+ cells were cultured for 15 min with IL-2 (1 μg/ml) and analyzed for colocalization (yellow when merged) of GM1 lipid rafts (green) and IL-2Rβ (red). Data (A–D) are representative of 2–3 experiments.
Collectively, the above findings indicated that the strong responsiveness of naive CD8+ cells to cytokines correlated with high expression of GM1 – and presumably lipid rafts – such expression being maintained by weak TCR signals arising from continuous TCR interaction with self-MHC-I ligands. Physiologically, sensitivity of naive CD8+ cells to IL-7 is known to be crucial for maintaining cell viability (Boyman et al., 2007; Guimond et al., 2005; Jameson, 2005). For IL-2, however, there is no evidence that this cytokine is involved in the normal homeostasis of naive CD8+ cells. As discussed below, hyperresponsiveness of CD8+ cells to IL-2 may only be physiologically important when these cells begin to respond to foreign antigens.
Sensitivity of naive CD8+ cells to IL-2 augments helper-dependent responses to foreign antigens
Optimal responses of CD8+ cells to antigen require the presence of “help” in the form of IL-2 from CD4+ cells (Malek, 2002; Rocha and Tanchot, 2004; Williams et al., 2006; Wilson and Livingstone, 2008). With strong antigens or TCR/CD3 ligation in vitro, CD8+ cells synthesize their own IL-2, and help from CD4+ cells is not needed for proliferation (Figure 3A). With weak antigens, however, IL-2 synthesis by CD8+ cells is limited and proliferation requires the addition of exogenous IL-2 (Cai and Sprent, 1994). Hence, effective responses to weak antigens must depend on the cells being highly sensitive to IL-2. This issue is addressed below.
To mimic responses to weak antigens, CD5lo vs. CD5hi CD44lo CD8+ cells were cultured with soluble (not cross-linked) anti-CD3 mAb in vitro together with graded low doses of IL-2 (< 10 ng/ml) (Figure 7A). With anti-CD3 mAb alone, proliferation was undetectable; likewise, there was negligible proliferation with low-dose IL-2 alone. With anti-CD3 mAb plus IL-2, by contrast, strong proliferative responses occurred. Significantly, CD5hi cells were 10–20-fold more sensitive to the added IL-2 than CD5lo cells. The conclusion therefore is that TCR ligation with mAb considerably augmented responsiveness to IL-2, both for CD5hi and CD5lo cells.
Figure 7.
Hypersensitivity of CD8+ cells to IL-2 augments their capacity to respond to foreign antigens. (A) Proliferation of B6 naive CD5lo and CD5hi CD8+ cells to a surrogate weak antigen; cells were cultured for 2 days with or without soluble anti-CD3 mAb (0.1 μg/ml) with graded concentrations of IL-2 (0.3–10 ng/ml). Data show the mean ± SD of triplicate samples. (B) Proliferation of CFSE-labeled 2C naive CD5lo (Ly5.1) vs. CD5hi (Thy1.1) CD8+ cells to a strong antigen; cells were cultured with allogeneic BALB/c splenocytes with or without IL-2 blockade and analyzed by flow cytometry. (C) Proliferation of CFSE-labeled 2C naive CD5lo (Ly5.1) vs. CD5hi (Thy1.1) CD8+ cells to a weak antigen; cells as in B were cocultured with CFSE-labeled OT-II naive CD4+ cells and syngeneic B6 splenocytes pulsed with 20 μM p2Ca peptide with or without OVA323-339 peptide (OT-IIp). (D) Surface markers on T cells after TCR stimulation were examined by culturing CD44lo CD8+ and CD4+ cells for 20 hr in plates coated with graded concentrations of anti-CD3 mAb and then stained for the markers shown. Data (A–D) are representative of 2–4 experiments.
To examine responses to cognate antigens, subsets of naive 2C CD8+ cells were cultured with strong vs. weak ligands recognized by the 2C TCR. For strong ligands, 2C cells (H-2b) were cultured with BALB/c (H-2d) spleen cells, which present highly-immunogenic endogenous p2Ca peptides bound to Ld (Sykulev et al., 1998); in this situation, the responding CD8+ cells synthesize their own IL-2 and do not need exogenous IL-2 to proliferate. As measured by CFSE dilution, proliferation was clearly stronger with CD5hi than CD5lo cells (Figure 7B). Proliferation was blocked by IL-2 blockade, indicating that the response was IL-2 dependent.
To examine helper-dependent responses, 2C cells were stimulated with a very weak ligand, namely exogenous p2Ca peptide presented by B6 (H-2b) spleen cells (Figure 7C); in this situation, p2Ca peptide is presented in poorly-immunogenic form bound to Kb (Sykulev et al., 1998). Both on day 3 and day 5 of culture, 2C CD8+ cells failed to proliferate, presumably because the peptide was too weak to induce the cells to synthesize IL-2. To provide a source of exogenous help, the 2C cells were supplemented with OT-II CD4+ cells with or without specific OVA323-339 peptide (which is presented bound to I-Ab by B6 spleen (Robertson et al., 2000)). Here, the key finding was that addition of OVA peptide to the mixture of 2C CD8+ cells and OT-II CD4+ cells led to strong proliferation of the 2C cells (as well as of the OT-II cells which were also CFSE-labeled), reflecting IL-2 synthesis by the CD4+ cells. Again, proliferation was more prominent for CD5hi than CD5lo cells (Fig 7C); for both cell types, proliferation was minimal with a low dose of p2Ca peptide (Figure S9A), indicating that the CD8+ response was p2Ca peptide dependent. Responses of 2C cells were abolished by IL-2 blockade (but not IL-21 blockade), indicating that help was IL-2 dependent (Figure S9B). Also, 2C responses were substantially reduced by pretreating these cells with MβCD, implicating lipid rafts (Figure S9B).
To examine helper-dependent responses in vivo, 2C CD8+ cells were injected into normal B6 mice together with moderately immunogenic Kb-restricted SIYRYYGL (SIYR) peptide (Udaka et al., 1996). At the very low concentrations of peptide used, there was no proliferation of 2C cells (Figures S10A and S10B). Significantly, when exogenous IL-2 was injected as a surrogate for CD4+ help, the 2C cells proliferated; proliferation was peptide dose dependent, higher for CD5hi than CD5lo cells (Figure S10A) and was not seen in the absence of peptide (Figure S10B). These in vivo findings thus correlated closely with the in vitro studies.
In the above experiments, the increased reactivity of CD8+ cells to IL-2 induced by TCR contact with antigen might reflect increased synthesis of lipid rafts. In support of this notion, weak CD3 mAb ligation of naive CD8+ cells (but not CD4+ cells) led to a marked (~50%) increase in expression of GM1 by 20 hr of culture but little change in CD122 or CD127 expression (Figure 7D). By confocal microscopy, TCR ligation also potentiated GM1 clustering after addition of IL-2 (Figure S11).
The conclusion from these experiments is that the strong responsiveness of resting naive CD8+ cells to IL-2 was further enhanced by TCR contact with foreign antigens, thereby improving the immune response to both strong and weak antigens. With strong antigens the cells produced their own IL-2, whereas with weak antigens the cells needed IL-2 from CD4+ cells. In both situations, CD5hi cells gave better responses than CD5lo cells.
DISCUSSION
Past studies showed that purified human naive CD4+ and CD8+ cells could be driven to proliferate by a cocktail of cytokines and was intensified by addition of APC or APC supernatants (Geginat et al., 2003; Geginat et al., 2001; Unutmaz et al., 1994). For CD8+ cells, naive cells responded to IL-15 alone in one study (Alves et al., 2003) but only to a mixture of IL-7 and IL-15 in another study (Geginat et al., 2003). In this paper, we show that culturing purified naive mouse CD8+ cells with IL-2 in vitro led to strong proliferation and differentiation into effector cells in the absence of APC. Similar results were seen with IL-15, but other cytokines were essentially nonmitogenic, with the exception of very weak stimulation by IL-7 – see below. These findings applied to CD8+ cells. For CD4+ cells, individually none of the cytokines tested, including IL-2 and IL-15, were able to stimulate purified populations of naive CD4+ cells.
Stimulation with IL-2 (or IL-15) alone in vitro was highly effective in causing naive CD8+ cells to differentiate into effector cells. This finding was surprising because the cells were not subjected to TCR ligation. Nevertheless, the studies with MHC-I−/− CD8+ cells indicated that IL-2 responses did require a covert TCR signal. Thus, IL-2 responses in vitro were low with MHC-I−/− CD8+ cells, and were even lower when these cells were deprived of all MHC-I contact by transfer to MHC-I−/− mice. Significantly, the unresponsiveness of MHC-I−/− CD8+ cells to IL-2 was completely restored by coculture with normal MHC-I+/+ CD8+ cells, implying that the TCR signals needed for IL-2 responses resulted from low-level “background” TCR contact with MHC-I on neighboring T cells. Further evidence that IL-2 responsiveness required TCR/MHC-I interaction came from the finding that parking normal B6 CD8+ cells briefly in TAP-1−/− mice led to a marked decline in IL-2 responses. Which particular cells present the MHC-I ligands recognized by resting CD8+ cells in vivo is unknown.
It is of interest that IL-2 responsiveness correlated with high expression of CD5. The prior finding that high CD5 expression on naive TCR Tg CD8+ cells was associated with strong homeostatic proliferation in T-depleted mice (Kieper et al., 2004) led to the current view that high CD5 expression is a manifestation of “above-average” TCR affinity for self-MHC epitopes. Since homeostatic proliferation in lymphopenic hosts is driven by elevated levels of IL-7, the implication is that T cells with strong self reactivity are hypersensitive to IL-7. The studies with CD5lo vs. CD5hi subsets of polyclonal B6 naive CD8+ cells were consistent with this possibility. Thus, homeostatic proliferation of CD5hi cells in vivo and proliferative responses to IL-7 in vitro were both much stronger with CD5hi cells than CD5lo cells; in vitro responses to IL-7 were very low unless supplemented with IL-12, implying that responses to IL-7 in vivo may require additional cytokines. The key point, however, is that the hypersensitivity of CD5hi cells to IL-7 also applied to IL-2 (and IL-15). Hence, the correlation between strong self-MHC reactivity and heightened sensitivity to cytokines applies to at least three different cytokines.
The observation that responses to cytokines correlated directly with cell-surface expression of GM1 implicated lipid rafts. Direct evidence in support of this notion came from the finding that treatment of naive CD8+ cells with MβCD to disrupt lipid rafts led to a marked decrease in IL-2 responses. Notably, confirming previous findings (de Mello Coelho et al., 2004) GM1 expression was far higher on naive CD8+ cells than CD4+ cells. By contrast, the reverse applied to CD5 expression. Hence, IL-2 responsiveness correlated well with GM1 expression but not with CD5 expression. Also, in marked contrast to the findings with GM1, IL-2 stimulation caused no association of CD5 with IL-2Rβ by confocal microscopy, implying that CD5 expression per se is not involved in cytokine responsiveness: high CD5 expression on CD8+ cells is simply a marker for high GM1 expression.
It is of interest that GM1 expression in the thymus was low on DP cells and SP CD4 cells but high on SP CD8 cells. For the latter, GM1 expression was higher on CD24lo cells than CD24hi cells, implying that expression reached maximal levels at a late stage of positive selection. Thereafter, GM1 expression remained constant when the cells were exported to the periphery. Significantly, GM1 levels declined after cell transfer to TAP-1−/− mice, indicating that maintenance of GM1 expression required continuous TCR/MHC-I interaction. Collectively, these findings indicate that positive selection to strong self-MHC-I ligands results in prominent upregulation of GM1 which, in turn, leads to hypersensitivity to cytokines. Precisely how TCR signals promote and maintain GM1 expression, however, is unclear.
The finding that cytokine responsiveness correlated with expression of GM1, a lipid raft marker, begs the question of the biological function of lipid rafts. For T cells, the prevailing view is that movement of signaling molecules such as Lck and other TCR-associated molecules into lipid rafts augments intracellular signaling (Harder, 2004). The distribution of cytokine receptors in lipid rafts, however, is controversial and information on this topic largely concerns activated T cells (Bodnar et al., 2008; Goebel et al., 2002; Marmor and Julius, 2001; Vamosi et al., 2004). For naive CD8+ cells, IL-2Rβ is found mostly in the soluble-membrane fraction in resting cells but in the lipid raft fraction after short-term culture with IL-2 (unpublished data of the authors). The colocalization studies shown here are in line with these findings. Thus, culturing naive CD8+ cells with IL-2 led to rapid colocalization of IL-2Rβ with GM1, implying entry of this receptor into lipid rafts. This could allow association with Lck, which is important for IL-2Rβ signaling (Hatakeyama et al., 1991; Minami et al., 1993). Although, definitive evidence on the role of lipid rafts in IL-2 signaling will need future studies, it is notable that the selective loss of GM1 and other complex gangliosides in GM2/GD2 synthase-deficient mice led to a marked reduction in whole spleen cell responses to IL-2 but normal responses to CD3 ligation (Zhao et al., 1999). This finding fits well with the data on naive CD8+ cells reported here.
With regard to physiological significance, contact with IL-7 is important for inducing expression of anti-apoptotic molecules such as Bcl-2, thereby maintaining cell viability. But what is the significance of CD5hi (and GM1hi) cells, i.e., cells with strong affinity for self ligands, being more responsiveness to IL-7 than CD5lo cells? On this point, CD5hi cells have substantially higher levels of pro-apoptotic molecules such as Bim than CD5lo cells (unpublished data of the authors). However, CD5hi cells also have higher levels of Bcl-2 than CD5lo cells. Hence, to counter the negative effects of high Bim expression, one can envisage that cells with above-average TCR affinity for self ligands need to be especially sensitive to IL-7 to ensure high Bcl-2 expression; thus, the cells have to express high levels of GM1. Conversely, cells with strong self reactivity need to express high levels of CD5, a negative regulator of TCR signaling, in order to prevent breakage of self tolerance. Therefore, for normal homeostasis, cells with strong self reactivity have to be both GM1hi (for viability) and CD5hi (for self tolerance).
With regard to other cytokines, numbers of naive CD8+ cells are reduced in IL-15−/− mice (Kennedy et al., 2000). Hence, CD8+ cell viability may be maintained in part by responsiveness to IL-15, as well as IL-7. For IL-2, however, contact with this cytokine is not known to influence homeostasis of naive CD8+ cells. So, what is the benefit of these cells being responsive to IL-2? Our suggestion is that the strong responsiveness of naive CD8+ cells to IL-2 is only important when these cells respond to foreign antigens. On this point, it is well established that CD8+ cell responses to antigen generally require help from CD4+ cells. CD4+ help involves several mechanisms, including stimulation (“licensing”) of APC (Ridge et al., 1998; Schoenberger et al., 1998) and release of cytokines, especially IL-2 (Williams et al., 2006; Wilson and Livingstone, 2008) but also IL-21 (Elsaesser et al., 2009). For primary responses, CD8+ cells produce their own IL-2 in response to strong antigens, and these responses are generally helper independent. With weak antigens, by contrast, CD8+ cell responses are heavily dependent on help from CD4+ cells. Here, help reflects the release of IL-2, both in vitro and in vivo (Cai and Sprent, 1994; Wilson and Livingstone, 2008).
As shown here, responses of naive CD8+ cells to antigen were heavily dependent on IL-2. With strong antigens, the CD8+ cells produced their own IL-2, whereas with weak antigens CD8+ cell responses relied on exogenous IL-2 produced by adjacent CD4+ T helper cells, these cells being engaged in responses to a different antigen. The helper-driven response of the CD8+ cells was antigen dependent, indicating that the response required TCR ligation and was not elicited by IL-2 alone. Hence, TCR contact of CD8+ cells with antigen enhanced their sensitivity to IL-2. Based on the expression of GM1, TCR ligation appeared to augment IL-2 responsiveness by inducing increased expression of lipid rafts. It is noticeable that CD8+ cell responses to antigen were higher with CD5hi than CD5lo cells. The interesting implication is that, especially for weak antigens, CD8+ cell responses may preferentially involve CD5hi cells, i.e., cells with high self reactivity. In vivo studies will be necessary to assess this possibility.
In conclusion, we show here that, after positive selection in the thymus, continuous contact of naive CD8+ cells with self-MHC-I ligands in the periphery induces covert TCR signals that promote sensitivity to several γc cytokines, including IL-7 and IL-2. Responsiveness to cytokines is most prominent for CD5hi cells, i.e., cells with strong self reactivity, and correlates with high expression of GM1, implicating a role for lipid rafts. Physiologically, sensitivity to IL-7 and also IL-15 is important for keeping naive CD8+ cells alive in interphase. Sensitivity of naive CD8+ cells to IL-2 becomes vital during the immune response. Thus, contact of CD8+ cells with foreign antigen induces a further increase in cytokine sensitivity, thereby boosting the capacity of CD8+ cells to receive help (IL-2) from CD4+ cells.
EXPERIMENTAL PROCEDURES
Mice
Mice used in this study are described in Supplemental Experimental Procedures. All mice were maintained under specific pathogen-free conditions and used at 6–12 weeks of age. All animal experiments were performed according to protocols approved by the Animal Experimental and Ethic Committee at the Garvan Institute.
Reagents, antibodies and flow cytometry
Reagents and antibodies are described in Supplemental Experimental Procedures. Cells were stained with antibodies according to standard protocols (Cho et al., 2007). Flow cytometry samples were run using a LSR II or FACSCanto II (BD Biosciences) and analyzed by FlowJo software (Tree Star).
T cell preparation, in vitro culture and proliferation assay
Various subsets of CD4+ and CD8+ T cells were purified by FACS or MACS sorting as described in Supplemental Experimental Procedures. Sorted cells were cultured with treatment of various stimuli as indicated and their proliferative responses were analyzed by [3H]thymidine incorporation or CFSE dilution as described in Supplemental Experimental Procedures.
Bone marrow chimeras
MHC-I−/− CD8+ cells were prepared by reconstituting heavily-irradiated B6 (Ly5.1) mice with T-depleted bone marrow (BM) from MHC-I−/− (Ly5.2) mice as described elsewhere (Cho et al., 2007). From these BM chimeras, MHC-I−/− CD44lo CD8+ cells (Ly5.2+) were purified by FACS sorting.
In vivo homeostatic proliferation and parking experiments
Homeostatic proliferation of various donor cells adoptively transferred into the indicated lymphopenic mice is described in Supplemental Experimental Procedures. For parking experiments in MHC-I-deficient hosts, MHC-I−/− CD44lo CD8+ cells purified from BM chimeras were transferred i.v. into MHC-I−/− mice (1–2 × 106 cells per mouse); at 3 days after transfer, purified donor cells were recovered from pooled SP and LN and used for in vitro culture with the indicated stimuli. For the parking experiments with TAP-1−/− hosts, normal B6 naive CD8+ cells (Ly5.1) were transferred i.v. into normal B6, IL-7−/− or TAP-1−/− mice (2 × 106 cells per mouse) and then recovered 3 days later, as for transfer into MHC-I−/− hosts.
Lipid raft disruption and confocal staining
Purified CD8+ cells were pretreated with MβCD for lipid raft disruption or analyzed for GM1 lipid raft clustering or colocalization with IL-2Rβ by confocal staining as described in Supplemental Experimental Procedures.
2C cell stimulation with or without CD4+ T cell help in vitro
For antigenic stimulation with or without CD4+ T cell help, a mixture of the indicated subsets of CD44lo 2C CD8+ cells was cultured with T-depleted irradiated allogeneic BALB/c splenocytes or syngeneic B6 splenocytes with or without OT-II CD4+ cells and peptides as described in Supplemental Experimental Procedures.
Statistical analysis
A two-tailed Student’s _t_-test was used to determine statistically significant differences. P values of less than 0.05 were considered statistically significant.
Supplementary Material
01
Acknowledgments
We thank A. Basten, C. King, K. Webster, R. Kohler and M. Palendira for helpful comments and suggestions; A. Hong for mice genotyping; R. Brink and C. Mackay for OT-II mice; I. Hwang (The Scripps Research Institute) for 2C.LFA-1−/− and 2C.CD28−/− mice; C. Brownlee, Y. Sontani and J. Darakdjian of the Garvan Flow Cytometry Core Facility for cell sorting; W. Hughes of the Garvan Pieter Huveneers Molecular Imaging Unit for confocal imaging; and the Garvan Biological Testing Facility for animal care. This work was supported by grants from the NIH (USA) and NHMRC (Australia).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar A, Nizsaloczki E, Mocsar G, Szaloki N, Waldmann TA, Damjanovich S, Vamosi G. A biophysical approach to IL-2 and IL-15 receptor function: localization, conformation and interactions. Immunol Lett. 2008;116:117–125. doi: 10.1016/j.imlet.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Cai Z, Sprent J. Resting and activated T cells display different requirements for CD8 molecules. J Exp Med. 1994;179:2005–2015. doi: 10.1084/jem.179.6.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello Coelho V, Nguyen D, Giri B, Bunbury A, Schaffer E, Taub DD. Quantitative differences in lipid raft components between murine CD4+ and CD8+ T cells. BMC Immunol. 2004;5:2. doi: 10.1186/1471-2172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel J, Forrest K, Morford L, Roszman TL. Differential localization of IL-2- and -15 receptor chains in membrane rafts of human T cells. J Leukoc Biol. 2002;72:199–206. [PubMed] [Google Scholar]
- Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol. 2001;13:687–698. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]
- Guimond M, Fry TJ, Mackall CL. Cytokine signals in T-cell homeostasis. J Immunother. 2005;28:289–294. doi: 10.1097/01.cji.0000165356.03924.e7. [DOI] [PubMed] [Google Scholar]
- Harder T. Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr Opin Immunol. 2004;16:353–359. doi: 10.1016/j.coi.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin SD, Perlmutter RM, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- Malek TR. T helper cells, IL-2 and the generation of cytotoxic T-cell responses. Trends Immunol. 2002;23:465–467. doi: 10.1016/s1471-4906(02)02308-6. [DOI] [PubMed] [Google Scholar]
- Marmor MD, Julius M. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood. 2001;98:1489–1497. doi: 10.1182/blood.v98.5.1489. [DOI] [PubMed] [Google Scholar]
- Marquez ME, Ellmeier W, Sanchez-Guajardo V, Freitas AA, Acuto O, Di Bartolo V. CD8 T cell sensory adaptation dependent on TCR avidity for self-antigens. J Immunol. 2005;175:7388–7397. doi: 10.4049/jimmunol.175.11.7388. [DOI] [PubMed] [Google Scholar]
- Minami Y, Kono T, Yamada K, Kobayashi N, Kawahara A, Perlmutter RM, Taniguchi T. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 1993;12:759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C, Rubinstein MP, Kim DM, Cho JH, Sprent J, Surh CD. The lymphopenic environment of CD132 (common gamma-chain)-deficient hosts elicits rapid homeostatic proliferation of naive T cells via IL-15. J Immunol. 2008;180:5320–5326. doi: 10.4049/jimmunol.180.8.5320. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr Opin Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001;194:1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh HL, Eisen HN. Peptide antagonism and T cell receptor interactions with peptide-MHC complexes. Immunity. 1998;9:475–483. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- Udaka K, Wiesmuller KH, Kienle S, Jung G, Walden P. Self-MHC-restricted peptides recognized by an alloreactive T lymphocyte clone. J Immunol. 1996;157:670–678. [PubMed] [Google Scholar]
- Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamosi G, Bodnar A, Vereb G, Jenei A, Goldman CK, Langowski J, Toth K, Matyus L, Szollosi J, Waldmann TA, Damjanovich S. IL-2 and IL-15 receptor alpha-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc Natl Acad Sci U S A. 2004;101:11082–11087. doi: 10.1073/pnas.0403916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Zhao J, Furukawa K, Fukumoto S, Okada M, Furugen R, Miyazaki H, Takamiya K, Aizawa S, Shiku H, Matsuyama T. Attenuation of interleukin 2 signal in the spleen cells of complex ganglioside-lacking mice. J Biol Chem. 1999;274:13744–13747. doi: 10.1074/jbc.274.20.13744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01