Rumination and Impaired Resource Allocation in Depression (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 11.
Published in final edited form as: J Abnorm Psychol. 2009 Nov;118(4):757–766. doi: 10.1037/a0017206
Abstract
Depression is characterized by a range of cognitive deficits that theorists posit are due to the resource capturing properties of rumination. The present study was designed to examine the relation between rumination and resource allocation in depression. Twenty-five depressed and 25 nondepressed participants completed a modified dual-task version of the recency-probes task, which assesses the controlled allocation of cognitive resources by comparing performance across low- and high-interference conditions. In low-interference conditions, participants performed either the recency-probes task or a tracking task, which required participants to track specific stimuli across trials (i.e., no dual-task interference). In the high-interference condition, participants performed both the recency-probes task and the tracking task, which required the controlled allocation of resources to resolve dual-task interference. Depressed participants performed significantly worse than did their nondepressed counterparts in only the high-interference condition; performance of the 2 groups was comparable in the low-interference conditions. Furthermore, the degree to which depressed participants were impaired in the high-interference condition was correlated .74 with rumination. These findings suggest that an association between rumination and impairments in resource allocation underlies the cognitive difficulties experienced by depressed individuals.
Keywords: depression, cognition, executive control, rumination, working memory
Major depressive disorder (MDD) is characterized by ruminative thoughts that involve negative, self-deprecating content and pessimistic ideas about the self, the world, and the future. Investigators have documented deficits in cognitive functioning in individuals diagnosed with MDD, including impairments in working memory, problem solving, planning, memory, and attention set shifting (Bearden et al., 2006; Must et al., 2006; Paelecke-Habermann, Pohl, & Leplow, 2005). The specific deficits in executive function that underlie this constellation of impairments, however, and the role of rumination in these deficits, are not well understood.
Investigators examining cognitive deficits in depression have attempted to distinguish between the roles of rumination and executive control in influencing depressive symptomatology (e.g., Hertel, 2000; Scheurich et al., 2008). For example, Hertel and Rude (1991) found that when depressed individuals completed unstructured learning and memory tasks, (i.e., tasks without specific goals or strategies to orient their attention), their performance was significantly worse than that of nondepressed persons. In contrast, depressed and nondepressed participants performed comparably when they were provided with specific goals and strategies to help them concentrate and direct their attention. These findings suggest that depressed individuals do not initiate controlled, task-focused attention when circumstances allow the intrusion of task-irrelevant thoughts, which is what likely occurs during rumination (Hertel, 2000). Indeed, Hertel (1998) posited that rumination weakens cognitive performance by capturing attention and cognitive resources, thereby preventing these resources from being allocated to effortful tasks.
Hertel’s finding that depressed individuals are impaired in initiating controlled, task-focused strategies is important but does not elucidate the underlying cognitive mechanisms by which rumination may interfere with task performance. Hertel (1998, 2000) used unstructured memory-span tasks to examine attentional difficulties in depression; it is clear, however, that effortful tasks involve complex structured cognitive processes. Outside the laboratory, individuals typically perform multiple cognitive tasks that compete for cognitive resources. These complex tasks often involve interference, which arises both from the conflicting demands of multiple tasks (cross-task interference) and from competing relevant and irrelevant stimuli representations within each task (within-task interference; Monsell, 2003; Nelson, Reuter-Lorenz, Sylvester, Jonides, & Smith, 2003).
In such contexts, it is possible that depression-associated difficulties in the ability to resolve interference arising from multiple sources are associated with two distinct but related processes. First, an underlying impairment in executive functioning may make it difficult for some individuals to allocate their cognitive resources in a controlled fashion once interference levels exceed a certain threshold. Indeed, such impairment in executive processing might explain not only the cognitive difficulties reported by depressed individuals but also their ruminative tendencies. Breaking the cycle of rumination requires a continual rerouting of cognitive resources away from intrusive thoughts and toward the relevant task at hand, a process that would be hindered by impairment in resource allocation.
Second, according to the dual-process model of cognitive vulnerability in depression, depressed persons’ cognitive resources might already be strained by competing mood-congruent and ruminative thought processes (Beevers, 2005). The brooding, mal-adaptive component of rumination, in particular, has been found to be associated with severity of depression (Treynor, Gonzalez, & Nolen-Hoeksema, 2003), as well as with greater negative attentional biases (Joormann, Dkane, & Gotlib, 2006), suggesting that depressed individuals with high levels of brooding have a tightly connected negative schema from which they have difficulty disengaging. The dual-process model posits further that depressed individuals are impaired in their ability to implement corrective reflective processing to overcome automatic negative biases because this processing is effortful and would require cognitive resources that are not available (Beevers, 2005). Thus, rumination, particularly the brooding component, might reduce cognitive flexibility and raise interference levels above a critical threshold, making it difficult for depressed individuals to disengage from rumination in order to perform a complex task.
We designed the present study to examine depression-associated impairments in allocating cognitive resources and resolving competing task demands under complex conditions that approximate the tasks people perform in their daily lives. On the basis of our formulation that an impairment in executive processes underlies depressed individuals’ difficulties in performing complex tasks as well as their ruminative tendencies, we generated two specific hypotheses: (a) Depressed individuals will exhibit difficulty allocating cognitive resources between two tasks under high levels of interference, but not under low levels of interference; and (b) the ability to effectively allocate cognitive resources under high levels of interference will be related to the tendency to ruminate and, more specifically, to engage in brooding.
To test these hypotheses, we assessed rumination and administered a dual-task version of the recency-probes task to depressed and nondepressed participants. The recency-probes task is a working memory paradigm that permits an examination of the effect of interference on task performance by inducing conflict between current and prior representations of a stimulus. To increase the complexity of the task, we modified the recency-probes paradigm by adding a tracking task to create a dual-task paradigm. Thus, participants had to perform both a primary task (the recency-probes task) and a secondary task (a tracking task), analogous to what might occur in a typical multitasking scenario. Performing the two tasks concurrently required participants to resolve competing task demands and reroute cognitive resources from one task to the other. This design allowed us to assess the effectiveness with which depressed individuals are able to resolve interference while rerouting cognitive resources between two tasks and, ultimately, to examine whether impairments in controlled resource allocation under high levels of interference are related to ruminative tendencies.
Method
Participants
Twenty-four individuals diagnosed with MDD and 24 never-disordered controls participated in this study. Participants were solicited from two outpatient psychiatry clinics in a university teaching hospital, as well as through advertisements posted in numerous locations within the community (e.g., Internet bulletin boards, university kiosks, supermarkets). Participants’ responses to a telephone interview provided initial selection information. This phone screen established that participants were fluent in English and were between 18 and 60 years of age. Individuals were excluded if they reported severe head trauma or learning disabilities, psychotic symptoms outside of a mood episode, bipolar disorder, or alcohol or substance abuse within the past 6 months. Eligible persons were invited to come to the laboratory for a more extensive interview. Participants were then scheduled for a second session within 2 weeks of the interview, during which they completed the task described here. Participants received $25 per hour for study participation.
Assessment of Depression
Trained interviewers administered the Structured Clinical Interview for the DSM–IV (First, Spitzer, Gibbon, & Williams, 1996) to participants during their first session in the study. All interviewers had extensive training in the use of the Structured Clinical Interview for the DSM–IV, with interrater reliability kappa coefficients of .93 for the MDD diagnosis and .92 for the “never-disordered” diagnosis (i.e., the absence of current or lifetime psychiatric diagnoses; Gotlib et al., 2004). Participants were included in the depressed group if they met the Diagnostic and Statistical Manual of Mental Disorders (4th ed; American Psychiatric Association, 1994) criteria for current MDD and were included in the nondepressed group if they had no current or past Axis I disorder. All participants completed the Beck Depression Inventory–II (BDI–II; Beck, Steer, & Brown, 1996; Steer, Ball, Ranieri, & Beck, 1999), a 21-item, self-report measure of the severity of depressive symptoms. The acceptable reliability and validity of the BDI–II has been well documented (Steer, Kumar, Ranieri, & Beck, 1998).
Assessment of Rumination
We administered the 22-item Ruminative Response Scale of the Response Style Questionnaire (Nolen-Hoeksema & Morrow, 1991) to participants and assessed the two components that are not confounded with depression severity: reflective pondering and brooding (Treynor et al., 2003). Whereas the Reflective Pondering Scale reflects an adaptive and purposeful turning inward to engage in cognitive problem solving to alleviate one’s depressive symptoms, the Brooding Scale reflects a maladaptive and passive comparison of one’s current situation with some unachieved standard. Previous studies using the Ruminative Response Scale have shown good test–retest reliability and acceptable convergent and predictive validity (Nolen-Hoeksema & Morrow, 1991; Nolen-Hoeksema, Parker, & Larson, 1994; Treynor et al., 2003).
Stimuli
A total of 473 words—370 neutral words and 103 category words—were used as stimuli. Of the neutral words, 190 were from the Affirmative Norms for English Words battery (Bradley & Lang, 1999; valence M = 5.19, SD = 0.80; arousal M = 4.57, SD = 0.73), and 280 were from the Francis and Kucera (1982) battery (valence M = 5.58, SD = 0.91; arousal M = 4.85, SD = 0.82). The category words were taken from 14 different ad hoc categories. A sample category is “Things to Save in Case of a Fire,” and sample category members include photos, children, jewelry, pets, heirlooms (for a complete listing of the 14 categories and their member words, see the Appendix). The order of the words presented throughout each condition was pseudorandom, and all words were presented one to three times.
Procedure
A computer running E-prime controlled stimulus presentation and response collection for all participants. Participants were run individually, and instructions were given both by the experimenter and by the computer. The recency-probes task (Monsell, 1978) was modified from Jonides, Smith, Marshuetz, Koeppe, and Reuter-Lorenz (1998) and D’Esposito, Postle, Jonides, and Smith (1999) to be a dual-task paradigm by the addition of a tracking task. The recency-probes and tracking tasks were performed concurrently. For the recency-probes task, each trial proceeded as follows: Participants viewed a target set of three words for 950 ms and remembered the set for a delay of 3,000 ms, during which a fixation cross appeared at the center of the screen. Next, participants viewed a single probe for 1,500 ms and indicated whether the probe was contained in the target set. Participants pressed one of two designated keys to respond to each individual trial: 1 for “yes” or 2 for “no.” Following the presentation of the probe word was an intertrial interval of 2,000 ms during which participants viewed a fixation cross in the center of the screen. Participants completed 232 trials (8 practice trials and 14 blocks of 16 trials each) and were given an opportunity for a break between each block.
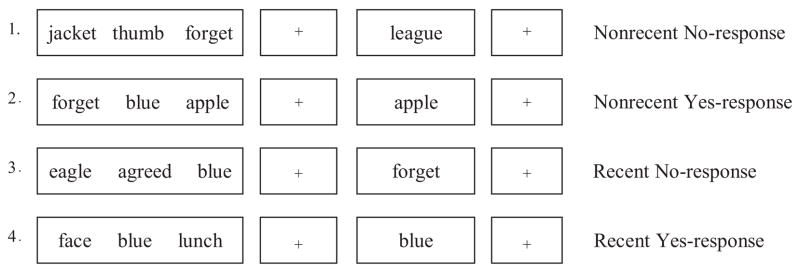
Four general trial types were derived from the recency-probes task: nonrecent no-response, nonrecent yes-response, recent no-response, and recent yes-response (see Figure 1 for an example). In nonrecent no-response trials, the probe matched no item from the current or the past two target sets. In nonrecent yes-response trials, the probe matched an item from the present target set but not from the preceding two target sets. In recent no-response trials, the probe did not match any items in the current target but did match an item from a target set of the preceding two trials. Finally, in recent yes-response trials, the probe matched an item from the current target set as well as an item from each of the preceding two target sets. We used two trial types, the recent no-response and nonrecent no-response trials, to measure within-task interference. Within-task interference is introduced in recent no-response trials because the probe word, although not in the current target set and consequently requiring a “no” response, is in the two previous target sets, thereby inducing familiarity and engendering an interfering “yes” response. Thus, familiarity and source recognition are placed into conflict, inducing within-task interference that participants must resolve to respond correctly to the trial. Consequently, reaction times (RTs) to recent no-response trials are generally longer than they are to nonrecent no-response trials (D’Esposito et al., 1999).
Figure 1.
Trial types of the recency-probes paradigm. Within-task interference is created in the recent no-response trials.
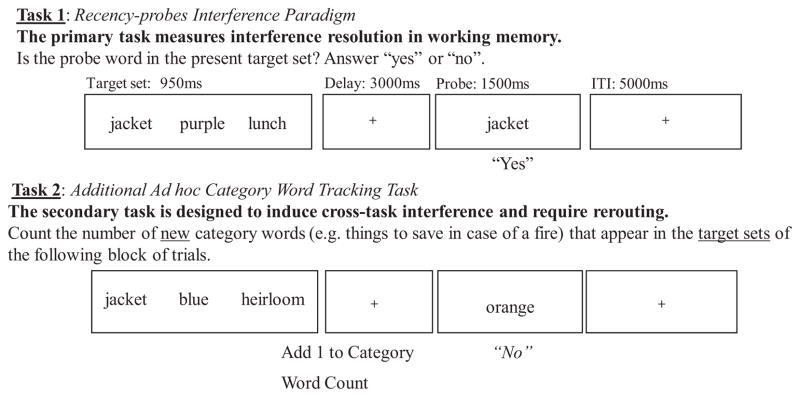
For the tracking task, at the onset of each block, an ad hoc category was presented (e.g., “Things to Save in Case of a Fire”; see Appendix for complete listing of categories). Stimuli within the block consisted of neutral words and corresponding category member words. Only neutral words that would not qualify as members of the category were used as stimuli. A different category was presented for each trial block. Participants were instructed to count the number of new category words presented within the target sets (not as probes) of the block, while still completing the primary recency-probes task (see Figure 2 for an example). Trials in which category words appear in the target set require the rerouting of cognitive resources because the participant must disengage from the tracking task and engage in the recency-probes task. On trials in which category words appear as probes, cross-task interference is induced because participants must inhibit counting the category word and must respond “yes” or “no” to the individual trial. After each block of trials ended, participants were prompted on the computer to enter the number of category words they counted in the target sets of the block using keys labeled 4 through 14.
Figure 2.
The recency-probes paradigm, altered to include a tracking task. The modification results in a dual-task paradigm that approximates goal-pursuit by requiring within-task and cross-task interference resolution and the rerouting of cognitive resources.
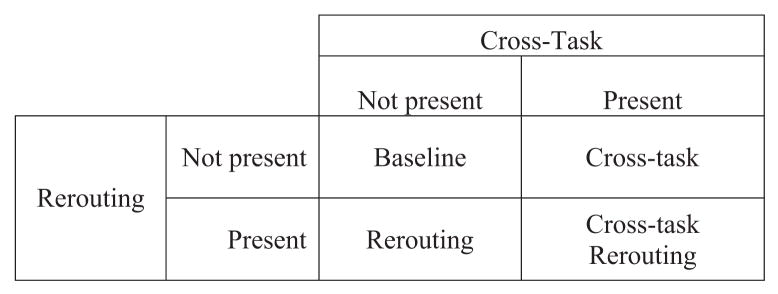
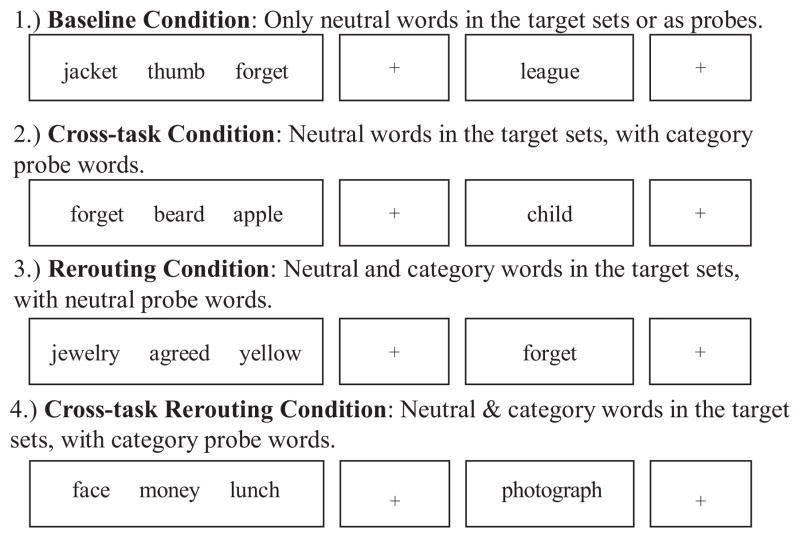
Within each block of 16 trials, we presented category words pseudorandomly to assess the interaction of rerouting and cross-task interference. The placement of the category word determined whether the trial included cross-task interference and/or whether it required the rerouting of cognitive resources from the tracking task to the primary task. In each trial, the category word could be placed (a) within the target set, (b) as a probe, (c) in both the target set and as a probe, or (d) in neither the target set nor as a probe. This 2 × 2 placement design (see Figure 3) resulted in four placement conditions: the baseline condition, the cross-task condition, the rerouting condition, and the cross-task rerouting condition. The baseline condition used only neutral words in the target set or as probes, and no category words were presented (see Figure 4, Type 1); the baseline condition had no cross-task interference and required no rerouting and would therefore measure the ability to resolve interference within a task. The cross-task condition used neutral words in the target set and a category word as the probe (see Figure 4, Type 2); the cross-task condition did not require rerouting but had cross-task interference because the probe word was a category word that could not be counted. Performance in the cross-task condition is an indicator of how effectively participants are able to resolve interference that arises between two tasks. This is analogous to a real-world scenario in which a student is listening to a lecture and the person sitting beside her poses a question to her, interfering with her ability to continue to follow the lecture. The rerouting condition had both neutral and category words in the target set and a neutral probe word (see Figure 4, Type 3). The rerouting condition required participants to count a category word and then direct their attention back to the recency-probes task. Performance in the rerouting condition reflects how effectively participants are able to reallocate resources between two tasks. An analogy here is a student trying to follow the details of a lecture while taking notes that summarize only the most salient points, a process that requires the student to switch continually from listening to note-taking, reallocating his or her cognitive resources each time. Finally, the cross-task rerouting condition had both neutral and category words in the target set and a category word as the probe (see Figure 4, Type 4). This was the high-interference condition, requiring participants to count the category word in the target set (as part of the tracking task), direct their attention back to the probe (which is also a category word), and resolve cross-task interference by inhibiting counting the category word probe (only target words are counted in the tracking task), while still responding “yes” or “no” to this word as part of the recency-probes task. Thus, performance in the cross-task re-routing condition reflects how effectively participants both reroute resources between two tasks and resolve cross-task interference. These four placement-condition trial types, combined with the four recency-probes trial types, resulted in 16 trial types.
Figure 3.
The two by two cross-task and rerouting recency-probes dual task design.
Figure 4.
Sample category placement condition trials for the ad hoc category: “Things to save in case of fire.”
Results
Participant Characteristics
Demographic and clinical characteristics of the MDD and non-depressed participants are presented in Table 1. Depressed and nondepressed participants did not differ significantly in age, t(46) = 1.2, or education, t(46) = 0.8, both _p_s > .05; as expected, depressed participants obtained significantly higher scores on the BDI–II than did nondepressed participants, t(46) = 18.4, p < .001. Seven of the 24 MDD participants were taking medication (five were taking an SSRI or other antidepressant; six were taking other types of psychotropic medications, such as benzodiazepines and anxiolytics). Finally, eight of the MDD participants were diagnosed with at least one comorbid disorder (five with social phobia, three with post-traumatic stress disorder, one with panic disorder, and one with agoraphobia).
Table 1.
Characteristics of Participants
| Nondepressed (N = 24) | Depressed (N = 24) | |||||
|---|---|---|---|---|---|---|
| Variable | M | SD | n | M | SD | n |
| n Female | 8 | 6 | ||||
| n Male | 16 | 18 | ||||
| Age (years) | 37 | 10 | 41 | 12 | ||
| Years of education | 16 | 16 | ||||
| Beck Depression Inventory–II | 1 | 1.1 | 33** | 9 | ||
| Ruminative Response Scale | 36 | 8.5 | 57** | 12.4 | ||
| Reflective pondering component | 10.4 | 3.6 | 11.5 | 3.8 | ||
| Brooding component | 7.7 | 2.6 | 13.5** | 3.7 |
Reaction Times
RTs and responses were recorded for each trial, and a mean was calculated for correct trials of each trial type. Trials on which participants made an incorrect response and individual RTs were more than 2.5 standard deviations from the trial type mean were excluded from analyses. It should be noted that, although task is complex, it is not difficult to perform; no participants were excluded because of high error rates or missed trials, and error rates for the task were low. Furthermore, depressed (_M_s = 3.5% and 9%, _SD_s = 1% and 2%) and nondepressed (_M_s = 3% and 9%, _SD_s = 1% and 2%) participants did not differ with respect to percentage of excluded trials, t(46) = 0.75, p > .1, or incorrect responses, t(46) = 0.06, p > .1, respectively. Moreover, performance of depressed individuals did not differ as function medication status1 or the presence or absence of a comorbid anxiety disorder.2 Mean RTs and accuracy rates for each trial type in each condition for depressed and nondepressed participants presented in Table 2.
Table 2.
Mean Reaction Times, Standard Deviations, and Accuracy Rates for Each Group for Each Trial Type in Each Condition
| Control | Depressed | |||||
|---|---|---|---|---|---|---|
| Response type | M | SD | Accuracy (%) | M | SD | Accuracy (%) |
| Baseline trial | ||||||
| Yes response | ||||||
| Nonrecent | 908 | 152 | 94 | 989 | 211 | 94 |
| Recent | 938 | 155 | 92 | 994 | 209 | 93 |
| No response | ||||||
| Nonrecent | 923 | 199 | 90 | 985 | 224 | 89 |
| Recent | 993 | 183 | 91 | 1,064 | 225 | 97 |
| Rerouting trial | ||||||
| Yes response | ||||||
| Nonrecent | 949 | 148 | 93 | 1,026 | 202 | 92 |
| Recent | 1,006 | 171 | 93 | 1,077 | 228 | 91 |
| No response | ||||||
| Nonrecent | 902 | 182 | 88 | 977 | 227 | 89 |
| Recent | 1,005 | 164 | 95 | 1,072 | 246 | 92 |
| Cross-task trial | ||||||
| Yes response | ||||||
| Nonrecent | 929 | 169 | 88 | 994 | 203 | 87 |
| Recent | 966 | 191 | 74 | 1,073 | 208 | 73 |
| No response | ||||||
| Nonrecent | 998 | 247 | 96 | 1,034 | 244 | 97 |
| Recent | 965 | 173 | 95 | 1,045 | 233 | 96 |
| Cross-task rerouting trial | ||||||
| Yes response | ||||||
| Nonrecent | 915 | 161 | 89 | 997 | 213 | 89 |
| Recent | 1,015 | 177 | 94 | 1,115 | 228 | 94 |
| No response | ||||||
| Nonrecent | 1,011 | 239 | 96 | 1,010 | 223 | 95 |
| Recent | 983 | 189 | 93 | 1,109 | 244 | 93 |
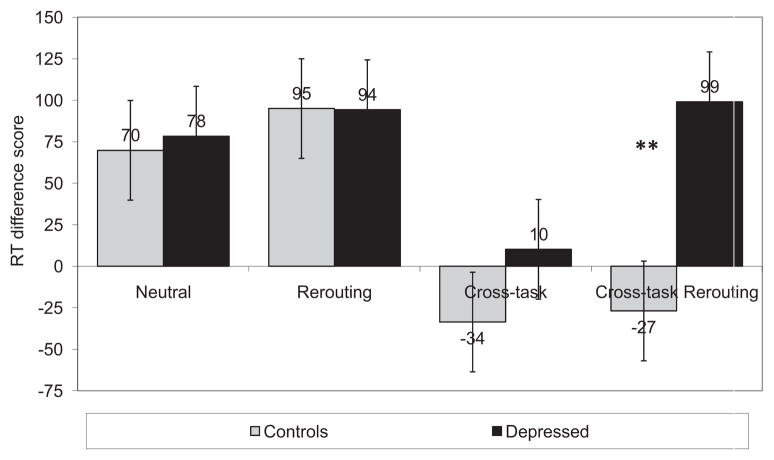
A five-way (Group [depressed, nondepressed] × Recency [non recent, recent] × Response [yes, no] × Cross-Task [category probe, neutral probe] × Rerouting [category word in target neutral target set]) analysis of variance (ANOVA) conducted RTs yielded a number of significant main effects and lower order interactions, all of which were qualified by a five-way interaction of group, recency, response, cross-task, and rerouting, F(1, 46) = 4.01, p < .05, η2 = .08. To decompose this five-way interaction, we calculated difference scores for both “no” and “yes” responses by subtracting nonrecent trial RTs from recent trial RTs. In no response trials, this difference score represents a measure of inter ference that, when compared across conditions, indicates how effectively participants allocate resources while rerouting and re solving cross- and within-task interference. No-response differ ence scores are presented in Figure 5. In contrast, no conflict present in yes-response trials. Consequently, yes-response differ ence scores reflect the effects of recency on rerouting and cross task interference in the absence of within-task interference.
Figure 5.
Mean difference scores for no-response trials across condition for depressed and nondepressed participants. Error bars represent standard error. **p < .001.
To assess interference resolution, we conducted separate three-way (Group × Cross-Task × Rerouting) ANOVAs on “no” and “yes” responses, which reflect the conjunction of within-task interference, cross-task interference and rerouting, and the conjunction of cross-task interference and rerouting, respectively. The ANOVA conducted on “no” responses yielded significant main effects for group, F(1, 46) = 4.15, p < .05, η2 = .083; cross-task, _F_(1, 46) = 25.82, _p_ < .001, η2= .36; and rerouting, _F_(1, 46) = 11.01, _p_ < .01, η2 = .19, as well as a significant interaction of group and cross-task, _F_(1, 46) = 8.5, _p_ < .01, η2 = .15. All of these effects were qualified by a significant interaction of group, cross-task, and rerouting, _F_(1, 46) = 4.49, _p_ < .05, η2 = .089. Follow-up tests indicated that depressed participants had significantly greater interference levels than did nondepressed participants in the cross-task rerouting condition, _t_(46) = 4.46, _p_ < .001, but comparable levels of interference in the baseline, cross-task, and rerouting conditions (_p_s > .1). Thus, compared with controls, depressed individuals are impaired at resolving interference while rerouting cognitive resources and resolving competing task demands.
The ANOVA conducted on “yes” responses yielded significant main effects for cross-task, F(1, 46) = 13.95, p < .001, η2= .23, and rerouting, F(1, 46) = 7.3, p < .01, η2 = .13; no other effects were significant. Follow-up tests indicated that cross-task difference scores were significantly greater than non-cross-task difference scores, t(47) = 3.64, and that rerouting difference scores were significantly greater than non-rerouting difference scores, t(47) = 2.73, both _p_s < .01. These results indicate that RTs were significantly longer for recent than for nonrecent yes-response trials in the cross-task and rerouting conditions than in the baseline conditions. Furthermore, combined with the no-response results presented above, it appears the performance of depressed individuals is impaired only when sufficiently high levels of interference (i.e., within-task interference, cross-task interference, and rerouting) are present.
Rumination
Our second hypothesis was that the ability to effectively allocate cognitive resources in the context of high levels of interference would be related to rumination and, specifically, to the brooding component of rumination. To examine the relations among brooding, depression, and performance in the cross-task rerouting condition, we conducted multiple linear regression analyses of brooding and reflective pondering scores across groups. Table 3 presents the results of these analyses. First, we conducted a multiple linear regression analysis in which cross-task rerouting interference levels were entered as the dependent variable, and brooding, reflective pondering, and group, as well as all two-way and three-way interactions, were entered as predictors. This analysis yielded main effects of group (β = .344, p < .05) and brooding (β = .427, p < .01), which were qualified by an interaction of brooding and group (β = .2.86, p < .05). Second, to examine brooding and reflective pondering independently, we conducted a multiple linear regression analysis on reflective pondering with cross-task rerouting interference levels entered as the dependent variable and reflective pondering, group, and the interaction entered as predictors. We also conducted a multiple linear regression analysis on brooding with cross-task rerouting interference levels entered as the dependent variable and brooding, group, and the interaction entered as predictors. The reflective pondering regression yielded a main effect of group (β = .598, p < .01) and an interaction of reflective pondering and group (β = .247, p < .05). The brooding regression yielded main effects of group (β = .371, p < .05) and brooding (β = .349, p < .05), which were qualified by an interaction of group and brooding (β = .297, p < .01).
Table 3.
Multiple Linear Regression Analyses of Cross-Task Rerouting Difference Scores, Brooding, and Reflective Pondering Scores
| Dependent variable: Cross-task rerouting | |||||
|---|---|---|---|---|---|
| Measure | B | SE | b | t | p |
| Brooding and reflective pondering across groups(N = 48) | |||||
| Group | −65.5 | 27.2 | −.34 | 2.4 | .02 |
| Ref | −5.6 | 4.57 | −.22 | 1.24 | .2 |
| Brd | 9.4 | 3.6 | .427 | 2.6 | .01 |
| Ref × Group | −8.32 | 10.8 | −.08 | 0.77 | .4 |
| Brd × Group | −37.34 | 115.42 | −.286 | 2.42 | .02 |
| Ref × Brd × Group | −2.49 | 15.89 | −.03 | 0.15 | .8 |
| Brooding across groups (N = 48) | |||||
| Group | −70.7 | 27.2 | −.37 | 2.6 | .01 |
| Brd | 7.7 | 3.62 | .34 | 2.3 | .02 |
| Brd × Group | −38.8 | 14.4 | −.29 | 2.7 | .01 |
| Reflective pondering across groups (N = 48) | |||||
| Group | −114.1 | 22.8 | −.59 | 4.99 | .001 |
| Ref | −0.63 | 3.1 | −.024 | 0.2 | .84 |
| Ref × Group | −24.4 | 11.7 | −.25 | −2.1 | .04 |
To decompose these two interactions, we correlated cross-task rerouting interference levels with brooding, reflective pondering, BDI–II scores, and rumination separately for the depressed and nondepressed participants. Table 4 presents the results of these analyses. The interaction of reflective pondering and group is due to a positive correlation between reflective pondering and cross-task rerouting interference in the depressed group (r = .27) and a negative correlation between reflective pondering and cross-task rerouting interference in the nondepressed group (r = −.36). Although neither of these correlations is statistically significant, they are significantly different from each other, t(20) = 4.36, p < .05. The interaction of brooding and group is due to a significant correlation between brooding and cross-task rerouting interference in the depressed group (_r_ = .75, _p_ < .001) but not in the nondepressed group (_r_ = .04, _p_ > .1); these correlations, too, differ significantly from each other, t(20) = 8.9, p < .01.
Table 4.
Correlations Between No-Response Cross-Task Rerouting Difference Scores and Reflective Pondering, Brooding, Rumination, and BDI–II Scores for Depressed and Nondepressed Participants
| Cross-task rerouting Interference | ||
|---|---|---|
| Measure | r | p |
| Depressed group (n = 24) | ||
| Ref | .266 | .2 |
| Brd | .745 | .001 |
| RRS | .625 | .001 |
| BDI–II | .47 | .05 |
| Nondepressed group (n = 24) | ||
| Ref | −.356 | .1 |
| Brd | −.04 | .86 |
| RRS | −.145 | .52 |
| BDI–II | −.012 | .95 |
Discussion
We designed the present study to examine depression-associated impairments in allocating cognitive resources and resolving competing task demands under conditions of high interference. Diagnosed depressed and nondepressed participants performed a dual-task version of the recency-probes task that manipulated cross-task interference, within-task interference, and rerouting requirements. To perform the task, participants were required to effectively allocate resources to resolve competing task demands and reroute cognitive resources from one task to the other. We hypothesized that depressed individuals would show impaired performance in the high-interference condition (i.e., the cross-task rerouting condition), because in this condition, cross-task and within-task interference resolution and rerouting were all required. We interpreted impairment in this high-interference condition as reflecting difficulties in the ability to allocate resources in a controlled fashion under complex task conditions. We further predicted that the task performance of depressed and nondepressed participants would be comparable under low-interference conditions.
Our findings supported these predictions. Response times were significantly delayed only when depressed participants were required to resolve both within-task and cross-task interference and to reroute cognitive resources during no-response cross-task rerouting trials. This delay in RTs reflects an impaired ability to allocate cognitive resources under conditions of high interference. As we predicted, depressed and nondepressed participants exhibited comparable performance in all other conditions, even showing the same reduction of within-task interference resolution in the recent no-response cross-task condition. In the cross-task condition, neutral words comprise the target set and the probe is a category word, so no rerouting is required, but cross-task interference is present because the probe is a category word that cannot be counted. The reduced interference in these trials, which replicates previous findings using this paradigm (Levens, Labouliere, Gollwitzer, & Phelps, 2008), occurs because the probe word is a category word that had first appeared two trials earlier as a member of the target set and, accordingly, had been counted for the tracking task. Prior tracking and counting of the category word served to strengthen the correct source response signal when the word appeared as a probe, thereby reducing the interference levels present in this condition.
The Cross-Task × Rerouting design of this task permitted us to assess performance on each of the component cognitive processes individually, as well as during trials in which within-task and cross-task interference resolution and rerouting processes intersected. The observed pattern of interference levels across condition and group (i.e., similar interference levels across all conditions and groups, except in the no-response cross-task rerouting condition) indicates that the abilities to resolve within-task and cross-task interference and to reroute information remain intact in depression. It is only when all three processes intersect that depressed individuals show impairment in interference resolution.
Executive control processes are required in those situations in which two or more tasks compete for access to limited cognitive resources. These processes are higher order operations that establish the timing, order, and priority of individual task requirements and carefully allocate the available cognitive resources among competing tasks in accordance with overall task requirements (Schubert, 2008). The fact that we found depressed individuals to exhibit impairment only in the no-response cross-task rerouting—the most complex—condition, and not when performing any of the isolated task components, suggests that their difficulty lies in the controlled ordering and partitioning of resources that is critical to the efficient and effective execution of complex tasks.
Our finding of selective impairment in executive control resource allocation, but not in individual cognitive processes, supports the dual-process model of cognitive vulnerability in depression (Beevers, 2005). This model suggests that negative mood is prevalent in depression because negatively biased self-referent associative processing is not able to be corrected or repaired by effortful reflective processes that could improve mood (Beevers, 2005; Carver, Johnson, & Joormann, 2008). Our findings broaden the model and indicate that even when depressed individuals are not actively trying to engage reflective processes to repair a negative mood, they still demonstrate impairment in the controlled allocation of their cognitive resources. This impairment in executive control, in the context of the dual-process model, may explain why depressed individuals experience difficulty performing complex tasks, how rumination may compound cognitive impairments, and why rumination might be so difficult to stop. An underlying impairment in executive functioning may make it difficult for some individuals to allocate their cognitive resources in a controlled fashion once interference levels exceed a certain threshold. Not only is rumination likely to draw on limited cognitive resources that could otherwise be directed toward performing a cognitive task but it might also have the effect of raising interference levels above a critical threshold, making it difficult for depressed individuals to reroute away from ruminative thought processes, in effect creating a more demanding task.
Thus, depressed individuals who ruminate may find themselves continually having to resolve interference between rumination and a task at hand and to reroute resources away from ruminative thoughts in a manner similar to that demonstrated in the high-interference condition in the present study. This conclusion is supported by the strong correlation observed between brooding rumination scores and interference levels among depressed individuals: Depressed persons who showed the strongest tendency to brood while ruminating exhibited the greatest impairment in the controlled allocation of resources under high-interference conditions. Because this task is sufficiently complex to make it unlikely that participants can ruminate during the task (Hertel, 2000; Pearson, Brewin, Rhodes, & McCarron, 2008), it is also unlikely that rumination during the cross-task rerouting condition underlies the strong correlation obtained between brooding scores and interference levels among depressed individuals. Instead, we think that the cross-task rerouting condition is a proxy for what happens during brooding. Brooding, consistently associated with maladaptive behaviors and poor depression outcome, has been shown to be negatively correlated with mastery, the belief that one has the power to change one’s outlook and behavior (Treynor et al., 2003). Impaired resource allocation may be a cognitive mechanism that underlies this association. That is, an impaired ability to control the allocation of cognitive resources could lead to a decreased sense of mastery, which could further exacerbate brooding. Thus, it is not difficult to see how an impairment in controlled resource allocation has the potential to exacerbate depressive symptoms: Without the ability to effectively disengage from brooding thoughts and reroute resources toward more adaptive cognitive processes that have the potential to improve mood, the maladaptive cycle of rumination is difficult to escape.
It is interesting that the relationship between brooding and impaired resource allocation was restricted to the depressed group, raising the question of the causal nature of this association. Although we cannot address the issue of causality in the current study, we can speculate about the interactions that might be taking place. One possibility is that the executive control impairment is primary, leading to poor resource allocation and thereby allowing a disproportionate amount of cognitive resources to be dedicated to rumination relative to other, more adaptive activities. In this formulation, nondepressed individuals would not have shown the brooding resource-allocation impairment exhibited by depressed individuals because they did not have the same underlying cognitive vulnerability. Alternatively, rumination or negative schemata (Beck, 1976) may underlie the impairment in executive processing observed in depressed individuals by adding to the overall cognitive load experienced at the onset of performing a task, thereby lowering the threshold for interference or making depressed individuals less cognitively flexible than controls. It is also reasonable to imagine a dynamic interplay between executive processing and rumination such that an underlying impairment in controlled resource allocation becomes exacerbated in the presence of rumination and leads to further difficulties in cognitive functioning and to the perpetuation of rumination. By administering the modified recency probes paradigm to remitted depressed individuals or to currently nondepressed individuals who, perhaps because of a personal or family history of depression, are at increased risk for developing this disorder, it would be possible in future research to examine whether the depression-associated impairment in resource allocation observed in the current study is a vulnerability factor for depression or whether it is the result of an ongoing depressive episode. Such studies would begin to provide a clearer picture of the causal role of executive processing impairments in the onset of depression.
The majority of research on cognitive functioning in depression to date has focused on loss of motivation and rumination (e.g., Fossati, Ergis, & Allilaire, 2002; Hertel, 2000; Scheurich et al., 2008), on cognitive impairments in effortful tasks (Wenzlaff & Eisenberg, 2001; Wenzlaff & Luxton, 2003), and on valence-specific deficits in working memory (Joormann & Gotlib, 2008; Levens & Gotlib, 2009). The findings of the present study, however, do not implicate a general cognitive or motivation impairment in depression or a valence-specific impairment in cognitive control but, rather, a focal impairment in the controlled allocation of cognitive resources that has the capacity to affect a range of behaviors and situations. We found that depressed participants did not differ from their nondepressed counterparts with respect to performance in lower load conditions or in their accuracy in the high-interference condition. This indicates that although differences in motivation between depressed and nondepressed individuals may contribute to cognitive impairments more generally, motivation was likely not a causal factor in the present findings. It is notable that our results do not rule out the possibility that rumination contributes to impaired cognitive performance in depression; rather, they point to a possible mechanism by which rumination interferes with efficient performance on cognitive tasks. Thus, our findings complement research reported by Hertel (1998) and the dual-process model of cognitive vulnerability in depression (Beevers, 2005), which posits that rumination weakens cognitive performance by capturing attention and cognitive resources, thereby preventing their allocation to effortful tasks. The impairment in executive processing found in the present study explains the performance deficits documented previously and suggests a more complex story about rumination in depression: Depressed individuals may experience difficulty disengaging from ruminative thoughts and rerouting toward more adaptive thoughts and activities in complex task scenarios because they are impaired at efficiently allocating their cognitive resources.
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH59259 to Ian H. Gotlib. We thank Lindsey Sherdell for her help in running the participants.
Appendix
Ad Hoc Categories and Stimuli
- For Practice Trials: Items needed to bake a pie: Fruit, Bowl, Oven, Spoon, Sugar
- Things to save in case of fire: Photograph, Child, Jewelry, Pets, Heirloom, Money
- Things to bring on a picnic: Blanket, Sandwich, Frisbee, Bread, Basket, Cookies, Drinks, Soda, Plates, Napkins
- Items needed for a new pet dog: Bed, Food, Bowl, Collar, Treat, Brush, Toys, Ball
- Activities or ways to lose weight: Medication, Diet, Aerobics, Operation, Exercise, Weights, Yoga, Bike
- Things commonly bought at the grocery store: Milk, Bread, Eggs, Butter, Juice, Cheese, Toothpaste
- Things to pack in your suitcase for a trip: Money, Jacket, Socks, Hairbrush, Shirts, Toothbrush
- Things you need to travel around Europe: Tickets, Suitcase, Passport, Guidebook, Wallet, Clothes, Shoes, Bus, Train
- Things to buy for a new baby: Blanket, Milk, Clothing, Crib, Bonnet, Bib, Diapers, Bottle
- Things necessary for your new office: Copier, Staples, Paper, Desk, Pens, Fax, Computer, Pencil
- Things needed to remodel your home: Tools, Budget, Carpenter, Paint, Contractor, Wood
- Items to bring to the beach: Swimsuit, Towel, Sun-screen, Shovel, Sunglasses, Bucket, Cooler, Food, Drinks, Sandals
- Items needed for a meeting, presentation, or interview: Suit, Resume, Notepad, Research, References, Pen
- Things to bring camping: Lantern, Tent, Matches, Knife, Coat, Pillow, Flashlight, Chairs
- Things needed to write a paper: Computer, Dictionary, Reference, Paper, Library, Journal, Book
Footnotes
1
We conducted a three-way (Medication [present, absent] × Cross-Task Interference [present, absent] × Rerouting [required, not required]) ANOVA on no-response RT difference scores to examine the effects of medication status on cross-task and rerouting abilities. This analysis did not yield either a significant main effect for medication or significant interactions involving medication (all _p_s > .05). We also conducted t tests to examine whether rumination scores or reflective and brooding scores differed as a function of medication status. The results of these analyses, too, yielded no significant group differences in rumination, reflective pondering, or brooding (all _p_s > .05).
2
We conducted a three-way (Comorbid Anxiety [present, absent] × Cross-Task Interference [present, absent] × Rerouting [required, not required]) ANOVA on no-response RT difference scores to examine the effects of comorbid anxiety on cross-task and rerouting abilities. This analysis did not yield either a significant main effect for comorbid anxiety or significant interactions involving comorbid anxiety (all _p_s > .05). We also conducted t tests to examine whether rumination scores or reflective and brooding scores differed as a function of the presence of comorbid anxiety. The results of these analyses, too, yielded no significant group differences in rumination, reflective pondering, or brooding (all _p_s > .05).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Villarreal V, Soares JC. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Research. 2006;142:139–150. doi: 10.1016/j.psychres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. New York: International Universities Press; 1976. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beevers CG. Cognitive vulnerability to depression: A dual process model. Clinical Psychology Review. 2005;25:975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective Norms for English Words (ANEW): Technical manual and affective ratings. Gainesville, FL: Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related fMRI. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders—Clinician Version (SCID–CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Fossati P, Ergis AM, Allilaire JF. Problem-solving abilities in unipolar depressed patients: Comparison of performance on the modified version of the Wisconsin and the California sorting tests. Psychiatry Research. 2002;104(2):145–156. doi: 10.1016/s0165-1781(01)00307-9. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Gotlib IH, Kasch KL, Traill SK, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004;113:386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Hertel PT. Relation between rumination and impaired memory in dysphoric moods. Journal Abnormal Psychology. 1998;107:166–172. doi: 10.1037//0021-843x.107.1.166. [DOI] [PubMed] [Google Scholar]
- Hertel PT. The cognitive-initiative account of depression-related impairments in memory. In: Medin D, editor. The psychology of learning and motivation. Vol. 39. New York: Academic Press; 2000. pp. 47–71. [Google Scholar]
- Hertel PT, Rude SS. Depressive deficits in memory: Focusing attention improves subsequent recall. Journal Experimental Psychology: General. 1991;120:301–309. doi: 10.1037/0096-3445.120.3.301. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behaviour Therapy. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Impaired selection of relevant positive information in depression. Depression and Anxiety. 2009;26:403–410. doi: 10.1002/da.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Labouliere CD, Gollwitzer P, Phelps EA. Interference resolution, cross-task interference and rerouting in working memory. 2008. Manuscript submitted for publication. [Google Scholar]
- Monsell S. Recency, immediate recognition memory, and reaction time. Cognitive Psychology. 1978;10:465–501. [Google Scholar]
- Monsell S. Task switching. Trends and Cognitive Neuroscience. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Must A, Szabó Z, Bódi N, Szász A, Janka Z, Kéri S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. Journal of Affective Disorders. 2006;90:209–215. doi: 10.1016/j.jad.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CYC, Jonides J, Smith EE. Dissociable neural mechanisms underlying response based and familiarity based conflict in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Parker LE, Larson J. Ruminative coping with depressed mood following loss. Journal of Personality and Social Psychology. 1994;67:92–104. doi: 10.1037//0022-3514.67.1.92. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 2005;89:125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Pearson M, Brewin CR, Rhodes J, McCarron G. Frequency and nature of rumination in chronic depression: A preliminary study. Cognitive Behaviour Therapy. 2008;37:160–168. doi: 10.1080/16506070801919224. [DOI] [PubMed] [Google Scholar]
- Scheurich A, Fellgiebel A, Schermuly I, Bauer S, Wolfges R, Muller MJ. Experimental evidence for a motivational origin of cognitive impairment in major depression. Psychological Medicine. 2008;38(2):237–246. doi: 10.1017/S0033291707002206. [DOI] [PubMed] [Google Scholar]
- Schubert T. The central attentional limitation and executive control. Frontiers in Bioscience. 2008;13:3569–3580. doi: 10.2741/2950. [DOI] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory–II in clinically depressed outpatients. Journal of Clinical Psychology. 1999;55:117–128. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Steer RA, Kumar G, Ranieri WF, Beck AT. Use of the Beck Depression Inventory–II with adolescent psychiatric outpatients. Journal of Psychopathology and Behavioral Assessment. 1998;20:127–137. [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Wenzlaff RM, Eisenberg AR. Mental control after dysphoria: Evidence of a suppressed, depressive bias. Behavior Therapy. 2001;32(1):27–45. [Google Scholar]
- Wenzlaff RM, Luxton DD. The role of thought suppression in depressive rumination. Cognitive Therapy and Research. 2003;27(3):293–308. [Google Scholar]