Emi2 Inhibition of the Anaphase-promoting Complex/Cyclosome Absolutely Requires Emi2 Binding via the C-Terminal RL Tail (original) (raw)
Both the D-box and the zinc-binding region (ZBR) of Emi2 are implicated in APC/C inhibition. This article shows that Emi2 binds the APC/C via the C-terminal tail, termed here the RL tail. The RL tail apparently promotes the inhibitory interactions of the D-box and the ZBR with the APC/C. The RL tail thus serves as a docking site for the APC/C.
Abstract
Emi2 (also called Erp1) inhibits the anaphase-promoting complex/cyclosome (APC/C) and thereby causes metaphase II arrest in unfertilized vertebrate eggs. Both the D-box and the zinc-binding region (ZBR) of Emi2 have been implicated in APC/C inhibition. However, it is not well known how Emi2 interacts with and hence inhibits the APC/C. Here we show that Emi2 binds the APC/C via the C-terminal tail, termed here the RL tail. When expressed in Xenopus oocytes and egg extracts, Emi2 lacking the RL tail fails to interact with and inhibit the APC/C. The RL tail itself can directly bind to the APC/C, and, when added to egg extracts, either an excess of RL tail peptides or anti-RL tail peptide antibody can dissociate endogenous Emi2 from the APC/C, thus allowing APC/C activation. Furthermore, and importantly, the RL tail–mediated binding apparently promotes the inhibitory interactions of the D-box and the ZBR (of Emi2) with the APC/C. Finally, Emi1, a somatic paralog of Emi2, also has a functionally similar RL tail. We propose that the RL tail of Emi1/Emi2 serves as a docking site for the APC/C, thereby promoting the interaction and inhibition of the APC/C by the D-box and the ZBR.
INTRODUCTION
The anaphase-promoting complex/cyclosome (APC/C) is a large and multisubunit E3 ubiquitin ligase that targets a variety of cell cycle regulators for proteolysis (Harper et al., 2002; Peters, 2006). At the metaphase to anaphase transition of M phase, the APC/C targets cyclin B, which activates the mitotic kinase Cdk1, and securin, which inhibits sister-chromatid separation, for degradation (Castro et al., 2005; Sullivan and Morgan, 2007). The APC/C also regulates entry into S phase via the degradation of cyclin A and geminin (Machida et al., 2005). APC/C activity is positively regulated by APC/C activators (Cdc20 and Cdh1) and negatively regulated by other proteins, such as spindle checkpoint proteins, Emi1 and Emi2 (Thornton and Toczyski, 2006; Pesin and Orr-Weaver, 2008).
Emi1, a vertebrate homolog of Drosophila Rca1 (Grosskortenhaus and Sprenger, 2002), is a primary inhibitor of the APC/C (APC/CCdh1) in interphase of the mammalian somatic cell cycle (Hsu et al., 2002; Thornton and Toczyski, 2006), and it is required for prevention of rereplication (Di Fiore and Pines, 2007; Machida and Dutta, 2007), as well as for entry into M phase (Reimann et al., 2001; Lee et al., 2006). Emi1 directly interacts with and inhibits the APC/C through the actions of the destruction box (D-box) and the zinc-binding region (ZBR) in the C-terminal region (Miller et al., 2006). The D-box competes with APC/C substrates for APC/C binding, enabling Emi1 to act as a pseudosubstrate inhibitor, whereas the ZBR somehow antagonizes APC/C ubiquitin ligase activity (Miller et al., 2006).
The meiotic cell cycle consists of two successive divisions: meiosis I (MI) and meiosis II (MII). In many animal species, full-grown immature oocytes are arrested at prophase of MI and undergo maturation after hormonal stimulation (Nebreda and Ferby, 2001; Kishimoto, 2003). In vertebrates, maturing oocytes progress from MI to MII without S phase and are arrested again at metaphase II (Meta-II) by cytostatic factor (CSF) (Masui and Markert, 1971; Sagata et al., 1989; Sagata, 1996). Emi2 (also called Erp1), a maternal paralog of Emi1, begins to be expressed immediately after MI and prevents cyclin B degradation partially (through the inhibition of APC/CCdc20), thereby enabling the MI/MII transition (without S phase) (Liu et al., 2006; Madgwick et al., 2006; Ohe et al., 2007). At Meta-II, accumulated Emi2 acts as a key effector of CSF and strongly inhibits the APC/C, thereby maintaining high Cdk1 activity and CSF arrest (Schmidt et al., 2005; Tung et al., 2005; Shoji et al., 2006). During maturation and CSF arrest of Xenopus oocytes, both the stability and the activity of Emi2 are up-regulated by the Mos-MAPK-p90rsk pathway (Inoue et al., 2007; Nishiyama et al., 2007; Wu et al., 2007b). On fertilization, however, Emi2 undergoes a rapid degradation in a manner dependent on CaMKII/Plk1 and SCFβ-TrCP ubiquitin ligase, thus allowing CSF release (Liu and Maller, 2005; Rauh et al., 2005; Hansen et al., 2006).
Although the mechanisms of expression, degradation, and activation of Emi2 are well known, how Emi2 interacts with and inhibits the APC/C is not well understood (Tung et al., 2007; Wu and Kornbluth, 2008). On the basis of the results with Emi1 (Miller et al., 2006), however, Emi2 has been thought to interact with and inhibit the APC/C via the C-terminal region containing the D-box and the ZBR. Indeed, it has been shown that both the D-box and the ZBR of Emi2 are required for APC/C inhibition and that the D-box is also required for Emi2-APC/C interaction (Schmidt et al., 2005; Nishiyama et al., 2007). However, the requirement of the D-box for Emi2-APC/C interaction is only partial (Nishiyama et al., 2007), suggesting an involvement of some other region(s) of Emi2 in Emi2-APC/C interactions.
In this study, using Xenopus egg extracts, we show that the C-terminal tail of Emi2 (termed here the RL tail) serves as a docking site for the APC/C and, thereby, promotes the inhibitory interactions of the D-box and the ZBR with the APC/C. The C-terminal tail of Emi1 is also required for Emi1 binding and inhibition of the APC/C. Thus, our data provide an important mechanistic insight into how Emi1/Emi2 interact with and inhibit the APC/C.
MATERIALS AND METHODS
Oocytes and CSF Extracts
Xenopus oocytes were prepared, cultured, matured, and microinjected as described previously (Ohe et al., 2007). CSF extracts were prepared and added with CaCl2, as described previously (Schmidt et al., 2005).
Cell Culture and Transfection
Human embryonic kidney (HEK) 293 cells cultured in 10-cm dishes (3 × 106) were transfected with 36 μg of expression plasmids by using a standard calcium phosphate-precipitation method.
cDNAs, In Vitro Transcription, and Morpholino Oligonucleotides
Full-length 3′UTR-containing cDNAs encoding Xenopus Emi2 (including proteolysis-resistant protein) and morpholino oligonucleotide (MO)-resistant Emi2 mRNA were described previously (Ohe et al., 2007). cDNAs encoding Xenopus and human Emi1 were isolated by PCR from appropriate cDNA libraries. All the cDNA constructs were subcloned into the N-terminally Myc3-tagged pT7G(UKII−) transcription vector (Ohe et al., 2007), except that the human Emi1 cDNA was subcloned into the pcDNA3.1(+) expression vector (Invitrogen, Carlsbad, CA). In vitro transcription of the cDNAs was performed as described previously (Inoue et al., 2007). Emi2 antisense MOs were prepared and used as described previously (Ohe et al., 2007).
Antibodies and Immunoblotting
Proteins from oocytes, CSF extracts, and HEK293 cells were analyzed by immunoblotting using Xenopus anti-Emi2(N) antibody (raised against residues 105-374 of Emi2), Xenopus anti-cyclin B1 antibody (gift from J. Maller, Howard Hughes Medical Institute, Aurora, CO), Xenopus anti-cyclin B2 antibody (gift from J. Maller), anti-Myc antibody (ab9106 or ab18185; Abcam, Cambridge, MA), anti-Cdc27 antibody (610455; BD Transduction Laboratories, Lexington, KY), anti-Cdc23 antibody (ab72206; Abcam), anti-Cdc20 antibody (ab18217; Abcam), anti-α-tubulin antibody (T9026; Sigma, St. Louis, MO), anti-geminin antibody (gift from H. Nishitani, University of Hyogo, Hyogo, Japan), or anti-cyclin A antibody (C4710; Sigma), essentially as described previously (Uto et al., 2004). To investigate the interaction between the RL tail of Emi2 and the APC/C, anti-Emi2-RL antibody raised against the C-terminal tail peptide (AQSKRNLKRL) was used.
Immunoprecipitation
For immunoprecipitation, CSF extracts (routinely 30 μl) were mixed with an equal volume of a binding buffer (20 mM Na2HPO4, 25 mM NaCl, 20 mM β-glycerophosphate, 4 mM EGTA, 20 μM leupeptin, 2 μM pepstatin A, 10 μg/ml aprotinin, 0.2 mM PMSF, 1 mM NaF, 1 mM Na3VO4, 5 mM 6-dimethylaminopurine (6DAP), and 1 μM okadaic acid), whereas HEK293 cells (routinely 3 × 106) were homogenized with 1 ml of a lysis buffer (20 mM Na2HPO4, 25 mM NaCl, 20 mM β-glycerophosphate, 4 mM EGTA, 1% Triton X-100, 20 μM leupeptin, 2 μM pepstatin A, 10 μg/ml aprotinin, 0.2 mM PMSF, 1 mM NaF, and 1 mM Na3VO4). The CSF extracts or cell lysates were then subjected to immunoprecipitation using beads coupled with anti-Myc antibody (ab1253; Abcam), protein G beads (22851; Pierce, Rockford, IL) coupled with anti-Cdc27 antibody (C7104; Sigma), anti-Emi2(N) antibody, or anti-Emi2-RL antibody for 30 min at 4°C. Coprecipitated proteins were analyzed by immunoblotting using appropriate antibodies.
RL Tail Peptide-APC/C–binding Assays
Synthetic peptides (CEALPGSAQSKRNLKRL, CEALPGSAQSKRNLKAA, CEALPGSKKSKQNLRRL, CEALPGSKKSKQNLRAA) were immobilized to SulfoLink coupling beads (20401; Pierce), with their quantitative couplings to the beads being verified by Ellman's test (Ellman, 1959). The beads (coupled with 30 μg of peptides) were incubated either with 30 μl of CSF extracts diluted with an equal volume of a binding buffer (20 mM Na2HPO4, 75 mM NaCl, 60 mM β-glycerophosphate, 4 mM EGTA, 20 μM leupeptin, 2 μM pepstatin A, 10 μg/ml aprotinin, 0.2 mM PMSF, 1 mM NaF, and 1 mM Na3VO4, 5 mM 6DAP, and 1 μM okadaic acid) or with immunopurified APC/C (derived from 80 μl of CSF extracts and dissolved in the binding buffer) for 20 min at 4°C and then pulled down for immunoblotting. To investigate the interaction of the RL tail of Emi2 and the APC/C, free synthetic peptides (adjusted to pH 7 with NaOH) were added to CSF extracts.
APC/C Purification
The APC/C was immunopurified from CSF extracts using Dynabeads protein G (Invitrogen) coupled with anti-Cdc27 antibody (C7104; Sigma) as described previously (Yamano et al., 2004), except that single AF3.1 epitope peptides (TQLHAAESDE; 3 mg/ml) were used to elute the APC/C from the beads. About 20% of the APC/C was recovered from the beads by this method.
RESULTS AND DISCUSSION
The C-Terminal Tail Is Required for Emi2 Activity during the MI/MII Transition
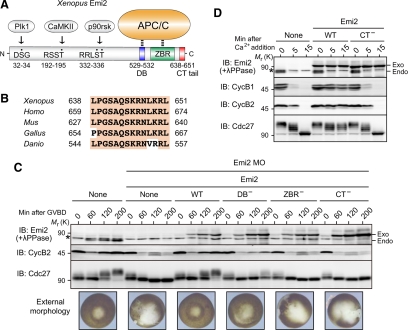
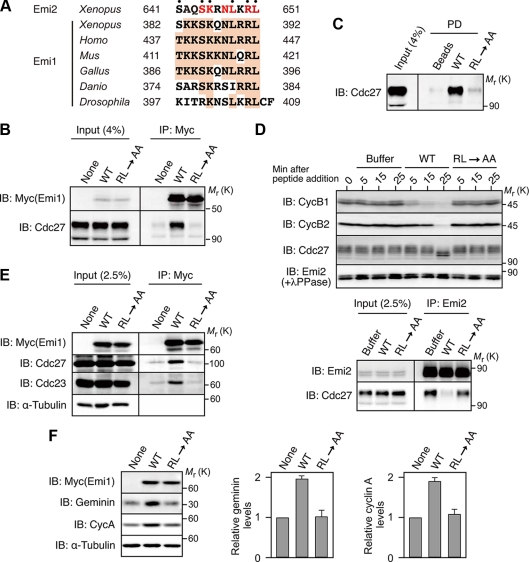
Emi2 inhibits the APC/C (APC/CCdc20) in vertebrate oocytes, and is essential for the MI to MII transition as well as for CSF arrest at Meta-II. Emi2 inhibition of the APC/C activity depends on both the C-terminal D-box, which likely competes with APC/C substrates for APC/C binding, and the ZBR, which somehow antagonizes APC/C ubiquitin ligase activity (Schmidt et al., 2005; Miller et al., 2006; Nishiyama et al., 2007). Emi2 has a C-terminal tail consisting of 14 amino acids, which immediately follows the ZBR (Figure 1A). Although this C-terminal tail is highly conserved in Emi2 proteins from various species (Figure 1B), its function, if any, has not been known. We first addressed whether the C-terminal tail would be required for normal Emi2 activity during progesterone-induced maturation of Xenopus oocytes. To this end, we ectopically expressed, by mRNA injection, Myc-tagged Emi2 mutants (deficient in the D-box, the ZBR, or the C-terminal tail) in oocytes in which the expression of endogenous Emi2 protein was inhibited by using Emi2 antisense MOs (Ohe et al., 2007). We then examined the expression of cyclin B2 and the external morphology of the oocytes after MI, to see whether the oocytes could enter MII and undergo arrest at Meta-II normally (Ohe et al., 2007). In the oocytes treated with Emi2 MO alone, reaccumulation of cyclin B2 was severely compromised after 1 h of germinal vesicle breakdown (GVBD) (or after MI; Furuno et al., 1994), and the external morphology was abnormal, with characteristic depigmentation of the animal hemisphere after 3 h of GVBD (Figure 1C, MO/None), consistent with a failure to enter MII and with parthenogenetic activation of the oocytes (Furuno et al., 1994; Inoue et al., 2007) (see Supplementary Figure S1 for quantification of the data of cyclin B2 in Figure 1C). Expression at endogenous levels of wild-type Emi2 in MO-treated oocytes was able to restore cyclin B2 reaccumulation and external morphology after GVBD (Figure 1C, WT), similar to previous results (Inoue et al., 2007). In contrast, expression of ZBR-deficient Emi2 (Cys583→Ala; Schmidt et al., 2005) could not restore such events at all (Figure 1C, ZBR−), whereas the expression of D-box–deficient Emi2 (Arg529/Leu532→Ala; Nishiyama et al., 2007) could do so, but only partially (Figure 1C, DB−). Intriguingly, expression of C-terminal tail–deficient (CT−) Emi2 (lacking the C-terminal 14 amino acids) also failed to restore cyclin B2 reaccumulation and external morphology after GVBD, although it was ∼2.5-fold greater than the expressions of other Emi2 constructs (Figure 1C, CT−). In these experiments, the levels of cyclin B2 correlated well with the electrophoretic mobility shifts of the APC/C core subunit Cdc27 (Figure 1C), in which the (up)shift is due primarily to phosphorylation by Cdk1/cyclin B and is a marker of APC/C inhibition at least during oocyte maturation (Gross et al., 2000; Kraft et al., 2003). Thus, these results show that the C-terminal tail is required for Emi2 activity (to inhibit the APC/C) during the MI to MII transition in Xenopus oocytes.
Figure 1.
Requirement of the C-terminal tail for Emi2 activity during the MI/MII transition and Meta-II arrest. (A) Domain organization of Xenopus Emi2 protein. Plk1, CaMKII, and p90rsk phosphorylate Ser or Thr residues (dotted) in the N-terminal region of Emi2 and regulate Emi2 stability and activity. The D-box (DB) and the ZBR in the C-terminal region serve to inhibit the APC/C, whereas the function of the C-terminal (CT) tail is not known. (B) Conservation of the C-terminal amino acid sequence in Emi2 proteins from various vertebrate species. (C) Immature oocytes were injected with Emi2 MO together with or without 300 pg of full-length 3′UTR-containing and MO-resistant mRNA encoding the indicated (Myc-)Emi2 constructs, cultured overnight, treated with progesterone, and subjected after GVBD to immunoblotting (IB) for the indicated proteins (for Emi2, oocyte extracts were treated with λ phosphatase before immunoblotting). Oocytes were also photographed 3 h after GVBD. Asterisk, background protein; exo, exogenous (Myc-)Emi2; endo, endogenous Emi2. (D) CSF extracts were incubated with or without 20 ng/μl mRNA encoding the indicated (Myc-)Emi2 constructs for 1 h, treated with cycloheximide for 5 min, further treated with calcium (CaCl2), and then subjected to immunoblotting for the indicated proteins. Four independent experiments were performed for both C and D, and, for each, a typical result is shown.
The C-Terminal Tail Is Required for Emi2 Activity during Meta-II Arrest
We also asked whether the C-terminal tail would be required for Emi2 activity during Meta-II (or CSF) arrest of mature oocytes. For this, we ectopically expressed either wild-type (WT) or CT− Emi2 proteins in Meta-II–arrested egg extracts (or CSF extracts) and added calcium (CaCl2) to the extracts to induce a release from CSF arrest (which is caused by the degradation of endogenous Emi2; Rauh et al., 2005). In this experiment, we used ectopic Emi2 proteins (WT or CT−) that were resistant to proteolysis upon calcium addition, to see the effect of the proteins on CSF release (Inoue et al., 2007). When expressed about threefold over endogenous levels, Emi2(WT) was able to block CSF release upon calcium addition, as evidenced by the maintenance of cyclin B1 and B2 levels and the persistence of slowly migrating (though partially dephosphorylated) Cdc27 even after calcium addition (Figure 1D). In contrast, Emi2(CT−) could not prevent CSF release at all, as shown by the very rapid degradation of cyclins B1/B2 and the apparently complete dephosphorylation of Cdc27 after calcium addition, just as in control CSF extracts treated with calcium alone (Figure 1D). Thus, Emi2(CT−) has virtually no activity to inhibit the APC/C in CSF extracts, indicating an absolute requirement for the C-terminal tail in Emi2 activity during Meta-II arrest. These results, together with the above results (Figure 1C), strongly indicate that the C-terminal tail is essential for Emi2 activity during maturation and Meta-II arrest of Xenopus oocytes.
The C-Terminal Tail Is Essential for Emi2-APC/C Interaction
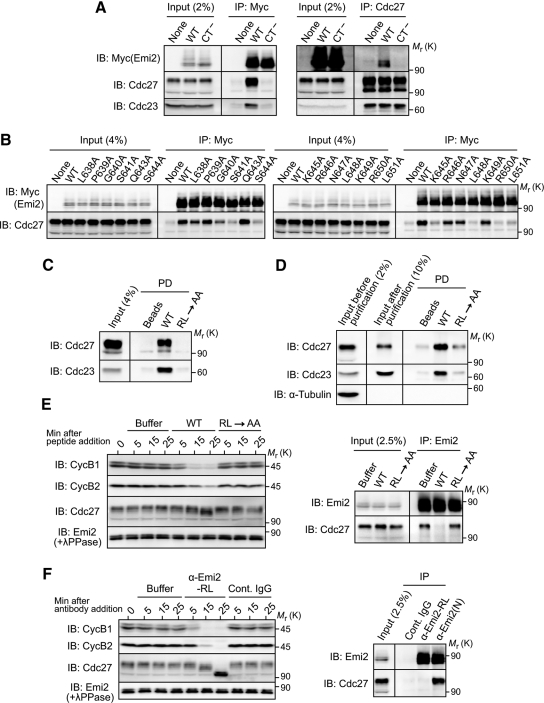
The activity or ability of Emi2 to inhibit the APC/C depends on the physical interaction of Emi2 with the APC/C (Wu et al., 2007a). Although the D-box and, possibly, the ZBR in Emi2 are both involved in Emi2-APC/C interactions as well as in APC/C inhibition (Miller et al., 2006; Nishiyama et al., 2007), the C-terminal tail might also be required for the Emi2-APC/C interaction. To test this possibility, we expressed Myc-tagged Emi2 proteins (WT or CT−) in CSF extracts and then subjected them to immunoprecipitation with anti-Myc antibody followed by immunoblotting with anti-Cdc27 antibody. Strikingly, Emi2(CT−) was not associated with endogenous Cdc27 (nor with another APC/C subunit Cdc23) at all, whereas Emi2(WT) was strongly associated with Cdc27 (as well as with Cdc23) (Figure 2A, left). Similar results were obtained by reciprocal immunoprecipitation with anti-Cdc27 antibody followed by immunoblotting with anti-Myc antibody (Figure 2A, right). Thus, these results show that the C-terminal tail is essential for Emi2-APC/C interaction, explaining why it was indispensable for Emi2 activity (Figure 1).
Figure 2.
Direct binding of Emi2 to the APC/C via the C-terminal RL tail. (A) CSF extracts were incubated with 20 ng/μl mRNA encoding the indicated (Myc-)Emi2 constructs for 2 h, subjected to immunoprecipitation (IP) either with anti-Myc antibody (left) or anti-Cdc27 antibody (right), and then immunoblotted for the indicated proteins. (B) CSF extracts were incubated with 30 ng/μl mRNA encoding the indicated (Myc-)Emi2 constructs for 1 h and subjected to Myc immunoprecipitation followed by Cdc27 immunoblotting. (C) CSF extracts were incubated with uncoupled control beads or beads coupled with Emi2 C-terminal tail peptides (residues 638-651 with or without an RL→AA mutation); the beads were then pulled down (PD) and subjected to immunoblotting for Cdc27 and Cdc23. (D) APC/C complexes immunopurified from CSF extracts were incubated with beads coupled with Emi2 C-terminal tail peptides and processed as in C. α-Tubulin served as a control of APC/C purification. (E) CSF extracts were added with buffer (as control) or an excess (2 mM) of C-terminal tail peptides and subjected either to immunoblotting for the indicated proteins at the indicated times (left) or to Emi2 immunoprecipitation followed by Cdc27 immunoblotting 5 min after the peptide addition (right). (F) CSF extracts were incubated with buffer, control rabbit IgG, or antibody against the C-terminal tail peptide (α-Emi2-RL; 40 ng/μl), and subjected to immunoblotting for the indicated proteins (left). CSF extracts were also incubated with control IgG, α-Emi2-RL, or anti-Emi2 (N-terminus) antibody (α-Emi2(N)) for 30 min and subjected to immunoprecipitation (with the respective antibodies) followed by Emi2 or Cdc27 immunoblotting (right). At least three independent experiments were performed for A–F, and for each a typical result is shown.
The 14-amino acid sequence of the C-terminal tail is nearly perfectly conserved in Emi2 proteins from various vertebrate species (see Figure 1B), so we determined which residue(s) in the C-terminal tail was required for Emi2-APC/C interaction, by expressing, in CSF extracts, Emi2 mutants with substituted alanine at the individual residues (except Ala642) of the C-terminal tail. Among the C-terminal 14 amino acids, the N-terminal three consecutive residues (Leu-Pro-Gly) were largely dispensable for Emi2-Cdc27 association, but at least six residues, including the ultimate Arg-Leu residues, of the C-terminal 11 residues were indispensable for the association (Figure 2B). Thus, the C-terminal 11-amino acid sequence of Emi2 apparently is required for Emi2-APC/C interaction and hence for Emi2 activity. Hereafter, we refer to this sequence as an RL tail or motif after the ultimate two amino acids of the sequence.
The RL Motif Directly Binds to the APC/C, with This Binding Being Essential for APC/C Inhibition by Endogenous Emi2
We addressed the important question of whether the RL motif could bind to the APC/C. To do this, we incubated bead-bound RL motif peptides (residues 638-651 with or without an RL→AA mutation) with CSF extracts, precipitated the beads, and subjected them to immunoblotting for Cdc27 (and Cdc23). These analyses showed that the WT RL motif peptides, but not the mutated peptides, coprecipitated with endogenous Cdc27 (as well as Cdc23) (Figure 2C). Furthermore, even when incubated with immunopurified APC/C, the WT but not the mutated RL motif peptides coprecipitated with Cdc27 (and Cdc23) (Figure 2D). Thus, these results strongly suggest that the RL motif can bind to the APC/C directly.
We next asked whether the RL motif–mediated binding was essential for the interaction of endogenous Emi2 with the APC/C, and hence for APC/C inhibition and Meta-II arrest in CSF extracts. For this, we tested whether an excess of (free) RL motif peptides added to CSF extracts could compete with endogenous Emi2 for APC/C binding and thereby could release CSF arrest (in the absence of calcium addition). Emi2 immunoprecipitation followed by Cdc27 immunoblotting revealed that the WT but not the mutated RL motif peptides were able to inhibit the association of endogenous Emi2 with Cdc27 (Figure 2E, right). The WT peptides, but not the mutated peptides, could also release CSF arrest, as indicated by the reduced levels of cyclins B1/B2 and the significantly decreased mobility shift of Cdc27 soon after addition of the peptides (Figure 2E, left). Furthermore, and interestingly, adding an antibody against the RL motif peptides to CSF extracts potently released CSF arrest (Figure 2F, left). As expected, immunoprecipitates using this anti-RL motif peptide antibody contained Emi2 but not Cdc27, whereas immunoprecipitates using anti-Emi2 (N-terminus) antibody contained both Emi2 and Cdc27 (Figure 2F, right). Thus, evidently, the anti-RL motif antibody stably bound to the RL motif (of endogenous Emi2) can prevent endogenous Emi2-APC/C interactions and thereby release CSF arrest. Altogether, these results strongly suggest that endogenous Emi2 directly interacts with the APC/C via the RL tail and that this interaction is essential for APC/C inhibition and hence for CSF arrest. Furthermore, and importantly, the fact that an excess of RL motif peptides can activate, rather than inactivate, the APC/C by competing with endogenous Emi2 for APC/C binding, does suggest that the RL tail of Emi2 serves as a docking site rather than an inhibitory site for the APC/C.
The RL Tail of Emi2 and the C-Terminal Tail of Cdc20 Bind to Different Sites within the APC/C
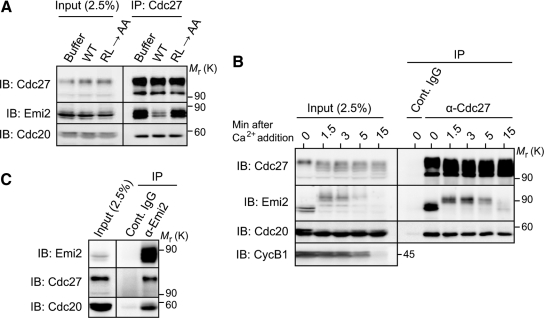
It has been shown that Cdc20, an activator of the APC/C (Yu, 2007), also binds to the APC/C via the C-terminal tail called an IR domain (Vodermaier et al., 2003). We asked whether the RL tail of Emi2 would bind to the same site(s) within the APC/C as the IR domain of Cdc20, thereby competitively inhibiting the activation of the APC/C by Cdc20. When added to CSF extracts, an excess of Emi2 WT-RL motif peptides could not appreciably affect the association of Cdc20 with Cdc27 (or the APC/C), whereas it could largely inhibit Emi2-Cdc27 association (Figure 3A). Furthermore, upon degradation of Emi2 by calcium treatment of CSF extracts, the association of Cdc20 with Cdc27 was not affected (or increased) at all (Figure 3B). Moreover, in CSF extracts, Emi2 was bound to the APC/C complexed with Cdc20 (Figure 3C), consistent with previous results (Wu et al., 2007a). Thus, it appears that the RL tail of Emi2 binds to the APC/C at a site(s) distinct from that occupied by the IR domain of Cdc20. This notion, however, is consistent with the fact that most of the essential residues in the RL motif of Emi2 (Figures 1B and 2B) are not conserved in the IR domain of Cdc20 (Vodermaier et al., 2003).
Figure 3.
Binding of the Emi2 RL motif to the APC/C at a site(s) distinct from that occupied by the Cdc20 IR domain. (A) CSF extracts were incubated with an excess (2 mM) of the indicated C-terminal RL motif peptides for 5 min and subjected to Cdc27 immunoprecipitation followed by Emi2 or Cdc20 immunoblotting. (B) CSF extracts were treated with calcium and then subjected to either control or Cdc27 immunoprecipitation followed by immunoblotting for the indicated proteins. On calcium treatment, Emi2 underwent hyperphosphorylation and degradation, as previously shown (Rauh et al., 2005). (C) CSF extracts were subjected to either control or Emi2 immunoprecipitation followed by immunoblotting for Cdc27 and Cdc20. The anti-Emi2 antibody used was anti-Emi2 (N-terminus) antibody, not anti-RL motif peptide antibody (which could not precipitate Cdc27 or Cdc20; data not shown, but see Figure 2F). Four, three, and four independent experiments were performed for A–C, respectively, and, for each a typical result is shown.
RL Motif Binding Likely Promotes the Interactions of the D-Box and the ZBR with the APC/C
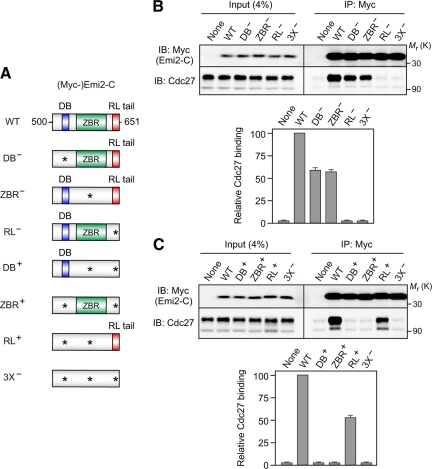
The RL motif is essential for the binding of Emi2 to the APC/C, as shown above. However, the D-box and the ZBR, which are both required for APC/C inhibition, are also likely to be involved in Emi2-APC/C interactions (Miller et al., 2006; Nishiyama et al., 2007). Then a question arises how the bindings of the three distinct motifs or domains to the APC/C are related to each other. To address this question, we used a (Myc-tagged) C-terminal fragment of Emi2 (residues 500-651; termed here Emi2-C) that contained only the D-box, the ZBR, and the RL tail (Figure 4A). We introduced Ala mutation in the essential residues of the D-box (R529/L532→A/A; DB−), the ZBR (C583→A; ZBR−), or the RL motif (RL→AA; RL−) of the Emi2-C construct (Figure 4A). When expressed in CSF extracts and immunoprecipitated with anti-Myc antibody, both the DB− and ZBR− mutants were associated with Cdc27 significantly (40–50%) less strongly than WT Emi2-C (Figure 4B). In contrast, the RL− mutant did not show any appreciable association with Cdc27 above the nonspecific background association, similar to the triple DB−/ZBR−/RL− (or 3X−) mutant (Figure 4B), suggesting a much stronger APC/C binding of the RL motif than those of the D-box and the ZBR (similar results were obtained using full-length Emi2 constructs; Supplementary Figure S2). We then constructed three more mutants of Emi2-C in which any two of the three motifs, in combination, were mutated; these mutants thus contained only one intact motif among the three motifs (hence DB+, ZBR+, and RL+ mutants) (Figure 4A). Somewhat surprisingly, neither the DB+ nor the ZBR+ mutants showed any detectable association with Cdc27 above the nonspecific background, similar to the 3X− mutant (Figure 4C). However, the RL+ mutant was associated with Cdc27, albeit significantly (∼50%) less strongly than the WT (or DB+/ZBR+/RL+) (Figure 4C). Thus, these results indicate that the RL motif can bind to the APC/C much more tightly than the D-box and the ZBR.
Figure 4.
Comparison of the binding affinities of the D-box, the ZBR, and the RL tail to the APC/C. (A) Schematic representation of various Emi2-C constructs. Each Emi2-C construct is N-terminally tagged with three Myc-epitopes. Asterisk, Ala mutation of the essential residue(s) in the indicated motifs (see text for details). (B and C) CSF extracts were incubated with 35 ng/μl mRNA encoding the indicated (Myc-)Emi2-C constructs for 1 h and subjected to Myc immunoprecipitation followed by Cdc27 immunoblotting (top). The levels of coprecipitated Cdc27 were quantitated and normalized to (Myc-)Emi2-C proteins; the value obtained for Cdc27 coprecipitated with WT Emi2-C was set at 100, and all values are means ± SD of five independent experiments (bottom).
The present results suggest that, although the APC/C binding affinity of the D-box alone or the ZBR alone is undetectably low (see DB+ and ZBR+ in Figure 4C), in the presence of the RL motif both the D-box and the ZBR can significantly contribute to Emi2-APC/C interactions (compare WT with DB− and ZBR− in Figure 4B). Thus, it is likely that the RL motif serves as a docking site for the APC/C (see also Figure 2) and thereby promotes the interactions of the D-box and the ZBR with the APC/C. Such events may well enable APC/C inhibition by the D-box and the ZBR. In this context, it is noteworthy that both the RL tail (which functions as a docking site) and the ZBR (which somehow inhibits APC/C ligase activity) are essential for Emi2 activity to inhibit the APC/C, whereas the D-box (which likely competes with substrate for APC/C binding) is not so critical (Figure 1C). Thus, the RL motif seems to be of primary importance for Emi2-APC/C interaction and, hence, for APC/C inhibition, which itself is mediated principally by the ZBR (Figure 1C; Schmidt et al., 2005).
Emi1 Also Binds the APC/C via the RL Motif
Emi1, a somatic paralog of Emi2, inhibits the APC/C (APC/CCdh1) in mammalian cells (Hsu et al., 2002; Di Fiore and Pines, 2007; Machida and Dutta, 2007). We noticed that the C-terminal tail of Emi1 is also well conserved in vertebrate species and shows a strong similarity to the C-terminal tail of Emi2. In particular, the Emi1 C-terminal tail contains most of the essential residues, including the ultimate RL residues, of the Emi2 RL tail (Figures 2B and 5A). We therefore investigated whether the RL-like motif of Emi1 could serve for Emi1-APC/C interaction. When expressed in CSF extracts and immunoprecipitated, wild-type Xenopus Emi1, but not its RL→AA mutant, was shown to be associated with endogenous Cdc27 (Figure 5B). Furthermore, when incubated with CSF extracts, (bead-bound) WT but not RL→AA peptides of the RL-like motif of Emi1 coprecipitated with Cdc27 (Figure 5C), similar to the RL motif peptides of Emi2 (Figure 2C). Even more interestingly, an excess of (free) WT but not RL→AA peptides of the RL-like motif was able to dissociate endogenous Emi2 from Cdc27 and release CSF arrest (Figure 5D), similar to the RL motif peptides of Emi2 (Figure 2E). Thus, these results strongly suggest that the C-terminal tail of Emi1 can serve as an RL motif for Emi1-APC/C interaction and binds to the same site(s) within the APC/C as the Emi2 RL motif.
Figure 5.
Binding of Emi1 to the APC/C via the RL tail. (A) Conservation of the C-terminal amino acid sequence in Emi1 proteins from various species. Emi2 (Xenopus) also has conserved amino acids (in red) at the corresponding sites of Emi1 (dotted residues are those essential for APC/C binding). (B) CSF extracts were incubated with 35 ng/μl mRNA encoding the indicated Xenopus (Myc-)Emi1 constructs (proteolysis-resistant forms; Ohsumi et al., 2004) for 2 h and subjected to Myc immunoprecipitation followed by Cdc27 immunoblotting. (C) CSF extracts were incubated with uncoupled control beads or beads coupled with the indicated Xenopus Emi1 C-terminal tail peptides (residues 379-392); the beads were then pulled down and subjected to immunoblotting for Cdc27. (D) CSF extracts were added with buffer (as control) or an excess (2 mM) of the indicated Xenopus Emi1 C-terminal peptides and subjected either to immunoblotting for the indicated proteins at the indicated times (top) or to Emi2 immunoprecipitation followed by Cdc27 immunoblotting 5 min after the peptide addition (bottom). (E) HEK293 cells were transfected with cDNA encoding the indicated human (Myc-)Emi1 constructs, cultured for 48 h, and subjected to Myc immunoprecipitation followed by immunoblotting for the indicated proteins (α-tubulin being a loading and IP control). (F) HEK293 cells transfected as in E were subjected to immunoblotting for the indicated proteins (left). The levels of geminin and cyclin A were quantitated and normalized to the level of α-tubulin; the value obtained for nontransfected cells was set at 1, and all values are means ± SD of five independent experiments (middle and right). At least three independent experiments were performed for B–E, and, for each a typical result is shown.
We then addressed whether the RL motif of Emi1 would be required for Emi1-APC/C interaction and APC/C inhibition in human somatic cells. When expressed in HEK293 cells and immunoprecipitated, wild-type human Emi1, but not its RL→AA mutant, was associated with endogenous Cdc27 (and Cdc23) (Figure 5E). Furthermore, overexpression of the WT but not RL→AA Emi1 in HEK293 cells significantly stabilized geminin and cyclin A, which are both natural substrates for the APC/CCdh1 (Machida et al., 2005; Peters, 2006) (Figure 5F). Thus, even in human cells, the RL motif of Emi1 apparently is required for Emi1 binding and inhibition of the APC/C. These results, together with the above results (Figure 5, B–D), strongly suggest that the RL tail of Emi1, like that of Emi2, serves as a docking site for the APC/C.
The RL tail of Emi1 is somewhat less well conserved in vertebrates than the RL tail of Emi2 (although it contains most of the essential residues in the Emi2 RL tail) (Figures 1B and 5A). This might, however, imply that Emi1, which functions principally in somatic cells and is also present in Drosophila (Grosskortenhaus and Sprenger, 2002), is evolutionarily older than Emi2, which appears to be specific to oocyte meiosis in vertebrates (Pesin and Orr-Weaver, 2008).
Concluding Remarks
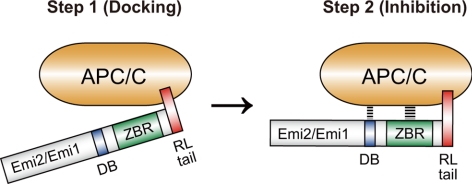
On the basis of the results with Emi1 (and partly Emi2 itself), the (physical) interaction of Emi2 with the APC/C has been thought to be mediated primarily by the D-box and possibly also by the ZBR. In this study, however, we clearly show that the C-terminal tail, termed here the RL tail or motif, is essential for the interaction of Emi2 (as well as Emi1) with the APC/C. The RL motif seems to serve as a docking site of Emi1/Emi2 for the APC/C, thereby promoting the interactions of the D-box and the ZBR with the APC/C and hence enabling the inhibition of the APC/C (Figure 6). Our results thus provide an important mechanistic insight into how Emi1/Emi2 interact with and inhibit the APC/C. In the future, elucidation of the precise binding site(s) of the RL motif, as well as those of the D-box and the ZBR, within the APC/C will greatly contribute to our better understanding of the molecular mechanism of APC/C regulation by Emi1/Emi2.
Figure 6.
A two-step model for the inhibition of the APC/C by Emi1/Emi2. Emi1/Emi2 inhibit the APC/C by two steps: (1) docking onto the APC/C via the RL tail, and (2) interaction and inhibition of the APC/C by the D-box and the ZBR.
Supplementary Material
[Supplemental Materials]
ACKNOWLEDGMENTS
We thank Drs. Jim Maller and Hideo Nishitani for anti-cyclin B1/B2 and anti-geminin antibodies, respectively. We also thank Drs. Takumi Koshiba and Toshiki Tsurimoto for technical advice on handling HEK293 cells, and Kazumi Ota for typing the manuscript. This work was supported by a scientific grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to N.S. M.O. and H.U. are research fellows of the Japan Society for the Promotion of Science.
Abbreviations used:
APC/C
anaphase-promoting complex/cyclosome
CSF
cytostatic factor
GVBD
germinal vesicle breakdown
D-box
destruction box
MI
meiosis I
MII
meiosis II
ZBR
zinc-binding region.
Present addresses: *Institute of Zoology, University of Heidelberg, 69120 Heidelberg, Germany;
†Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba Science City, Ibaraki 305-8572, Japan.
REFERENCES
- Castro A., Bernis C., Vigneron S., Labbe J. C., Lorca T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- Di Fiore B., Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J. Cell Biol. 2007;177:425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Furuno N., Nishizawa M., Okazaki K., Tanaka H., Iwashita J., Nakajo N., Ogawa Y., Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. D., Schwab M. S., Taieb F. E., Lewellyn A. L., Qian Y. W., Maller J. L. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90Rsk. Curr. Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Grosskortenhaus R., Sprenger F. Rca1 inhibits APC-Cdh1Fzr and is required to prevent cyclin degradation in G2. Dev. Cell. 2002;2:29–40. doi: 10.1016/s1534-5807(01)00104-6. [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Tung J. J., Jackson P. K. CaMKII and Polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl. Acad. Sci. USA. 2006;103:608–613. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Burton J. L., Solomon M. J. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- Hsu J. Y., Reimann J.D.R., Sorensen C. S., Lukas J., Jackson P. K. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nat. Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Inoue D., Ohe M., Kanemori Y., Nobui T., Sagata N. A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature. 2007;446:1100–1104. doi: 10.1038/nature05688. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Cell-cycle control during meiotic maturation. Curr. Opin. Cell Biol. 2003;15:654–663. doi: 10.1016/j.ceb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kraft C., Herzog F., Gieffers C., Mechtler K., Hagting A., Pines J., Peters J. M. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Lee D. J., Oh S. P., Park H. D., Nam H. H., Kim J. M., Lim D. S. Mouse emi1 has an essential function in mitotic progression during early embryogenesis. Mol. Cell. Biol. 2006;26:5373–5381. doi: 10.1128/MCB.00043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Grimison B., Lewellyn A. L., Maller J. L. The anaphase-promoting complex/cyclosome inhibitor Emi2 is essential for meiotic but not mitotic cell cycles. J. Biol. Chem. 2006;281:34736–34741. doi: 10.1074/jbc.M606607200. [DOI] [PubMed] [Google Scholar]
- Liu J., Maller J. L. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Machida Y. J., Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida Y. J., Hamlin J. L., Dutta A. Right place, right time, and only once: replication initiation in metazoans. Cell. 2005;123:13–24. doi: 10.1016/j.cell.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Madgwick S., Hansen D. V., Levasseur M., Jackson P. K., Jones K. T. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J. Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Miller J. J., Summers M. K., Hansen D. V., Nachry M. V., Lehman N. L., Loktev A., Jackson P. K. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda A. R., Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol. 2001;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Ohsumi K., Kishimoto T. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature. 2007;446:1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- Ohe M., Inoue D., Kanemori Y., Sagata N. Erp1/Emi2 is essential for the meiosis I to meiosis II transition in Xenopus oocytes. Dev. Biol. 2007;303:157–164. doi: 10.1016/j.ydbio.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Ohsumi K., Koyanagi A., Yamamoto T. M., Gotoh T., Kishimoto T. Emi1-mediated M-phase arrest in Xenopus eggs is distinct from cytostatic factor arrest. Proc. Natl. Acad. Sci. USA. 2004;101:12531–12536. doi: 10.1073/pnas.0405300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesin J. A., Orr-Weaver T. L. Regulation of APC/C activators in mitosis and meiosis. Annu. Rev. Cell Dev. Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Rauh N. R., Schmidt A., Bormann J., Nigg E. A., Mayer T. U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Reimann J. D., Freed E., Hsu J. Y., Kramer E. R., Peters J. M., Jackson P. K. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;106:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Sagata N. Meiotic metaphase arrest in animal oocytes: its mechanisms and biological significance. Trends Cell Biol. 1996;6:22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Duncan P. I., Rauh N. R., Sauer G., Fry A. M., Nigg E. A., Mayer T. U. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Yoshida N., Amanai M., Ohgishi M., Fukui T., Fujimoto S., Nakano Y., Kajikawa E., Perry A. C. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M., Morgan D. O. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 2007;11:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- Thornton B. R., Toczyski D. P. Precise destruction: an emerging picture of the APC/C. Genes Dev. 2006;20:3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- Tung J. J., Hansen D. V., Ban K. H., Loktev A. V., Summers M. K., Adler J. R., III, Jackson P. K. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. J., Padmanabhan K., Hansen D. V., Richter J. D., Jackson P. K. Translational unmasking of Emi2 directs cytostatic factor arrest in meiosis II. Cell Cycle. 2007;6:725–731. doi: 10.4161/cc.6.6.3936. [DOI] [PubMed] [Google Scholar]
- Uto K., Inoue D., Shimuta K., Nakajo N., Sagata N. Chk1, but not Chk2, inhibits Cdc25 phosphatases by a novel common mechanism. EMBO J. 2004;23:3386–3396. doi: 10.1038/sj.emboj.7600328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodermaier H. C., Gieffers C., Maurer-Stroh S., Eieenhaber F., Peters J. M. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 2003;13:1459–1468. doi: 10.1016/s0960-9822(03)00581-5. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr. Biol. 2007a;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Q., Hansen D. V., Guo Y., Wang M. Z., Tang W., Freel C. D., Tung J. J., Jackson P. K., Kornbluth S. Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc. Natl. Acad. Sci. USA. 2007b;104:16564–16569. doi: 10.1073/pnas.0707537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Q., Kornbluth S. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 2008;121:3509–3514. doi: 10.1242/jcs.036855. [DOI] [PubMed] [Google Scholar]
- Yamano H., Gannon J., Mahbubani H., Hunt T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol. Cell. 2004;13:137–147. doi: 10.1016/s1097-2765(03)00480-5. [DOI] [PubMed] [Google Scholar]
- Yu H. Cdc20, a WD40 activator for a cell cycle degradation machine. Mol. Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Materials]