Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4 (original) (raw)
Abstract
Type 2 diabetes has been coined “a two-hit disease,” as it involves specific defects of glucose-stimulated insulin secretion from the pancreatic beta cells in addition to defects in peripheral tissue insulin action required for glucose uptake. Both of these processes, insulin secretion and glucose uptake, are mediated by SNARE (soluble _N_-ethylmaleimide-sensitive factor attachment protein receptor) protein core complexes composed of syntaxin, SNAP-23/25, and VAMP proteins. The SNARE core complex is regulated by the Sec1/Munc18 (SM) family of proteins, which selectively bind to their cognate syntaxin isoforms with high affinity. The process of insulin secretion uses multiple Munc18-syntaxin isoform pairs, whereas insulin action in the peripheral tissues appears to use only the Munc18c-syntaxin 4 pair. Importantly, recent reports have linked obesity and Type 2 diabetes in humans with changes in protein levels and single nucleotide polymorphisms (SNPs) of Munc18 and syntaxin isoforms relevant to these exocytotic processes, although the molecular mechanisms underlying the observed phenotypes remain incomplete (5, 104, 144). Given the conservation of these proteins in two seemingly disparate processes and the need to design and implement novel and more effective clinical interventions, it will be vitally important to delineate the mechanisms governing these conserved SNARE-mediated exocytosis events. Thus, we provide here an up-to-date historical review of advancements in defining the roles and molecular mechanisms of Munc18-syntaxin complexes in the pathophysiology of Type 2 diabetes.
Keywords: Sec1/Munc18 proteins, glucose homeostasis, diabetes, insulin resistance, soluble _N_-ethylmaleimide-sensitive factor attachment protein receptor proteins, glucose-stimulated insulin secretion
Coordination of Whole Body Glucose Homeostasis
Circulating blood glucose levels are tightly regulated in mammals and are maintained at about 5 mM (∼80–100 mg/dl). Following intake of high-carbohydrate food, blood glucose levels rise to ∼8 mM (∼120–140 mg/dl), which under normal homeostatic circumstances, induces the pancreas to secrete insulin from the beta cells within the islets of Langerhans. This release of insulin subsequently signals to the liver to reduce glucose output, while simultaneously inducing clearance of excess glucose from the blood by the skeletal muscle and adipose tissue. Normally, this process restores the blood glucose levels to 5 mM within 2 h after the meal. However, during pathological progression from a normal to a clinically defined Type 2 diabetic phenotype, there are clear and progressive aberrations in both insulin secretion, as well as glucose uptake/clearance mechanisms. Given that a significant number of proteins required for insulin secretion and glucose clearance are identical, alterations in their abundance and/or function would materially impact both mechanisms and increase susceptibility to aberrant glucose homeostasis.
SNARE-Mediated Exocytosis
The SNARE core complex is composed of three proteins in a heterotrimeric 1:1:1 ratio: 1) syntaxin, a 35-kDa protein containing an N-terminal regulatory domain, a C-terminal SNARE domain, and a far C-terminal transmembrane domain anchoring it to the plasma membrane (PM); 2) SNAP23 or SNAP25, two complementary isoforms that lack transmembrane domain anchors but localize to the PM due to palmitoylated cysteine residues; and 3) VAMP2 (also known as synaptobrevin), an 18-kDa protein containing a far C-terminal transmembrane domain, anchoring it to the vesicle membrane. As depicted in Fig. 1, the vesicle (v-) SNARE VAMP2 pairs with the two target PM (t-) SNAREs syntaxin and SNAP23/25 forming the SNARE core complex. Together, the three SNARE proteins produce a stable bundle of the four α-helices, with one α-helix from VAMP, one from syntaxin, and the remaining two from SNAP23/25 (for a review, see Ref. 52). This complex is remarkably SDS resistant once formed. To date, 6 v-SNARE isoforms and 13 t-SNARE isoforms have been identified in cell types relevant to insulin secretion and insulin action (Table 1). Interestingly, the underlying vesicle/granule exocytosis events of insulin secretion and glucose uptake share numerous commonalities with those of neuronal synaptic vesicle exocytosis (Table 2). Glucose-stimulated insulin secretion (GSIS) from the pancreatic beta cell and glucose uptake in the muscle and adipose are both mediated by the same SNARE protein isoforms: syntaxin 4, SNAP23, and VAMP2 (for reviews, see Refs. 29, 131). Additionally, beta cells use t-SNARE isoforms syntaxin 1 and SNAP25 to mediate the first phase of insulin secretion (27). However, key differences exist that distinguish the specialization of events in each cell type (summarized in Table 2).
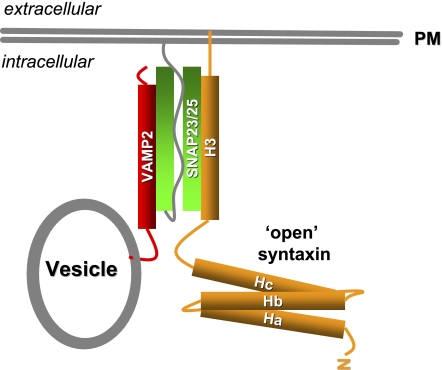
Fig. 1.
Soluble _N_-ethylmaleimide sensitive factor attachment protein receptor (SNARE) core complex formation. Upon stimulation, syntaxin (orange) adopts an “open” conformation exposing its H3 domain to form the SNARE core complex with VAMP2 (red) and SNAP23/25 (green).
Table 1.
Expression of v- and t-SNARE isoforms in adipose, skeletal muscle, and pancreatic β-cells
| Tissue (Localization) | Function | References | |
|---|---|---|---|
| v-SNARE | |||
| VAMP2/Synaptobrevin | Pancreatic β-cells, adipocyte, muscle (vesicles) | Exocytosis of insulin granules (β-cells), GLUT4 vesicles at the PM | (87, 96, 149, 160) |
| VAMP3/Cellubrevin | Pancreatic β-cells, adipocyte, muscle (vesicles) | Exocytosis of insulin granules (β-cells), GLUT4 vesicles at the PM | (125, 149, 158, 160) |
| VAMP4 | Adipocyte, muscle (TGN) | ND | (123, 160) |
| VAMP5/myobrevin | Adipocyte, muscle (PM) | Myogenesis | (160, 171) |
| VAMP7/TI-VAMP | Adipocyte (PM, endosome) | Osmotic shock-induced GLUT4 translocation | (160) |
| VAMP8/Endobrevin | Pancreatic beta cells, adipocyte (endosome) | GLUT4 endocytosis, insulin secretion (β-cells) | (77, 160) |
| t-SNARE | |||
| Syntaxin 1A | Pancreatic β-cells (PM) | First phase insulin secretion in pancreatic β-cells | (76, 85, 91) |
| Syntaxin 2/Epimorphin | Pancreatic β-cells, adipocyte (PM) | ND | (137, 151, 173) |
| Syntaxin 3 | Pancreatic β-cells, adipocyte (PM) | ND | (137, 156, 158) |
| Syntaxin 4 | Pancreatic β-cells, adipocyte, muscle (PM) | Both phases of insulin secretion (β-cells), GLUT4 translocation | (87, 120, 122, 151) |
| Syntaxin 5 | Adipocyte (TGN) | GLUT4 endocytosis in adipocyte | (156) |
| Syntaxin 6 | Adipocyte, muscle (TGN) | Putative involvement in GLUT4 endocytosis (adipocytes) | (92, 117, 133) |
| Syntaxin 7 | Pancreatic β-cells, adipocyte (endosome) | ND | (90, 92) |
| Syntaxin 8 | Adipocyte (endosome) | ND | (92) |
| Syntaxin 10 | Muscle | ND | (127) |
| Syntaxin 12 | Adipocyte | ND | (92) |
| Syntaxin 16 | Adipocyte (TGN) | GLUT4 intracellular trafficking | (117) |
| SNAP23 | Pancreatic β-cells, adipocyte, muscle (PM) | Exocytosis of insulin granules (β-cells), GLUT4 vesicles at the PM | (94, 153) |
| SNAP25 | Pancreatic β-cells (PM) | Exocytosis of insulin granules (β-cells) | (108) |
Table 2.
Comparison of exocytosis events of insulin granules, GLUT4 vesicles and synaptic vesicles
| Insulin Secretion | GLUT4 Vesicle Translocation | Neurotransmitter Release |
|---|---|---|
| Large secretory granules | Large secretory granules | Small synaptic vesicles |
| (>100-nm radius) | (>100-nm radius) | (<25-nm radius) |
| Recycling via Golgi complex | Recycling via Golgi complex | Local recycling |
| Two secretion phases: 1st fast (spans 6-10 min); 2nd, slow and sustained (10 min–hours) | Slow (5–15 min) and sustained | Fast, short-lasting secretion |
| (0.1–6 ms) | ||
| Small number of predocked granules | Few predocked vesicles | Large number of predocked vesicles |
| Exocytosis targeted to large plasma membrane section | Exocytosis targeted to large plasma membrane section | Exocytosis restricted to synaptic active zone |
| Complex secretory mixes (e.g., multiple peptides, catecholamines, nucleotides) | Cargo proteins to integrate into the plasma membrane | Release of one or two low-molecular-weight compounds |
| SNARE isoforms: syntaxins 1 and 4, | SNARE isoform: syntaxin 4 | SNARE isoform: syntaxin 1 |
| SNAP23 and SNAP25, VAMP2 and VAMP3 | SNAP23, VAMP2 | SNAP25, VAMP2 |
| SM isoforms: Munc18-1, Munc18c | SM isoform: Munc18c | SM isoform: Munc18-1 |
| Latrunculin potentiates secretion | Latrunculin inhibits GLUT4 translocation | Latrunculin potentiates secretion |
Insulin Granule Exocytosis
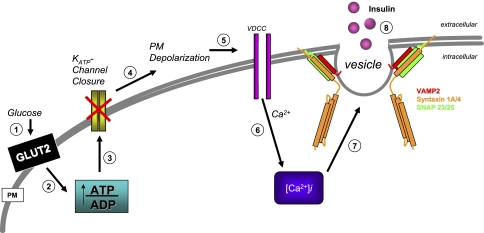
Elevated postprandial glucose levels trigger a signaling cascade in the pancreatic islet beta cells to elicit insulin release (Fig. 2), depicted as an ∼8-step process (for reviews, see Refs. 64 and 100). First, glucose entry into beta cells is facilitated via the plasma membrane-localized glucose transporter, GLUT2. GLUT2 has a relatively low affinity (_K_M ∼30 mM) for glucose, is constitutively present within the plasma membrane, and does not require SNARE proteins for translocation and membrane localization. Following GLUT2-facilitated uptake, glucose is phosphorylated by glucokinase to generate glucose-6-phosphate, which is subsequently metabolized via mitochondrial oxidative phosphorylation to effect an increase in intracellular ATP:ADP ratio (step 2). Elevated beta cell ATP levels induce closure of ATP-dependent potassium channels (step 3), resulting in cell depolarization (step 4) and an influx of calcium ions (Ca2+) through voltage-dependent calcium channels (step 5) to yield a net increase in the intracellular Ca2+ concentration ([Ca2+]i; step 6). The increase in [Ca2+]i signals SNARE complex formation (step 7) to facilitate insulin release from the granules (step 8), although the precise mechanism(s) by which Ca2+ triggers granule fusion remains somewhat unresolved.
Fig. 2.
Glucose-stimulated insulin secretion from the islet beta cell. An 8-step model: step 1: Glucose enters the cell through the constitutively active PM localized GLUT2 transporter and gets metabolized, which, in turn, (step 2) increases the ATP:ADP ratio resulting in (step 3) the closure of the ATP-dependent potassium channels (KATP). The closure of the KATP channels leads to (step 4) plasma membrane (PM) depolarization, (step 5) opening of the voltage-dependent calcium channels (VDCC), causing calcium influx into the cell (step 6). As a result, intracellular calcium [Ca2+]i levels rise, and through a largely uncharacterized series of events, the SNARE proteins mediate (step 7) vesicle fusion to facilitate (step 8) insulin release.
Insulin secretion occurs in pulsatile fashion in sync with Ca2+ influxes during two major phases, and is termed “biphasic.” First-phase insulin secretion occurs within 5–10 min following beta cell stimulation. Second-phase insulin secretion is less robust than the first phase, but can be sustained for several hours if elevated blood-glucose levels persist (21, 40, 45, 46). These two phases of secretion are thought to use separate pools of insulin-containing granules. First-phase secretion appears to arise from plasma membrane-predocked granules, termed the “readily releasable pool” (RRP), while second-phase secretion is believed to involve release from a granule pool deeper within the cell, the “storage-granule pool,” which presumably replenishes the RRP (4, 99). In addition, KCl and other nonnutrient secretagogues can induce a first-phase type release, while only fuel-type secretagogues, such as glucose, can produce a sustained second-phase insulin release (36). First- and second-phase release events also differ in their requisite SNARE protein isoforms. First-phase insulin release uses syntaxin 1A, syntaxin 4, SNAP25 or SNAP23, and the v-SNARE VAMP2, whereas second-phase secretion is managed by syntaxin 4, SNAP25 or SNAP23, and VAMP2, but specifically not syntaxin 1A (Table 3).
Table 3.
Genetically engineered mouse models of SNARE protein ablation/overexpression for studies of glucose homeostasis in vivo
| Genotype | Aberration | Phenotype | References |
|---|---|---|---|
| Syntaxin | |||
| Syntaxin 1A (−/−) | No syntaxin 1A expression | Fewer docked granules during only first phase of insulin secretion | (85) |
| Syntaxin 1B (−/−, open) | Syntaxin 1B LE expression only, no endogenous | ND | (37) |
| Syntaxin 1A (−/−), Syntaxin 1B (−/−, LE) | No syntaxin 1A or 1B, LE mutant expression | ND | (37) |
| Syntaxin 1A Tg | Beta cell-specific syntaxin 1A overexpression, decreased Munc18-1 expression | Fasting hyperglycemia, impaired glucose tolerance, and insulin exocytosis | (63) |
| Syntaxin 4 (−/+) | Reduced syntaxin 4 and Munc18c expression, null lethal | Insulin resistant; reduced GLUT4 translocation; defective insulin secretion | (122, 168) |
| Syntaxin 4 Tg | Increased syntaxin 4 and Munc18c expression in pancreas, skeletal muscle, and adipose tissues only | Insulin sensitive; enhanced GLUT4 translocation and insulin secretion | (120, 122) |
| Munc18 | |||
| Munc18-1 (−/−, −/+) | Reduced expression of Munc18-1, null lethal | ND | (147, 148) |
| Munc18-1 Tg | Overexpression of Munc18-1 in neuron | ND | (142) |
| Munc18c Tg | Overexpression of Munc18c | Insulin resistant; impaired insulin secretion | (121) |
| Munc18c (−/−) | No Munc18c expression | Enhanced GLUT4 uptake in MEF-derived adipocytes | (56) |
| Munc18c (−/+) | Reduced Munc18c expression, null lethal by E7.5 | Insulin resistant; impaired insulin secretion; reduced GLUT4 translocation in skeletal muscle | (82, 83) |
| VAMP | |||
| VAMP2 (−/−) | No VAMP2 expression, lethal | ND | (114) |
| VAMP3 (−/−) | No VAMP3 expression | Normal insulin and glucose tolerance, and normal glucose uptake | (169) |
| VAMP8 (−/−) | No VAMP8 expression | ND | (98, 152) |
| SNAP25 (−/−, −/+) | Reduced SNAP25 expression, null lethal at birth | ND | (154) |
GLUT4 Vesicle Translocation
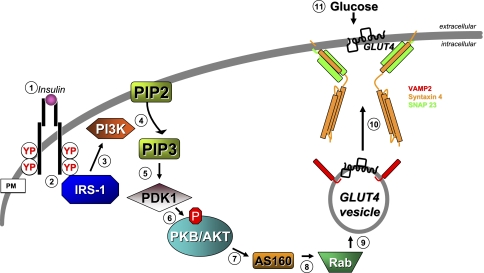
Approximately 80% of homeostatic glucose clearance is handled by skeletal muscle, with the remainder by adipose and other insulin-responsive tissues. Skeletal muscle glucose clearance involves transduction of the extracellular insulin signal into intracellular signaling events to induce translocation of intracellular GLUT4 vesicles to the surface of muscle cell t-tubule and sarcolemmal membranes. In this process, SNARE-regulated GLUT4 vesicle fusion is the most distal event (for reviews, see Refs. 14, 50, 155). Under basal conditions, GLUT4 protein is localized to intracellular vesicles within cells, and unlike GLUT2, GLUT4 has a higher affinity for glucose (_K_M ∼4 mM), thus providing a steep gradient for rapid glucose clearance. Insulin signaling to evoke GLUT4-mediated glucose clearance entails at least 11 steps, as modeled in Fig. 3. The process ensues with insulin binding to extracellular α-subunits of the insulin receptor (IR) present on the surface of muscle cells and adipocytes (step 1), inducing tyrosine autophosphorylation within the IR protein, and increased kinase activity of the IR β-subunits (step 2). Tyrosine autophosphorylation of the IR fosters recruitment of IR substrates, including the canonical insulin receptor substrate (IRS), typically through a phosphotyrosine binding domain. Once bound, substrates are themselves tyrosine phosphorylated. Phosphorylated receptor substrates then serve as additional recruitment targets for specific proteins containing Src Homology 2 domains, including phosphatidylinositide kinase (PI3K) (step 3). Once recruited, PI3K is activated to catalyze the phosphorylation of phosphatidylinositol (steps 4 and 5) bisphosphate (PIP2) at the 3′ position, yielding phosphatidylinositol (steps 3, 4, and 5) trisphosphate (PIP3) (step 4). 3-Phosphoinositide-dependent kinase-1 (PDK-1) is able to recognize the 3′ position of PIP3 with its Pleckstrin homology (PH) domain and is recruited to the plasma membrane, where it is activated (step 5). At the plasma membrane, PDK-1 phosphorylates and activates AKT/PKB (also known as protein kinase B, step 6) along with atypical PKC isoforms zeta and lambda (aPKC). As well defined as these initial signaling steps are, the identities of downstream substrates/targets of these serine/threonine kinases, which result in GLUT4 vesicle translocation remain unclear, although some recent progress has been made: Akt signals downstream (step 7) to AS160 (a Rab GTPase-activating protein) (112), and AS160 signals downstream to multiple Rab targets (step 8) (72). Rab proteins have been described in GLUT4-containing vesicles (step 9) and are presumed to facilitate their trafficking to and docking at the plasma membrane. However, a gap exists beyond this step to link to the SNARE complex at the plasma membrane: t-SNARE isoforms syntaxin 4 and SNAP23 with the v-SNARE VAMP2 are known to be required for GLUT4 vesicles to fuse with the plasma membrane (step 10) to facilitate glucose uptake into the skeletal muscle cell (step 11).
Fig. 3.
Glucose uptake via insulin-stimulated GLUT4 translocation and fusion in muscle and adipose tissues. An 11-step model: step 1: extracellular insulin binds to the α-subunit of the insulin receptor (IR), triggering autophosphorylation and activation of the β-subunit kinase activity; step 2: this induces recruitment of IRS-1, and (step 3) IRS-1 recruits PI3K. Step 4: PI3K phosphorylates PIP2 to yield PIP3. Step 5: PIP3 recruits PDK1 to the PM, where it (step 6) phosphorylates and activates AKT. Step 7: AKT phosphorylates AS160, and (step 8) AS160 targets multiple Rabs present on GLUT4-containing vesicles (step 9), although the precise mechanisms beyond this remain unclear. Step 10: vesicle fusion occurs via the SNARE proteins, resulting in GLUT4 integration into the PM to facilitate (step 11) glucose uptake.
Independent of insulin, exercise and muscle contraction have also been shown to increase glucose uptake into skeletal muscle, although far less is known regarding the requirements and mechanisms of SNARE proteins in this process. It is clear that exercise increases the translocation of GLUT4 and VAMP2 to the plasma membrane of human and rat skeletal muscle (61, 105). VAMP3 was also initially implicated in contraction-stimulated GLUT4 translocation; however, subsequent studies of skeletal muscle from the VAMP3 (−/−) mice failed to support this role (169). With the plethora of knockout mouse models of SNARE proteins now available, it is anticipated that the requirements and roles for these proteins in exercise-stimulated glucose uptake will be soon forthcoming.
Differential SNARE Isoform Function in Exocytosis Events of Glucose Homeostasis
Insulin-secreting islet beta cells and insulin-responsive muscle and adipose cells contain multiple isoforms of each of the SNARE proteins required for the distal exocytosis events occurring at the plasma membrane (Table 1).
Syntaxin family.
The pancreatic beta cell expresses plasma membrane-localized syntaxin isoforms 1A, 2, 3, and 4, though additional nonplasma membrane syntaxin isoforms are also expressed, albeit their function in exocytosis remains untested (Table 1). To date, only isoforms 1A and 4 are clearly known to be required for insulin exocytosis (85, 122), primarily from data obtained using knockout mouse models. Mice with syntaxin 1A deficiency show selectively impaired insulin release during the first phase, while islets from syntaxin 4 heterozygous (−/+) knockout mice display defects in both first and second phases of GSIS. Furthermore, islets isolated from transgenic mice overexpressing syntaxin 4 in the islet secrete ∼30% more insulin in both phases, implying a positive role for syntaxin 4 in both first- and second-phase insulin secretion (122). Oddly, syntaxin 1-overexpressing transgenic mice show insulin resistance with impaired insulin secretion (63). Although the molecular basis for this phenotype is unclear, it has been hypothesized that stoichiometry of particular SNARE proteins in cells is crucial for optimal function in exocytosis.
In contrast to pancreatic beta cells and neuronal cells, neither muscle nor adipose tissues—two of the primary insulin-responsive tissues—express syntaxin 1, but instead appear to rely upon syntaxin 4 (51, 137, 151). Syntaxin 4 (−/+) mice exhibit a blunted insulin-stimulated GLUT4 translocation and decreased glucose uptake into skeletal muscle (168). Consistent with this, transgenic mice overexpressing syntaxin 4 in skeletal muscle tissue show a twofold increase in GLUT4 translocation into the sarcolemmal and t-tubule membranes of hindlimb muscle (120). Syntaxin 4 is thus the only syntaxin isoform currently known to be required for insulin-stimulated GLUT4 vesicle translocation. Recently, GLUT4 was found to be expressed in the hypothalamus, suggesting that syntaxin 4 has a role in the brain as well (9). Syntaxin isoforms 2, 3, 5, 6, 7, 8, and 12 are reportedly expressed in adipocytes but not as critical participants in insulin-stimulated GLUT4 vesicle exocytosis (Table 1).
SNAP25, SNAP23, and SNAP29.
SNAP25, the principal neuronal isoform, is present in the beta cell but absent from muscle and adipose cells (108). SNAP23 is also expressed in beta cells and is capable of compensating for an absence of SNAP25 (107). However, skeletal muscle and adipose tissue express and use only SNAP23 (2, 57, 95). SNAP29 is also widely expressed (124), although it differs from SNAP23 and SNAP25 in that it binds to intracellularly localized syntaxin isoforms, in addition to the plasma membrane-bound syntaxins (48). SNAP29 has not yet been reported to function in insulin exocytosis or GLUT4 vesicle exocytosis.
VAMP family.
There are seven currently identified VAMP isoforms, all of which are attached by a C-terminal transmembrane domain to vesicle/granule membranes, including insulin granules, synaptic vesicles, GLUT4-containing vesicles, or ER-Golgi compartments (17). Islet beta cells express VAMP2/synaptobrevin, VAMP3/cellubrevin, and VAMP8, with VAMP2 as the predominant isoform required for GSIS (77, 96, 106). Remarkably, 3T3L1 adipocytes express all VAMP isoforms except for VAMP1 (160), but only VAMP2, VAMP3, and VAMP7 have been directly linked to GLUT4 vesicle exocytosis (69, 169). In skeletal muscle, VAMP2, 3, 5, and 7 coimmunoprecipitate with GLUT4 vesicles, and all but VAMP3 translocate to the plasma membrane with GLUT4 in response to contraction (105). In L6 myoblasts, VAMP7/TI-VAMP is expressed and required for both insulin stimulated and osmotic shock triggered-GLUT4 vesicle translocation (67).
Sec1/Munc18 protein family.
In the early 1990s, the yeast Sec1 protein was implicated as a regulator of SNARE assembly and exocytosis function, through its ability to directly interact with syntaxin. Homologues were subsequently identified in Caenorhabditis elegans (unc18), Drosophila melanogaster (Rop), and mammals (Munc18) (for a review, see Ref. 103). Collectively, proteins of this type are referred to as SM proteins, for Sec1/Munc18 (SM). The mammalian members of this family, Munc18 proteins, are ∼66- to 68-kDa soluble proteins with no apparent transmembrane domain, yet they are frequently found at the plasma membrane through direct interaction with their cognate syntaxins (43, 132). Plasma membrane-associated SM proteins present in mammalian cells include Munc18a, Munc18b, and Munc18c (also referred to as −1, −2, and −3, respectively); nonplasma membrane-associated mammalian SM proteins are mVps45 and mSly1. Endogenous Munc18a (referred to as Munc18-1 hereafter) and Munc18b bind to the syntaxin isoforms 1–3, whereas Munc18c binds with high affinity solely to syntaxin 4 (Table 4).
Table 4.
SM-Syntaxin binding specificities
| SM Proteins | Syntaxin Partner | Function | Reference |
|---|---|---|---|
| Mus musculus | |||
| Munc18a/-1 | Syntaxin 1, 2, 3 | Synaptic vesicle exocytosis | (43) |
| Munc18b/-2 | Syntaxin 1, 2, 3 | Apical membrane trafficking | (101, 102) |
| Munc18c/-3 | Syntaxin 4 | GLUT4 vesicle exocytosis, insulin granule secretion | (84, 126, 129, 132, 134) |
| mSly1 | Syntaxin 5, 18 | ER to Golgi transport | (23, 166) |
| mVPS45 | Syntaxin 16/Tlg2p | TGN transport | (26, 128) |
| Homo sapiens | |||
| Munc18a/-2 | Syntaxin 3 | Primary neutrophil granule fusion | (10) |
| Munc18c/-3 | Syntaxin 4 | Insulin action in human skeletal muscle | (5) |
| ND | Syntaxin 1A | Neuronal disorder (migraine predisposition) | (19) |
| ND | Syntaxin 7,8 | Heterotypic fusion of late endosome | (44) |
The regions/residues within the SM proteins that are responsible for syntaxin partnering specificity are still undetermined, remarkable given the high degree of similarity that exists among Munc18 isoforms (Munc18b and Munc18c show 62% and 51% amino acid identity, respectively, to Munc18-1). Of the many proteins known to bind directly to syntaxins in both insulin-secreting and insulin-responsive cell types, Munc18 proteins bind with highest affinity (54). Munc18-syntaxin complexes are found principally at the plasma membrane. However, the Munc18 proteins are soluble and equally abundant in the cytosolic compartment as they are at the membrane, though cytosolic Munc18 proteins are not associated with syntaxins (30, 132, 134). Munc18 proteins are presumed to localize to the plasma membrane by association with membrane-localized proteins, such that increased expression of syntaxin 4 selectively attracts Munc18c to the plasma membrane (132). The purpose or function of soluble Munc18 within the cytosolic cellular compartment is currently unknown. Islet beta cells express all three isoforms, while adipocytes and skeletal muscle express only Munc18b and Munc18c (130, 158). Depletion studies using RNAi or genetic ablation of either Munc18-1 or Munc18c typically show loss of exocytic function, indicative of their conserved functional importance in SNARE-mediated exocytosis events (82, 83, 147).
Munc18 and SNARE Protein Mouse Models: Alterations of Glucose Homeostasis
Reduced protein and/or mRNA levels of syntaxin 1A, syntaxin 4, and/or Munc18c have been reported in islets and skeletal muscle of diabetic and obese human patients (5, 89). Similarly, rodent models of obesity and diabetes, including the Goto-Kakizaki rat, Zucker rat, ob/ob, and streptozotocin-induced diabetes mouse models exhibit significantly lower levels of these same SNARE isoforms (35, 59, 78, 170). In corroborating fashion, numerous knockout and transgenic mouse models selectively targeted for SNARE and Munc18 proteins have defects in glucose homeostasis (Table 3). As nearly all of the classic whole body SNARE and Munc18 protein homozygous knockout mice die either in embryogenesis or at birth, the majority of the current understanding arises from studies utilizing haploinsufficient mouse models. Overall, data generally support the concept that Type 2 diabetes is a polygenic disease, likely emanating from haploinsufficiencies. Since these are in vivo models of altered glucose homeostasis, effects upon whole body homeostasis, as well as the tissue-specific effects underlying the whole body phenotypes are discussed together below.
Syntaxin mouse models.
Shown in Table 3, mouse models of syntaxin 1A protein overexpression (beta cell specific transgenic), syntaxin 4 protein overexpression (pancreas, skeletal muscle, and adipose specific transgenic), syntaxin 1A and/or syntaxin 1B deficient, and syntaxin 4 haploinsufficient mice have been generated, and many characterized for glucose homeostatic control.
SYNTAXIN 1.
Consistent with clonal cell studies and syntaxin 1A ablation/interference, islets isolated from classic whole-body syntaxin 1A knockout mice display impaired first-phase insulin release associated with a decrease in predocked granules, as determined by total internal reflection fluorescence microscopy and electron microscopy, and show normal expression levels of Munc18-1 and Munc18b (33, 85). Surprisingly, mice with beta-cell-specific overexpression of syntaxin 1A display fasting hyperglycemia, hypoinsulinemia, and impaired glucose tolerance (63). One possible explanation for this disparate phenotype may be reduced Munc18-1 levels in the syntaxin 1A-overexpressing islets, but the cause of the paucity of Munc18-1 is unclear. Additionally, nontissue specific knock-in/knockout mice engineered with two mutations in syntaxin 1B (L165A/E166A) presumed to confer an “open” state syntaxin 1B molecule, on a syntaxin 1A knockout background have been generated (37), though effects upon insulin secretion have not yet been reported.
SYNTAXIN 4.
Syntaxin 4 homozygous (−/−) null mice die early in embryogenesis, apparently due to a requirement for syntaxin 4 in the fusion of the GLUT8-containing vesicle with the plasma membrane in the mouse blastocyst (162). However, syntaxin 4 heterozygous (−/+) knockout mice are viable and exhibit insulin resistance and impaired insulin secretion (122, 168). This insulin resistance is largely due to significantly reduced skeletal muscle glucose uptake and GLUT4 translocation, while insulin secretion deficit is attributed to decreased first- and second-phase insulin release (122). Notably, in addition to the expected 50% decrease in syntaxin 4 protein in the syntaxin 4 (−/+) mouse tissues, Munc18c protein levels were decreased in parallel, while no other protein levels were altered (168). In consistent fashion, syntaxin 4-overexpressing transgenic mice show a parallel upregulation of endogenous Munc18c protein abundance in the three tissues overxpressing the syntaxin 4 transgene: adipose, skeletal muscle, and pancreas (120). Syntaxin 4-transgenic mice show increased insulin sensitivity, which is likely linked to their increased GLUT4 translocation and enriched GLUT4 deposition in the sarcolemmal and t-tubule membranes of skeletal muscle (120). Islets isolated from syntaxin 4-transgenic mice also exhibit 30% greater GSIS during both phases (122). These data corroborate studies that correlate reduced syntaxin 4 and Munc18c protein levels with aberrant insulin action in human and mouse skeletal muscle (5, 170). Collectively, these findings raise the possibility that strategies that increase syntaxin 4 protein levels may coordinately protect against the development of insulin secretion and insulin resistance defects.
Unlike skeletal muscle and islet cells, primary adipocytes do not exhibit significant changes to glucose uptake, either in the syntaxin 4-overexpressing transgenic mice or the syntaxin 4 (−/+) knockout mice (120, 168). Although this seemingly contrasts with the earlier 3T3-L1 adipocyte studies, implicating syntaxin 4 in glucose uptake, it is important to note that those early studies used a dominant-negative mutant form of syntaxin 4 to interfere with endogenous VAMP2 trafficking and ablate insulin-stimulated GLUT4 translocation (87), an approach that does not necessarily reflect requirement for syntaxin 4. As such, whether or not there is an absolute requirement for syntaxin 4 in GLUT4 vesicle translocation in adipocytes, or assuming syntaxin 4 is required, the minimum “threshold” syntaxin 4 required, remain questions open for investigation.
Munc18 mouse models.
SM proteins in yeast, flies, and worms have been universally characterized as positive and essential regulators of exocytosis events. Moreover, the impairment phenotypes of Munc18-1 (−/+) knockout (142, 147) and Munc18c (−/+) knockout (82, 83) mouse models, indeed, support a positive required role of these SM proteins in exocytosis events in vivo (Munc18-1 and Munc18c homozygous knockouts are lethal). In terms of glucose homeostasis, the Munc18c heterozygous knockout mouse model exhibits glucose intolerance due to peripheral insulin resistance coupled with deficient GSIS (82, 83). Insulin-stimulated GLUT4 vesicle translocation in the Munc18c (−/+) hindlimb skeletal muscle was dramatically abolished, indicating that Munc18c deficit is likely responsible for the peripheral insulin resistance (82). In terms of the GSIS impairment, RNAi-mediated depletion of Munc18c from isolated islets or clonal beta cells in culture thoroughly recapitulate the defective GSIS seen in the Munc18c (−/+) knockout islets (83). In contrast to previously discussed studies of SM proteins as positive factors in exocytosis, a line of MEF-derived adipocytes from a second Munc18c (−/−) mouse model showed increased GLUT4 presence in plasma membrane subcellular fractions (56). However, because this model consists of derived rather than primary cells or tissues, direct comparisons cannot be made. With regard to the role of Munc18-1 in glucose homeostasis, the Munc18-1 (−/+) have yet to be assessed for defects. However, intriguing data gained by RNAi-mediated depletion of Munc18-1 from clonal beta cells suggest they would likely have defects in GSIS that would contribute to glucose intolerance (139).
A transgenic mouse model of Munc18c protein overexpression in adipose, skeletal muscle, and pancreas, akin to the syntaxin 4 overexpression transgenic mouse model (Table 3), exhibits peripheral insulin resistance resulting from impaired insulin-stimulated glucose uptake and GLUT4 vesicle exocytosis, and decreased GSIS (121). This in vivo phenotype fully recapitulated data from Munc18c protein overexpression in 3T3-L1 adipocytes, skeletal muscle, and islet beta cells (60, 84, 126, 132). Notably, Munc18c transgenic mice and Munc18c (−/+) knockout mice have normal syntaxin 4 protein abundance, suggesting that syntaxin 4 expression directs Munc18c expression, but not vice versa (82, 121).
The negative effect of SM protein overexpression has been recapitulated in Drosophila (42). Consensus interpretation of these data postulates that the overly abundant SM proteins bind to and sequester cognate endogenous syntaxins, preventing interaction with the v-SNARE proteins, and in doing so, impair exocytosis. However, Munc18-1 overexpression in neuronal cells in culture rather enhances exocytosis (141), and overexpression of Munc18-1 in clonal beta cells had no effect upon insulin secretion (139). With regard to whether SM proteins have a clear positive or negative role, these disparate results may arise from differential protein abundance and stoichiometry, since overexpression studies rarely control for protein expression level on a per cell basis. Alternatively, the differences may be related to the different types of exocytosis reactions in which Munc18-1 and Munc18c reportedly participate, with Munc18-1 functioning in rapid vesicle release (synaptic neurotransmission and first-phase insulin release) and Munc18c serving in sustained second-phase insulin release and GLUT4 vesicle exocytosis events (Table 2).
VAMP and SNAP mouse models.
Of the two v-SNARE isoforms found in insulin-secreting and insulin-responsive cell types, only VAMP3 (−/−) knockout mice have been characterized for glucose homeostasis. Although VAMP3 was initially implicated in GLUT4 vesicle translocation in 3T3-L1 adipocytes (87, 125, 150), using dominant-negative and toxin cleavage approaches, VAMP3 null mice show normal insulin and glucose tolerance (169). Insulin-stimulated glucose uptake into adipocytes is normal in these mice, suggesting that VAMP3 is dispensable for GLUT4 translocation, perhaps because of compensation from VAMP2. VAMP2 (−/+) knockout mice thrive, while the null mice die immediately after birth (114). Furthermore, calcium-triggered synaptic vesicle exocytosis in neurons of VAMP2 haploinsufficient mice is significantly impaired, and given the numerous overlaps between this neuronal process and that of first-phase insulin release from the beta cell, it is anticipated that VAMP2 would be required for glucose homeostasis in vivo, provided it remains the only other v-SNARE protein in these tissue types. VAMP8 (−/−) knockout mice survive and have apparent defects in pancreatic acinar cell zymogen granule content and platelet secretion (98, 152), although they have yet to be characterized for glucose homeostasis.
Although SNAP25 homozygous (−/−) knockout mice fail to thrive beyond birth, studies conducted using embryonic and fetal tissue have demonstrated a lack of evoked synaptic vesicle exocytosis (154). While SNAP25 heterozygous (−/+) knockout mice do thrive, no characterizations of potential effects upon whole body glucose homeostasis or insulin release from isolated islets are reported to date. No SNAP23 knockout mice are reported in the current literature.
Munc18-Syntaxin Complexes: Molecular Mechanisms
Protein-protein interaction studies in vitro.
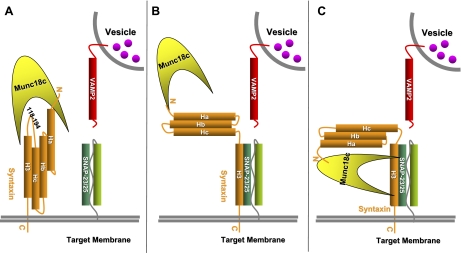
The functional in vivo models and cell-based studies of SM and syntaxin proteins indicate SM-syntaxin coupling is the key to understanding exocytosis events and has direct pertinence to many diseases. By 1999, the first structures of Munc18-1 bound to syntaxin 1A were revealed, solved using crystallographic and NMR approaches (8, 25, 74), where Munc18-1 was seen to hold syntaxin in a “closed” conformation in a 1:1 stoichiometric complex, and interpreted as a conformation precluding syntaxin participation in SNARE core complex assembly (modeled in Fig. 4_A_). Studies of SNARE proteins in cell and tissue lysates support this model, along with in vitro ultracentrifugation studies showing the exclusion of Munc18-1/nSec1 protein from the SNARE core complex (32). More recently, the Munc18c isoform has been cocrystallized with the N-terminal 19 residue peptide of syntaxin 4, indicating the importance of this particular site for protein-protein interaction (49). Although it was initially argued that this interaction fostered a new conformation (modeled in Fig. 4_B_), subsequent studies in adipocytes and beta cells cast doubt on its relative importance in relevant exocytosis events, as the same peptide failed to confer binding to Munc18c and is ineffective as a competitive inhibitor (22, 54).
Fig. 4.
Proposed models of Munc18c-syntaxin 4 interaction. A: unstimulated state model of Munc18 holding syntaxin in a “closed” conformation, which, in turn, inhibits syntaxin from being able to participate in the SNARE tertiary complex, thus inhibiting exocytosis. B: new model in the field derived from in vitro data; in stimulated state, Munc18 remains bound to “open” syntaxin at the N terminus, allowing syntaxin to participate in the SNARE core complex. C) Munc18 interacts with the SNARE core complexes through the four α-helical bundle.
A third binding mode has recently been proposed, whereby the SM protein associates with the four alpha-helical bundle comprising the SNARE core complex (modeled as Fig. 4_C_). Support for this new binding mode comes from in vitro reconstitution assays using recombinant Munc18-1, syntaxin 1A, SNAP-25, and VAMP2 proteins (115). This model relies upon the hypothesis that Munc18-1 functions principally as a positive and necessary factor to promote exocytosis, consistent with the mouse model data. The latest mechanistic hypothesis incorporates the SNARE binding protein complexin (38, 70). Complexins (CPXs), also named synaphins, were originally described in neuronal cells to play an essential role in Ca2+-dependent neurotransmitter release (71, 97, 138). In vitro, complexins bind to assembled heterotrimeric SNARE complexes (16). However, the question of whether they promote or inhibit SNARE-regulated exocytosis is unresolved due to conflicting in vivo data (11). Complexins do not appear to bind individual SNARE proteins, but a recent study shows that CPX1 can bind to syntaxin/SNAP25 binary complexes (157). Furthermore, it has been reported that Munc18-1 and CPX1 can bind simultaneously to the SNARE complex (24). Complexin expression in beta cells was noted prior to the emergence of the current mechanistic model (1), and as such, its linkage to the insulin exocytosis mechanism has not yet been determined.
Notably, detection of Munc18-SNARE complex association, as depicted in Fig. 4, B and C, requires low-stringency assay conditions, where little to no detergent is included (<0.1% Triton X-100). In contrast, older titration studies performed under higher stringency conditions demonstrated the ability of Munc18-1 to displace SNAP25 and VAMP2 from syntaxin 1 (43, 93, 167). Similarly, Munc18c reduces the binding of SNAP23 to syntaxin 4 in a concentration-dependent manner when evaluated in 1% Triton X-100 solubilized cell lysates (2), yet under low-stringency detergent conditions, Munc18c binding to syntaxin 4-bound SNARE core complexes was detected (65). Although the benefit of low-stringency buffers has enabled the ability to detect the otherwise elusive transient docking complex, coimmunoprecipitation data obtained from cell or tissue lysates currently support only model A (Fig. 4_A_). Interestingly, a novel immunofluorescent approach conducted in cells supports the concept of an intermediate transition complex of syntaxin 1 bound simultaneously to Munc18-1 and SNAP25 and that the addition of VAMP2 subsequently displaced Munc18-1 (174). Biochemical validation of the formation of this and the macromolecular complexes in models B and C by endogenous proteins in these cell types awaits further investigation.
Ultrastructural analyses of Munc18-1 in complex with syntaxin 1A (74), and Munc18c complexed with the N-terminal peptide of syntaxin 4 (but not the entire soluble region of syntaxin 4 protein) (49), have yielded tremendous insight into the potential function(s) of these complexes. Syntaxin 4 contains four cytosolic alpha helices; from the N-terminus, the domains are Ha, Hb, Hc, and H3, followed by a transmembrane domain, as determined by its homology to syntaxin 1. The C-terminal H3 domain is the canonical SNARE protein motif, which participates in the SNARE core complex (25), and on the basis of homology, the bundled coils of syntaxin 4 are predicted to fit into the cleft of an arch/crescent-shaped Munc18c (Fig. 5_A_). The Hc and H3 domains are connected by a flexible linker region believed to catalyze syntaxin's transition from a “closed” to an “open” conformation, with the open form being active for engagement in the SNARE core complex. Consistent with this, residues within the 118–194 region constituting the Hc and linker domains of syntaxin 4 are critical Munc18c interaction contacts (54), with the N-terminal peptide of syntaxin 4 fitting into a small pocket nearer the top of the overall Munc18c “crescent” shape (49). Interestingly, mutations in Munc18c, which significantly impair binding to syntaxin 4, are situated at the apex of the crescent (22, 134, 135), distanced from the arched cleft, and in close proximity to the binding sites for Doc2β and the syntaxin 4 N-terminal peptide (Fig. 5_B_). The significance of Doc2β is discussed in a subsequent section below.
Fig. 5.
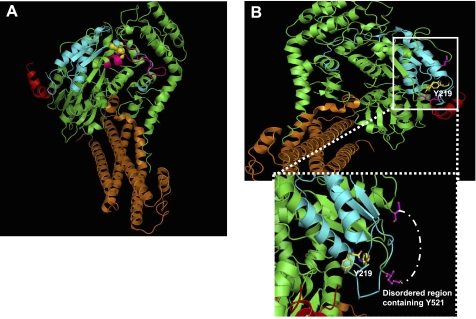
Modeling of Munc18c-syntaxin 4 interaction. A: depicting full-length Munc18c (green) interacting with syntaxin 4 (orange). The peptide termed 18c/pep3 (residues 460–483) is illustrated in pink and arginine 240, shown in yellow; both have been shown to play important roles in Munc18c-syntaxin 4 interaction (134, 135). B: upper panel-Munc18c Tyr219 sits in the Doc2β-binding region on Munc18c (blue) juxtaposed to the binding cleft for the N terminus of syntaxin 4 (red). Lower panel-enlarged view of the boxed region, illustrating the close proximity between Tyr219 and Tyr521, though Tyr521 is not present in the crystal structure due to its location within a disorder region (drawn into the model with white dashed line).
Protein-protein interaction studies in cells and tissues—impact of posttranslational modifications.
Munc18-syntaxin complexes are regulated through posttranslational modifications, including serine/threonine phosphorylation, tyrosine phosphorylation, _O_-linked glycosylation, and nitrosylation of either or both proteins. Serine phosphorylation of Munc18-1 by PKC and/or threonine phosphorylation by Cdk5 (cyclin-dependent kinase 5) disrupts Munc18-syntaxin interaction and promotes secretory granule exocytosis (3, 7, 28, 31, 118). Munc18c has been shown recently to be phosphorylated by PKC, causing dissociation from syntaxin 4 and increasing SNARE complex assembly in pancreatic acinar cells (20). Syntaxin 1A can also be serine phosphorylated by the death-associated protein kinase (DAPK), which significantly decreases its interaction with Munc18-1 (136). The catalytic subunit of the serine/threonine phosphatase protein 2B has been shown to directly interact with Munc18c in human umbilical vein endothelial cells (80).
While serine/threonine phosphorylation of Munc18 or syntaxin isoforms in insulin-secreting or insulin-responsive cell types has yet to be demonstrated, both proteins can undergo tyrosine phosphorylation in a stimulus-dependent manner. In clonal MIN6 beta cells, Munc18c becomes tyrosine phosphorylated at residue Tyr219, and as a result, dissociates from syntaxin 4 (54, 84). In 3T3-L1 adipocytes, Munc18c residue Tyr521 was modified in response to either insulin or PDGF stimulations (113, 145). Interestingly, Tyr521 resides in a disordered region, although prediction mapping places it in close proximity to Tyr219 (Fig. 5_B_), suggesting that this vicinity of Munc18c may be an important site for stimulus-induced conformational changes. In addition, syntaxin 4 becomes phosphorylated at residues Tyr115 and Tyr251 in insulin-stimulated 3T3-L1 adipocytes (113). Remarkably, syntaxin 4 does not appear to undergo tyrosine phosphorylation in clonal beta cells (84), perhaps indicating a form of mechanistic “bifurcation.” Still further, Munc18c can be modified by _O_-linked glycosylation in 3T3-L1 adipocytes under insulin-resistant conditions with glucosamine, concurrent with impaired insulin-stimulated GLUT4 translocation (15) and deficient Munc18c localization to the plasma membrane (79).
Impact of additional binding partners on SM-syntaxin interactions.
While in vitro studies are invaluable for detailing kinetic and binding site information, cellular studies have elucidated a major role for posttranslational modifications in how the SM-syntaxin complexes associate and dissociate. Moreover, SM-syntaxin complex accessory proteins, which are relevant to insulin secretion and insulin action, have been identified, as discussed below.
DOC2β.
As a soluble 45-kDa double-C2 domain-containing protein, Doc2β is expressed in adipocytes and islet beta cells, and it exerts positive effects upon GLUT4 vesicle and insulin granule exocytosis events, respectively (34, 54, 58, 75). In islet beta cells, Doc2β protein overexpression increases GSIS by ∼40%, and the siRNA-mediated depletion of Doc2β attenuates insulin release (58, 75), with similar effects observed in 3T3-L1 adipocytes (34). Doc2β can mediate similar outcomes in synaptic vesicle exocytosis through association of its first C2 binding domain (C2A) with Munc18-1 (146). In islet beta cells, the second C2 domain (C2B) mediates its association with residues 173–255 of Munc18c, including the regulatory Y219 phosphorylation site (58). Tyrosine phosphorylation of Munc18c decreases Munc18c-syntaxin 4 interactions with a concomitant twofold increase in Munc18c-Doc2β binding (54). Doc2β effectively competes with syntaxin 4 for Munc18c binding, and its endogenous association with Munc18c is required for GSIS. In contrast, it has been suggested that Doc2β instead exerts its effects through interaction with syntaxin 4 in a calcium-dependent manner (75). Although calcium was present in both beta cell studies, methodological differences such as use of low-stringency binding conditions, use of calcium-chelators, and use of a transmembrane-containing insoluble syntaxin protein may have permitted detection of syntaxin 4 association with Doc2β. Thus, although there is full agreement that Doc2β plays a positive role in syntaxin 4-mediated exocytosis, details of the underlying mechanism must await further examination.
MUNC13-1.
Munc13-1 is a soluble 200-kDa protein expressed in pancreatic islet beta cells (89, 116), but not in adipocytes or skeletal muscle. Munc13-1 is composed of one C1 and two C2 domains that mediate phorbol ester and diacylglycerol binding and phospholipid-dependent Ca2+ binding, respectively. Munc13-1 can pair directly with Munc18-1, Doc2β, or syntaxin 1A (6, 146). Munc13-1 overexpression amplifies insulin secretion and is proposed to function in granule priming (116). Reduced expression of Munc13-1 is observed in islets isolated from diabetic humans or Zucker fa/fa rats (89, 116), consistent with glucose intolerance and the deficient insulin release characteristics of Munc13-1 (−/+) knockout mice (62) and is therefore believed to have a required role in insulin exocytosis.
WNK1.
A unique member of the serine/threonine kinase family, WNK1 [With No K (lysine)] has been linked to the inherited hypertension syndrome pseudohypoaldosteronism II (161, 163). WNK1 is a soluble 230-kDa kinase expressed in 3T3-L1 adipocytes and islet beta cells (55, 66) and is a Munc18c-binding protein (81). WNK1 and Munc18c associate via direct interaction of the N-terminal 172 residues of Munc18c (distinct from residues bound by Doc2β) and the kinase domain of WNK1, and competitive inhibition of this complex impairs syntaxin 4-mediated insulin granule exocytosis (81). Also, WNK1-Munc18c complexes are found localized to the plasma membrane, although complexes are also found in the cytosol, a unique feature among Munc18c binding proteins to date. Two other unique features of this complex exist: 1) despite the requirement for the kinase domain of WNK1, its intrinsic kinase activity is apparently dispensable for interaction (i.e., Munc18c does not serve as a WNK1 substrate) and 2) siRNA-mediated WNK1 depletion does not impact insulin secretion from clonal beta cells or glucose uptake into adipocytes (55), complicating specific designation of its role in exocytosis events pertinent to glucose homeostasis.
80K-H.
The 80K-H protein (80 kDa) was originally identified as a PKCζ binding partner and is widely expressed, especially at the plasma membrane of insulin-sensitive 3T3L1 adipocytes and L6 myotubes (47). 80K-H has been implicated in vesicle trafficking events via a close relationship to the protein VASAP-60 (13), as well as in GLUT4 vesicle transport through its ability to interact with both Munc18c and PKCζ in an insulin-dependent manner (47, 119). The requirement of 80K-H in glucose uptake in vivo and/or primary cells and its putative role as a signaling link between PKCζ and Munc18c await further investigation.
RAB3A.
Rab proteins are a large family of small GTPases responsible for the regulation of many membrane trafficking events and are thought to participate in insulin exocytosis, as well as GLUT4 vesicle exocytosis through interaction with Munc18-syntaxin complexes. However, despite efforts over the past decade, only Rab3A, a Munc18-1-binding protein, has been identified to date (39), with no Munc18c-binding candidate to modulate insulin action as of yet. Rab3-null mice exhibit glucose intolerance coupled to ablated first-phase insulin release but without insulin resistance (164), consistent with its role as solely a Munc18-1 and not a Munc18c binding factor.
SYNIP.
Synip (syntaxin-interacting protein) is a 62-kDa protein that was initially discovered as a novel syntaxin 4-binding protein and has been implicated in the control of glucose transport and GLUT4 vesicle translocation in 3T3-L1 adipocytes (73). It binds only to the syntaxin 4 isoform in an insulin-sensitive manner. This mechanism appears to function through Synip phosphorylation at Ser99 in response to activation of Akt2 and subsequent dissociation from syntaxin 4 to promote GLUT4 vesicle exocytosis (86, 165), although this is a disputed finding (111). Synip expression in βHC-9 clonal beta cells has also been reported, and a role for it is implicated in syntaxin 4-mediated insulin exocytosis using an overexpression paradigm (109). Data on Synip knockout or RNAi-mediated knockdown mice will be required to determine whether Synip is necessary during syntaxin 4-based exocytosis events relevant to glucose homeostasis.
TOMOSYN.
Tomosyn proteins are syntaxin binding factors, with 7 different isoforms expressed from two genes, tomosyn-1 and tomosyn-2 (41). b-Tomosyn-1 (b stands for big), a cytosolic protein, was identified as a syntaxin 4-binding partner in 3T3-L1 adipocytes, and its overexpression inhibited GLUT4 translocation to the plasma membrane (159). Similarly, in beta cells depletion of an analogous syntaxin 1A-binding isoform of tomosyn-1 was found to decrease stimulated exocytosis (18). In contrast, depletion of a related m-tomosyn-1 (m stands for medium) isoform (which also binds to syntaxin 1A) in clonal beta cells was shown to increase insulin release while overexpression was inhibitory, suggesting that it functions as a negative regulator of insulin exocytosis (172). Although tomosyn-1 knockout mice exist and have enhanced synaptic transmission (110), the mice are not yet characterized for effects upon glucose homeostasis, and it remains unclear which isoforms are ablated.
CAB45B.
Cab45b was recently identified as a soluble 42-kDa calcium binding protein associated with Munc18b in pancreatic islet beta cells (173). In clonal INS-1E beta cells, antibody-mediated interference of endogenous Cab45b or RNAi-knockdown of Munc18b expression reduced depolarization-evoked membrane capacitance, implicating potential roles for each protein in insulin exocytosis.
Novel roles for Munc18 and syntaxin proteins in granule mobilization and pool refilling.
New evidence suggests that nontraditional roles exist for SM and syntaxin proteins in exocytosis. Both Munc18-1 and Munc18c (−/+) knockout mouse models reveal the necessity of these proteins in granule localization to the plasma membrane (83, 141). Munc18-1-depleted clonal beta cells also exhibit defective docking of insulin granules to the plasma membrane (139). Although this might suggest that the soluble fraction of SM proteins somehow directs granule mobilization through the cytoskeletal matrix, pilot in vitro F-actin binding studies argue against Munc18c protein as a direct binding factor of F-actin (M. Kalwat and D. C. Thurmond, unpublished results). In contrast, syntaxin 4 was recently shown to, indeed, bind directly to F-actin, through an N-terminal “spectrin-like” domain that is relevant to insulin exocytosis in clonal beta cells (53). In contrast, syntaxin 1A failed to directly associate with F-actin, although it does coimmunoprecipitate with F-actin in beta cell lysates and dissociate transiently in response to glucose stimulation (133). Syntaxin 4 can also associate with α-fodrin, an F-actin binding factor, in primary rat adipocytes (68). Given that syntaxin 4 and Munc18c, in particular, are required for the mobilization phase of insulin exocytosis, and are also responsible for the relatively long-range trafficking of GLUT4 vesicles in adipocytes and myocytes, future exploration regarding their interactions with cytoskeletal elements should prove very exciting. However, it is important to note that stimulus-induced actin remodeling appears to be different for exocytosis in beta cells than it is in adipocytes and myocytes. Current data suggest that disruption of the actin cytoskeleton using agents such as latrunculin in beta cells potentiates insulin exocytosis, but in adipocytes inhibits GLUT4 vesicle exocytosis (12, 88, 133, 140, 143).
Perspectives and Significance
Munc18c and syntaxin 4 are clearly common links in the known mechanisms of insulin granule exocytosis and GLUT4 vesicle translocation, and yet the molecular details of their actions in these processes remain incomplete. With regard to Munc18c, significant progress has been made in its characterization as a positive effector of both processes, as well as the recent identifications of novel binding factors that implicate it in both syntaxin 4-dependent (vesicle docking/fusion at the plasma membrane) and syntaxin 4-independent (granule mobilization/localization) mechanisms. The possibility that Munc18c functions in a syntaxin 4-independent role in facilitating insulin granule delivery is particularly important, given that granule recruitment to the readily releasable pool of a β-cell is a rate-limiting component of insulin release. Pharmacological targeting of Munc18c function, directly or indirectly, via a binding partner implicated in that mechanism, could presumably exert profound effects upon the capacity of the β-cell to sustain insulin release beyond the first few minutes. The ability to sustain insulin release in a regulated biphasic manner, as opposed to the constitutive release triggered by current popular oral medications, which cause hypoglycemia and hasten beta cell failure, would be of tremendous advantage as it would allow for restoration of glucose homeostasis with lower resting insulin levels and hence less risk of hypoglycemic episodes.
In addition to gaining more insight into how Munc18c-syntaxin 4 complexes are regulated, it will be particularly important to determine how and why syntaxin 4 and Munc18c protein levels decrease under conditions of obesity and Type 2 diabetes in humans and rodent models. Interestingly, knockout and transgenic mouse model studies suggest that Munc18c expression is controlled by syntaxin 4. Thus, future studies aimed toward gaining the ability to control syntaxin 4 expression could be advantageous in improving whole body glucose homeostasis.
GRANTS
This work was supported by grants from the National Institutes of Health (DK067912 and DK076614) to D. C. Thurmond, and a predoctoral fellowship from the American Heart Association to J. L. Jewell.
ACKNOWLEDGMENTS
We thank Dr. Dean Wiseman and Michael Kalwat for assistance with the figures and critical reading of this manuscript.
REFERENCES
- 1.Abderrahmani A, Niederhauser G, Plaisance V, Roehrich ME, Lenain V, Coppola T, Regazzi R, Waeber G. Complexin I regulates glucose-induced secretion in pancreatic beta-cells. J Cell Sci 117: 2239–2247, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, Niki T, Okazawa H, Kubota T, Kasuga M. Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c. Biochem Biophys Res Commun 234: 257–262, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJ, Morgan A, Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J Biol Chem 278: 10538–10545, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Barg S, Eliasson L, Renstrom E, Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse β-cells. Diabetes 51: S74–S82, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bergman BC, Cornier MA, Horton TJ, Bessesen DH, Eckel RH. Skeletal muscle munc18c and syntaxin 4 in human obesity. Nutr Metab (Lond) 5: 21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J Biol Chem 272: 2520–2526, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Bhaskar K, Shareef MM, Sharma VM, Shetty AP, Ramamohan Y, Pant HC, Raju TR, Shetty KT. Co-purification and localization of Munc18-1 (p67) and Cdk5 with neuronal cytoskeletal proteins. Neurochem Int 44: 35–44, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bracher A, Perrakis A, Dresbach T, Betz H, Weissenhorn W. The X-ray crystal structure of neuronal Sec1 from squid sheds new light on the role of this protein in exocytosis. Struct Fold Des 8: 685–694, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun 192: 1297–1302, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Brochetta C, Vita F, Tiwari N, Scandiuzzi L, Soranzo MR, Guerin-Marchand C, Zabucchi G, Blank U. Involvement of Munc18 isoforms in the regulation of granule exocytosis in neutrophils. Biochim Biophys Acta 1783: 1781–1791, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Brose N. For better or for worse: complexins regulate SNARE function and vesicle fusion. Traffic 9: 1403–1413, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Brozinick JT, Jr, Hawkins ED, Strawbridge AB, Elmendorf JS. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J Biol Chem 279: 40699–40706, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brule S, Rabahi F, Faure R, Beckers JF, Silversides DW, Lussier JG. Vacuolar system-associated protein-60: a protein characterized from bovine granulosa and luteal cells that is associated with intracellular vesicles and related to human 80K-H and murine beta-glucosidase II. Biol Reprod 62: 642–654, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3: 267–277, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Liu P, Thurmond DC, Elmendorf JS. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett 534: 54–60, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron 33: 397–409, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Cheviet S, Bezzi P, Ivarsson R, Renstrom E, Viertl D, Kasas S, Catsicas S, Regazzi R. Tomosyn-1 is involved in a post-docking event required for pancreatic beta-cell exocytosis. J Cell Sci 119: 2912–2920, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Corominas R, Ribases M, Cuenca-Leon E, Cormand B, Macaya A. Lack of association of hormone receptor polymorphisms with migraine. Eur J Neurol 16: 413–415, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Cosen-Binker LI, Lam PP, Binker MG, Reeve J, Pandol S, Gaisano HY. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem 282: 13047–13058, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology 83: 572–584, 1968 [DOI] [PubMed] [Google Scholar]
- 22.D'Andrea-Merrins M, Chang L, Lam AD, Ernst SA, Stuenkel EL. Munc18c interaction with syntaxin 4 monomers and SNARE complex intermediates in GLUT4 vesicle trafficking. J Biol Chem 282: 16553–16566., 2007 [DOI] [PubMed] [Google Scholar]
- 23.Dascher C, Balch WE. Mammalian Sly1 regulates syntaxin 5 function in endoplasmic reticulum to Golgi transport. J Biol Chem 271: 15866–15869, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Deak F, Xu Y, Chang WP, Dulubova I, Khvotchev M, Liu X, Sudhof TC, Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol 184: 751–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J 18: 4372–4382, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dulubova I, Yamaguchi T, Gao Y, Min SW, Huryeva I, Sudhof TC, Rizo J. How Tlg2p/syntaxin 16 ‘snares’ Vps45. EMBO J 21: 3620–3631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P. Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol 586: 3313–3324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher AI, Shuang R, Giovannucci DR, Zhang L, Bittner MA, Stuenkel EL. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J Biol Chem 274: 4027–4035, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Foster LJ, Klip A. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am J Physiol Cell Physiol 279: C877–C890, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Fujita H, Tuma PL, Finnegan CM, Locco L, Hubbard AL. Endogenous syntaxins 2, 3 and 4 exhibit distinct but overlapping patterns of expression at the hepatocyte plasma membrane. Biochem J 329: 527–538, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita Y, Sasaki T, Fukui K, Kotani H, Kimura T, Hata Y, Sudhof TC, Scheller RH, Takai Y. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J Biol Chem 271: 7265–7268, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, Scheller RH, Takai Y. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron 20: 905–915, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Fujiwara T, Mishima T, Kofuji T, Chiba T, Tanaka K, Yamamoto A, Akagawa K. Analysis of knock-out mice to determine the role of HPC-1/syntaxin 1A in expressing synaptic plasticity. J Neurosci 26: 5767–5776, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda N, Emoto M, Nakamori Y, Taguchi A, Miyamoto S, Uraki S, Oka Y, Tanizawa Y. DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes 58: 377–384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaisano HY, Ostenson CG, Sheu L, Wheeler MB, Efendic S. Abnormal expression of pancreatic islet exocytotic soluble _N_-ethylmaleimide-sensitive factor attachment protein receptors in Goto-Kakizaki rats is partially restored by phlorizin treatment and accentuated by high glucose treatment. Endocrinology 143: 4218–4226, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 89: 1288–1295, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber SH, Rah JC, Min SW, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, Verhage M, Rosenmund C, Sudhof TC. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science 321: 1507–1510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science 323: 512–516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham ME, Handley MT, Barclay JW, Ciufo LF, Barrow SL, Morgan A, Burgoyne RD. A gain-of-function mutant of Munc18-1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18-1 with Rab3. Biochem J 409: 407–416, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Grodsky GM. Kinetics of insulin secretion: underlying metabolic events. In: Diabetes Mellitus: A Fundamental and Clinical Text, edited by LeRoith D, Taylor S, Olefsky J. Philadelphia, PA: Lippincott Williams & Wilkins, 2000 [Google Scholar]
- 41.Groffen AJ, Jacobsen L, Schut D, Verhage M. Two distinct genes drive expression of seven tomosyn isoforms in the mammalian brain, sharing a conserved structure with a unique variable domain. J Neurochem 92: 554–568, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Harrison SD, Broadie K, van de Goor J, Rubin GM. Mutations in the Drosophila rop gene suggest a function in general secretion and synaptic transmission. Neuron 13: 555–566, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366: 347–351, 1993 [DOI] [PubMed] [Google Scholar]
- 44.He Y, Linder ME. Differential palmitoylation of the endosomal SNAREs syntaxin 7 and syntaxin 8. J Lipid Res 50: 398–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55: 3470–3477, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Henquin JC, Nenquin M, Stiernet P, Ahren B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in beta-cells. Diabetes 55: 441–451, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Hodgkinson CP, Mander A, Sale GJ. Identification of 80K-H as a protein involved in GLUT4 vesicle trafficking. Biochem J 388: 785–793, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohenstein AC, Roche PA. SNAP-29 is a promiscuous syntaxin-binding SNARE. Biochem Biophys Res Commun 285: 167–171, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci USA 104: 8773–8778, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Jagadish MN, Fernandez CS, Hewish DR, Macaulay SL, Gough KH, Grusovin J, Verkuylen A, Cosgrove L, Alafaci A, Frenkel MJ, Ward CW. Insulin-responsive tissues contain the core complex protein SNAP-25 (synaptosomal-associated protein 25) A and B isoforms in addition to syntaxin 4 and synaptobrevins 1 and 2. Biochem J 317: 945–954, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J Biol Chem 283: 10716–10726, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jewell JL, Oh E, Bennett SM, Meroueh SO, Thurmond DC. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J Biol Chem 283: 21734–21746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang ZY, Zhou QL, Holik J, Patel S, Leszyk J, Coleman K, Chouinard M, Czech MP. Identification of WNK1 as a substrate of Akt/protein kinase B and a negative regulator of insulin-stimulated mitogenesis in 3T3–L1 cells. J Biol Chem 280: 21622–21628, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Kanda H, Tamori Y, Shinoda H, Yoshikawa M, Sakaue M, Udagawa J, Otani H, Tashiro F, Miyazaki J, Kasuga M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest 115: 291–301, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M. Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3–L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2. J Biol Chem 275: 8240–8247, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Ke B, Oh E, Thurmond DC. Doc2beta is a novel Munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J Biol Chem 282: 21786–21797, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18: 706–716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan AH, Thurmond DC, Yang C, Ceresa BP, Sigmund CD, Pessin JE. Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle. J Biol Chem 276: 4063–4069, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristiansen S, Hargreaves M, Richter EA. Exercise-induced increase in glucose transport, GLUT-4, and VAMP-2 in plasma membrane from human muscle. Am J Physiol Endocrinol Metab 270: E197–E201, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki M, Betz A, Brose N, Gaisano HY. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes 55: 1421–1429, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Lam PP, Leung YM, Sheu L, Ellis J, Tsushima RG, Osborne LR, Gaisano HY. Transgenic mouse overexpressing syntaxin-1A as a diabetes model. Diabetes 54: 2744–2754, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem 259: 3–17, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Latham CF, Lopez JA, Hu SH, Gee CL, Westbury E, Blair DH, Armishaw CJ, Alewood PF, Bryant NJ, James DE, Martin JL. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic 7: 1408–1419, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Lee BH, Min X, Heise CJ, Xu BE, Chen S, Shu H, Luby-Phelps K, Goldsmith EJ, Cobb MH. WNK1 phosphorylates synaptotagmin 2 and modulates its membrane binding. Mol Cell 15: 741–751, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Li D, Randhawa VK, Patel N, Hayashi M, Klip A. Hyperosmolarity reduces GLUT4 endocytosis and increases its exocytosis from a VAMP2-independent pool in l6 muscle cells. J Biol Chem 276: 22883–22891, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Liu L, Jedrychowski MP, Gygi SP, Pilch PF. Role of insulin-dependent cortical Fodrin/Spectrin remodeling in glucose transporter 4 translocation in rat adipocytes. Mol Biol Cell 17: 4249–4256, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin LB, Shewan A, Millar CA, Gould GW, James DE. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3–L1 adipocytes. J Biol Chem 273: 1444–1452, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323: 516–521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83: 111–119, 1995 [DOI] [PubMed] [Google Scholar]
- 72.Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391: 87–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Min J, Okada S, Kanzaki M, Elmendorf JS, Coker KJ, Ceresa BP, Syu LJ, Noda Y, Saltiel AR, Pessin JE. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol Cell 3: 751–760, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404: 355–362, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Miyazaki M, Emoto M, Fukuda N, Hatanaka M, Taguchi A, Miyamoto S, Tanizawa Y. DOC2b is a SNARE regulator of glucose-stimulated delayed insulin secretion. Biochem Biophys Res Commun 384: 461–465, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Nagamatsu S, Fujiwara T, Nakamichi Y, Watanabe T, Katahira H, Sawa H, Akagawa K. Expression and functional role of syntaxin 1/HPC-1 in pancreatic beta cells. Syntaxin 1A, but not 1B, plays a negative role in regulatory insulin release pathway. J Biol Chem 271: 1160–1165, 1996 [DOI] [PubMed] [Google Scholar]
- 77.Nagamatsu S, Nakamichi Y, Watanabe T, Matsushima S, Yamaguchi S, Ni J, Itagaki E, Ishida H. Localization of cellubrevin-related peptide, endobrevin, in the early endosome in pancreatic beta cells and its physiological function in exo-endocytosis of secretory granules. J Cell Sci 114: 219–227, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S, Furukawa H, Ishida H. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes 48: 2367–2373, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Nelson BA, Robinson KA, Buse MG. Insulin acutely regulates Munc18-c subcellular trafficking: altered response in insulin-resistant 3T3–L1 adipocytes. J Biol Chem 277: 3809–3812, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Nolasco LH, Gushiken FC, Turner NA, Khatlani TS, Pradhan S, Dong JF, Moake JL, Vijayan KV. Protein phosphatase 2B inhibition promotes the secretion of von Willebrand factor from endothelial cells. J Thromb Haemost 7: 1009–1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh E, Heise CJ, English JM, Cobb MH, Thurmond DC. WNK1 is a novel regulator of Munc18c-syntaxin 4 complex formation in soluble NSF attachment protein receptor (SNARE)-mediated vesicle exocytosis. J Biol Chem 282: 32613–32622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh E, Spurlin BA, Pessin JE, Thurmond DC. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 54: 638–647, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Oh E, Thurmond DC. Munc18c depletion selectively impairs the sustained phase of insulin release. Diabetes 58: 1165–1174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oh E, Thurmond DC. The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J Biol Chem 281: 17624–17634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, Matsushima S, Kawakami H, Watanabe T, Akagawa K, Nagamatsu S. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol 177: 695–705, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okada S, Ohshima K, Uehara Y, Shimizu H, Hashimoto K, Yamada M, Mori M. Synip phosphorylation is required for insulin-stimulated Glut4 translocation. Biochem Biophys Res Commun 356: 102–106, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol 17: 2425–2435, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omata W, Shibata H, Li L, Takata K, Kojima I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem J 346: 321–328, 2000 [PMC free article] [PubMed] [Google Scholar]
- 89.Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble _N_-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes 55: 435–440, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Parton LE, McMillen PJ, Shen Y, Docherty E, Sharpe E, Diraison F, Briscoe CP, Rutter GA. Limited role for SREBP-1c in defective glucose-induced insulin secretion from Zucker diabetic fatty rat islets: a functional and gene profiling analysis. Am J Physiol Endocrinol Metab 291: E982–E994, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem 279: 4234–4240, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Perera HK, Clarke M, Morris NJ, Hong W, Chamberlain LH, Gould GW. Syntaxin 6 regulates Glut4 trafficking in 3T3–L1 adipocytes. Mol Biol Cell 14: 2946–2958, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pevsner J, Hsu SC, Scheller RH. n-Sec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA 91: 1445–1449, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem 271: 13300–13303, 1996 [DOI] [PubMed] [Google Scholar]
- 95.Rea S, Martin LB, McIntosh S, Macaulay SL, Ramsdale T, Baldini G, James DE. Syndet, an adipocyte target SNARE involved in the insulin-induced translocation of GLUT4 to the cell surface. J Biol Chem 273: 18784–18792, 1998 [DOI] [PubMed] [Google Scholar]
- 96.Regazzi R, Wollheim CB, Lang J, Theler JM, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca2+-but not for GTP gamma S-induced insulin secretion. EMBO J 14: 2723–2730, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104: 71–81, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang CC, Hong W, Whiteheart SW. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell 18: 24–33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Renstrom E, Eliasson L, Bokvist K, Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol 494: 41–52, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhodes CJ. Processing of the insulin molecule. In: Diabetes Mellitus: a Fundamental and Clinical Text, edited by LeRoith T, Olefsky Philadelphia, PA: Lippincott Williams & Wilkins, 2000, p. 20–38 [Google Scholar]
- 101.Riento K, Jantti J, Jansson S, Hielm S, Lehtonen E, Ehnholm C, Keranen S, Olkkonen VM. A sec1-related vesicle-transport protein that is expressed predominantly in epithelial cells. Eur J Biochem 239: 638–646, 1996 [DOI] [PubMed] [Google Scholar]
- 102.Riento K, Kauppi M, Keranen S, Olkkonen VM. Munc18–2, a functional partner of syntaxin 3, controls apical membrane trafficking in epithelial cells. J Biol Chem 275: 13476–13483, 2000 [DOI] [PubMed] [Google Scholar]
- 103.Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci 3: 641–653, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Romeo S, Sentinelli F, Cavallo MG, Leonetti F, Fallarino M, Mariotti S, Baroni MG. Search for genetic variants of the SYNTAXIN 1A (STX1A) gene: the −352 A>T variant in the STX1A promoter associates with impaired glucose metabolism in an Italian obese population. Int J Obes (Lond) 32: 413–420, 2008 [DOI] [PubMed] [Google Scholar]
- 105.Rose AJ, Jeppesen J, Kiens B, Richter EA. Effects of contraction on localization of GLUT4 and v-SNARE isoforms in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297: R1228–R1237, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Rossetto O, Gorza L, Schiavo G, Schiavo N, Scheller RH, Montecucco C. VAMP/synaptobrevin isoforms 1 and 2 are widely and differentially expressed in nonneuronal tissues. J Cell Biol 132: 167–179, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sadoul K, Berger A, Niemann H, Weller U, Roche PA, Klip A, Trimble WS, Regazzi R, Catsicas S, Halban PA. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J Biol Chem 272: 33023–33027, 1997 [DOI] [PubMed] [Google Scholar]
- 108.Sadoul K, Lang J, Montecucco C, Weller U, Regazzi R, Catsicas S, Wollheim CB, Halban PA. SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J Cell Biol 128: 1019–1028, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saito T, Okada S, Yamada E, Ohshima K, Shimizu H, Shimomura K, Sato M, Pessin JE, Mori M. Syntaxin 4 and Synip (syntaxin 4 interacting protein) regulate insulin secretion in the pancreatic beta HC-9 cell. J Biol Chem 278: 36718–36725, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Sakisaka T, Yamamoto Y, Mochida S, Nakamura M, Nishikawa K, Ishizaki H, Okamoto-Tanaka M, Miyoshi J, Fujiyoshi Y, Manabe T, Takai Y. Dual inhibition of SNARE complex formation by tomosyn ensures controlled neurotransmitter release. J Cell Biol 183: 323–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sano H, Kane S, Sano E, Lienhard GE. Synip phosphorylation does not regulate insulin-stimulated GLUT4 translocation. Biochem Biophys Res Commun 332: 880–884, 2005 [DOI] [PubMed] [Google Scholar]
- 112.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Schmelzle K, Kane S, Gridley S, Lienhard GE, White FM. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes 55: 2171–2179, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 294: 1117–1122, 2001 [DOI] [PubMed] [Google Scholar]
- 115.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128: 183–195, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Sheu L, Pasyk EA, Ji J, Huang X, Gao X, Varoqueaux F, Brose N, Gaisano HY. Regulation of insulin exocytosis by Munc13-1. J Biol Chem 278: 27556–27563, 2003 [DOI] [PubMed] [Google Scholar]
- 117.Shewan AM, van Dam EM, Martin S, Luen TB, Hong W, Bryant NJ, James DE. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol Biol Cell 14: 973–986, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, Stuenkel EL. Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J Biol Chem 273: 4957–4966, 1998 [DOI] [PubMed] [Google Scholar]
- 119.Smithers NP, Hodgkinson CP, Cuttle M, Sale GJ. 80K-H acts as a signaling bridge in intact living cells between PKCzeta and the GLUT4 translocation regulator Munc18c. J Recept Signal Transduct Res 28: 581–589, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Spurlin BA, Park SY, Nevins AK, Kim JK, Thurmond DC. Syntaxin 4 transgenic mice exhibit enhanced insulin-mediated glucose uptake in skeletal muscle. Diabetes 53: 2223–2231, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Spurlin BA, Thomas RM, Nevins AK, Kim HJ, Noh HJ, Kim JA, Shulman GI, Thurmond DC. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes 52: 1910–1917, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic β-cells. Mol Endocrinol 20: 183–193, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Steegmaier M, Klumperman J, Foletti DL, Yoo JS, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell 10: 1957–1972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, Luo Y, Scheller RH. Three novel proteins of the syntaxin/SNAP-25 family. J Biol Chem 273: 34171–34179, 1998 [DOI] [PubMed] [Google Scholar]
- 125.Tamori Y, Hashiramoto M, Araki S, Kamata Y, Takahashi M, Kozaki S, Kasuga M. Cleavage of vesicle-associated membrane protein (VAMP)-2 and cellubrevin on GLUT4-containing vesicles inhibits the translocation of GLUT4 in 3T3–L1 adipocytes. Biochem Biophys Res Commun 220: 740–745, 1996 [DOI] [PubMed] [Google Scholar]
- 126.Tamori Y, Kawanishi M, Niki T, Shinoda H, Araki S, Okazawa H, Kasuga M. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3–L1 adipocytes. J Biol Chem 273: 19740–19746, 1998 [DOI] [PubMed] [Google Scholar]
- 127.Tang BL, Low DY, Tan AE, Hong W. Syntaxin 10: a member of the syntaxin family localized to the trans-Golgi network. Biochem Biophys Res Commun 242: 345–350, 1998 [DOI] [PubMed] [Google Scholar]
- 128.Tellam JT, James DE, Stevens TH, Piper RC. Identification of a mammalian Golgi Sec1p-like protein, mVps45. J Biol Chem 272: 6187–6193, 1997 [DOI] [PubMed] [Google Scholar]
- 129.Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James DE. Characterization of Munc-18c and syntaxin-4 in 3T3–L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J Biol Chem 272: 6179–6186, 1997 [DOI] [PubMed] [Google Scholar]
- 130.Tellam JT, McIntosh S, James DE. Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. J Biol Chem 270: 5857–5863, 1995 [DOI] [PubMed] [Google Scholar]
- 131.Thurmond DC. Regulation of insulin action and insulin secretion by SNARE-mediated vesicle exocytosis. In: Mechanisms of Insulin Action, edited by Saltiel A. R., Pessin J. E. Georgetown, TX: Landes Bioscience, 2007, p. 52–70 [Google Scholar]
- 132.Thurmond DC, Ceresa BP, Okada S, Elmendorf JS, Coker K, Pessin JE. Regulation of insulin-stimulated GLUT4 translocation by munc18c in 3T3L1 adipocytes. J Biol Chem 273: 33876–33883, 1998 [DOI] [PubMed] [Google Scholar]
- 133.Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble _N_-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol Endocrinol 17: 732–742, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Thurmond DC, Kanzaki M, Khan AH, Pessin JE. Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol Cell Biol 20: 379–388, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thurmond DC, Pessin JE. Discrimination of GLUT4 vesicle trafficking from fusion using a temperature-sensitive Munc18c mutant. EMBO J 19: 3565–3575, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tian JH, Das S, Sheng ZH. Ca2+-dependent phosphorylation of syntaxin-1A by the death-associated protein (DAP) kinase regulates its interaction with Munc18. J Biol Chem 278: 26265–26274, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Timmers KI, Clark AE, Omatsu-Kanbe M, Whiteheart SW, Bennett MK, Holman GD, Cushman SW. Identification of SNAP receptors in rat adipose cell membrane fractions and in SNARE complexes co-immunoprecipitated with epitope-tagged _N_-ethylmaleimide-sensitive fusion protein. Biochem J 320: 429–436, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tokumaru H, Umayahara K, Pellegrini LL, Ishizuka T, Saisu H, Betz H, Augustine GJ, Abe T. SNARE complex oligomerization by synaphin/complexin is essential for synaptic vesicle exocytosis. Cell 104: 421–432, 2001 [DOI] [PubMed] [Google Scholar]
- 139.Tomas A, Meda P, Regazzi R, Pessin JE, Halban PA. Munc 18–1 and granuphilin collaborate during insulin granule exocytosis. Traffic 9: 813–832, 2008 [DOI] [PubMed] [Google Scholar]
- 140.Tomas A, Yermen B, Min L, Pessin JE, Halban PA. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J Cell Sci 119: 2156–2167, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Toonen RF, Kochubey O, de Wit H, Gulyas-Kovacs A, Konijnenburg B, Sorensen JB, Klingauf J, Verhage M. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J 25: 3725–3737, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Toonen RF, Wierda K, Sons MS, de Wit H, Cornelisse LN, Brussaard A, Plomp JJ, Verhage M. Munc18-1 expression levels control synapse recovery by regulating readily releasable pool size. Proc Natl Acad Sci USA 103: 18332–18337, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsakiridis T, Vranic M, Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem 269: 29934–29942, 1994 [PubMed] [Google Scholar]
- 144.Tsunoda K, Sanke T, Nakagawa T, Furuta H, Nanjo K. Single nucleotide polymorphism (D68D, T to C) in the syntaxin 1A gene correlates to age at onset and insulin requirement in Type II diabetic patients. Diabetologia 44: 2092–2097, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Umahara M, Okada S, Yamada E, Saito T, Ohshima K, Hashimoto K, Yamada M, Shimizu H, Pessin JE, Mori M. Tyrosine phosphorylation of Munc18c regulates platelet-derived growth factor-stimulated glucose transporter 4 translocation in 3T3L1 adipocytes. Endocrinology 149: 40–49, 2008 [DOI] [PubMed] [Google Scholar]
- 146.Verhage M, de Vries KJ, Roshol H, Burbach JP, Gispen WH, Sudhof TC. DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adapter proteins in early stages of secretion. Neuron 18: 453–461, 1997 [DOI] [PubMed] [Google Scholar]
- 147.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC, de Vries KJ, Geijtenbeek A, Brian EC, de Graan PN, Ghijsen WE. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Dynamics of Munc18-1 phosphorylation/dephosphorylation in rat brain nerve terminals. Science 287: 864–869, 2000 [DOI] [PubMed] [Google Scholar]
- 148.Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Sudhof TC, Neher E, Verhage M. Munc18-1 promotes large dense-core vesicle docking. Neuron 31: 581–591, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Volchuk A, Mitsumoto Y, He L, Liu Z, Habermann E, Trimble W, Klip A. Expression of vesicle-associated membrane protein 2 (VAMP-2)/synaptobrevin II and cellubrevin in rat skeletal muscle and in a muscle cell line. Biochem J 304: 139–145, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volchuk A, Sargeant R, Sumitani S, Liu Z, He LJ, Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3–L1 adipocytes. J Biol Chem 270: 8233–8240, 1995 [DOI] [PubMed] [Google Scholar]
- 151.Volchuk A, Wang Q, Ewart HS, Liu Z, He L, Bennett MK, Klip A. Syntaxin 4 in 3T3–L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell 7: 1075–1082, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell 7: 359–371, 2004 [DOI] [PubMed] [Google Scholar]
- 153.Wang G, Witkin JW, Hao G, Bankaitis VA, Scherer PE, Baldini G. Syndet is a novel SNAP-25 related protein expressed in many tissues. J Cell Sci 110: 505–513, 1997 [DOI] [PubMed] [Google Scholar]
- 154.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5: 19–26, 2002 [DOI] [PubMed] [Google Scholar]
- 155.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal 19: 2209–2217, 2007 [DOI] [PubMed] [Google Scholar]
- 156.Watson RT, Pessin JE. Transmembrane domain length determines intracellular membrane compartment localization of syntaxins 3, 4, and 5. Am J Physiol Cell Physiol 281: C215–C223, 2001 [DOI] [PubMed] [Google Scholar]
- 157.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure 16: 308–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wheeler MB, Sheu L, Ghai M, Bouquillon A, Grondin G, Weller U, Beaudoin AR, Bennett MK, Trimble WS, Gaisano HY. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology 137: 1340–1348, 1996 [DOI] [PubMed] [Google Scholar]
- 159.Widberg CH, Bryant NJ, Girotti M, Rea S, James DE. Tomosyn interacts with the t-SNAREs syntaxin4 and SNAP23 and plays a role in insulin-stimulated GLUT4 translocation. J Biol Chem 278: 35093–35101, 2003 [DOI] [PubMed] [Google Scholar]
- 160.Williams D, Pessin JE. Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J Cell Biol 180: 375–387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 162.Wyman AH, Chi M, Riley J, Carayannopoulos MO, Yang C, Coker KJ, Pessin JE, Moley KH. Syntaxin 4 expression affects glucose transporter 8 translocation and embryo survival. Mol Endocrinol 17: 2096–2102, 2003 [DOI] [PubMed] [Google Scholar]
- 163.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000 [DOI] [PubMed] [Google Scholar]
- 164.Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, Poitout V, Baskin DG, Wang Y, Philipson LH, Rhodes CJ. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem 278: 9715–9721, 2003 [DOI] [PubMed] [Google Scholar]
- 165.Yamada E, Okada S, Saito T, Ohshima K, Sato M, Tsuchiya T, Uehara Y, Shimizu H, Mori M. Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4-containing vesicles. J Cell Biol 168: 921–928, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell 2: 295–305, 2002 [DOI] [PubMed] [Google Scholar]
- 167.Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol 148: 247–252, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang C, Coker KJ, Kim JK, Mora S, Thurmond DC, Davis AC, Yang B, Williamson RA, Shulman GI, Pessin JE. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest 107: 1311–1318, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang C, Mora S, Ryder JW, Coker KJ, Hansen P, Allen LA, Pessin JE. VAMP3 null mice display normal constitutive, insulin- and exercise-regulated vesicle trafficking. Mol Cell Biol 21: 1573–1580, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rauniyar R, Kahn CR. Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci USA 101: 16525–16530, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zeng Q, Subramaniam VN, Wong SH, Tang BL, Parton RG, Rea S, James DE, Hong W. A novel synaptobrevin/VAMP homologous protein (VAMP5) is increased during in vitro myogenesis and present in the plasma membrane. Mol Biol Cell 9: 2423–2437, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang W, Lilja L, Mandic SA, Gromada J, Smidt K, Janson J, Takai Y, Bark C, Berggren PO, Meister B. Tomosyn is expressed in beta-cells and negatively regulates insulin exocytosis. Diabetes 55: 574–581, 2006 [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y, Kang YH, Chang N, Lam PP, Liu Y, Olkkonen VM, Gaisano HY. Cab45b, a Munc18b-interacting partner, regulates exocytosis in pancreatic β-cells. J Biol Chem 284: 20840–20847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zilly FE, Sorensen JB, Jahn R, Lang T. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS Biol 4: e330, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]