MMP-9/Gelatinase B Is a Key Regulator of Growth Plate Angiogenesis and Apoptosis of Hypertrophic Chondrocytes (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 16.
Summary
Homozygous mice with a null mutation in the MMP-9/ gelatinase B gene exhibit an abnormal pattern of skeletal growth plate vascularization and ossification. Although hypertrophic chondrocytes develop normally, apoptosis, vascularization, and ossification are delayed, resulting in progressive lengthening of the growth plate to about eight times normal. After 3 weeks post-natal, aberrant apoptosis, vascularization, and ossification compensate to remodel the enlarged growth plate and ultimately produce an axial skeleton of normal appearance. Transplantation of wild-type bone marrow cells rescues vascularization and ossification in gelatinase B–null growth plates, indicating that these processes are mediated by gelatinase B–expressing cells of bone marrow origin, designated chondro clasts. Growth plates from gelatinase B–null mice in culture show a delayed release of an angiogenic activator, establishing a role for this proteinase in controlling angiogenesis.
Introduction
Skeletal development requires the exquisite coordination of programs for cellular growth, differentiation, apoptosis, extracellular matrix (ECM) remodeling, and angiogenesis. Bones form by at least two distinct mechanisms: intramembranous and endochondral ossification (Caplan, 1988). Endochondral bone formation presents a particularly interesting developmental challenge. During this process an avascular tissue (cartilage) is replaced by one of the most highly vascularized tissues (bone) in the vertebrate body. Endochondral ossification occurs in the growth plates and epiphyses of long bones. The growth plates are characterized by the orderly proliferation and maturation of chondrocytes in longitudinal columns, forming stratified zones of reserve, proliferative, maturing, and hypertrophic cartilage (Poole, 1991). Hypertrophic chondrocytes secrete large amounts of a specialized ECM rich in collagen type X, which becomes calcified. Ossification begins with invasion of the calcified hypertrophic cartilage by capillaries, accompanied by apoptosis of terminal hypertrophic chondrocytes, degradation of cartilage matrix, and deposition of bone matrix by osteoblasts. Remodeling of bone matrix by osteoclasts results in a cavity filled with vascular channels containing hematopoietic cells.
Little is known about the control of angiogenesis at the growth plate. Cartilage is highly resistant to vascularization, except for hypertrophic cartilage, which becomes a target for capillary invasion and angiogenesis during endochondral ossification (Kuettner and Pauli, 1983). Hypertrophic cartilage may produce angiogenic activators, whereas other types of cartilage produce angiogenic inhibitors (Descalzi Cancedda et al., 1995). A delicate balance between the rate of formation of calcified cartilage and its vascularization must be maintained in order for bone development to proceed normally.
Angiogenesis requires invasion by endothelial cells and localized proteolytic modification of the ECM (Ingber and Folkman, 1989). Proteolysis of the ECM allows cell migration and may also release stored signaling molecules. Matrix metalloproteinases (MMP) are implicated in these processes owing to their ability to cleave ECM components (Birkedal-Hansen et al., 1993; Werb, 1997). MMPs are expressed by and around forming blood vessels (Karelina et al., 1995), and modification of MMP activity modulates endothelial cell proliferation or tubule formation in several in vitro models of angio-genesis (Murphy et al., 1993; Schnaper et al., 1993; Fisher et al., 1994). Hyaline cartilage, which is resistant to vascular invasion, is rich in angiogenic inhibitors, including members of the tissue inhibitor of the metalloproteinases (TIMP) family (Moses and Langer, 1991).
Gelatinase B (92 kDa gelatinase, MMP-9) degrades components of the ECM with high specific activity for denatured collagens (gelatin). It can cleave native collagens of type IV, V, and XI, and elastin, but not native type I collagen, proteoglycans, or laminins. Gelatinase B can also cleave a variety of non-ECM molecules such as IL-1β, substance P, myelin basic protein, and amyloid β peptide (reviewed in Vu and Werb, 1998).
Gelatinase B has a restricted pattern of expression that changes from development to maturity. It is frequently expressed at sites of active tissue remodeling and neovascularization. It is highly expressed during embryonic development by trophoblast cells at the implantation site and by osteoclasts (Reponen et al., 1994; Alexander et al., 1996). Later, it is expressed mainly by inflammatory cells and in pathological processes such as inflammatory arthritis, tumor invasion, skin blistering diseases, corneal ulcers, and Alzheimer's disease (re-viewed in Vu and Werb, 1998). Gelatinase B is also implicated in invasive behavior in trophoblasts and metastatic tumor cells.
In this study we have generated mice deficient in gelatinase B by targeted mutagenesis and determined the consequences upon endochondral bone formation.
Results
Gelatinase B–Null Mice Are Viable
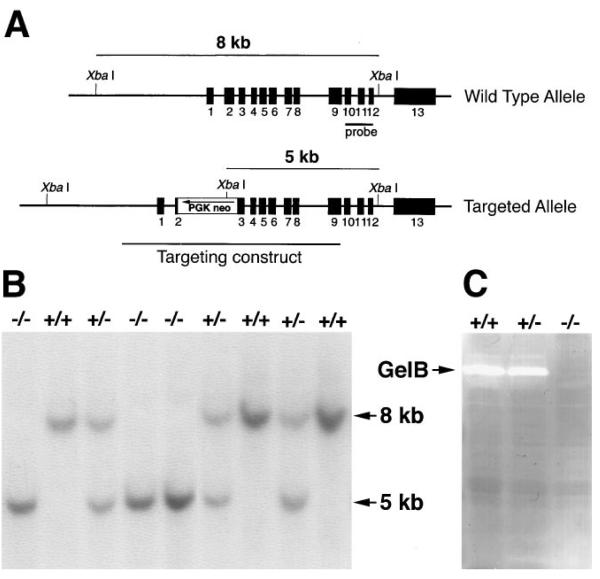
To inactivate the gelatinase B gene, part of exon 2 and all of intron 2 were replaced with a cassette containing the neomycin phosphotransferase cDNA (_neo_r) driven by the phosphoglycerate kinase (PGK) promoter (Figure 1A). The targeting construct was introduced into ZW4 (129/Sv) ES cells by electroporation, and resistant colonies were selected with G418. Eight homologously targeted clones were identified out of 103 clones screened. Two independent correctly targeted clones were aggregated with morulas of CD1 mice or injected into blasto-cysts of C57BL/6 mice. Chimeric males were mated with either CD1 or Swiss Black mice, and offspring were screened with Southern blots of tail DNA for germline transmission of the targeted allele (Figure 1B). All of the studies were done using animals derived from the two independent ES clones.
Figure 1. Generation of Gelatinase B–Null Mice.
(A) Genomic organization of the wild-type and targeted alleles after homologous recombination. The location of the fragment used as a probe in Southern blotting is shown, as well as the sizes of the two XbaI fragments detected for wild-type and targeted alleles. (B) Southern blot analysis of genomic DNA. Fragments corresponding to wild-type (8 kb) and targeted (5 kb) alleles are shown. (C) Gelatin zymographic analysis of speen cell lysates. Gelatinolytic activity corresponding to gelatinase B is shown.
Intercrossing of heterozygous animals gave rise to live homozygous offspring with expected Mendelian ratios and with no obvious phenotypic defects. Homozygous null animals were fertile and survived for at least 24 months. These mice were also viable on pure 129/Sv, C57BL/6, and FVB/N backgrounds. Homozygous null animals lacked gelatinase B activity in spleen cell lysates (Figure 1C).
Gelatinase B–Null Animals Show Abnormal Development of Growth Plates in the Long Bones
Gelatinase B is highly expressed in osteoclasts; therefore, we first examined whether these mice exhibited signs of defective osteoclast function such as osteopetrosis or altered trabecular bone density. Initial histo-logic and radiographic examination of young adult gelatinase B–null mice (2 months) revealed no obvious phenotype in the skull or axial skeleton. However, the long bones (tibia and femurs) of gelatinase B–null mice were about 10% shorter than those of wild-type littermates (data not shown).
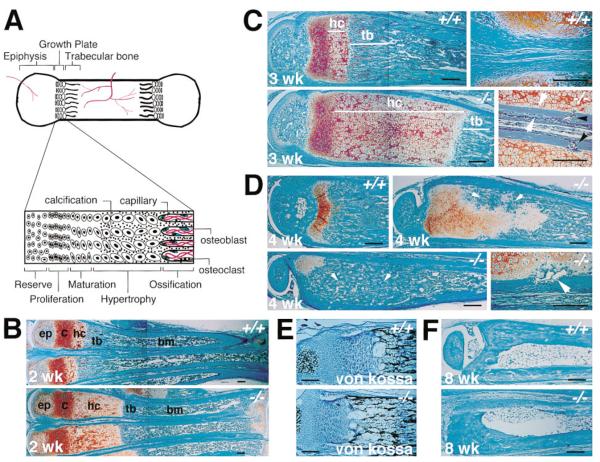
Bone length is determined in part by the activity of the growth plate during endochondral bone formation. Therefore, we examined growth plates of gelatinase B–null animals. Tibias, femurs, and metatarsals of homozygous animals had microscopic abnormalities in their growth plates. We focused on the metatarsals of postnatal mice because of their small size and convenience; other appendicular bones (tibia, femur) had similar findings. Growth plates of gelatinase B–null mice had a lengthened zone of hypertrophic cartilage with no difference in the reserve or proliferating zones (Figure 2B). The same abnormalites were seen in the metatarsal growth plates of over 50 mice examined from two different backgrounds (129Sv/CD1 and 129Sv/Swiss Black). The hypertrophic cartilage zone in the gelatinase B–null meta-tarsals was about twice that of wild-type metatarsals at birth (data not shown) and became more pronounced with the growth of the bones. By 3 weeks postnatal, it was six to eight times as long in the null animals as in wild-type animals (Figure 2C). The cells in the lengthened hypertrophic zone were morphologically normal, and the matrix calcified normally (Figure 2E). There was no difference in the thickness of the cortical bone. However, the area of metaphyseal trabecular bone was somewhat shorter in the gelatinase B–null bones (Figure 2C). Cortical bone formed and extended normally and independently of trabecular bone in the null animals, resulting in a collar of bone surrounding the nonossified lengthened hypertrophic cartilage, whereas wild-type hypertrophic cartilage is not surrounded by cortical bone (Figure 2C).
Figure 2. Histological Analysis of the Metatarsals of Wild-Type and Gelatinase B–Null Mice.
(A) Diagram of the growth plate showing different cartilage zones.
(B) Histological sections of 2-week-old metatarsals stained with hematoxylin/fast green/safranin O. (ep) epiphysis; (c) reserve, proliferation, and maturation zones; (hc) hypertrophic cartilage; (tb) trabecular bone; (bm) bone marrow. Scale bar = 200 μm.
(C) Histological sections of 3-week-old metatarsals. Magnified view on the right lower panel shows cortical bone surrounding the hypertrophic cartilage in gelatinase B–null mice (outlined by white arrows). Black arrows point to capillaries invading from the cortical bone. (hc) hypertrophic cartilage; (tb) trabecular bone.
(D) Histological sections of 4-week-old metatarsals showing aberrant ossification process in the gelatinase B–null mice (arrows in upper right panel). The lower left panel shows a bone whose hypertrophic cartilage has been almost completely ossified giving the appearance of osteopetrosis (arrows). Magnified view on the lower right panel shows a vessel invading from the side (arrow).
(E) Von Kossa staining of 2-week-old metatarsal growth plates. Calcium deposits stain black.
(F) Histological sections of 8-week-old metatarsals.
At 4 weeks of age, ossification in the gelatinase B–null animals was no longer limited to the distal end of the hypertrophic cartilage. Ectopic areas of ossification began to appear within the hypertrophic zone (Figure 2D). This aberrant process appeared to be initiated by invasion of blood vessels from the periphery (Figure 2D), which could be seen as early as 3 weeks of age (Figure 2C). Ectopic ossification proceeded rapidly, so that in some bones the entire zone of hypertrophic cartilage was ossified, leading to a large area of trabecular bone reminiscent of osteopetrosis (Figure 2D). However, this osteopetrosis resolved with subsequent remodeling, producing normal appearing bones by 8 weeks (Figure 2F).
Hypertrophic Chondrocyte Differentiation Is Normal in Gelatinase B–Null Mice
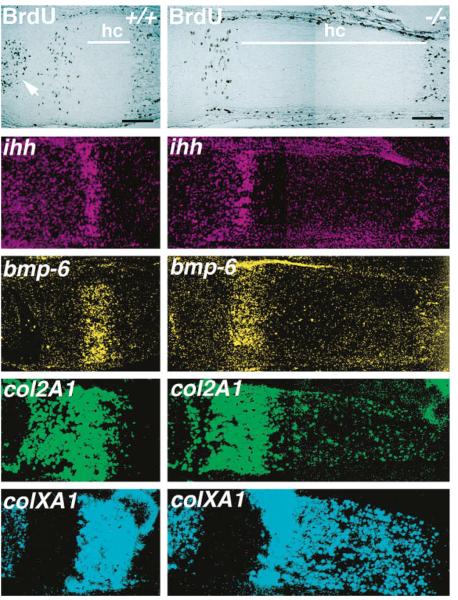
One mechanism for the lengthened zone of hypertrophic cartilage is an alteration in the proliferation or maturation of chondrocytes. The number of proliferating cells incorporating bromo-deoxyuridine (BrdU) in the growth plates was similar in the homozygous null and wild-type animals at all ages examined (Figure 3; data not shown), indicating that increased proliferation was not a mechanism leading to the expanded zone of hypertrophic cartilage. BrdU-positive cells were also seen in the epiphyses of wild-type metatarsals at 2 weeks of age, where the secondary (epiphyseal) site of ossification had already started. In the gelatinase B–null animals, the secondary ossification sites were delayed until 2.5 weeks of age (data not shown). However, by 3 weeks of age these sites were completely ossified, as in wild-type animals (Figure 2B). Interestingly, there was no discernable increase in the hypertrophic cartilage zone in these epiphyseal sites.
Figure 3. BrdU Labeling and In Situ Hybridization with Chondrocyte Markers.
Left column panels show metatarsals from 2-week-old wild type and right column panels show those from gelatinase B–null mice. Cells that have incorporated BrdU stain brown. Arrow points to the secondary site of osssification. Zones of expression of Indian hedgehog (ihh), bone morphogenetic protein-6 (bmp-6), collagen type II (col2A1), and collagen type X (colXA1) are shown by dark-field photography of in situ hybridization with antisense mRNA probes. (hc) hypertrophic cartilage. Scale bar = 200 μm.
A distinct pattern of stage-specific gene expression characterizes chondrocyte differentiation. Indian hedgehog (ihh) is expressed by mature and upper hypertrophic chondrocytes and is important in the control of chondrocyte maturation (Lanske et al., 1996; Vortkamp et al., 1996). Bone morphogenetic protein-6 (bmp-6) is also expressed by upper hypertrophic chondrocytes, and collagen type 2 (col2A1) is expressed by chondrocytes in the reserve, proliferative, mature, and upper hypertrophic zones. In situ hybridizations showed no differences in the pattern of expression of mRNA transcripts for ihh, bmp-6, and col2A1 between the wild-type and gelatinase B–null growth plates at 1 and 2 weeks postnatal (Figure 3; data not shown). The expression of ihh and bmp-6 was not detected in epiphyseal sites or in gelatinase B–null growth plates after 3 weeks of age (data not shown) as observed previously in wild-type bones (Iwasaki et al., 1997). In contrast to these markers of early differentiation, collagen type X (colXA1), a specific marker of all hypertrophic chondrocytes (Elima et al., 1993), showed an expanded domain of expression corresponding to the lengthened zone of hypertrophic cartilage (Figure 3). This finding indicates that the cells present in the greatly expanded hypertrophic cartilage zone remained metabolically active.
Apoptosis of Hypertrophic Chondrocytes Is Delayed in the Gelatinase B–Null Mice
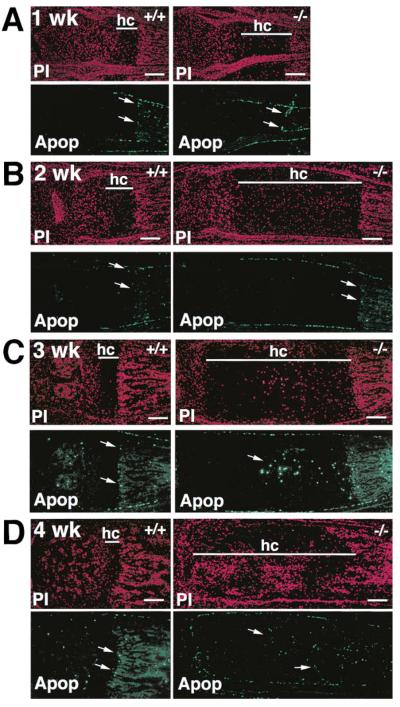
During endochondral ossification, terminal hypertrophic chondrocytes adjacent to the invading capillaries undergo apoptosis (Farnum and Wilsman, 1989; Hatori et al., 1995). The enlargement of the hypertrophic cartilage zone could be due to altered hypertrophic chondrocyte death or tissue remodeling (ossification) at the calcified cartilage–bone interface. In both wild-type and gelatinase B–null growth plates, apoptotic chondrocytes were found only in the terminal row adjacent to the ossification front (Figures 4A and 4B). This indicates that in the gelatinase B–null growth plates there was a delay in hypertrophic chondrocyte apoptosis coupled with delayed ossification of hypertrophic cartilage. Gelatinase B could either have a direct effect on apoptosis or on ossification.
Figure 4. Apoptotic Cells in the Metatarsal Growth Plate.
(A and B) Propidium iodide (PI) and ApopTag (Apop) staining of 1- and 2-week-old metatarsal growth plates from wild-type and gelatinase B–null mice. Arrows point to terminal hypertrophic chondrocytes that are apoptotic. Apoptotic cells are also present in the bone marrow, perichondrium, and periosteum. (hc) hypertrophic cartilage.
(C and D) PI and Apop staining of 3-week-old (C) and 4-week-old (D) metatarsal growth plates. Arrows in the gelatinase B–null growth plates show apoptotic chondrocytes in the middle of the hypertrophic cartilage at three weeks (C) or in the vascularized and ossified hypertrophic cartilage at 4 weeks (D). Scale bars = 200 μm.
By 3 weeks of age, when the hypertrophic zone had lengthened up to 8-fold, aberrant apoptosis began in the middle of the hypertrophic cartilage (Figure 4C). This was followed by ectopic ossification, evident by 4 weeks of age, with chondrocyte apoptosis around the areas of ossification throughout the hypertrophic zone (Figure 4D).
Cells at the Hypertrophic Cartilage–Bone Junction Express Gelatinase B
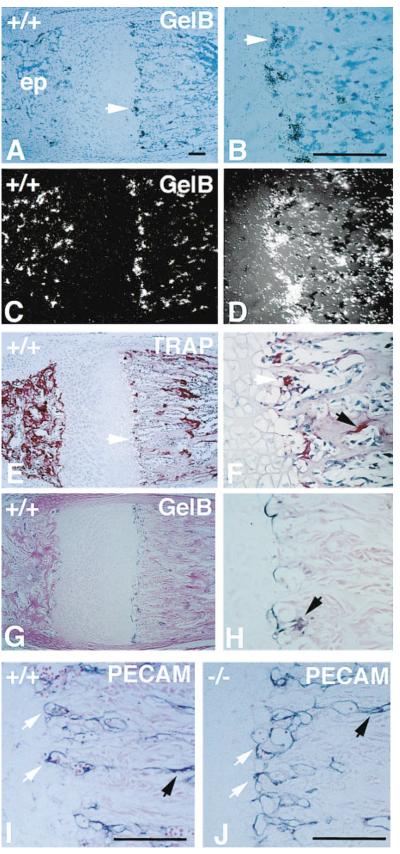
High expression of gelatinase B was seen in two populations of cells: multinucleated osteoclasts on trabecular bone surfaces and smaller, mostly mononuclear cells at the transverse septae of the cartilage–bone junction that lead the vascular invasion front (Figures 5A–5D). Although the distribution of cells expressing gelatinase B was similar to that of cells expressing tartrate-resistant acid phosphatase (TRAP), a marker for the osteoclast cell lineage (Figures 5E and 5F), there were also TRAP-negative cells expressing gelatinase B. Gelatinase B expression did not colocalize with staining with an antibody to PECAM-1, an endothelial cell surface marker (Figures 5I and 5J), suggesting that cells expressing gelatinase B are not endothelial cells. Gelatinase B protein was present in small stellate cells and also tightly associated with the ECM localized to the junction between hypertrophic cartilage and the vascular invasion front (Figures 5G and 5H).
Figure 5. Expression of Gelatinase B at the Growth Plate.
(A–D) Bright-field (A and B) and dark-field (C and D) photographs of a section of 3-week-old wild-type metartarsal growth plate hybridized with a gelatinase B antisense mRNA probe. Arrows point to gelatinase B expressing cells at the cartilage–bone junction. (B) and (D) are magnified views of (A) and (C). (ep) epiphysis. Scale bar = 100 μm.
(E and F) TRAP staining of an adjacent section (E) and magnified view (F) showing TRAP positive (red) cells at the cartilage–bone junction (white arrows) and on the trabecular bone surfaces (black arrows). Note the distribution of TRAP-positive cells is similar to that of gelatinase B–expressing cells.
(G and H) Immunohistochemistry of 3-week-old wild type metatarsal growth plate with an antibody to gelatinase B, showing staining at the cartilage bone juction and trabecular surfaces. Magnified view (H) shows staining of ECM and stellate cells (arrow).
(I and J) Immunohistochemistry of 3-week-old wild-type and gelatinase B–null metatarsal growth plates with an antibody to PECAM-1 showing staining of blood vessels at the cartilage–bone junction (white arrows) and in between trabecular bones (black arrows).
Bone Marrow Transplantation Corrects the Gelatinase B–Null Phenotype
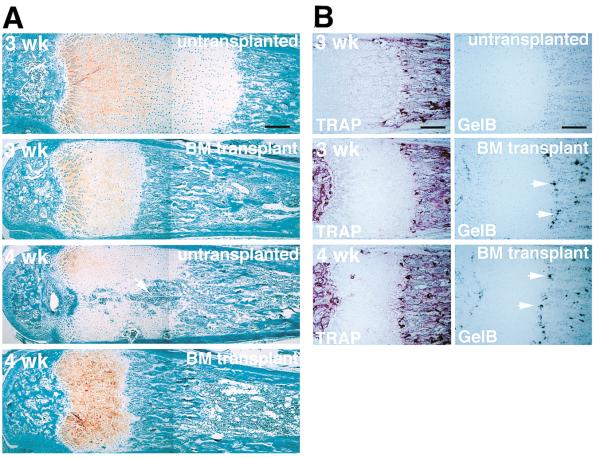
Three major cell types are present in the ossifying growth plate: mesenchymal cells that give rise to osteoblasts, hematopoietic cells that give rise to osteoclasts and blood cells, and endothelial cells. To determine the origin of the critical cells whose lack of gelatinase B expression leads to the defect in the gelatinase B–null mice, we irradiated newborn gelatinase B–null mice and reconstituted their bone marrow with wild-type cells. The presence of engrafted functional wild-type bone marrow cells in the transplanted mice was confirmed by the presence of gelatinolytic activity corresponding to gelatinase B in their spleens (data not shown). At 3 weeks after transplantation, the zones of hypertrophic cartilage in the metatarsal growth plates of treated mice were reduced in size, intermediate between untreated gelatinase B–null and wild-type mice (Figure 6A). At 4 weeks after transplantation, the growth plates of treated mice did not show the aberrant ossification seen in their untransplanted littermates (Figure 6A). Cells expressing gelatinase B appeared at the cartilage–bone junction in the transplanted mice, some of which corresponded to TRAP-positive cells (Figure 6B). These data indicate that the critical cells are of bone marrow origin.
Figure 6. Growth Plates of Gelatinase B–Null Mice after Bone Marrow Transplantation.
(A) Histological sections of metatarsals from untransplanted and bone marrow (BM) transplanted mice at 3 and 4 weeks after transplantation. Arrow points to area of ectopic ossification in the untransplanted 4-week-old growth plate. Scale bar = 200 μm.
(B) TRAP staining and in situ hybridization with an antisense mRNA gelatinase B probe showing that gelatinase B–expressing cells, some of which correspond to TRAP-positive cells, appear at the cartilage–bone junction and on the trabecular bone surfaces after transplantation (arrows).
Release of an Angiogenic Activator from Gelatinase B–Null Growth Plate in Culture Is Delayed
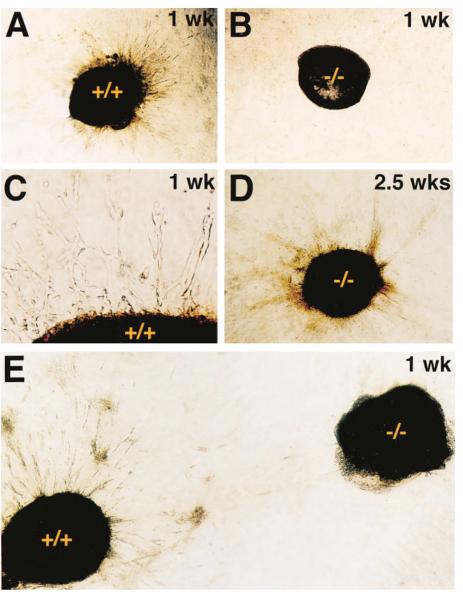
The delay in ossification of the growth plates in gelatinase B–null animals may be due to either defective ECM remodeling or impaired angiogenesis, or both. We used an in vitro angiogenesis assay to compare the angiogenic activity of growth plates from wild-type versus gelatinase B–null mice. Hypertrophic cartilage, including the immediately adjacent vascular invasion front where gelatinase B is expressed, was dissected out of 2-week-old wild-type and gelatinase B–null metatarsals. These were embedded into collagen gels containing randomly distributed bovine microvascular endothelial cells. In this assay, wild-type cartilage induced a dramatic response within a few days in culture, with the radial alignment, proliferation, and migration of endothelial cells toward the embedded cartilage (Figures 7A and 7C). In contrast, endothelial cells remained in a random distribution around the gelatinase B–null cartilage (Figure 7B). When both wild-type and gelatinase B–null cartilage were placed side by side on the same collagen gel, the presence of gelatinase B–null cartilage did not affect the angiogenic response to wild-type cartilage (Figure 7E). When the gelatinase B–null cartilage was maintained in the collagen gel for more than a week, it began to induce an angiogenic response (Figure 7D) consistent with the in vivo observation that the vascularization and ossification of hypertrophic cartilage in the gelatinase B–null growth plate were only delayed until 3 weeks post-natal.
Figure 7. In Vitro Angiogenesis Assay.
(A–C) Hypertrophic cartilage from 2-week-old wild-type (A) and gelatinase B–Null (B) mice after one week in a collagen gel coculture with endothelial cells, showing an angiogenic response from the endothelial cells only to wild-type cartilage. Magnified view (C) shows tubular structures formed by endothelial cells surrounding the wild-type hypertrophic cartilage.
(D) Gelatinase B–Null hypertrophic cartilage after 2.5 weeks in coculture with endothelial cells, showing an angiogenic response. (E) Wild-type and gelatinase B–Null cartilage after one week side by side in coculture with endothelial cell, showing angiogenic response only to wild-type cartilage that is not abrogated by the presence of the gelatinase B–Null cartilage.
Discussion
In this study we have demonstrated a developmentally restricted function for gelatinase B. Our data show that gelatinase B functions in the control of hypertrophic cartilage vascularization and ossification. Thus, we have identified a gene that specifically affects cartilage vascularization. This specific function of gelatinase B was unexpected. A role in implantation and bone resorption has been postulated for gelatinase B owing to its high expression in trophoblasts and osteoclasts. Surprisingly, lack of gelatinase B did not result in failure to implant or in osteopetrosis. The gelatinase B–Null animals developed to term, survived normally after birth, and were fertile. Therefore, while gelatinase B may play a role in implantation, bone resorption, and other physiological processes, other enzymes either play a more dominant role or are able to compensate for the lack of gelatinase B. Interestingly, deficiency of the closely related gelatinase A (MMP-2) does not appear to result in any significant developmental or physiological impairment (Itoh et al., 1997).
Gelatinase B Is Involved in Endochondral Bone Formation
The lack of gelatinase B causes a delay in endochondral ossification. This is a complex and tightly regulated process. Normal long bone development requires proper growth and differentiation of cartilage cells (chondro-genesis), the regulated replacement of cartilage by bone (osteogenesis), and normal bone remodeling. Many gene products are involved in this highly orchestrated network of events. Parathyroid hormone-related peptide (PTHrP), its receptor, and the ihh gene product regulate chondrocyte growth and maturation (Amizuka et al., 1996; Lanske et al., 1996; Vortkamp et al., 1996). The transcription factor Cbfa1 is necessary for the differentiation of osteoblasts and the formation of bone matrix (Ducy et al., 1997; Komori et al., 1997; Otto et al., 1997). Defects in genes such as csf-1, c-fos, and c-src, which affect the function or development of osteoclasts, lead to defects in bone remodeling with consequent development of osteopetrosis (Yoshida et al., 1990; Soriano et al., 1991; Grigoriadis et al., 1994).
The gelatinase B–null mice displayed an abnormally large hypertrophic cartilage zone that was not due to abnormal growth and differentiation of chondrocytes, as shown by the normal pattern of BrdU incorporation and of the expression of ihh, bmp-6, and col2A1, markers of proliferating and maturing chondrocytes. In contrast, mice deficient in FGFR-3 and cGMP-dependent protein kinase II have enlarged hypertrophic cartilage zones secondary to abnormal chondrocyte proliferation and differentiation (Deng et al., 1996; Pfeifer et al., 1996). The gelatinase B–Null mice had delayed endochondral ossification. This defect was confined to the ossification of growth plate cartilage. There were no obvious effects on ossification of the epiphyses or of intramembranous bones. The initiation of the epiphyseal ossification sites in the gelatinase B–Null animals was slightly delayed, but ossification was completed timely and there was no abnormal accumulation of hypertrophic cartilage.
Angiogenesis Is Rate Limiting in the Process of Endochondral Ossification
A critical step in the process of endochondral ossification is the vascularization of hypertrophic cartilage. This process has three distinct components: the initial invasion of capillaries into the cartilaginous anlage to establish the marrow cavity and the growth plates; continuing vascularization coupled with ossification of the growing growth plates; and vascularization and ossification of the epiphysis. The initial capillary invasion appears to require the formation of a bone collar around the cartilage anlage. The cartilageous limbs in mice with a null mutation in Cbfa1, a gene essential for osteoblast development, have calcified hypertrophic cartilage but no bone formation and are not vascularized (Komori et al., 1997; Otto et al., 1997). Lack of gelatinase B does not appear to affect significantly the initial capillary invasion. In E18 embryos the cartilaginous metatarsals are not yet vascularized, and in newborn mice the growth plates are already well established in both wild-type and gelatinase B–Null animals (data not shown). This does not exclude the possibility of a short delay in the initial capillary invasion that resolves within this period. The vascularization and ossification of the epiphysis were also not significantly affected by the lack of gelatinase B.
Gelatinase B deficiency greatly affected the vascularization and ossification of the growth plates. This process requires the resorption of cartilage matrix and the growth and invasion of capillaries followed by the deposition of bone matrix by osteoblasts onto the partially resorbed cartilage scaffold. Capillary ingrowth into the cartilage occurs by angiogenesis, as suggested by electron microscopic study of vascular casts of growth plates (Aharinejad et al., 1995). This vascularization has to be tightly controlled and coordinated with the formation of hypertrophic cartilage for normal bone development. Our data indicate that gelatinase B participates in this regulation, since its absence leads to a delay in hypertrophic chondrocyte apoptosis, vascularization, and ossification, thereby leading to an abnormal accumulation of hypertrophic cartilage.
One possible function of gelatinase B is to degrade cartilage matrix. Lack of ECM resorption would then lead to retardation of capillary ingrowth for a purely mechanical reason, i.e., lack of a cleared path. Alternatively, cartilage matrix may act as a specific inhibitor to vascular invasion. However, it is unlikely that the defect in gelatinase B deficiency is a deficit in generic ECM degradation. There are other proteinases expressed in the growth plates, including collagenase-3, stromelysin-1, gelatinase A, and the cathepsins (Brown et al., 1989; Ohsawa et al., 1993; Rantakokko et al., 1996), that should produce ECM degradation. Cathepsin B and interstitial collagenase have been shown to be sufficient for the degradation of type X collagen, the major collagen of hypertrophic cartilage (Sires et al., 1995). An alternative hypothesis is that gelatinase B is required to generate specific signals that are essential for the control of growth plate hypertrophic chondrocyte apoptosis and/or vascularization and ossification.
Hypertrophic Chondrocyte Apoptosis Is Regulated by Gelatinase B at the Growth Plate
During endochondral ossification, the terminal hypertrophic chondrocytes, the cells situated in the last row of hypertrophic cartilage in proximity to the invading capillary loops, undergo apoptosis. Apoptosis of the terminal chondrocytes may serve as a signal leading to vascular invasion. Alternatively, vascular invasion may cause apoptosis of the chondrocytes in contact with invading vessels, perhaps from dissolution of the surrounding ECM. One significant finding in the gelatinase B–Null growth plates is that even though there is an expanded area of hypertrophic cartilage, still only the terminal cells undergo apoptosis. This suggests that chondrocytes do not go through an automatic progression of proliferation, maturation, hypertrophy, and apoptosis, but that apoptosis of terminal hypertrophic chondrocytes is functionally coupled to vascularization and ossification so that only the cells in contact with the vascular invasion front undergo cell death. Gelatinase B could function to cause chondrocyte apoptosis and indirectly allow vascularization and ossification. Protein-ases, and associated ECM degradation, lead to apoptosis in several systems including development, neuronal death, and mammary gland involution (Chen and Strickland, 1997; as reviewed in Werb, 1997) and would provide a rational and straightforward role for gelatinase B in the growth plate. However, another possibility is that gelatinase B controls angiogenesis by generating an angiogenic signal. In either case, lack of gelatinase B would result in the same phenotype: delayed chondrocyte apoptosis coupled with delayed vascularization and ossification.
Gelatinase B Is a Key Regulator of Growth Plate Angiogenesis
We found that explants of 2-week-old wild-type hyper-tropic cartilage (and the adjacent area of trabecular bone where gelatinase B is located) immediately released potent angiogenic activity in in vitro angiogenesis assays, whereas there was a delay in the release of angiogenic activity from explants of the corresponding tissue from gelatinase B–Null animals. Thus, one function of gelatinase B is to generate angiogenic activators or to inactivate angiogenic inhibitors. Gelatinase B may release angiogenic molecules sequestered in the ECM. There is precedent for proteolytic release and activation of growth factors. For example, stromelysin, interstitial collagenase, and plasmin can degrade perlecan and release bound FGF (Whitelock et al., 1996), and hepatocyte growth factor (HGF) is proteolytically activated by urokinase (Naldini et al., 1995).
What angiogenic activity might be regulated by gelatinase B? Several putative angiogenic factors have been identified in hypertrophic cartilage, while resting hyaline cartilage is rich in antiangiogenic activities. aFGF, bFGF, and endothelial cell stimulating factor (ESAF) have been found in hypertrophic cartilage (Brown et al., 1987; Jingushi et al., 1995). Other angiogenic activities secreted by hypertrophic chondrocytes in culture have been reported, including transferrin and a 120 kDa factor that is up-regulated by vitamin D (Alini et al., 1996; Carlevaro et al., 1997). Gelatinase B may release these from intra-cellular or ECM storage sites, or it may generate (a) yet uncharacterized factor(s).
The delay in the release of an angiogenic activity from gelatinase B–null growth plates in culture is consistent with the observation that the ossification of hypertrophic cartilage in gelatinase B–null mice was only delayed, not completely inhibited. Thus, other mechanisms for regulating angiogenic activities at the growth plates exist. The normal development of bone must depend on a delicate balance of angiogenic activators and inhibitors.
Excess Hypertrophic Cartilage Is Remodeled by Aberrant Chondrocyte Apoptosis and Vascularization in the Gelatinase B–Null Growth Plate
At 3 weeks postnatal, aberrant apoptosis was evident in the enlarged gelatinase B–null hypertrophic cartilage. Although it is possible that this zone has become so large that nutrients in the center become limiting, this seems unlikely given the size of growth plates in larger species such as human and bovine. The gelatinase B–null hypertrophic cartilage was also surrounded by a collar of cortical bone because intramembranous bone formation is unaffected in these mice, and cortical bone formation proceeded in an autonomous manner independently of the ossification of the hypertrophic cartilage. This collar of cortical bone could also limit the delivery of nutrients.
By 4 weeks of age, an abnormal pattern of vascularization and ossification took place in the hypertrophic cartilage of gelatinase B–null mice. This vascularization and ossification occurred haphazardly within the zone and appeared to be initiated by capillaries invading from the peripheral cortical bone, very much like the initial vascular invasion of the cartilage anlage. Although the signals that initiate this abnormal angiogenesis are unknown, apoptosis of the hypertrophic chondrocytes preceded vascularization with the appearance of apoptotic chondrocytes in the center of hypertrophic cartilage of 3-week-old growth plates and without any obvious capillaries in the vicinity.
Are There Two Types of Hypertrophic Cartilage?
Why does ossification of growth plate cartilage require gelatinase B? One notable finding is that ihh is expressed by chondrocytes at the growth plate but not by those in the epiphyses (Iwasaki et al., 1997). The expression of ihh therefore distinguishes growth plate cartilage, whose conversion into bone appears to be different from the other sites in the requirement for gelatinase B. Moreover, when ihh is down-regulated in the growth plate at three weeks postnatal, an abnormal ossification process occurs in the enlarged gelatinase B–null growth plates. This gelatinase B–independent ossification may be similar to the normal ossification of sites that do not have ihh expression, such as the epiphyses. These observations lead to a model of two distinct endochondral ossification processes. Endochondral bone formation from hypertrophic cartilage that expresses ihh, such as the growth plate, would require gelatinase B, whereas ossification of hypertrophic cartilage that does not express ihh, such as in the epiphysis, would not require gelatinase B.
In Search of the Chondroclast
Gelatinase B is expressed by osteoclasts and by cells at the junction between cartilage and bone in the normal growth plates. Gelatinase B–expressing cells at the chondro-osseous junction have several interesting features. They lead the invading capillaries. They are derived from bone marrow cells. The osteopetrotic c-_fos_-null and the op/op mice, which lack osteoclasts, show a milder growth plate defect than that of gelatinase B–null mice (unpublished data). These data suggest that the majority of gelatinase B-expressing cells at the chondroosseous junction belong to the macrophage/osteoclast lineage. Although some of these cells express TRAP and may be osteoclast precursors, they may also be of a different mononuclear phagocyte lineage divergent from the osteoclast line prior to a commited precursor. Perivascular cells have been observed at the growth plates associated with invading vascular loops and have been postulated to be chondroclasts, a hypothesized cell type of obscure origin whose function is to resorb cartilage (Anderson and Parker, 1966; Schenk et al., 1968; Lewinson and Silbermann, 1992). Because gelatinase B–expressing cells are intimately associated with the capillary invasion front and are important for the remodeling of cartilage, we propose that these cells are chondroclasts. However, the possibility remains that cells expressing gelatinase B are responsible for the regulation of angiogenesis and that other cells, perhaps the endothelial cells themselves as has been suggested (Schenk et al., 1968), are responsible for cartilage resorption and thus are the true “chondroclasts.”
Experimental Procedures
Construction of Targeting Vector
The mouse gelatinase B gene was cloned from a 129/Sv genomic library in a bacteriophage P1 vector (Genome Systems, St. Louis, MO). A targeting construct was generated in pBluescript SK (Stratagene) that included 5.3 kb of 5′ homology and 3.1 kb of 3′ homology. The 5′ homology included 4.7 kb of promoter sequence, exon 1, intron 1, and 28 bp of exon 2. The 3′ homology begins at the 5′ end of exon 3 and extends to a point within intron 9. A cassette containing the neomycin phosphotransferase cDNA driven by the phosphoglycerate kinase promoter (PGK-Neo) was used to replace most of exon 2 and all of intron 2. The targeting construct was linearized with NotI prior to electroporation into embryonic stem cells.
Generation of Gelatinase B–Null Mice
The targeting construct was introduced by electroporation into ZW4 (129/Sv, Taconic) ES cells isolated by standard methods. Selected G418-resistant clones were screened by Southern blot analysis of XbaI-digested genomic DNA, probed with a PvuII–XbaI fragment that was not part of the targeting construct. Heterozygous ES cells were aggregated with morulas of CD1 mice or injected into blastocysts of C56BL/6Jmice and transferred into uteri of pseudopregnant females. Chimeric males were mated with either CD1 or Swiss Black females, and offspring were screened by Southern blot analysis of tail genomic DNA. Heterozygous littermates were mated to obtain homozygous animals. Spleen lysates were analyzed for loss of gelatinase B activity by gelatin SDS-substrate gel zymography (Fisher and Werb, 1995).
Histological Analysis
Bones were fixed in 4% paraformaldehyde overnight and decalcified in 0.5 M EDTA (pH 7.4) for 7–10 days at 4°C prior to embedding in paraffin. Sections (4 μm) were stained with hematoxylin/fast green/ safranin O. TRAP staining was done using a leukocyte acid phosphatase kit (Sigma, St. Louis, MO). For frozen sections, freshly dissected bones were snap frozen in liquid nitrogen, embedded in OCT (Tissue-Tek) at −20°C, and cut into 10 μm sections. Von Kossa staining for mineralization was done on undecalcified frozen sections by incubating the slides in 5% silver nitrate for 5 min under UV light, followed by 3 min in 5% sodium thiosulfate, and subsequent counterstaining with hematoxylin.
In Situ Hybridization
Paraffin sections were placed on acid-etched, TESPA-treated slides and prepared for in situ hybridization as described (Albrecht et al., 1997). Plasmids were linearized with the appropriate restriction enzymes to transcribe either sense or antisense 33P- or 35S-labeled riboprobes (ihh, bmp-6, colIIA1, and colXA1 probes were described in Albrecht et al., 1997; gelatinase B probe was described in Reponen et al., 1994). Slides were washed at a final stringency of 65°C in 2× SSC, dipped in emulsion, and exposed for 3–7 days (for 33P-labeled probes) or 1–2 weeks (for 35S-labeled probes). Slides were counterstained with hematoxylin or Hoechst 33342.
Detection of BrdU-Labeled and Apoptotic Cells
Mice were injected intraperitoneally with 10 mg/ml BrdU (Sigma, St. Louis, MO) at doses of 0.01 ml/g 1 hr prior to sacrifice. Bones were fixed and processed for paraffin sections as above. Slides were immunostained with an antibody against BrdU using a kit from Zymed (South San Francisco, CA). Apoptotic cells were identified with the TUNEL method with fluorescein-conjugated antibody using a kit from Oncor (Gaithersburg, MD).
Immunohistochemistry
Sections were deparaffinized, baked at 60°C for 1 hr, immersed in 5% H2O2 in PBS for 5 min, washed in PBS, treated with 0.1 M glycine for 1 min, washed in PBS, then blocked in the following solutions in a stepwise fashion with PBS washes in between: 5% nonfat dry milk in PBS for 10 min, 1 mg/ml ovalbumin in PBS for 10 min, and 5% sheep serum in PBS for 30 min. After incubation in 5% sheep serum, the blocking solution was removed and primary antibody was added. Rabbit anti-mouse gelatinase B was generated as described previously (Behrendtsen et al., 1992); monoclonal rat anti-mouse PECAM-1 was a gift from Dr. S. Baldwin (University of Pennsylvania). Antibody was diluted in PBS with 1 mg/ml BSA. Slides were incubated in primary antibody overnight at 4°C, then washed in PBS, blocked again with 5% sheep serum in PBS, and incubated in secondary antibody (biotinylated anti-rabbit or anti-rat Ig from Amersham, Cleveland, OH) at ambient temperature for 1 hr. Slides were subsequently washed in PBS and incubated with horseradish peroxidase–conjugated streptavidin (Amersham, Cleveland, OH) at room temperature for 1 hr, then washed in PBS and developed with True Blue substrate (Kirkeggard and Perry Laboratory, Gaithersburg, MD).
Bone Marrow Transplantation
Newborn pups were sublethally X-irradiated with 300 cGy. Wild-type bone marrow cells were obtained by flushing the cavity of freshly dissected syngeneic adult femurs and tibias with PBS. Flushed cells were dispersed by pipetting, washed, and resuspended in PBS. Nucleated cells (5 × 107) in 50 μl were injected intraperitoneally into irradiated mice. Neomycin at 2 mg/ml was added to the water of the nursing mother. Pups were killed at 3 and 4 weeks after transplantation. To verify engraftment, the spleens were used to test for gelatinase B expression by gelatin zymography.
Collagen Gel Angiogenesis Assays
Bovine capillary endothelial cells (BCE) were trypsinized and resuspended in DMEM with 10% calf serum. Cells (4 × 105/ml) were mixed 1:1 with a chilled collagen solution (Vitrogen 100, Collagen Corp., Palo Alto, CA) in MEM medium containing 0.1% NaHCO3, 2 mM glutamine, and 10 mM HEPES. Aliquots (250 μl) of the collagen-cell mixture were pipetted into 48-well tissue culture plates, and cartilage pieces were added to each well before the solution was allowed to gel at 37°C. Culture plates were incubated in a 10% CO2, balance air atmosphere and observed for endothelial cell growth, migration, and tube formation.
Acknowledgments
We thank Adrian Erlebacher for helpful discussions, Juanito Meneses and Roger Pedersen for assistance with embryonic stem cells and blastocyst injection, Anh Le for assistance with in situ hybridizations, Qin Han for assistance with immunohistochemistry, Ole Behrendtsen for assistance with enzymology and graphics, and Catherine Butterfield for the gift of bovine capillary endothelial cells. This study was supported by grants from the National Institutes of Health (DE10306 and HD26732 to Z. W. and HL47328 to R. M. S), by a grant from the National Cancer Institute (CA37395) to D. H., by a National Research Service Award (HL07185) and a fellowship from the American Lung Association California Affliate to T. H. V., a Parker B. Francis Fellowship to J. M. S., a Deutsche Forschungsgemeinschaft fellowship to G. B., and by the Alan A. and Edith L. Wolff Charitable Trust and the Marc and Marjorie Seldin Pulmonary Research Fund to R. M. S.
References
- Aharinejad S, Marks SC, Jr., Bock P, MacKay CA, Larson EK, Tahamtani A, Mason-Savas A, Firbas W. Microvascular pattern in the metaphysis during bone growth. Anat. Rec. 1995;242:111–122. doi: 10.1002/ar.1092420115. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Eichele G, Helms JA, Lu H. Visualization of gene expression patterns by in situ hybridization. In: Daston GP, editor. Molecular and Cellular Methods in Developmental Toxicology. CRC Press; Boca Raton: 1997. pp. 23–48. [Google Scholar]
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- Alini M, Marriott A, Chen T, Abe S, Poole AR. A novel angiogenic molecule produced at the time of chondrocyte hypertrophy during endochondral bone formation. Dev. Biol. 1996;176:124–132. doi: 10.1006/dbio.1996.9989. [DOI] [PubMed] [Google Scholar]
- Amizuka N, Henderson JE, Hoshi K, Warshawsky H, Ozawa H, Goltzman D, Karaplis AC. Programmed cell death of chondrocytes and aberrant chondrogenesis in mice homozygous for parathyroid hormone-related peptide gene deletion. Endocrinology. 1996;137:5055–5067. doi: 10.1210/endo.137.11.8895380. [DOI] [PubMed] [Google Scholar]
- Anderson CE, Parker J. Invasion and resorption in endochondral ossification. An electron microscopic study. J. Bone Joint Surger. Am. 1966;48:899–914. [PubMed] [Google Scholar]
- Behrendtsen O, Alexander CM, Werb Z. Metalloproteinases mediate extracellular matrix degradation by cells from mouse blastocyst outgrowths. Development. 1992;114:447–456. doi: 10.1242/dev.114.2.447. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brown RA, Taylor C, McLaughlin B, McFarland CD, Weiss JB, Ali SY. Epiphyseal growth plate cartilage and chondrocytes in mineralising cultures produce a low molecular mass angiogenic procollagenase activator. Bone Miner. 1987;3:143–58. [PubMed] [Google Scholar]
- Brown CC, Hembry RM, Reynolds JJ. Immunolocalization of metalloproteinases and their inhibitor in the rabbit growth plate. J. Bone Joint Surg. Am. 1989;71:580–593. [PubMed] [Google Scholar]
- Caplan AI. Bone development. Ciba Found. Symp. 1988;136:3–21. doi: 10.1002/9780470513637.ch2. [DOI] [PubMed] [Google Scholar]
- Carlevaro MF, Albini A, Ribatti D, Gentili C, Benelli R, Cermelli S, Cancedda R, Cancedda FD. Transferrin promotes endothelial cell migration and invasion: implication in cartilage neovascularization. J. Cell Biol. 1997;136:1375–1384. doi: 10.1083/jcb.136.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippo-campus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Descalzi Cancedda F, Melchiori A, Benelli R, Gentili C, Masiello L, Campanile G, Cancedda R, Albini A. Production of angiogenesis inhibitors and stimulators is modulated by cultured growth plate chondrocytes during in vitro differentiation: dependence on extracellular matrix assembly. Eur. J. Cell Biol. 1995;66:60–68. [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Elima K, Eerola I, Rosati R, Metsaranta M, Garofalo S, Perala M, De Crombrugghe B, Vuorio E. The mouse collagen X gene: complete nucleotide sequence, exon structure and expression pattern. Biochem. J. 1993;289:247–253. doi: 10.1042/bj2890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum CE, Wilsman NJ. Cellular turnover at the chondro-osseous junction of growth plate cartilage: analysis by serial sections at the light microscopical level. J. Orthop. Res. 1989;7:654–666. doi: 10.1002/jor.1100070505. [DOI] [PubMed] [Google Scholar]
- Fisher SJ, Werb Z. The catabolism of extracellular matrix components. In: Haralson MA, Hassell JR, editors. Extracellular Matrix Molecules: A Practical Approach. IRL Press at Oxford University Press; Oxford, UK: 1995. pp. 261–287. [Google Scholar]
- Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poor-man R, Mitchell MA. Interstitial collagenase is required for angiogenesis in vitro. Dev. Biol. 1994;162:499–510. doi: 10.1006/dbio.1994.1104. [DOI] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Hatori M, Klatte KJ, Teixeira CC, Shapiro IM. End labeling studies of fragmented DNA in the avian growth plate: evidence of apoptosis in terminally differentiated chondrocytes. J. Bone Miner. Res. 1995;10:1960–1968. doi: 10.1002/jbmr.5650101216. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Le AX, Helms JA. Expression of indian hedgehog, bone morphogenetic protein 6 and gli during skeletal morphogenesis. Mech. Dev. 1997;69:197–202. doi: 10.1016/s0925-4773(97)00145-7. [DOI] [PubMed] [Google Scholar]
- Jingushi S, Scully SP, Joyce ME, Sugioka Y, Bolander ME. Transforming growth factor-beta 1 and fibroblast growth factors in rat growth plate. J. Orthop. Res. 1995;13:761–768. doi: 10.1002/jor.1100130516. [DOI] [PubMed] [Google Scholar]
- Karelina TV, Goldberg GI, Eisen AZ. Matrix metalloproteinases in blood vessel development in human fetal skin and in cutaneous tumors. J. Invest. Dermatol. 1995;105:411–417. doi: 10.1111/1523-1747.ep12321097. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kuettner KE, Pauli BU. Vascularity of cartilage. In: Hall BK, editor. Cartilage. Academic Press; New York: 1983. pp. 281–312. [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LHK, Ho C, Mulligan RC, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Lewinson D, Silbermann M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat. Rec. 1992;233:504–514. doi: 10.1002/ar.1092330403. [DOI] [PubMed] [Google Scholar]
- Moses MA, Langer R. A metalloproteinase inhibitor as an inhibitor of neovascularization. J. Cell. Biochem. 1991;47:230–235. doi: 10.1002/jcb.240470308. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J. Cell. Physiol. 1993;157:351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio PM. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J. Biol. Chem. 1995;270:603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- Ohsawa Y, Nitatori T, Higuchi S, Kominami E, Uchiyama Y. Lysosomal cysteine and aspartic proteinases, acid phosphatase, and an endogenous cysteine proteinase inhibitor, cystatin-beta, in rat osteoclasts. J. Histochem. Cytochem. 1993;41:1075–1083. doi: 10.1177/41.7.8515049. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- Poole AR. The growth plate: cellular physiology, cartilage assembly and mineralization. In: Hall BK, Newman SA, editors. Cartilage: Molecular Aspects. CRC Press; Boca Raton: 1991. pp. 179–211. [Google Scholar]
- Rantakokko J, Aro HT, Savontaus M, Vuorio E. Mouse cathepsin K: cDNA cloning and predominant expression of the gene in osteoclasts, and in some hypertrophying chondrocytes during mouse development. FEBS Lett. 1996;393:307–313. doi: 10.1016/0014-5793(96)00907-6. [DOI] [PubMed] [Google Scholar]
- Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J. Cell Biol. 1994;124:1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk RK, Wiener J, Spiro D. Fine structural aspects of vascular invasion of the tibial epiphyseal growth plate of growing rats. Acta Anat. 1968;69:1–17. doi: 10.1159/000143059. [DOI] [PubMed] [Google Scholar]
- Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D'Orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J. Cell. Physiol. 1993;156:235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- Sires UI, Schmid TM, Fliszar CJ, Wang ZQ, Gluck SL, Welgus HG. Complete degradation of type X collagen requires the combined action of interstitial collagenase and osteoclast-derived cathepsin-B. J. Clin. Invest. 1995;95:2089–2095. doi: 10.1172/JCI117896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Gelatinase B: structure, regulation, and function. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. Academic Press; San Diego: 1998. pp. 115–148. [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]