Genetic approach for intracerebroventricular delivery (original) (raw)
Abstract
Administration of synthetic or purified peptides directly into the brain ventricles is a method commonly used by neuroscientists for exploring physiological and behavioral functions of gene products. i.v. administration is controlled by the blood–brain barrier, which limits its effectiveness, and current approaches for acute or chronic intracerebroventricular delivery have significant technical drawbacks resulting from both the chemical properties of the delivered substance and the experimental procedures. Here we describe a genetic approach for the delivery of secreted peptides or proteins into the cerebrospinal fluid (CSF). Using a choroid plexus-specific promoter, we established a lentiviral-based system, which offers inducible and reversible delivery of a gene product into the CSF. The functionality of this system was demonstrated by using the overexpression of the two established neuropeptides, corticotropin-releasing factor and gonadotropin-releasing hormone, modulating anxiety-like behavior and estrus cycle, respectively. We show that this choroid plexus-specific lentiviral-based system is a reliable, effective, and adaptable research tool for intracerebroventricular delivery.
Keywords: blood–brain barrier, choroid plexus, Lentiviruses, secreted peptides, cerebrospinal fluid
Pharmacological administration of synthetic peptides or secreted recombinant proteins into the brain ventricles is a common method used by neuroscientists for exploring physiological and behavioral functions of novel or known gene products. Cerebroventricular, rather than systemic administration of these proteins is required to bypass the blood–brain barrier (BBB), and allow the nonselective transport of peptides or proteins from the periphery into the central nervous system (CNS) (1–3).
Current solutions for delivery of peptides or secreted proteins to the CNS, for short-term acute administration, include a stereotaxic injection into the ventricular space, known as intracerebroventricular (ICV) administration, or for chronic delivery, an ICV microinjection pump. These methods rely heavily on the solubility and half-life of the injected substance; require chemical or in vitro synthesis of the administered ligand, and may require different purification procedures. The ICV administration is difficult to use in studies requiring repeated or prolonged administration of the ligand, because the microinjection pump procedure depends on the ligand stability and capacity of the reservoir; and complex surgical procedures are required for installation and manipulation of the pump. Furthermore, the current procedures require extensive handling of the experimental animals, which may create behavioral or physiological disturbances.
The choroid plexus plays a critical role in the barrier mechanism regulating the exchange of molecules between the brain’s internal milieu, and the periphery (4–6). This blood–cerebrospinal fluid (CSF) barrier is composed of epithelial cells with apical tight-junctions that restrict intercellular passage of molecules from fenestrated blood vessels (1, 4–6). The CSF circulatory system’s function is to provide micronutrients, neurotrophins, hormones, neuropeptides, and growth factors extensively to neuronal networks (4–6). Therefore, neuromodulators directed to CSF can modify and adapt a variety of behavioral, neuroendocrine, and immunologic processes (7).
In the current study, we established a choroid plexus-specific and lentiviral-based genetic system, which offers inducible and reversible delivery of peptides or proteins into the CSF, and shows significant advantages over the current available methodologies. We demonstrate the functionality of this unique system, using two well-established neuropeptide systems, as a powerful tool for addressing research questions involving acute or chronic effects of gene products within the CNS.
Results
Design and Construction of Choroid Plexus-Specific Lentiviral Constructs for Inducible Overexpression.
Our aim was to generate a genetic system for inducible delivery of peptides or secreted proteins into the CSF. To do so we genetically targeted the choroid plexus tissue by using a combination of lentiviral delivery, to ensure stability of the inserted gene, and the bacterial Tet-On transcriptional regulation system (8, 9) to achieve inducibility and reversibility of the expression of the delivered gene.
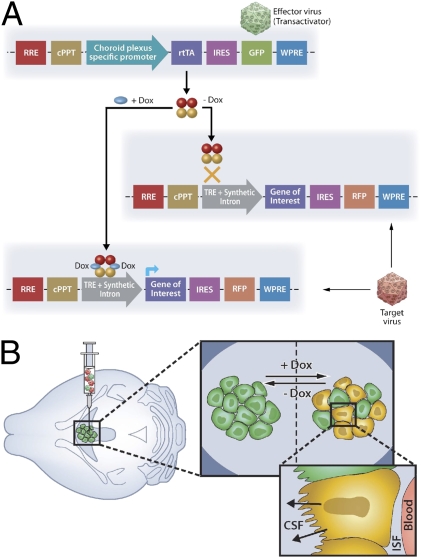
The system is composed of two complimentary lentiviral vectors. The “Effector” construct consists of a choroid plexus-specific promoter that drives the expression of reverse tetracycline trans activator (rtTA) protein and the reporter green fluorescent protein (GFP) (Fig. 1_A_ Upper). The “Target” construct includes the tetracycline-responsive element (TRE) DNA sequence, upstream to the nucleotide coding sequence of the requested gene of interest, followed by the reporter red fluorescent protein (RFP) (Fig. 1_A Lower_). Transcription initiation of the gene of interest and the RFP is mediated only in the presence of the inducer, doxycycline (Dox) (Fig. 1_A_).
Fig. 1.
Schematic representation of a lentiviral-based system designed for inducible overexpression of peptides or proteins of interest by the choroid plexus cells. (A) Schematic representation of the Effector (Upper) and Target (Lower) constructs. (B) ICV injection of the Effector and Target viruses (Left) results in a Dox inducible transcription of the transgene by the choroid plexus cells (Large Inset) and delivery of the gene product into the CSF (Small Inset). rtTA, reverse tetracycline trans activator; TRE, tetracycline-responsive element; ISF, interstitial fluid.
A mixture of the two lentiviruses is injected ICV and the delivered genes are incorporated into the DNA of the choroid plexus cells. Initiation of transcription, limited to the choroid plexus cells by the choroid plexus-specific promoter, is induced by administrating Dox-containing drinking water, and results in secretion of the final processed gene product into the CSF (Fig. 1_B_). Dox is the inducer of choice for our purposes as it has been demonstrated to cross the BBB (10).
Choroid Plexus Specificity and in Vivo Validation of Constructed Lentiviruses.
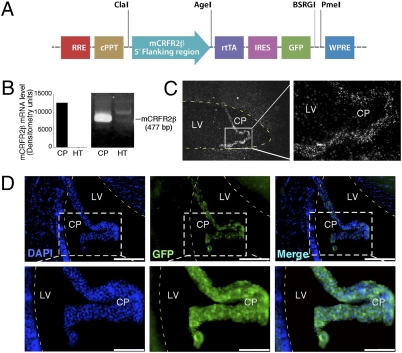
To ensure that transgene expression is limited to cells of the choroid plexus, we isolated ≈2.5 kb of the 5′-flanking region of the corticotropin releasing factor receptor type 2 beta (CRFR2β) gene, which was demonstrated (11, 12) to be expressed in the brain specifically by the choroid plexus cells, and used it to drive the transcription of the rtTA within the choroid plexus cells (Fig. 2_A_). The CRFR2 gene has three functional splice variants in humans (α, β, and γ) and two in rodents (α and β) that are produced by the use of alternate 5′ exons (12, 13). The CRFR2α is the CRFR2 splice variant expressed by neurons in the rodent’s brain, whereas CRFR2β mRNA is widely expressed in peripheral tissues, with highest levels in the skeletal muscle, heart, and skin and in the brain only by the choroid plexus cells (11, 12).
Fig. 2.
Choroid plexus specificity and in vivo validation of constructed lentiviruses. (A) Schematic representation of the choroid plexus-specific Effector lentiviral construct, containing 2.5 kb of the 5′-flanking region of the CRFR2β gene, which drives the transcription of the rtTA and the GFP proteins. (B) Semiquantitative RT-PCR analysis, using specific primers for mouse CRFR2β, demonstrates abundant expression of CRFR2β in the choroid plexus but not in hypothalamic tissue (HT). (C) Dark-field photomicrographs showing positive hybridization signal for mouse CRFR2β in the choroid plexus. (D) ICV injection of the choroid plexus-specific Effector lentiviruses resulted in GFP expression specifically by the choroid plexus and not the ependymal cells. Higher magnification images demonstrate the ability of these lentiviruses to infect the choroid plexus cells with high efficiency. (Scale bars: Upper panels 0.2 mm; lower panels 0.1 mm.) CP, choroid plexus; LV, lateral ventricle.
To further confirm the specificity of central CRFR2β expression to the choroid plexus, total RNA was extracted from mouse choroid plexus and hypothalamic tissues and was reverse-transcribed to generate cDNA pools. The cDNA products were used as templates for semiquantitative RT-PCR analysis, using specific primers for mouse CRFR2β (Fig. 2_B_). The RT-PCR data demonstrate expression of CRFR2β that is abundant and restricted to the choroid plexus tissue (Fig. 2_B_). To further demonstrate the mRNA distribution of mouse CRFR2β in the choroid plexus cells, we used specific in situ probe and a positive hybridization signal for mouse CRFR2β was found only in the choroid plexus (Fig. 2_C_).
Intracerebroventricular injection of the choroid plexus-specific lentiviruses shows GFP expression specifically by the choroid plexus cells (Fig. 2_D_ and Fig. S1). Costaining with the nuclear marker DAPI clearly demonstrates that only the choroid plexus-cells and not the ependymal cell (cells which line the brain ventricles) or other surrounding regions of the brain are expressing GFP (Fig. 2_D Upper_ and Fig. S1). Higher magnification images demonstrate the ability of these lentiviruses to infect the choroid plexus cells with high efficiency (Fig. 2_D Lower_). Strong and extensive GFP expression in infected choroid plexus was revealed even 4 months after lentiviruses administration, suggesting a stable and constant expression of the transgene.
Precursor Processing and Posttranscriptional Modifications by the Choroid Plexus Tissue.
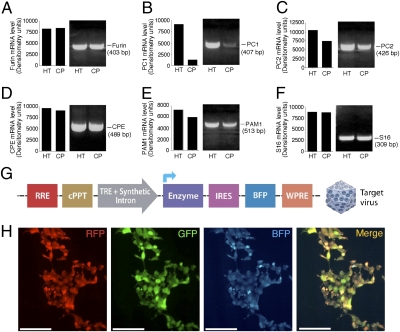
Most neuropeptides are derived from larger biologically inactive polypeptide precursors and require posttranslational processing to become biologically active. Specific proteases/endopeptidases are thought to process precursors during transit through the ER/golgi secretory pathway (14). After cleavage, the residual basic amino acids are removed by an exopeptidase (carboxypeptidase B/H/E), which is often followed by other posttranslational modifications such as glycosylation, sulphation, and amidation to obtain full bioactivity (14).
To ensure that the target proteins are processed correctly by the choroid plexus cells, we screened total RNA isolated from the mouse choroid plexus for a set of key processing enzymes known to be necessary for processing of most neuropeptides, and expressed by neuroendocrine neurons. Semiquantitative RT-PCR analysis of the cleaving enzyme Furin, a paired basic amino acid cleaving enzyme (Fig. 3_A_), and the exopeptidase E (CPE), which is responsible for the removal of an amino acid from the end of a polypeptide chain (Fig. 3_D_), showed similar expression levels in the choroid plexus cells, compared with the positive control tissue obtained from the mouse hypothalamus. The mRNA levels of the prohormone convertase 2 (PC2) (Fig. 3_C_) and the peptidylglycine alpha-amidating monooxygenase 1 (PAM1), which catalyze peptides to active alpha-amidated products (Fig. 3_E_), were only slightly reduced compared with the levels in the hypothalamic tissue. The PC1 expression levels, although detected, were significantly lower in the choroid plexus tissue than in hypothalamic tissue (Fig. 3_B_). Because both PC1 and PC2 cleave paired basic amino acids, the high levels of PC2 are likely to be sufficient for proper processing.
Fig. 3.
Precursors processing and posttranscriptional modifications by the choroid plexus tissue. Semiquantitative RT-PCR analysis of the cleaving enzyme Furin (A), PC1 (B), PC2 (C), CPE (D), PAM1 (E), and the ribosomal protein S16 (F), performed on cDNA isolated from the mouse choroid plexus (CP) and hypothalamic tissue (HT). (G) Schematic representation of the Target lentiviral construct, designed to conditionally express additional enzyme or cofactor, followed by a blue florescent protein (BFP). (H) Infection of HEK-293T cells with the three reporter lentiviruses showed the feasibility of a triple infection, as can be seen in the fluorescent visualization. (Scale bars: 0.15 mm.)
To make the experimental system applicable for peptides that require processing enzymes and cofactors not expressed by the choroid plexus tissue, we designed and constructed a third lentiviral DNA construct that will express, if needed, the necessary protein followed by a blue fluorescent protein (BFP) in an inducible manner (Fig. 3_G_). Infection of HEK-293T cells with the three reporter viruses showed the feasibility of a triple-infection, as seen in the fluorescent visualization (Fig. 3_H_). Cells infected with the individual viruses demonstrated the distinct visualization of their reporter proteins only in the appropriate color channel (Fig. S2).
Proof of Principle Experiment 1: Overexpression of Corticotropin-Releasing Factor and the Subsequent Anxiogenic Behavior.
To demonstrate the functionality of the established system we studied the overexpression of the two established neuropeptides, corticotropin-releasing factor (CRF) and gonadotropin-releasing hormone (GnRH), known to modulate anxiety-like behavior and estrus cycle, respectively.
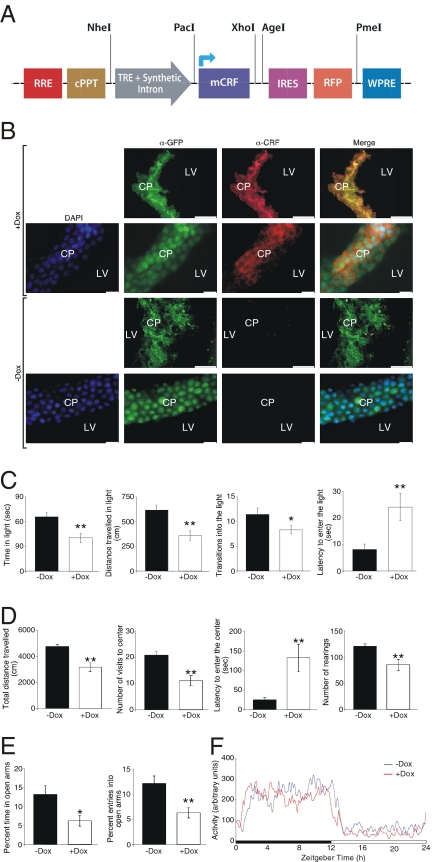
A Target lentiviral vector expressing the mouse CRF under the transcriptional control of TRE was constructed (Fig. 4_A_) and lentiviruses were generated. A mixture of the Effector and “CRF-Target” lentiviruses were injected ICV to C57BL/6 mice. Semiquantitative RT-PCR, as well as real-time PCR revealed a robust increase in CRF mRNA levels in choroid plexus tissue, as early as 2 hours after Dox administration (Fig. S3). Brains were collected under induced (+Dox) or noninduced (-Dox) conditions and processed for immunohistochemistry. Immunohistochemical analysis of brain slices showed a choroid plexus-specific staining for GFP under both induced and noninduced conditions (Fig. 4_B_) and a specific Dox-dependent staining for mouse CRF (red fluorescence, Fig. 4_B_). Fig. S4 further demonstrates that the red fluorescence represent the CRF immunoreactivity and not the visualization of the expressed RFP.
Fig. 4.
Inducible overexpression of mCRF in the CSF using the choroid plexus-specific lentiviral-based system and the subsequent anxiogenic behavior. (A) Schematic representation of the CRF-Target lentiviral construct, designed to conditionally express mCRF, followed by a RFP. (B) Immunohistochemical analysis of brain slices obtained from mice injected with a mixture of the Effector and CRF-Target lentiviruses and kept under induced (+Dox) or noninduced (-Dox) conditions show a choroid plexus-specific staining for GFP under both induced and noninduced conditions (green fluorescence) and a specific Dox-dependent staining for mouse CRF (red fluorescence). (Scale bars: long: 0.1 mm; short: 0.15 mm.) (C) Mice injected with a mixture of the Effector and CRF-Target lentiviruses and kept under induced (+Dox) conditions for 3 days, showed a significant increase in anxiety-like behavior measured by the light/dark transfer test, the open field test (D) and the elevated plus maze test (E). (F) No significant differences were found between the experimental groups in their home cage locomotor activity. Values are expressed as mean ± SEM. **, P < 0.005; *, P < 0.05. n = 10–12 male C57BL/6 mice were used in each of the behavioral tests.
To demonstrate the capability of the CRF produced by the choroid plexus cells to reach and activate its cognate receptors in the brain, we evaluated the central activation of c-Fos after Dox administration. Mice ICV-injected with a mixture of the Effector and the CRF-Target viruses were treated with or without Dox in their drinking water and c-Fos immunoreactivity was used to evaluate neuronal activation. The data summarized in Fig. S5 clearly demonstrate specific c-Fos immunoreactivity in CRFR1 expressing brain nuclei after Dox administration, including the piriform cortex, paraventricular nucleus of the thalamus, dentate gyrus, lateral septum, and dorsal hypothalamus, as demonstrated by Bittencourt and Sawchenko (15).
To evaluate the anxiety-like behavior of mice conditionally overexpressing CRF at the choroid plexus, we performed a set of related behavioral tests, with or without the presence of the inducer Dox. Three days after Dox induction, mice showed an increase in anxiety-like behavior measured by the light/dark transfer test, with significantly less time spent in the light compartment, reduced exploration of light area, fewer transitions to the light compartment and an increase in the latency to first enter the light compartment (Fig. 4_C_). Results from the open field test were consistent with the results obtained from the light/dark transfer test. Mice overexpressing CRF showed a reduced number of entries to the center, a greater latency entering the center, a decrease in rearing events, and lower exploratory behavior as measured by the shorter distance traveled during the test (Fig. 4_D_). In accordance with the open field and dark/light transfer tests, the results from the elevated plus maze test show significant differences between the control and the induced group of mice. CRF overexpressing mice show a decrease in the percent of time spent in the open arm and the percent of entries into open arms (Fig. 4_E_). No significant differences were found between the experimental groups in their home cage locomotor activity (Fig. 4_F_), suggesting that the observed phenotype is not a consequence of locomotion deficit but rather a genuine change in the mice anxiety-like behavior. These results clearly demonstrate that mice overexpressing CRF after being treated with Dox, display increased levels of anxiety-like behavior as expected for central overexpression of CRF.
Proof of Principle Experiment 2: Overexpression of Gonadotropin-Releasing Hormone and Estrus Cycle Modulation.
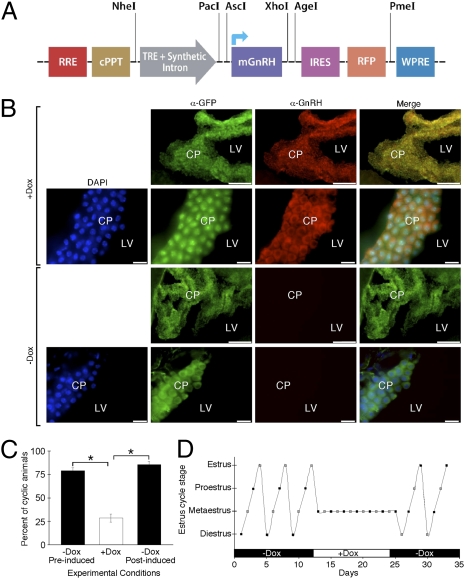
Intact estrus cycle is maintained via pulsatile release of hypothalamic GnRH, which is essential for proper functioning of the pituitary-gonadal axis. A constant delivery of high concentration of GnRH will lead to gonadal hypoactivity and cessation or abnormalities of the estrous cycle (16). A lentiviral Target vector expressing the mouse GnRH under the transcriptional control of TRE was constructed (Fig. 5_A_) and lentiviruses were generated. A mixture of the Effector and “GnRH-Target” lentiviruses were injected ICV to ICR female mice. Brains were collected under induced (+Dox) or noninduced (-Dox) conditions and processed for immunohistochemistry. Immunohistochemical analysis of brain slices showed a choroid plexus-specific staining for GFP under both induced and noninduced conditions (Fig. 5_B_) and a specific Dox-dependent staining for mouse GnRH (red fluorescence, Fig. 5_B_). Fig. S6 further demonstrates that the red fluorescence represent the GnRH immunoreactivity and not the visualization of the expressed RFP. It is important to note that the GnRH-specific antibody used in this study recognizes the mature processed form of GnRH only (17), which further supports a proper precursors processing by the choroid plexus cells.
Fig. 5.
Inducible overexpression of mGnRH in the CSF using a choroid plexus-specific lentiviral based system and the subsequent estrus cycle modulation. (A) Schematic representation of the GnRH-Target lentiviral construct, designed to conditionally express mGnRH, followed by a RFP. (B) Immunohistochemical analysis of brain slices obtained from mice injected with a mixture of the Effector and GnRH-Target lentiviruses and kept under induced (+Dox) or noninduced (-Dox) conditions show a choroid plexus-specific staining for GFP under both induced and noninduced conditions (green fluorescence) and a specific Dox-dependent staining for mouse GnRH (red fluorescence). (Scale bars: long: 0.1 mm; short: 0.15 mm) (C) The estrus cycle length of female mice injected with a mixture of the Effector and GnRH-Target lentiviruses was determined prior, during, and after Dox administration. Estrous cycle determination showed that while most of the injected animals show a normal 4-day estrous cycle under noninduced conditions, Dox administration significantly disrupted the cycle integrity. After removal of Dox from drinking water most of the mice reestablished an intact 4-day cycle (D) A representative estrous cycle profile of a female mouse throughout the experiment. Values are expressed as mean ± SEM. *, P < 0.0001. n = 30 female ICR mice were used for the estrous cycle studies.
To determine the estrus cycle length of ICR female mice conditionally overexpressing GnRH at the choroid plexus, we performed vaginal smears prior, during and after removal of Dox administration. Estrous cycle determination showed that although most of the injected animals showed a normal 4-day estrous cycle under noninduced conditions (Fig. 5_C_), Dox administration significantly disrupted the cycle. Interestingly, after removal of Dox from drinking water, most of the mice re-established an intact 4-day cycle (Fig. 5_C_). Fig. 5_D_ shows the estrous cycle of a representative female mouse throughout the experiment.
Discussion
The ICV route of administration is used frequently to assess central effects of neuropeptides and secreted proteins. Currently available methods for the delivery of peptides or pharmacological agents into the CSF present researchers with limitations, concerning both the natural properties of the delivered substance (solubility, stability, synthesis, purifications, etc) and the experimental needs (prolonged and/or repeated administration, inducible delivery, minimal handling of the experimental animals, etc).
In the current study, we demonstrate a genetic approach for ICV delivery. Using the endogenous properties of the choroid plexus tissue to generate and secrete CSF, we constructed an inducible lentiviral-based system, which was specifically designed to be active and overexpress the gene-of-interest product only at the choroid plexus cells. The modular design of the Target construct enables simple modification of the system to suit the researcher’s experimental needs. This flexible system can easily be adapted for various methods such as chronic exposure, inducible expression, and minimal handling of animals, as well as alleviating the need for complex synthesis and purification protocols and the use of expensive micropumps.
The molecules secreted by the choroid plexus gain proximal and distal access to the brain parenchyma via volume transmission, convective distribution, and receptor-mediated retrograde transport to neurons via nerve endings located near the ependyma or the pia-astroglial membrane (4, 5). The use of regulatory DNA sequences of genes specifically expressed (in the brain) by the choroid plexus cells provides the ability to genetically control the choroid plexus and not neuronal or glial cells transcription. In the current study, we used the 5′-flanking region of the CRFR2β gene, which was demonstrated to be highly and specifically expressed by the choroid plexus cells (11, 12). However, the use of different choroid plexus-specific transcripts, such as the transthyretin (18), GPR125 (19), or ones revealed from BBB-specific transcriptome or proteomic screens (20–22), may provide an alternative for controlling the levels of the expressed transgene.
Replication-defective lentiviral vector particles, used in this study, are capable of infecting a wide variety of dividing and nondividing cells, and with stable integration into the host genome, resulting in long-term expression of the transgene (23–25). The choroid plexus epithelial cells are derived from the ependymal lining of the ventricles and earlier studies that evaluated the proliferative activity of the forebrain ependyma and choroid plexus epithelium (using [3H]-thymidine) in young adult rats, demonstrated the persistence of low level of proliferation, with a turnover time of >130 days (26). Coupling the lentiviruses features, with the tight and reversible control of expression offered by the Tet-On system (8, 9), provides a powerful genetic tool for the generation of site-specific and inducible expression of the inserted transgene. The ability to control expression of the transgene via the drinking water (or alternatively, via food) alleviates the need for frequent handling of experimental animals, allowing for a more neutral experimental environment and reducing artifacts and “noise” in the experimental setup.
Research focused on the delivery of therapeutics across the BBB conducted over the last several years demonstrated the potential use of specific endogenous transporters localized within the brain capillary endothelium (2, 3). Several endogenous polypeptides such as insulin, insulin-like growth factors, leptin, and transferrin, undergo receptor-mediated transport (RMT) across the BBB. Peptidomimetic monoclonal antibodies can also cross the BBB via RMT by using the endogenous transporters and may carry pharmaceutical molecules such as recombinant proteins, antibodies, RNA interference drugs, or nonviral gene medicines (2, 3, 27). A reported adaptation of this method using lentiviruses can be used for chronic administration (28).
Most biologically active peptides are derived from large inactive polypeptide precursors, which require selective proteolysis (14, 29, 30). Therefore, the choroid plexus cells must express the appropriate processing enzymes. Comparing the expression profile of the main processing enzymes between the mouse choroid plexus tissue and the mouse hypothalamus, which served as positive control, we demonstrated the presence of mRNA for all tested enzymes. The presence of mRNA and protein for Furin, CPE, and PAM1 in the rat choroid plexus cells were demonstrated (31–34). The above expression profile, together with our functional neuroanatomical data (c-Fos activation) and the behavioral and physiological changes, strongly support the capacity of the choroid plexus cells to produce biologically active peptides. In addition, it is also important to note that the choroid plexus cells are known to endogenously express several secreted neuropeptides and proteins such as the insulin-like growth factor II (35), and secretogranin I and II (31). The described system offers the option of using additional induced lentiviral constructs that can express other exogenous enzymes or cofactors, in addition to the reporter BFP, when needed in the experimental design.
To demonstrate the functionality of the generated system we used two well-studied neuropeptides with an established central effect as a proof-of-principle. Target lentiviruses containing the mouse CRF or GnRH cDNA sequences were generated and injected together with the Effector rtTA virus. The results of the immunohistochemical analysis of these animals clearly demonstrate a Dox-dependent induction of CRF or GnRH expression specifically in the choroid plexus cells. Further behavioral and physiological characterization of these mice demonstrated the biological activity of the delivered gene.
CRF administration via the ICV route is able to access CRF receptor-expressing cells as clearly demonstrated by Fos induction (15). CRF plays an important and well-established role in the regulation of the HPA axis under basal and stress conditions (36, 37). In addition to its hypophysiotropic action, CRF is proposed to integrate the autonomic, metabolic, and behavioral responses to stressors (38–40). CRF and its receptors are implicated in the control of arousal, anxiety, cognitive functions, and appetite (41–45). Chronic hyperactivation of the CRF system has been linked to stress-related emotional disorders such as anxiety, anorexia nervosa, and depression as well as learning and memory deficits (41–45). Central administration of CRF (46) was demonstrated to increase anxiogenic-like behavior, through an adrenal nondependent mechanism, whereas the use of CRF receptor antagonists reduced anxiogenic-like behavior (47). In accordance with the published literature, mice treated with Dox, inducing overexpression of CRF at the choroid plexus cells, showed a clear increase in anxiety-like behavior in three different behavioral paradigms.
GnRH, also known as luteinizing hormone-releasing hormone (LHRH), is a decapeptide that plays a pivotal role as the physiologic regulator of reproduction (48, 49). This neuropeptide is synthesized by hypothalamic neurosecretory cells and is released in a pulsatile manner. It is then carried via a specialized portal blood system to the anterior pituitary gland and stimulates the secretion of the gonadotropic hormones, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) (48–50). GnRH interacts with high-affinity receptors on the gonadotropes in the anterior pituitary. The pulse-timing and concentration levels of GnRH control the production of FSH (low frequency) and LH (high frequency) and are critical for the maintenance of gonadal steroidogenesis, normal reproductive function, and estrous cycle. Chronic, high concentrations of GnRH induce regulatory changes that lead to gonadal hypoactivity and cessation or abnormalities of the estrous cycle (16). As expected, using our system for inducible overexpression of GnRH by the choroid plexus cells significantly disrupted the estrus cycle integrity.
The choroid plexus expresses, in addition to secretory biologically active compounds, a large number of receptors and transport systems, providing it with the ability to monitor the brain extracellular milieu (5, 7) via currently unknown mechanisms. In addition, changes in the anatomy and physiology of the choroid plexus have been linked to aging and neurodegenerative diseases, such as Alzheimer’s, motor neuron disease, and multiple sclerosis (1, 5). However, it is not clear whether these changes are causal contributors to the disease or a consequence, which strengthen the initial pathological process. The genetic, choroid plexus-specific system, presented in the current study, can also serve as a useful experimental tool for studying the biology of the choroid plexus tissue.
Methods
Design and Construction of Choroid Plexus-Specific and Inducible Lentiviral Vectors.
All constructs were assembled by using standard cloning methods and confirmed by DNA sequencing. For detailed description of the cloning process, see SI Methods.
Production of Lentiviral Vectors and ICV Injections.
Recombinant lentiviruses were produced by transient transfection in HEK293T cells, as described (51). See SI Methods for the ICV injection details.
RNA Preparation and Semiquantitative and Real-Time PCR Analysis.
The presence of the relevant enzymes in the choroid plexus that are required for posttranslational processing of protein precursors and a quantitative analysis of CRF mRNA by the choroide plexus cells were performed by using semiquantitative and real-time PCR analysis, as detailed in the SI Methods.
Animals and Experimental Groups.
Detailed description of the animals and experimental groups used in this study can be found in SI Methods.
Behavioral Studies.
Behavioral studies were performed as described (52, 53)· For detailed description of the behavioral tests see SI Methods.
Immunohistochemistry and in Situ Hybridization.
Specific in situ hybridization and immunohistochemistry were performed as described (12, 52). For detailed description of the protocols, see SI Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank Dr. Inder Verma, The Salk Institute for Biological Studies, La Jolla, CA, for providing us with lentiviral vectors, Dr. Wylie Vale, The Salk Institute for Biological Studies, for the CRF-specific antiserum, and Dr. Yitzhak Koch, Department of Neurobiology, Weizmann Institute of Science, for the GnRH-specific antiserum. This work is supported by grants from Roberto and Renata Ruhman, Israel Science Foundation, and Israel Ministry of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM. Barriers in the brain: A renaissance? Trends Neurosci. 2008;31:279–286. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. Drug and gene delivery to the brain: The vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 4.Johanson CE, Duncan JA, Stopa EG, Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res. 2005;22:1011–1037. doi: 10.1007/s11095-005-6039-0. [DOI] [PubMed] [Google Scholar]
- 5.Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays. 2005;27:262–274. doi: 10.1002/bies.20193. [DOI] [PubMed] [Google Scholar]
- 6.Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chodobski A, Szmydynger-Chodobska J. Choroid plexus: Target for polypeptides and site of their synthesis. Microsc Res Tech. 2001;52:65–82. doi: 10.1002/1097-0029(20010101)52:1<65::AID-JEMT9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossen M, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 10.Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 11.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 12.Chen A, et al. Mouse corticotropin-releasing factor receptor type 2alpha gene: Isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- 13.Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: Identification of multiple splice variants. Mol Endocrinol. 2003;17:395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 14.Docherty K, Steiner DF. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- 15.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: An analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickery BH. Comparisons of the potential utility of LHRH agonists and antagonists for fertility control. J Steroid Biochem. 1985;23(5B):779–791. doi: 10.1016/s0022-4731(85)80014-5. [DOI] [PubMed] [Google Scholar]
- 17.Koch Y, et al. Production and characterization of an antiserum to synthetic gonadotropin-releasing hormone. Biochem Biophys Res Commun. 1973;55:616–622. doi: 10.1016/0006-291x(73)91188-1. [DOI] [PubMed] [Google Scholar]
- 18.Costa RH, Lai E, Darnell JE., Jr. Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986;6:4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering C, et al. The Adhesion GPCR GPR125 is specifically expressed in the choroid plexus and is upregulated following brain injury. BMC Neurosci. 2008;9:97–110. doi: 10.1186/1471-2202-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardridge WM. Blood–brain barrier genomics. Stroke. 2007;38(2)(Suppl):686–690. doi: 10.1161/01.STR.0000247887.61831.74. [DOI] [PubMed] [Google Scholar]
- 21.Thouvenot E, et al. The proteomic analysis of mouse choroid plexus secretome reveals a high protein secretion capacity of choroidal epithelial cells. Proteomics. 2006;6:5941–5952. doi: 10.1002/pmic.200600096. [DOI] [PubMed] [Google Scholar]
- 22.Enerson BE, Drewes LR. The rat blood–brain barrier transcriptome. J Cereb Blood Flow Metab. 2006;26:959–973. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- 23.Naldini L, Blömer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsson J, Lundberg C. Lentiviral vectors for use in the central nervous system. Mol Ther. 2006;13:484–493. doi: 10.1016/j.ymthe.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan AN, Lewis PD. A quantitative study of cell proliferation in ependyma and choroid plexus in the postnatal rat brain. Neuropathol Appl Neurobiol. 1979;5:303–309. doi: 10.1111/j.1365-2990.1979.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev. 2007;59:141–152. doi: 10.1016/j.addr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain- barrier. Proc Natl Acad Sci USA. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidah NG, Chrétien M. Proprotein and prohormone convertases: A family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 30.Fugère M, Day R. Cutting back on pro-protein convertases: The latest approaches to pharmacological inhibition. Trends Pharmacol Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gee P, Rhodes CH, Fricker LD, Angeletti RH. Expression of neuropeptide processing enzymes and neurosecretory proteins in ependyma and choroid plexus epithelium. Brain Res. 1993;617:238–248. doi: 10.1016/0006-8993(93)91091-6. [DOI] [PubMed] [Google Scholar]
- 32.MacCumber MW, Snyder SH, Ross CA. Carboxypeptidase E (enkephalin convertase): mRNA distribution in rat brain by in situ hybridization. J Neurosci. 1990;10:2850–2860. doi: 10.1523/JNEUROSCI.10-08-02850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer MK, Stoffers DA, Eipper BA, Watson SJ. Expression of peptidylglycine alpha-amidating monooxygenase (EC 1.14.17.3) in the rat central nervous system. J Neurosci. 1992;12:222–234. doi: 10.1523/JNEUROSCI.12-01-00222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schäfer MK, et al. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis. J Neurosci. 1993;13:1258–1279. doi: 10.1523/JNEUROSCI.13-03-01258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynes MA, Brooks PJ, Van Wyk JJ, Lund PK. Insulin-like growth factor II messenger ribonucleic acids are synthesized in the choroid plexus of the rat brain. Mol Endocrinol. 1988;2:47–54. doi: 10.1210/mend-2-1-47. [DOI] [PubMed] [Google Scholar]
- 36.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 37.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 38.Sutton RE, Koob GF, Le Moal M, Rivier J, Vale WW. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 39.Brown MR, et al. Corticotropin-releasing factor: Actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- 40.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 41.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 42.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 43.Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 45.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 46.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 47.Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents. Comparison with diazepam and buspirone. Psychopharmacology (Berl) 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- 48.Amoss M, et al. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44:205–210. doi: 10.1016/s0006-291x(71)80179-1. [DOI] [PubMed] [Google Scholar]
- 49.Matsuo H, Baba Y, Nair RMG, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 50.Okon E, Koch Y. Localisation of gonadotropin-releasing and thyrotropin-releasing hormones in human brain by radioimmunoassay. Nature. 1976;263:345–347. doi: 10.1038/263345a0. [DOI] [PubMed] [Google Scholar]
- 51.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 52.Chen A, et al. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neufeld-Cohen A, et al. Mol Psychiatry. 2009. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information