Regulation of Adiponectin Secretion by Adipocytes in the Polycystic Ovary Syndrome: Role of Tumor Necrosis Factor-α (original) (raw)
Abstract
Context: Adipose tissue dysfunction associated with low-grade chronic inflammation and dysregulation of adipokine secretion might significantly contribute to the pathogenesis of polycystic ovary syndrome (PCOS).
Objective: The objective of the study was to determine whether the effect of TNF-α, IL-6, monocyte chemoattractant protein-1, or coculture of adipocytes and adipose tissue macrophages (ATMs), on the secretion of adiponectin by adipocytes, differs in PCOS compared with controls.
Design and Participants: Primary cultures of sc adipocytes and coculture of adipocytes and ATMs from overweight and obese patients with PCOS and healthy control women were used.
Main Outcome Measures: Adiponectin secretion by adipocytes was measured.
Results: The baseline secretion of adiponectin by isolated adipocytes did not differ between PCOS and control samples. The net change in adiponectin secretion in response to IL-6, monocyte chemoattractant protein-1, and TNF-α differed between PCOS (decreasing) and control (increasing) adipocytes, although the difference reached significance only for TNF-α (P < 0.04). Coculture of isolated adipocytes and ATMs resulted in a decrease in adiponectin secretion by PCOS (P < 0.05) but not control adipocytes, and the difference between the net change in adiponectin secretion in PCOS vs. control samples was significant (P < 0.03).
Conclusions: Our results suggest that adiponectin secretion by adipocytes in response to cytokines/chemokines and most notably in response to coculturing with ATMs differs between PCOS and control women, favoring greater suppression of adiponectin in PCOS. The mechanisms underlying these defects and the role of concurrent obesity remain to be determined.
Adiponectin secretion by adipocytes in response to cytokines/chemokines and to co-culturing with adipose tissue macrophages differs between PCOS and control women, favoring greater suppression of adiponectin in PCOS.
Polycystic ovary syndrome (PCOS) is a common endocrine-metabolic-reproductive disorder, affecting about 6–10% of reproductive-aged women (1). Insulin resistance, and the development of compensatory hyperinsulinemia, is a frequent finding in PCOS (2,3,4,5). The resulting compensatory hyperinsulinemia stimulates androgen secretion from the ovarian theca cells, acting synergistically with LH (6,7) and inhibits the hepatic production of SHBG (8,9), accounting for much of the hyperandrogenic features and ovulatory dysfunction of these patients. Likewise, evidence of subclinical inflammation is present in PCOS, regardless of the degree of obesity (10,11,12). These features result in an increased risk for developing type 2 diabetes (13) and cardiovascular disease (14) in PCOS.
Insulin resistance in PCOS is in part due to the high prevalence of obesity in the disorder. Nonetheless, we should note that approximately 40% of PCOS women, even in the United States, are not obese (1), that obesity in PCOS appears to primarily reflect environmental factors and to have a modest impact on the prevalence of the disorder (15), and that PCOS women are insulin resistant above and beyond that determined by their body mass index (BMI) (5). However, whereas obesity per se may not be the primary driver of PCOS, it is possible that adipose tissue dysfunction may play a role in the insulin resistance and the subclinical inflammation and consequently the metabolic and cardiovascular consequences of the disorder.
Adipose tissue is an endocrinologically active organ producing a number of peptides, notably adipokines (which includes cytokines, chemokines, growth factors, and neurally active hormones among others), lipids, and steroids (16,17,18,19). Adipokines act in an autocrine and paracrine fashion on adipose tissue itself and in an endocrine manner to affect other tissues and organs, including skeletal muscle, adrenal cortex, and the central and sympathetic nervous systems (19), modulating a number of functions, including insulin action and subclinical inflammation (20,21,22). Overall, adipose tissue, through its endocrine role, is an important determinant of total body in vivo insulin sensitivity (18,19).
Some adipokines are synthesized only by adipocytes, including the antiinflammatory molecule adiponectin. Adiponectin demonstrates insulin-sensitizing and antiinflammatory properties, and dysregulation of adiponectin has been implicated in the pathogenesis of obesity-related insulin resistance and increased subclinical inflammation (23). Other adipokines, such as TNF-α, are mainly secreted by adipose tissue macrophages (ATMs), whereas IL-6 and monocyte chemoattractant protein (MCP)-1 are produced by both adipocytes and ATMs (19,22,24); TNF-α and MCP-1 have proinflammatory properties that lead insulin resistance in adipocytes (25,26), whereas IL-6 may have dual properties (27,28,29,30,31).
A recent metaanalysis of 16 studies in PCOS women reported that circulating adiponectin levels are lower in PCOS than controls after controlling for BMI, albeit with significant heterogeneity across individual studies (32). Furthermore, adiponectin mRNA levels appear to be decreased in both visceral and sc fat in PCOS women compared with weight-matched controls (33), although Fain et al. (34) noted that adiponectin is mainly produced in sc, as opposed to visceral, fat. Adiponectin secretion in PCOS is potentially regulated by several mechanisms, including the paracrine effects of adipokines and direct interactions between adipocytes and ATMs (35). In the present study, we aimed to determine in PCOS and matched control women: 1) the effects of individual adipokines (TNF-α, IL-6, and MCP-1) on adiponectin secretion by adipocytes; and 2) the direct effect of ATMs on the secretion of adiponectin by adipocytes.
Subjects and Methods
Study subjects
Ten patients with PCOS (BMI range 26–40 kg/m2) were recruited from the Center for Fertility Reproductive Medicine and the Center for Androgen-Related Disorders at Cedars-Sinai Medical Center. Twelve healthy women were recruited as matched controls (BMI range 26–40 kg/m2). The diagnosis of PCOS was made according to the National Institutes of Health 1990 criteria (1). These include: 1) clinical evidence of hyperandrogenism and/or hyperandrogenemia; 2) oligoovulation; and 3) the exclusion of related disorders, including nonclassic 21-hydroxylase-deficient adrenal hyperplasia, hyperprolactinemia, thyroid dysfunction, Cushing’s syndrome, or androgen-producing tumors (1). The criteria for defining hirsutism, hyperandrogenemia, ovulatory dysfunction, and exclusion of related disorders have been previously reported (36). Controls were women with regular menstrual cycles and without family history of endocrine abnormality or hirsutism. These women had no evidence of hirsutism, acne, or alopecia or endocrine dysfunction.
All subjects underwent a brief physical examination, including hirsutism scoring using a modification of the modified Ferriman-Gallwey method (mFG) (37). Subjects were deemed hirsute if their mFG score was 6 or greater (38); controls had an mFG score of 3 or less. Blood sampling was performed in the fasting state between d 3 and 8 (follicular phase) after a spontaneous menstrual cycle or a progesterone-induced withdrawal bleed. No subjects had used hormonal preparations, including oral contraceptives, for 3 or more months preceding the study, and none were pregnant. All had normal TSH and prolactin levels. All subjects gave informed written consent, according to the guidelines of the Cedars-Sinai Medical Center Institutional Review Board.
Hormonal analyses
Hormonal measures, including total and free testosterone (T), dehydroepiandrosterone and sulfate (DHEAS) were obtained. Total T was measured using high-turbulence liquid chromatography tandem mass spectrometry and free T determined by equilibrium dialysis (Quest Diagnostics, San Juan Capistrano, CA). Insulin was assayed by chemiluminescence (ADVIA Centaur chemiluminescent immunoassay system; Siemens Healthcare, Deerfield, IN). DHEAS analysis was performed by a competitive immunoassay (Modular E170; Roche Diagnostics, Indianapolis, IN). Glucose levels were measured using the hexokinase/glucose-6-phosphate dehydrogenase method (Roche Applied Sciences, Indianapolis, IN).
Isolation of human adipocytes
Adipose tissue samples (∼5 gm) were obtained from sc adipose tissue through a small incision in the lower abdomen. Specimens were transported immediately to the laboratory in a HEPES salts buffer containing 4% BSA and 2 mm pyruvate (pH 7.4) and were finely minced. A small amount of tissue was fixed in 4% PBS-buffered paraformaldehyde for immunofluorescence (see below), and the remainder was used for adipocyte and macrophage isolation. The minced adipose tissue was treated with collagenase at a ratio of 3.5 mg/g of tissue and incubated for 60 min at 37 C in a rotary shaking bath at 100 rpm. The cell suspension was then filtered through a premoistened 400-μm nylon mesh (Small Parts, Inc., Miami Lakes, FL) to isolate the adipocytes, and the cells were washed twice for 2 min at 50 × g at room temperature. After the second wash, the cells were refiltered through nylon mesh. At this point the cells were ready for experimentation.
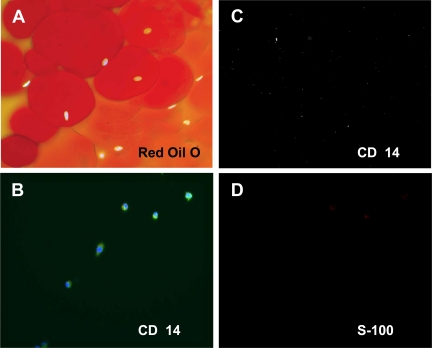
To confirm the purity of the adipocyte isolates, Oil Red O staining, nuclear 4′,6′-diamidino-2-phenylindole (DAPI) labeling, and immunostaining with an antibody to the macrophage marker CD14 were performed. Untreated adipocytes were fixed in 4% paraformaldehyde, rinsed twice for 10 min in PBS, and then stained with 0.35% Oil Red O (Sigma Aldrich, St. Louis, MO) in a 3:2 isopropanol-water solution for 10 min and rinsed in PBS. Adipocytes (positive Oil Red O and DAPI staining) were clearly distinguishable from free lipid (positive Oil Red O and negative DAPI staining). Little free lipid remained in the adipocyte isolates (Fig. 1A). Furthermore, immunostaining of the isolates with a CD14 antibody demonstrated that the adipocyte fraction was free from contamination with macrophages (Fig. 1C).
Figure 1.
Characterization of isolated human adipose tissue adipocytes and macrophages. Untreated adipocytes from cell culture were fixed in 4% paraformaldehyde, and Oil Red O staining and nuclear DAPI labeling were performed to distinguish adipocytes from free lipid (A). Immunofluorescent labeling with an antibody to CD14 was performed to test for the presence of macrophages (C). Oil Red O (red) and DAPI (light blue) colabeling demonstrate that the isolated cells are mature adipocytes (×100 magnification) (A), and lack of CD14 staining indicates that the isolated adipocytes fraction is free from adipocytes (C). ATMs isolated from adipose tissue were subjected to immunofluorescent labeling with antibodies to CD14 (green) (B) and S-100 (D) and mounted using DAPI after 3 d in culture. The cells are CD14(+), consistent with the identification of these cells as macrophages (B), and S-100(−), indicating that the ATM fraction is free from adipocytes (×200) (D).
Adipocyte culture and determination of secreted adiponectin levels
Isolated adipocytes (107 cell/ml) were maintained in 24-well clusters in the presence of DMEM containing 10% fetal calf serum and antibiotics and incubated at 37 C in humidified 5% CO2-95% air atmosphere for 16 h. For all assays, duplicate wells were plated for each sample and assay condition. Cells were treated with adipokines or left untreated as appropriate, and 48 h after treatment, the cell culture media were collected for the detection of adiponectin. The concentrations of adiponectin (picograms per milliliter) in the culture media were determined using a solid phase ELISA (Dousset; R&D Systems, Minneapolis, MN). Sample concentrations were determined using an ELISA reader (Sunrise, T-can, Vmax kinetic microplate reader; Molecular Devices, Sunnyvale, CA). Duplicates were prepared and read for every sample. Sample concentrations were interpolated from a standard curve calculated by linear regression of the color development of known concentrations of known recombinant human standards (ranging from 62.5 to 4000 pg/ml, prepared fresh for each assay). Initially, three different sample dilutions were made and each dilution was assayed in duplicate. The sample concentrations were then compared with the standard curve, and the results of the dilution with values closest to the middle (straightest) portion of the control curve were used. Log transformation of the adiponectin concentrations was used to facilitate the use of parametric data analysis methods.
To validate the ELISA itself, intra- and interassay comparisons were performed. Intraassay percent coefficients of variation, obtained by measuring three different samples 30 times each, ranged from 4.2 to 5.8%. Interassay percent coefficients of variation, obtained by measuring three different samples in 30 different experiments, ranged from 4.7 to 5.7%. Assay specificity was tested using three different human recombinant adipokines (C-reactive protein, IL-6, MCP-1) at concentrations ranging from 50 to 50,000 pg/ml, all of which failed to yield positive measurements for adiponectin. We determined the linearity of the assay by diluting human recombinant adiponectin (Dousset; R&D Systems) in either assay dilution buffer [PBS containing 1% BSA (Sigma) or adipocyte culture medium (see above)]. The linearity of standard curves obtained using dilution buffer and conditioned medium were very similar (y = 0.0028 × +0.0166, r2 = 0.999; and y = 0.0028 × +0.0156, r2 = 0.999, respectively). In addition, we determined the percentage of recovery by measuring known concentrations of recombinant adiponectin (ranging from 62.5 to 4000 pg/ml) by ELISA. The adiponectin concentrations detected by ELISA were approximately the same as those of the standard curve at each dilution, with a range of recovery between 91 and 106 (n = 6) using either dilution buffer or conditioned medium (data not shown).
Incubation of adipocytes in the presence or absence of adipokines
To identify the optimal concentrations of adipokines to use, TNF-α or MCP-1 was added to adipocyte cell culture medium at concentrations ranging from 0.1, 1.0, 10, and 100 ng/ml, and after 48 h the medium was harvested for the detection of adiponectin by ELISA as described above. Pair-wise comparisons of the results were performed under an ANOVA model.
Isolation of human ATMs
ATMs were partially purified from the resuspension of the stromal pellet fraction obtained during adipocyte isolation as described above, using a Ficoll gradient. This ATM fraction was then diluted in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, 1% non-essential amino acids, 1% sodium pyruvate, and 10 ng/ml granulocyte macrophage-stimulating factor, and kept in culture for up to 96 h. Cells in the macrophage fraction expressed CD14, also labeled with DAPI (Fig. 1B), and also expressed CD68, another macrophage marker (data not shown). To confirm that the ATM fraction was free from contamination with adipocytes, immunostaining with S-100, a marker for preadipocytes and newly formed adipocytes (39,40), was performed. The results indicated that the isolated macrophage fraction was free from contaminating adipocytes (Fig. 1D).
Coculturing of adipocytes and ATMs
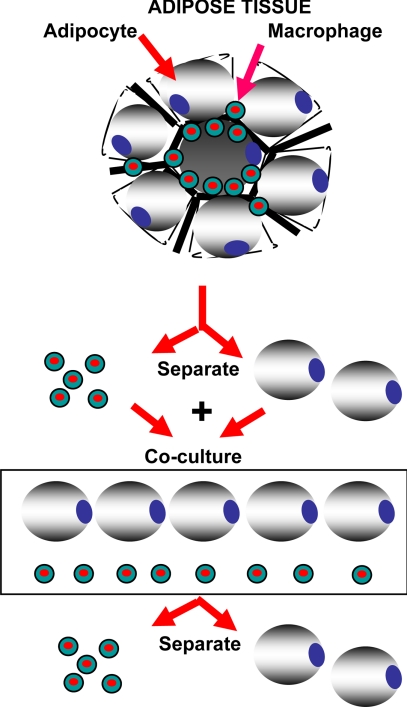
To determine the role that ATMs play in regulating adiponectin secretion from adipocytes, we devised a coculture system (Fig. 2). Isolated adipocytes and ATMs (see above) were allowed to equilibrate overnight in their respective cell culture media. The following day, adipocytes were added to the wells containing ATMs in a 1:1 ratio. Again, duplicate wells were plated for each sample and assay condition. The cells were then cocultured for 24 h in a coculture plate (Corning Costar Co., Lowell, MA). This is a direct coculture system that allows contact between the cell types. After 24 h of coculture, the adipocytes were removed and placed in new wells containing adipocyte medium, and the adipocytes were cultured in isolation again for an additional 48 h. At the end of this 48-h time period, the media from the adipocytes was collected for measurement of adiponectin levels. We investigated the effects of different ATM to adipocyte ratios in our coculture studies (e.g. 1:1, 1:3, 1:6) and found that a 1:1 ratio of adipocytes and ATMs maximized the production of adiponectin (data not shown).
Figure 2.
Graphical depiction of the adipocyte-ATM coculture system and coculture of adipocytes and ATMs. Adipocytes and ATMs were isolated from adipose tissue as described in Materials and Methods. After a 16-h equilibration period, the adipocytes and ATMs are cocultured with direct contact between the cell types for 24 h. The adipocytes are then removed and cultured separately for an additional 48 h, after which the culture media were harvested for analysis by ELISA.
Statistical analysis
Statistical analysis was performed using the Statistical Analysis System program (SAS Institute, Inc., Cary, NC). All values were log transformed to achieve a more normal distribution. For the dose-response curves performed for TNF-α and MCP-1, pair-wise comparisons were made using an ANOVA model. For measurements of the effects of individual adipokines or coculture with ATMS on adiponectin secretion, comparisons were carried out parametrically using t tests. We chose to use log scale analysis followed by t tests, rather than nonparametric analysis because the sds increase systematically as the means increase on the original scale, which is evidence that a log transformation is needed. Residual quantile-quantile plots confirmed that there were no major violations of the parametric normality assumptions. Due to the small amount of adipose tissue isolated from each subject, not all of the subjects contributed to each of the experiments performed. The number of samples per group used for each experiment is indicated in the figures and figure legends. Insulin resistance and β-cell function were estimated using the homeostasis model assessment (41).
Results
Hormonal and biochemical features of the PCOS patients
Of the 12 control women studied, 70% were white, whereas 80% of the 10 PCOS subjects were white. There were no significant differences in BMI between PCOS and controls (Table 1), although controls were slightly older than PCOS women. As expected, waist to hip ratio, mFG score, and free T were greater in PCOS women; although fasting insulin, homeostasis model assessment insulin resistance index, and homeostasis model assessment of percent β-cell function tended to be higher in PCOS women than controls (Table 1), these differences did not reach significance, likely a reflection of sample size.
Table 1.
Baseline data comparing controls vs. PCOS
| Controls (n = 12) | PCOS (n = 10) | P value | |
|---|---|---|---|
| Age (yr) | 35.8 ± 7.1 | 27.7 ± 5.6 | 0.013 |
| BMI (kg/m2) | 30.5 ± 5.1 | 33.5 ± 3.2 | NS |
| mFG hirsutism score | 1.5 ± 1.8 | 7.9 ± 3.8 | 0.001 |
| Waist to hip ratio | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.018 |
| Total T (ng/ml) | 19.6 ± 5.7 | 49.1 ± 40.1 | NS |
| Free T (pg/ml) | 2.5 ± 1.5 | 5.9 ± 2.9 | 0.013 |
| DHEAS (μg/dl) | 198.7 ± 91.1 | 253.0 ± 97.0 | NS |
| Fasting insulin (μIU/ml) | 12.3 ± 10.1 | 36.6 ± 40.6 | NS |
| Fasting glucose (mg/dl) | 85.0 ± 8.2 | 89.1 ± 11.3 | NS |
| HOMA-IR | 2.2 ± 1.5 | 7.6 ± 9.5 | NS |
| HOMA-%β-cell | 165 ± 55 | 600 ± 629 | NS |
Adipose tissue contains resident or ATMs
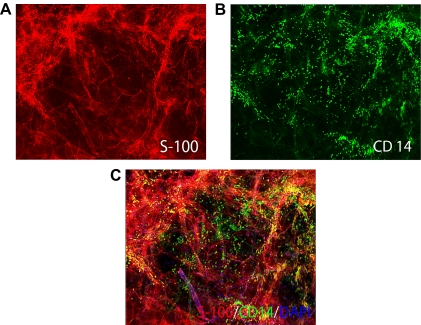
To identify cell types present in human adipose tissue, we performed immunofluorescent staining with specific antibodies. We demonstrated the presence of preadipocytes and adipocytes, identified by positive staining for S-100, a marker found in preadipocytes and recently matured adipocytes (Fig. 3A), and also identified cells positive for CD14, a macrophage/monocyte-specific marker, clearly indicating the presence of macrophages in the adipose tissue (Fig. 3B). Areas of CD14/S100 overlap (Fig. 3B, yellow in the merged image) indicate potential regions of interaction between adipocytes and ATMs (Fig. 3C).
Figure 3.
ATMs in adipose tissue from an obese control subject. Immunofluorescent staining of adipose tissue from an obese control subject demonstrating the presence of abundant ATMs. Fixed whole adipose tissues were immunolabeled with antibodies to S-100, a selective marker of preadipocytes and newly formed adipocytes (red) (A) and CD14, a macrophage/monocyte-specific marker (green) (B), and counterstained with the nuclear label DAPI. Combined S-100/CD14/DAPI staining (×200) demonstrates significant infiltration by macrophages into the adipose tissue (C). Multiple direct contacts between the resident macrophages (green) and preadipocytes or adipocytes (red) are apparent (C). Similar results were observed in adipose tissues from a PCOS patient (data not shown).
Adiponectin secretion by adipocytes in response to IL-6, MCP-1, and TNF-α
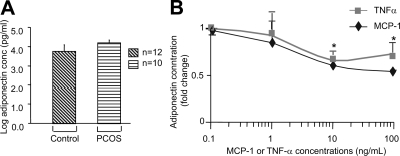
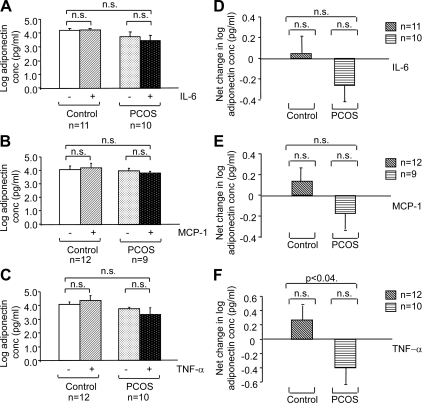
To assess the effect of cytokines/chemokines on adiponectin secretion by adipocytes, we first determined the basal levels of adiponectin secretion by adipocytes from PCOS subjects and BMI-matched controls. No significant differences were observed in the basal levels of adiponectin secretion between the two groups after 48 h in culture (Fig. 4A). We then performed dose-response experiments with TNF-α and MCP-1. Adiponectin secretion in response to TNF-α and MCP-1 was measured in duplicate in two different adipocyte samples after preincubation with 0.1, 1.0, 10, and 100 ng/ml of TNF-α or MCP-1. TNF-α and MCP-1 at concentrations of 10 ng/ml were found to produce maximal inhibition of adiponectin secretion (P < 0.05, Fig. 4B). These results are consistent with the findings of other researchers, who also found adipokine concentrations of 10 ng/ml to be optimal for use (42,43). We then tested the effects of treatments with individual adipokines on adiponectin secretion, performing group-to-group (aggregate) analyses. The baseline secretion of adiponectin by isolated adipocytes did not differ between PCOS and control samples (data not shown). The net change in adiponectin secretion (treated minus baseline) in response to incubation with 10 ng/ml of IL-6, MCP-1, and TNF-α was found to differ between PCOS (decreasing) and control (increasing) adipocytes, although the difference in the response of PCOS vs. control adipocytes reached significance only for TNF-α (P < 0.04) (Fig. 5, A–F).
Figure 4.
Changes in adiponectin secretion by adipocytes in response to TNF-α and MCP-1. The graphs depict the log scale means of the basal levels of adiponectin secreted by PCOS and control adipocytes in picograms per milliliter (A). The graphs depict the fold change in log concentration of adiponectin (picograms per milliliter) in response to incubation with 1, 1.0, 10, and 100 ng/ml of the adipokines TNF-α (diamonds) and MCP-1 (squares). Each value was expressed as the mean of two separate experiments perform in different batches of human adipocytes. All samples were assayed in duplicate. All values were log transformed before analysis, and pairwise comparisons were performed using an ANOVA model. A concentration of 10 ng/ml of either MCP-1 or TNF-α was found to result in maximum suppression of adiponectin secretion by adipocytes. *, P < 0.05 compared with baseline (B).
Figure 5.
Change in adiponectin secretion by adipocytes in response to treatment with adipokines/chemokines. The graphs depict the log scale means of the absolute adiponectin levels in picograms per milliliter (A–C) and net changes in log adiponectin concentration (D–F) secreted by PCOS and control adipocytes in response to incubation with 10 ng/ml IL-6 (A and D), MCP-1 (B and E), and TNF-α (C and F). All values were log transformed before analysis. Sample numbers (n) per group are indicated for each experiment.
Adiponectin secretion by adipocytes in response to coculturing with ATMs
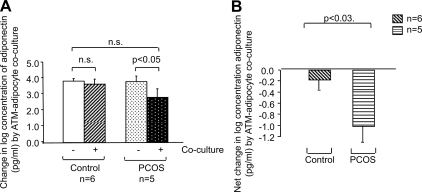
At the end of coculture period (Fig. 2), the culture media were collected for analysis by ELISA. We found that direct coculture of isolated adipocytes and ATMs resulted in a decrease in adiponectin secretion by PCOS adipocytes (P < 0.05) but not control adipocytes (Fig. 6A) and that the difference between the net change in adiponectin secretion in PCOS vs. control samples was significant (P < 0.03) (Fig. 6B).
Figure 6.
Change in adiponectin secretion by adipocytes in response to adipocyte-ATM coculture. The graphs depict the log scale means of the absolute adiponectin levels in picograms per milliliter (A) and net change in log adiponectin concentration (B) in response to ATM-adipocyte coculture for control (n = 6) and PCOS (n = 5) adipocytes. All values were log transformed before analysis.
Discussion
Available data on adiponectin secretion by adipocytes suggest that adiponectin production in adipose tissue is regulated by various paracrine and endocrine factors and that inflammation-related adipokines TNF-α (19) and IL-6 (44,45) are involved in the decreased expression of adiponectin in adipocytes and in the development of insulin resistance. Understanding the cellular and molecular mechanisms involved in regulating adiponectin secretion in PCOS adipocytes is critical to understanding the etiology of the insulin resistance in PCOS. Our results suggest that adiponectin secretion by adipocytes in response to cytokines/chemokines, and most notably in response to TNF-α and direct coculture with ATMs, differs between PCOS and control women, favoring greater suppression of adiponectin in PCOS.
Circulating adiponectin levels and adiponectin mRNA levels in both visceral and sc fat are decreased in PCOS women compared with healthy women (8,10), whereas adiponectin receptors 1 and 2 expression are increased in both of these adipose compartments. These data, together with our findings that adiponectin secretion by adipocytes is suppressed to a greater extent in PCOS than in controls in response to cytokines/chemokines, or direct coculturing with ATMs, suggest that the regulation of adiponectin secretion by adipose tissues differs between PCOS and control women. Because adiponectin is an important insulin-sensitizing hormone (40), the greater suppression of adiponectin by ATM-secreted factors (e.g. TNF-α) in PCOS suggests that this mechanism may account, at least in part, for the decreased insulin sensitivity of PCOS women.
These results are consistent with recent data indicating that, of the cytokine/chemokines secreted by macrophages, TNF-α is a crucial determinant of adipokine dysregulation in the metabolic syndrome, and that immunoneutralization of TNF-α abrogates adipokine dysregulation in adipocytes of obese subjects (46). TNF-α exerts its effects through two distinct receptors, TNF receptor-1 and -2. Through complex signaling cascades and networks, these effectors lead to the activation of caspases and two transcription factors, activation protein-1 (C-Jun-C-Fos complex) and nuclear factor κB, which may inhibit the secretion of adiponectin gene expression and secretion (26,47,48). Thus, dysregulation of these effectors may be a potential molecular mechanism by which TNF-α acts to overly decrease adiponectin secretion from PCOS adipocytes.
In conclusion, our results suggest that adiponectin secretion by adipocytes in response to cytokines/chemokines, and most notably in response to TNF-α, differs between PCOS and control women, favoring greater suppression of adiponectin in PCOS. In addition, adipocyte-macrophage cross talk has an even greater inhibitory effect on adiponectin secretion by adipocytes from women with PCOS, suggesting that other ATM-secreted factors may also play important, and possibly synergistic, effects in regulating adiponectin secretion, both in general and in PCOS in particular. Further research into the molecular mechanisms underlying the dysregulation of adiponectin secretion in PCOS will provide vital clues to the etiology of insulin resistance in PCOS and possibly other metabolic disorders and to the nature of paracrine signaling between adipocytes and macrophages in adipose tissue. These studies may eventually lead to the identification of therapeutic targets that may decrease obesity-induced inflammation and diminish the metabolic dysfunction of obesity, PCOS, and related disorders.
Footnotes
This work was supported in part by the Helping Hand of Los Angeles and Grants RO1-DK073632 (to R.A.) and M01-RR00425 (to the Cedars-Sinai Medical Center General Clinical Research Center) from the National Institutes of Health.
Disclosure Summary: G.C., B.S.T., B.O.Y., C.B., R.M., and S.H. have nothing to declare. R.A. has received consulting fees from Bionovo and Merck & Co.
First Published Online January 20, 2010
Abbreviations: ATM, Adipose tissue macrophage; BMI, body mass index; DAPI, 4′,6′-diamino-2-phenylindole; DHEAS, dehydroepiandrosterone and sulfate; MCP, monocyte chemoattractant protein; mFG, modified Ferriman-Gallwey; PCOS, polycystic ovary syndrome; T, testosterone.
References
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA 1992 Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol 167:1807–1812 [DOI] [PubMed] [Google Scholar]
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Legro RS, Finegood D, Dunaif A 1998 A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 83:2694–2698 [DOI] [PubMed] [Google Scholar]
- DeUgarte CM, Bartolucci AA, Azziz R 2005 Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 83:1454–1460 [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ 1986 Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab 62:904–910 [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F 1998 Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83:2001–2005 [DOI] [PubMed] [Google Scholar]
- Plymate SR, Matej LA, Jones RE, Friedl KE 1988 Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67:460–464 [DOI] [PubMed] [Google Scholar]
- Nestler JE, Barlascini CO, Matt DW, Steingold KA, Plymate SR, Clore JN, Blackard WG 1989 Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 68:1027–1032 [DOI] [PubMed] [Google Scholar]
- Tarkun I, Cetinarslan B, Türemen E, Cantürk Z, Biyikili M 2006 Association between circulating tumor necrosis factor-α, interleukin-6, and insulin resistance in normal-weight women with polycystic ovary syndrome. Metab Syndr Relat Disord 4:122–128 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Trakada G, Bixler EO, Lin HM, Pejovic S, Zoumakis E, Chrousos GP, Legro RS 2006 Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism 55:1076–1082 [DOI] [PubMed] [Google Scholar]
- Hu WH, Qiao J, Zhao SY, Zhang XW, Li MZ 2006 [Monocyte chemoattractant protein-1 and its correlation with lipoprotein in polycystic ovary syndrome]. Beijing Da Xue Xue Bao 38:487–491 [PubMed] [Google Scholar]
- Ovalle F, Azziz R 2002 Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril 77:1095–1105 [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ, Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ 2008 Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health-National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yildiz BO, Knochenhauer ES, Azziz R, Yildiz BO, Knochenhauer ES, Azziz R 2008 Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 93:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Flier JS 2000 Adipose tissue as an endocrine organ. Trends Endocrinol Metab 11:327–332 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS 2004 Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355 [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS 2004 Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2546–2556 [DOI] [PubMed] [Google Scholar]
- Ronti T, Lupattelli G, Mannarino E 2006 The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 64:355–365 [DOI] [PubMed] [Google Scholar]
- Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H 2002 Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028 [DOI] [PubMed] [Google Scholar]
- Chen H 2006 Cellular inflammatory responses: novel insights for obesity and insulin resistance. Pharmacol Res 53:469–477 [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H 2003 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A, D'Agostino Jr R, Howard G, Mykkänen L, Tracy RP, Haffner SM 2000 Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102:42–47 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM 1993 Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- Wang B, Jenkins JR, Trayhurn P 2005 Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-α. Am J Physiol Endocrinol Metab 288:E731–E740 [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK 1998 IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A 2003 IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 4:551–556 [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA 2006 Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55:2688–2697 [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK 2005 The anti-inflammatory effect of exercise. J Appl Physiol 98:1154–1162 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Pedersen BK 2008 Skeletal muscle as an immunogenic organ. Curr Opin Pharmacol 8:346–351 [DOI] [PubMed] [Google Scholar]
- Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, Papadimas I, Panidis D 2009 Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update 15:297–307 [DOI] [PubMed] [Google Scholar]
- Carmina E, Chu MC, Moran C, Tortoriello D, Vardhana P, Tena G, Preciado R, Lobo R 2008 Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertil Steril 89:642–648 [DOI] [PubMed] [Google Scholar]
- Fain JN, Buehrer B, Tichansky DS, Madan AK 2008 Regulation of adiponectin release and demonstration of adiponectin mRNA as well as release by the non-fat cells of human omental adipose tissue. Int J Obes 32:429–435 [DOI] [PubMed] [Google Scholar]
- Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR 2008 Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab 295:E842–E850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R 2005 Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril 83:1343–1346 [DOI] [PubMed] [Google Scholar]
- Hatch R, Rosenfield RL, Kim MH, Tredway D 1981 Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140:815–830 [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R 1998 Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ 2004 Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res 19:256–264 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, Mineyama T, Ishikawa M, Moroi M, Sugi K, Yamauchi T, Ueki K, Tobe K, Noda T, Nagai R, Kadowaki T 2006 Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem 281:8748–8755 [DOI] [PubMed] [Google Scholar]
- Matthews DE., Farewell VT, Pyke R 1985 Asymptotic score-statistic processes and tests for constant hazard against a change-point alternative. Ann Stat 13:583–591 [Google Scholar]
- Bruun JM, Pedersen SB, Kristensen K, Richelsen B 2002 Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol Cell Endocrinol 190:91–99 [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, Vidal-Puig A, Jones R, Considine RV 2005 Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor α. Obes Res 13:662–669 [DOI] [PubMed] [Google Scholar]
- Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y 2001 PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094–2099 [DOI] [PubMed] [Google Scholar]
- Skurk T, Alberti-Huber C, Herder C, Hauner H 2007 Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92:1023–1033 [DOI] [PubMed] [Google Scholar]
- Maury E, Noël L, Detry R, Brichard SM 2009 In vitro hyperresponsiveness to tumor necrosis factor-α contributes to adipokine dysregulation in omental adipocytes of obese subjects. J Clin Endocrinol Metab 94:1393–1400 [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M 2001 Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11:372–377 [DOI] [PubMed] [Google Scholar]
- Kim HB, Kong M, Kim TM, Suh YH, Kim WH, Lim JH, Song JH, Jung MH 2006 NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3–L1 adipocytes. Diabetes 55:1342–1352 [DOI] [PubMed] [Google Scholar]